Introduction
Previous studies have demonstrated that elevated
levels of homocysteine (Hcy) have been implicated as an independent
risk factor for atherosclerosis (AS) (1–3).
Studies have focused on the involvement of Hcy on the dysfunction
and injury of vascular cells, such as vascular smooth muscle cells
(VSMCs) (4,5). It has been previously observed that
Hcy induced VSMCs proliferation, however the underlying mechanisms
remain to be fully elucidated (6).
Hcy is a non-protein, sulfur containing amino acid,
which is a metabolic intermediate of the methionine cycle. It is a
precursor of S-adenosylmethionine (SAM), the unique methyl group
donor for DNA methylation (7).
Subsequent to transfer of the methyl group, SAM is transformed into
S-adenosylhomocysteine (SAH), which can be hydrolyzed to form Hcy
(8). DNA methylation refers to the
addition of a methyl group to the 5 position of cytosine in the
context of a CpG dinucleotide. Increasing evidence indicates that
human diseases, including AS, are either caused or impacted by
abnormal methylation (9). A
previous study suggested that Hcy may also be involved in
disturbing the expression of AS-associated genes through the
interference of DNA epigenetic phenotype modification, such as DNA
methylation (10). An additional
previous study demonstrated that abnormal DNA methylation of genes
including peroxisome proliferator-activated receptor α,
apolipoprotein E (ApoE) and genomic DNA contribute to the
development of AS induced by Hcy (11,12).
Studies have identified DNA methylation profiling of the vascular
tissues in the setting of AS or atherosclerotic plaques (13,14).
However, the methylation status of several key genes that
characterized the VSMC proliferation induced by Hcy in single VSMCs
remains to be elucidated. In addition, it remains unclear whether
Hcy affected the methylation status and caused proliferation.
In the present study, platelet-derived growth factor
(PDGF), p53, phosphatase and tensin homologue on chromosome 10
(PTEN) and mitofusin 2 (MFN2), which are associated with cell
proliferation regulation, and two global methylation indicators,
aluminium (Alu) and long interspersed nucleotide element-1 (Line-1)
were selected to analyze the methylation status in the VSMCs treat
with Hcy, which may clarify the mechanisms of VSMCs proliferation
in AS induced by Hcy and provide a potential diagnostic marker for
AS induced by Hcy.
Materials and methods
Cell culture
Primary culture of VSMCs was obtained from the media
of the umbilical vein of human as previously described (15). Experiments were approved by the
Ningxia Medical University Medical Ethical Committee (Yinchuan,
China). Cells were cultured in Dulbecco's modified Eagle's
medium-Han's F12 media (DMEM-F12; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 20% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 U/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in an incubator with 5% CO2. When cells grew to
80% confluence, serum was media-deprived for 24 h to become
synchronous, then 5% FBS (Gibco; Thermo Fisher Scientific, Inc.)
for another 24 h before Hcy addition. Hcy was applied at the
concentrations of 30, 50, 100, 200 and 500 µM folate
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 5 µM
5-aza-2′-deoxycytidine (AZC; Sigma-Aldrich; Merck KGaA),
respectively.
Nested methylation-specific-polymerase
chain reaction (nMS-PCR) for methylation analysis
The detection of methylation levels was conducted as
previously described (16). The
summary of primers and product sizes of the nMS-PCR assays are
presented in Table I. The PCR
products were separated by electrophoresis through a 2% agarose gel
containing ethidium bromide. DNA bands were visualized by Gel
Documentation and Analysis System ChemiDoc XRS (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and calculated by the
formula: Methylation % = methylation/(methylation + unmethylated) ×
100%.
 | Table I.Summary of nested
methylation-specific-polymerase chain reaction primers. |
Table I.
Summary of nested
methylation-specific-polymerase chain reaction primers.
| Gene | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) | Size (bp) | Annealing
temperature (°C) |
|---|
| PDGF-O |
AAGGTTGTTTTTATTTATTTTTTGT |
AACTACAAACTACAACTACTCCAAT | 305 | 52 |
| PDGF-M |
GTTTGTTTGTTTTTTTGCGTATTC |
CTACTCCGATTTTCTCTTTACAACG | 193 | 59 |
| PDGF-U |
GGTTTGTTTGTTTTTTTGTGTATTT |
ACTCCAATTTTCTCTTTACAACAAA | 192 | 57 |
| p53-O |
GTTTTGGTTTGAAGGATAGTAGTT |
AAAAACCCTAAAACTTAATAAAAAC | 404 | 55 |
| p53-M |
TTAGTTTTAGTTAGGATGGTTTCGA |
GAAAAATAAACCGAAATCCCG | 212 | 56 |
| p53-U |
ATTAGTTTTAGTTAGGATGGTTTTGA |
CAAAAAATAAACCAAAATCCCAC | 214 | 55 |
| PTEN-O |
TTGGAAAGTTTTTTAATTAGGGATA |
ATTTCAAAAACCCAAAAAACAC | 445 | 55 |
| PTEN-M |
GTGATTTTTTTCGGAAAGTAGTTTC |
TAAAAACCCGACAAAATAAATCG | 211 | 57 |
| PTEN-U |
ATTTTTTTTGGAAAGTAGTTTTGA |
AAAAACCCAACAAAATAAATCACC | 207 | 56 |
| MFN2-O |
ATAGAATGTAAATTTGGATTTTAGA |
ACTAATAAACCCTAAACCCAACC | 355 | 53 |
| MFN2-M |
TTTGTTTCGTTTTTTTAGTTTCG |
CTAAACCCAACCGACTCG | 210 | 55 |
| MFN2-U |
TTTGTTTTGTTTTTTTAGTTTTGG |
TAAACCCAACCAACTCACC | 209 | 55 |
| Alu-O |
TATTTTGGTTAATAAGGTGAAATTT |
TCCAACAACTATAAAAAAACTTTTT | 256 | 55 |
| Alu-M |
GGGCGTTTGTAGTTTTAGTTATTC |
TAAAACGAAATCTCGCTCTATCG | 143 | 56 |
