Introduction
Renal cell carcinoma (RCC) accounts for 90% of renal
malignancies and 2–3% of cases of cancer in adults worldwide
(1,2), the incidence of which has been
increasing at a rate of 2% per year. RCC is usually asymptomatic,
with a 5-year survival rate of >90% for stage I disease and of
20–30% for stage IV disease (2,3). RCC
is resistant to conventional chemotherapy and radiation. The 2004
World Health Organization classified RCC into multiple
morphological subtypes: Clear cell RCC, papillary RCC, chromophobe
RCC, collecting duct RCC and unclassified forms, which account for
80, 10–15, 5, 1 and 2% of cases, respectively (1,4).
SPARCL1, also known as MAST9 or hevin, is a member
of the SPARC protein family (5).
The SPARC family of proteins, includes SPARC, testicans (5), tsc36 (6) and QR1 (7,8).
SPARCL1 is downregulated in several types of cancer. In cases of
colorectal (9), prostate (10–14),
urinary bladder (15), lung
(13) and pancreatic cancer
(16), SPARCL1 is expressed at low
levels. Several studies have demonstrated that SPARCL1 reduces cell
proliferation, anchorage-independent growth, migration and invasion
in vitro (5,8,9,13)
and in vivo (17,18), suggesting that SPARCL1 may function
as a tumor suppressor. However, whether SPARCL1 contributes to the
progression of RCC remains to be elucidated.
In the present study, it was found that SPARCL1 was
downregulated in human RCC tissues compared with para-carcinoma
tissues. When SPARCL1 was overexpressed in RCC cell lines, it was
confirmed that SPARCL1 reduced RCC cell migration and invasion, but
did not affect growth. Mitogen-activated protein kinase (MAPK)
signaling is important in human RCC (19–22).
The results of the present study showed that SPARCL1 inhibited the
expression of phosphorylated p38/c-Jun N-terminal kinase
(JNK)/extracellular signal-regulated kinase (ERK) MAPKs, suggesting
that SPARCL1 suppressed RCC cell migration and invasion via the
inactivation of p38/JNK/ERK MAPKs. Therefore, targeting SPARCL1 may
provide an applicable approach for treating RCC.
Materials and methods
Cells and media
The Caki-1, 768-p, ACTH and HKC cell lines were
purchased from the Cell Bank of the Shanghai Institute for
Biological Science (Shanghai, China). The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM), respectively,
supplemented with 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C and 5%
CO2.
Tissue microarrays
The RCC tissue microarray, purchased from Wuhan
Boster Biological Technology Co., Ltd. (Wuhan, China) was used for
the immunohistochemical analysis of the expression of SPARCL1. RCC
and para-carcinoma tissue samples have different
clinicopathological characteristics including age, gender, tumor
volume, Fuhrman classification, pathological tumor stage (pT),
pathological lymph node stage (pN), pathological