| Alu-U |
GGTGTTTGTAGTTTTAGTTATTTGG |
TTAAAACAAAATCTCACTCTATCACC | 143 | 56 |
| Line-1-O |
TTTATTAGGGAGTGTTAGATAGTGGG |
TACCCAAACAAACCTAAACAATAAC | 359 | 57 |
| Line-1-M |
TATTAGGGAGTGTTAGATAGTGGGC |
AACCCGATTTTCCAAATACGT | 162 | 59 |
| Line-1-U |
TTAGGGAGTGTTAGATAGTGGGTGT |
AAAAAACCCAATTTTCCAAATACA | 164 | 59 |
Reverse transcription-quantitative PCR
(RT-qPCR) for measurement of mRNA expression
The total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). And the RNA (2 ml)
was reverse transcribed using the Revert Aid First Strand cDNA
synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) for 60 min at 42°C and 70°C for 5 min. The
primer nucleotide sequences of PDGF are as presented in Table II. The RT-qPCR was conducted with
the FTC-3000 Real-Time PCR detection system (Funglyn Biotech, Inc.,
Toronto, ON, Canada). The qPCR reaction system as follows: 25 µl 2X
SYBR mixture, 1 µl forward primer, 1 µl reverse primer, 2 µl cDNA
and then RNase-free water up to 50 µl. The thermocycling conditions
were as follows: Initial activation at 95°C for 5 min, followed by
30 cycles of 95°C for 20 sec, annealing temperatures for 20 sec (as
stated in Table II) and at 72°C
for 30 sec. The mRNA level of each gene was acquired from the value
of the quantification cycle (Cq) of the qPCR as associated to that
of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the
following equation (17):
ΔCq=Cq(GAPDH)-Cq(sample). Final results,
expressed as N-fold differences in the target gene expression and
relative to the calibrator termed ‘Ntarget’, were
determined according to the following equation:
Ntarget=2Cq(sample)-Cq(calibrator), where Cq
values of the calibrator and sample were determined by subtracting
the Cq value of the target gene from the Cq value. Each qPCR assay
was performed in duplicate and included positive, negative and no
template reagent controls.
 | Table II.Summary of reverse
transcription-quantitative polymerase chain reaction primers. |
Table II.
Summary of reverse
transcription-quantitative polymerase chain reaction primers.
| Gene | Forward primer
sequence (5′→3′) | Reverse primer
sequence (5′→3′) | Size (bp) | Annealing
temperature (°C) |
|---|
| PDGF |
CCACTCGATCCGCTCCTTTGA |
GAACCCAGGCTCCTTCTTCCAC | 150 | 60 |
| p53 |
CGTGTTTGTGCCTGTCCTG |
TGCTCGCTTAGTGCTCCCT | 105 | 58 |
| PTEN |
AAGACCATAACCCACCACAGC |
ACCAGTTCGTCCCTTTCCAG | 125 | 57 |
| MFN2 |
TACACTGGCTCCAACTGC |
AACCAACCGGCTTTATTC | 188 | 55 |
Western blotting assay for measurement
of proteins expression
Whole-cell proteins were extracted with cell lysis
buffer (KeyGene, Shanghai, China) that included the protease
inhibitor phenylmethanesulfonyl fluoride (KeyGene) at 4°C for 30
min, then were separated by 12% sulfate-polyacrylamide gel
electrophoresis. The proteins and the pre-stained marker
(Fermentas; Thermo Fisher Scientific, Inc.) were then transferred
onto an Immobilon-P transfer polyvinylidene fluoride membrane (EMD
Millipore, Billerica, MA, USA) with a Semi-dry Transfer Cell (model
755; Bio-Rad Laboratories, Inc.) for 90 min, allowing the
pre-stained marker to be completely transferred from the gel to the
membrane. The gel was then discarded and the membrane was incubated
at room temperature in 5% non-fat milk prepared with
phosphate-buffered saline with 0.05% v/v Tween-20 (PBS-T) buffer. A
total of 2 h later, the membrane was cut as required, placed in a
suitable hybridization bag with 1 ml of primary antibody [all
primary antibodies were diluted to 1:1,000 with 1% non-fat milk;
anti-PDGF (cat. no. ab23914), anti-p53 (cat. no. ab26), anti-PTEN
(cat. no. ab32199), anti-MFN2 (cat. no. ab56889) and anti-β-actin
(cat. no. ab8227) antibodies were obtained from Abcam, Cambridge,
UK] and were incubated at 4°C. The membrane was washed three times
with PBS-T and the mouse anti-goat horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2354;
1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was added
for 2 h at room temperature. Blots were developed on X-ray film
(Kodak, Shanghai, China). The protein bands were visualized and
analyzed using the Gel Documentation and Analysis System ChemiDoc
XRS system with Image Lab software (version 4.1; Bio-Rad
Laboratories, Inc.), and calculated by the gray value of the
bands.
SAM and SAH concentrations examined by
HPLC
The concentrations of SAM and SAH were determined by
high performance liquid chromatography (HPLC). Samples were loaded
into a C18 column (Shimadzu, Tokyo, Japan), run by a Hitachi L2000
HPLC system (HPLC; D-2000 Elite HPLC; Hitachi High Technologies,
Tokyo, Japan). Absorption of eluted compounds was monitored at (λ)
ex=254 nm. Elution of SAM and SAH was achieved at a flow rate of
1.0 ml/min with the mobile phase ammonium format solution.
Chromatograms were recorded using a D-2000 Elite integrator. SAM
and SAH standards were used to identify the elution peaks, and the
SAM and SAH values of the tissues were calculated with the standard
curve.
Statistical analysis
Each experiment was repeated 3 times. Results were
expressed as the mean ± standard deviation. One-way analysis of
variance was used to compare the means of multiple groups, followed
by Newman-Keuls test. P≤0.05 was considered to indicate a
statistically significant difference.
Results
The bioinformatics analysis of
promoters in PDGF, p53, PTEN and MFN2
As a key epigenetic modification of the genome, DNA
methylation occurs almost exclusively in the context of CpG
dinucleotides, particularly in the CpG islands (18). Ranging from 0.5–5 kb and occurring
on average every 100 kb, CpG islands are GC rich (50–70%) and have
a ratio of CpG:GpC of at least 0.6 (19). Collectively, CpG islands account
for 1–2% of the genome and their location is primarily in the 5′
regulatory regions of all housekeeping genes as well as up to 40%
of tissue-specific genes (20).
CpG methylation is associated with gene silencing, and to identify
whether CpG islands exist in the chosen genes PDGF, p53, MFN2 and
PTEN, the 5′-flanking region of the genes was analyzed using an
online search engine (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi).
The criteria of the CpG island is a CpG-rich region length more
than 100 bp, GC percentage >50%, and observe/expect ratio
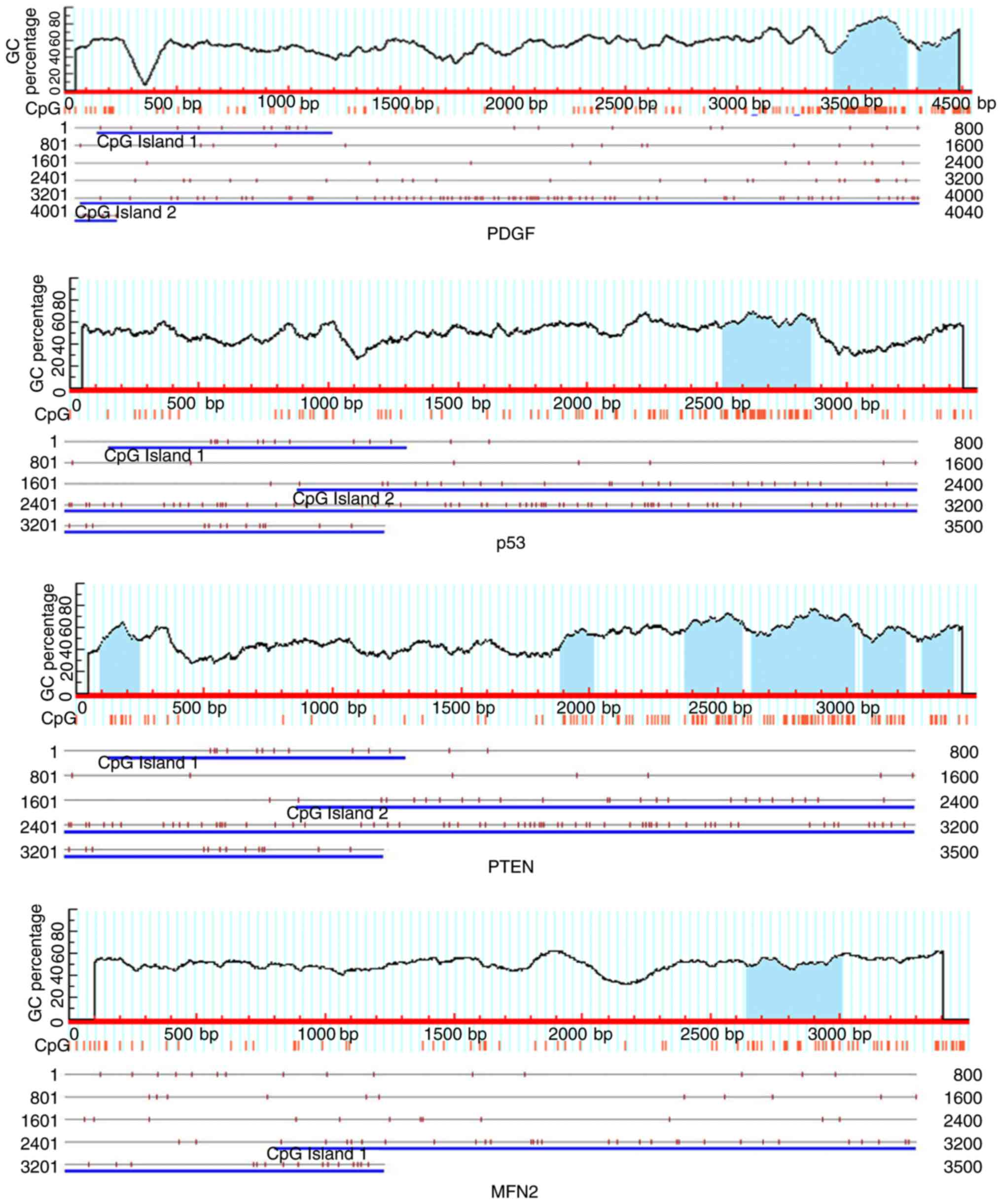
>0.60. As presented in Fig. 1,
at least one CpG island was identified in the DNA promoter regions
of all the 4 genes. In the PTEN gene promoter, 6 CpG islands were
observed, which indicated that they had greater potential to be
modified by DNA methylation and regulated their expression.
PDGF, P53, PTEN, MFN2, Alu and line-1
methylation levels in VSMCs
To ascertain whether DNA methylation occurred in
these genes, the methylation status of genes was analyzed by
nMS-PCR. For PDGF, as presented in Fig. 2A, the methylation status of PDGF is
increased, particularly, in the 100 µM Hcy group, in which it was
increased 7.6-fold, compared with the untreated group (P<0.01).
For p53, as presented in Fig. 2B,
p53 methylation levels were increased in a dose-dependent manner
with the Hcy concentration. For PTEN and MFN2, the methylation
levels were elevated by treatment with Hcy (Fig. 2C and D), however, it was not
dose-dependent. Notably, the most marked effect was observed in the
100 µM group. When treated with folate, the methylation levels of
PDGF, PTEN and MFN2 were decreased compared with that of the 100 µM
group (P<0.05).
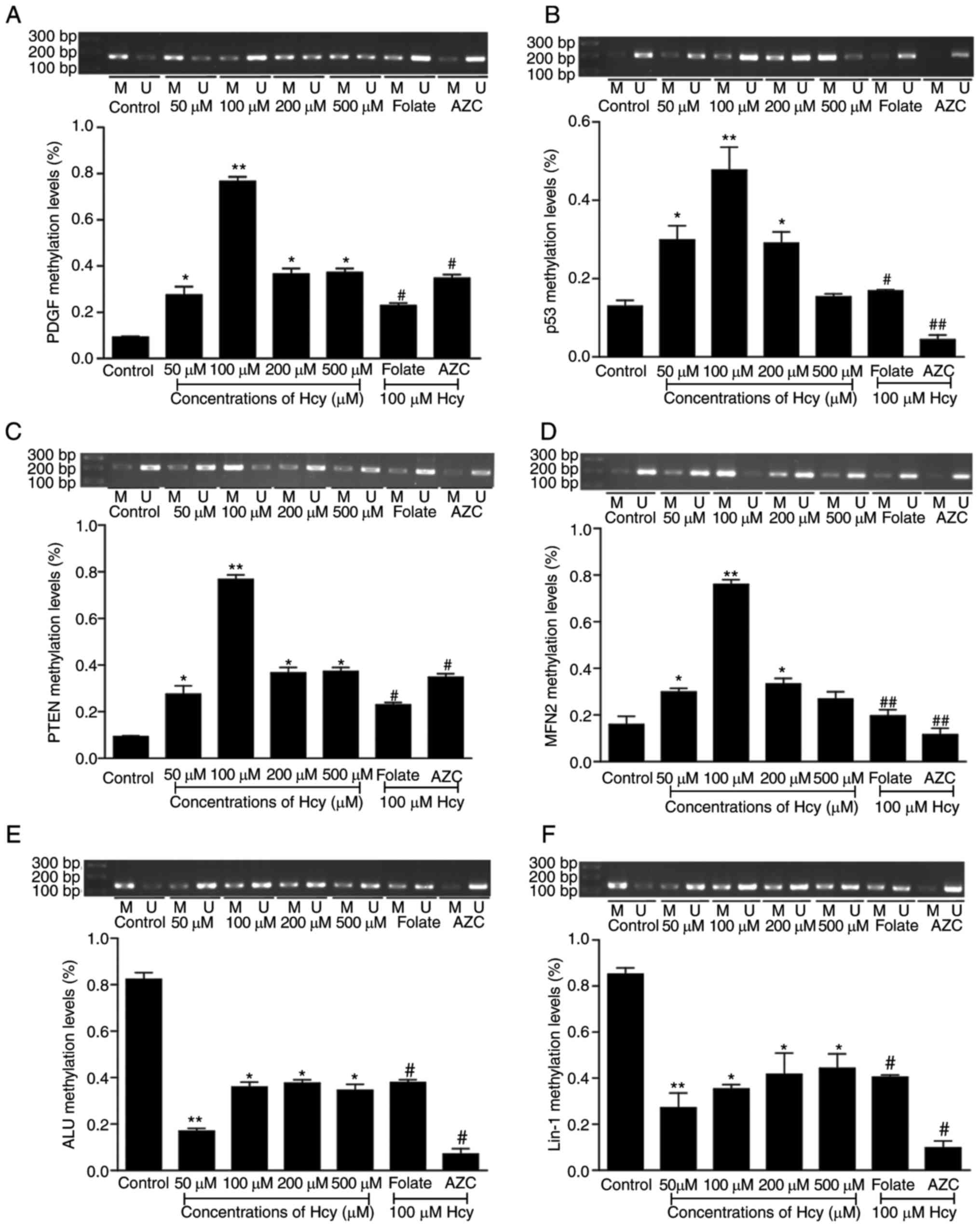 | Figure 2.The methylation levels of PDGF, P53,
PTEN, MFN2, Alu and Line-1. The methylation status was detected in
VSMCs treated with different concentrations of Hcy by nMS-PCR. (A)
The methylation of PDGF was detected by nMS-PCR in VSMCs, following
co-incubation with different concentrations of Hcy for 72 h; (B)
The methylation of P53 was detected by nMS-PCR in VSMCs, following
co-incubation with different concentrations of Hcy for 72 h; (C)
The methylation of PTEN was detected by nMS-PCR in VSMCs, following
co-incubated with different concentrations of Hcy for 72 h; (D) The
methylation of MFN2 was detected by nMS-PCR in VSMCs, following
co-incubation with different concentrations of Hcy for 72 h; (E)
The methylation of Alu was detected by nMS-PCR in VSMCs, following
co-incubation with different concentrations of Hcy for 72 h; (F)
The methylation of Line-1 was detected by nMS-PCR in VSMCs,
following co-incubation with different concentrations of Hcy for 72
h. M, methylation; U, unmethylated; Folate group, 30 µM folate in
100 µM Hcy; AZC group, 5 µM AZC in 100 µM Hcy. Data are presented
as the mean ± standard deviation from 3 independent experiments
performed in triplicate. *P<0.05, **P<0.01, vs. the control
group; #P<0.05, ##P<0.01, vs. the 100
µM Hcy group. PDGF, platelet-derived growth factor; PTEN,
phosphatase and tensin homologue on chromosome 10; MFN2, mitofusin
2; Alu, alumninium; Line-1, long interspersed nucleotide element-1;
nMS-PCR, nested methylation-specific-polymerase chain reaction;
VSMCs, vascular smooth muscle cells; Hcy, homocysteine; AZC,
5-aza-2′-deoxycytidine. |
It was known that Hcy impacted the gene-specific
methylation, however it remained unclear whether it affected the
global methylation status, Alu and LINE-1 methylation status is
considered as a good indicator of global methylation (21), thus these methylation levels were
detected in VSMCs treated by Hcy. The results indicated that both
Alu and LINE-1 were in a hypo-methylated state (Fig. 2E and F; P<0.01). No significant
difference was observed in 100 µM group, however the results in the
50 µM group were significant. In addition, the folate group
exhibited an antagonistic effect (P<0.05).
AZC, an inhibitor of DNA methylation, was added, and
co-incubated VSMCs were treated with different concentrations of
Hcy, then the methylation levels of Alu and Line-1 were measured
with nMS-PCR. When compared with untreated cells, the methylation
levels of Alu and Line-1 were suppressed in the Hcy treated groups.
These data indicated that Hcy impacted the methylation status of
these genes and that this may contribute to the proliferation of
VSMCs induced by Hcy.
PDGF, P53, PTEN and MFN2 expression
levels in Hcy-treated VSMCs
It was identified that Hcy affected the methylation
levels of genes, however whether this further impacted the
expression remained unclear. In order to investigate whether this
occurred in VSMCs treated by Hcy, the mRNA and protein levels of
the genes were assayed by RT-qPCR and western blotting. As
presented in Fig. 3A, PDGF mRNA
and protein expression levels were increased in a dose-dependent
manner. p53, PTEN and MFN2 were decreased in the 50, 100, 200 and
500 µM Hcy groups (Fig. 3B-D). The
expression levels of p53, PTEN and MFN2 consistently exhibited the
greatest effects in the 100 µM group. In addition, more folate
suppressed the changes induced by Hcy. When treated with AZC, the
antagonist of DNA methylation, the expression of PDGF, p53, PTEN
and MFN2 increased significantly (P<0.05). These data suggested
that Hcy affected the expression of the genes, which may be
involved in the DNA methylation process.
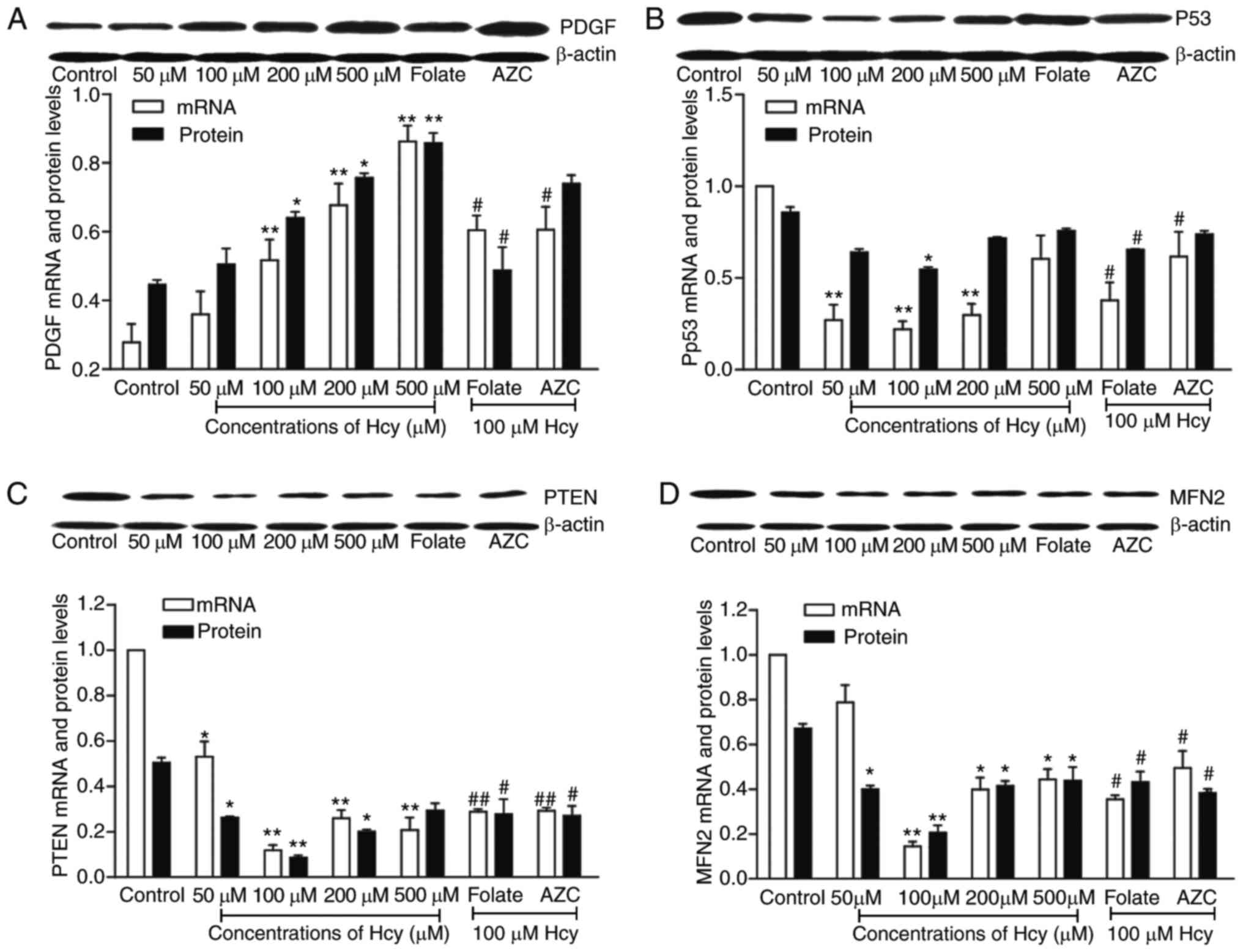 | Figure 3.The expression of PDGF, P53, PTEN and
MFN2 in Hcy-treated VSMCs. VSMCs were exposed to 0, 50, 100, 200
and 500 µM of Hcy for 72 h, mRNA and protein were extracted from
VSMCs and detected by reverse transcription-quantitative polymerase
chain reaction and western blotting, respectively. Expression
levels of (A) PDGF, (B) p53, (C) PTEN and (D) MFN2 in VSMCs
co-incubated with Hcy for 72 h are presented. Folate group, 30 µM
folate in 100 µM Hcy; AZC group, 5 µM AZC in 100 µM Hcy Data are
presented as the mean ± standard deviation from 3 independent
experiments performed in triplicates. *P<0.05 and **P<0.01,
vs. the control group; #P<0.05 and
##P<0.01, vs. the 100 µM Hcy group. PDGF,
platelet-derived growth factor; PTEN, phosphatase and tensin
homologue on chromosome 10; MFN2, mitofusin 2; Hcy, homocysteine;
VSMCs, vascular smooth muscle cells. |
SAM and SAH levels in VSMCs treated
with Hcy
Similar to Hcy, SAM and SAH are intermediates in the
methionine metabolism, and are important factors in the
transmethylation process (22).
The SAM and SAH levels were measured using HPLC in the VSMCs, which
were treated with Hcy. As presented in Fig. 4, the results demonstrated that the
intracellular levels of SAH were significantly increased, compared
with the control group, while the concentrations of SAM and the
ratio of SAM/SAH were decreased, when Hcy concentrations increased
(P<0.05; Fig. 4). SAH levels
increased and SAM levels decreased (P<0.05), when VSMCs were
treated with Hcy.
 | Figure 4.The SAM and SAH levels in VSMCs
treated by Hcy. (A) A chromatogram of SAH and SAM of all groups
detected by high-performance liquid chromatography, chromatograms
were recorded by an integrator. a, Untreated group; b, 50 µM Hcy
group; c, 100 µM Hcy group; d, 200 µM Hcy group; e, 500 µM Hcy
group; and f, folate group. (B) The concentrations of SAM and SAH
in VSMCs. (C) The ratio of SAH and SAH in VSMCs. Folate group, 30
µM folate in 100 µM Hcy. The data were obtained using automatic
peak area integration. Data are presented mean ± standard deviation
from 3 independent experiments performed in triplicate. *P<0.05
and **P<0.01, vs. the control group; #P<0.05 vs.
the 100 µM Hcy group. SAM, S-adenosylmethionine; SAH,
S-adenosylhomocysteine; VSMCs, vascular smooth muscle cells; Hcy,
homocysteine. |
Discussion
VSMCs are major components of the arterial wall, and
abnormal proliferation of VSMCs serves a pivotal role in the
pathogenesis of AS (23). As an
important independent risk factor of AS, Hcy stimulated the VSMCs
proliferation, which was identified in previous studies (6,24).
Cellular proliferation is associated with the regulation of key
genes. Thus in the present study, 4 genes were selected for
analysis; PDGF, p53, PTEN and MFN2. PDGF is a potent mitogen, which
serves crucial roles in developmental and physiological processes,
in addition to being directly implicated in proliferative
disorders, and it is a potent stimulator of VSMC growth (25). p53 is a key tumor suppressor and a
key regulator of various signaling pathways, including cell-cycle
regulation, induction of apoptosis, development and differentiation
(26). In vivo, p53
expression is negatively correlated with markers of cell
proliferation in human atherosclerosis; adenoviral expression of
p53 reduces cellular proliferation in the rat carotid artery
(27), and by contrast, siRNA of
p53 increases cellular proliferation (28). In
p53−/−/ApoE−/− mice, p53 have been
demonstrated to increase aortic plaque formation, with increased
rates of cell proliferation and reduced rates of apoptosis in
brachiocephalic artery plaques (29). PTEN is well known tumor suppressor
and is additionally involved in regulation of a variety of
physiological and pathological processes, including cell
proliferation, differentiation, apoptosis, adhesion and migration
(30). PTEN was expressed
endogenously in VSMCs, and overexpression of PTEN significantly
inhibited basal and PDGF-mediated VSMC proliferation and migration
(31). PTEN overexpression in
VSMCs using adenoviral transfection resulted in inhibition of cell
proliferation and migration induced by angiotensin II (32). MFN2 was first cloned from VSMCs in
spontaneous hypertensive rats using the differential display
technique (33), which restrained
the proliferation of VSMCs mediated by inhibition of extracellular
signal-related kinase/mitogen-activated protein kinase signaling
and subsequent cell-cycle arrest. The expression changes of these
key genes may involve in the imbalance of cell proliferation. The
present study indicated an elevation of PDGF, and a reduction of
p53, PTEN and MFN2 in VSMCs treated with Hcy, which indicated that
Hcy impacted the expression of these genes and resulted in the
proliferation of VSMCs.
DNA methylation is an important epigenetic
modification at the transcriptional level, which commonly occurs at
the CpG dinucleotides of gene promoter regions, particularly in the
promoter-associated CpG islands (34). Consistent with these important
roles, a growing number of human diseases have been identified to
be associated with aberrant DNA methylation. In the present study,
CpG islands were searched for in the promoters of the selected
genes, PDGF, p53, PTEN and MFN2, using two CpG island search
engines. It was identified that at least one CpG island exists in
the promoter regions of all 4 genes. In the promoter region of
PTEN, 6 CpG islands were identified, which indicated that they have
potential to be modified by DNA methylation and regulate their
expression.
It was previously reported that Hcy caused
cell-type-specific hypomethylation in endothelial cells, VSMCs and
foam cells (35) and it was
hypothesized that Hcy metabolism and methylation may be associated,
resulting in tissue-specific pathology in AS. In addition, DNA
methylation also impacts the selected genes PDGF, p53, PTEN and
MFN2. In the present study, the key genes associated with cell
proliferation regulation were selected in order to investigate the
methylation changes in the single VSMCs. In the VSMCs treated with
Hcy, hypermethylation or hypomethylation was observed to occur in
the promoters of the selected genes, of which PDGF was
hypomethylated and p53, PTEN, MFN2 were hypermethylated.
Furthermore, the expression levels were altered from normal to
aberrant levels. When treated with AZC, an antagonist of DNA
methylation, both the methylation status and the expression of the
four genes was altered. These results demonstrate that Hcy affected
the expression of the genes, which may be involved in the DNA
methylation process.
In order to try to confirm the methylation status
characteristics in the VSMCs affected by Hcy, we detected global
methylation levels were measured. In general, Alu and LINE-1
elements are methylated in somatic tissues, however are
hypomethylated in human cancer (36). In the present study, Hcy was
observed to reduce the methylation levels of Alu and LINE-1. In the
VSMCs, Hcy not only affected the methylation levels of particular
genes, however additionally impacted the global methylation status.
Hcy-associated methylation changes are also suggested as a
biomarker for use in the prevention and therapy of AS in the
future.
In the methionine cycle, SAM is the key methyl donor
for cellular methylation events including DNA methylation, and this
process produces SAH. SAH is converted to Hcy in a reversible
reaction, and elevated levels of SAH and concomitant increases in
the SAH/SAM ratio are suggested to inhibit cellular methylation
reactions (37). The present study
observed an increase of SAM and a decrease in the SAM/SAH ratio,
which demonstrated that Hcy amassment accumulated in the methionine
cycle, and caused the SAM, SAH and SAM/SAH ratio changes, then
affected methylation process. Furthermore, in the cycle, folate is
integrally involved in both substrate synthesis and product removal
via its role in methionine synthesis from Hcy. Folate insufficiency
leads to a decrease in SAM synthesis (38), which may compromise SAM-dependent
methylation reactions, however additionally also leads to an
increase in cellular concentrations of SAH by promoting Hcy
accumulation via reversal of SAH hydrolase. In the present study,
folate was added to reduce the Hcy effect. The results indicated an
antagonistic effect against Hcy both in the methylation levels and
in the gene expression levels. These data indicated that Hcy may
impact the methylation status partly through the methionine
cycle.
In conclusion, Hcy impacted the methylation status
of the genes involved in the cell proliferation, leading to a loss
of cellular control in VSMCs, and induced the proliferation of
VSMCs. Hcy not only affected the methylation levels of special
genes, however additionally impacted the global methylation status,
which may be a characteristic of VSMCs treated with Hcy. The data
provided evidence for the mechanisms of VSMC proliferation in AS
induced by Hcy and may provide a potential diagnostic marker for AS
induced by Hcy.
References
|
1
|
Devasia AJ, Joy B and Tarey SD: HSerum
homocysteine as a risk factor for carotid intimal thickening in
acute stroke: A cross sectional observational study. Ann Indian
Acad Neurol. 19:48–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu K, Xuekelati S, Zhang Y, Yin Y, Li Y,
Chai R, Li X, Peng Y, Wu J and Guo X: Expression levels of
atherosclerosis-associated miR-143 and miR-145 in the plasma of
patients with hyperhomocysteinaemia. BMC Cardiovasc Disord.
17:1632017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiaoling Y, Li Z, ShuQiang L, Shengchao M,
Anning Y, Ning D, Nan L, Yuexia J, Xiaoming Y, Guizhong L and
Yideng J: Hyperhomocysteinemia in ApoE−/− Mice leads to
overexpression of enhancer of Zeste homolog 2 via miR-92a
regulation. PLoS One. 11:e01677442016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Lin J, Ju T, Chu L and Zhang L:
Vascular smooth muscle cell differentiation to an osteogenic
phenotype involves matrix metalloproteinase-2 modulation by
homocysteine. Mol Cell Biochem. 406:139–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Shen J, Zhan R, Wang X, Wang X,
Zhang Z, Leng X, Yang Z and Qian L: Proteomic analysis of
homocysteine induced proliferation of cultured neonatal rat
vascular smooth muscle cells. Biochim Biophys Acta. 1794:177–184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han XB, Zhang HP, Cao CJ, Wang YH, Tian J,
Yang XL, Yang AN, Wang J, Jiang YD and Xu H: Aberrant DNA
methylation of the PDGF gene in homocysteine-mediated VSMC
proliferation and its underlying mechanism. Mol Med Rep.
10:947–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taysi S, Keles MS, Gumustekin K, Akyuz M,
Boyuk A, Cikman O and Bakan N: Plasma homocysteine and liver tissue
S-adenosylmethionine, S-adenosylhomocysteine status in vitamin
B6-deficient rats. Eur Rev Med Pharmacol Sci. 19:154–160.
2015.PubMed/NCBI
|
|
8
|
Elshorbagy AK, Jernerén F, Samocha-Bonet
D, Refsum H and Heilbronn LK: Serum S-adenosylmethionine, but not
methionine, increases in response to overfeeding in humans. Nutr
Diabetes. 6:e1922016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Fu X, Peng X, Xiao Z, Chen G and
Wang X: DNA methylation profiling reveals correlation of
differential methylation patterns with gene expression in human
epilepsy. J Mol Neurosci. 59:68–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mandaviya PR, Stolk L and Heil SG:
Homocysteine and DNA methylation: A review of animal and human
literature. Mol Genet Metab. 113:243–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang AN, Zhang HP, Sun Y, Yang XL, Wang N,
Zhu G, Zhang H, Xu H, Ma SC, Zhang Y, et al: High-methionine diets
accelerate atherosclerosis by HHcy-mediated FABP4 gene
demethylation pathway via DNMT1 in ApoE(−/-) mice. FEBS Lett.
589:3998–4009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yideng J, Zhihong L, Jiantuan X, Jun C,
Guizhong L and Shuren W: Homocysteine-mediated PPARalpha, gamma DNA
methylation and its potential pathogenic mechanism in monocytes.
DNA Cell Biol. 27:143–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Y, Zhang J, Xiong J, Cao J, Li G and
Wang S: Ligands of peroxisome proliferator-activated receptor
inhibit homocysteine-induced DNA methylation of inducible nitric
oxide synthase gene. Acta Biochim Biophys Sin (Shanghai).
39:366–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greißel A, Culmes M, Napieralski R, Wagner
E, Gebhard H, Schmitt M, Zimmermann A, Eckstein HH, Zernecke A and
Pelisek J: Alternation of histone and DNA methylation in human
atherosclerotic carotid plaques. Thromb Haemost. 114:390–402. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang HP, Wang YH, Cao CJ, Yang XM, Ma SC,
Han XB, Yang XL, Yang AN, Tian J, Xu H, et al: A regulatory circuit
involving miR-143 and DNMT3a mediates vascular smooth muscle cell
proliferation induced by homocysteine. Mol Med Rep. 13:483–490.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma S, Zhang H, Sun W, Gong H, Wang Y, Ma
C, Wang J, Cao C, Yang X, Tian J and Jiang Y: Hyperhomocysteinemia
induces cardiac injury by up-regulation of p53-dependent Noxa and
Bax expression through the p53 DNA methylation in ApoE(−/-) mice.
Acta Biochim Biophys Sin (Shanghai). 45:391–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
How-Kit A, Daunay A, Mazaleyrat N, Busato
F, Daviaud C, Teyssier E, Deleuze JF, Gallusci P and Tost J:
Accurate CpG and non-CpG cytosine methylation analysis by
high-throughput locus-specific pyrosequencing in plants. Plant Mol
Biol. 88:471–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Camacho-Arroyo I, Hansberg-Pastor V and
Rodríguez-Dorantes M: DNA methylation analysis of steroid hormone
receptor genes. Methods Mol Biol. 1165:89–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chatterjee R and Vinson C: CpG methylation
recruits sequence specific transcription factors essential for
tissue specific gene expression. Biochim Biophys Acta.
1819:763–770. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pramio DT, Pennacchi PC, Maria-Engler SS,
Campos AH, Duprat JP, Carraro DM and Krepischi AC: LINE-1
hypomethylation and mutational status in cutaneous melanomas. J
Investig Med. 64:899–904. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang H, Liu Z, Ma S, Zhang H, Kong F, He
Y, Yang X, Wang Y, Xu H, Yang A, et al: Ratio of
S-adenosylmethionine to S-adenosylhomocysteine as a sensitive
indicator of atherosclerosis. Mol Med Rep. 14:289–300.
2016.PubMed/NCBI
|
|
23
|
Qi L, Zhi J, Zhang T, Cao X, Sun L, Xu Y
and Li X: Inhibition of microRNA-25 by tumor necrosis factor α is
critical in the modulation of vascular smooth muscle cell
proliferation. Mol Med Rep. 11:4353–4358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng L, Longbin L, Zhou C, Pan S, Zhai X,
Jiang C, Guo Y, Ji Z, Chi J, Peng F and Guo H: Polyphenols and
Polypeptides in Chinese rice wine inhibit homocysteine-induced
proliferation and migration of vascular smooth muscle cells. J
Cardiovasc Pharmacol. 67:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin SY, Jung JY, Yong Y, Cho HJ, Lim Y
and Lee YH: Inhibition of PDGF-induced migration and TNFα-induced
ICAM-1 expression by maltotetraose from bamboo stem extract (BSE)
in mouse vascular smooth muscle cells. Mol Nutr Food Res.
60:2086–2097. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jain AK, Allton K, Iacovino M, Mahen E,
Milczarek RJ, Zwaka TP, Kyba M and Barton MC: p53 regulates cell
cycle and microRNAs to promote differentiation of human embryonic
stem cells. PLoS Biol. 10:e10012682012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varela A, Piperi C, Sigala F, Agrogiannis
G, Davos CH, Andri MA, Manopoulos C, Tsangaris S, Basdra EK and
Papavassiliou AG: Elevated expression of mechanosensory polycystins
in human carotid atherosclerotic plaques: Association with p53
activation and disease severity. Sci Rep. 5:134612015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shan X, Fu YS, Aziz F, Wang XQ, Yan Q and
Liu JW: Ginsenoside Rg3 inhibits melanoma cell proliferation
through down-regulation of histone deacetylase 3 (HDAC3) and
increase of p53 acetylation. PLoS One. 9:e1154012014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun GB, Qin M, Ye JX, Pan RL, Meng XB,
Wang M, Luo Y, Li ZY, Wang HW and Sun XB: Inhibitory effects of
myricitrin on oxidative stress-induced endothelial damage and early
atherosclerosis in ApoE−/− mice. Toxicol Appl Pharmacol.
271:114–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY and Fu L:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu C, Liu S, Sun Y, Shi G and Li Y: Effect
of recombinant hPTEN gene expression on PDGF induced VSMC
proliferation. Cell Biochem Biophys. 70:1185–1190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen WJ, Pang JH, Lin KH, Lee DY, Hsu LA
and Kuo CT: Propylthiouracil, independent of its antithyroid
effect, promotes vascular smooth muscle cells differentiation via
PTEN induction. Basic Res Cardiol. 105:19–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shu Z, Yu M, Zeng G, Zhang X, Wu L and Tan
X: Epigallocatechin-3-gallate inhibits proliferation of human
aortic smooth muscle cells via up-regulating expression of
mitofusin 2. Eur J Cell Biol. 93:137–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vogel Ciernia A and LaSalle J: The
landscape of DNA methylation amid a perfect storm of autism
aetiologies. Nat Rev Neurosci. 17:411–423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang Y, Yang X, Ma L, Cai X, Wang L, Yang
C, Li G, Zhang M, Sun W and Jiang Y: Homocysteine-mediated
cholesterol efflux via ABCA1 and ACAT1 DNA methylation in THP-1
monocyte-derived foam cells. Acta Biochim Biophys Sin (Shanghai).
45:220–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yüksel Ş, Kucukazman SO, Karataş GS,
Ozturk MA, Prombhul S and Hirankarn N: Methylation Status of Alu
and LINE-1 interspersed repetitive sequences in Behcet's disease
patients. Biomed Res Int. 2016:13930892016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J and Zheng YG: SAM/SAH analogs as
versatile tools for SAM-Dependent Methyltransferases. ACS Chem
Biol. 11:583–597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Howe CG, Niedzwiecki MM, Hall MN, Liu X,
Ilievski V, Slavkovich V, Alam S, Siddique AB, Graziano JH and
Gamble MV: Folate and cobalamin modify associations between
S-adenosylmethionine and methylated arsenic metabolites in
arsenic-exposed Bangladeshi adults. J Nutr. 144:690–697. 2014.
View Article : Google Scholar : PubMed/NCBI
|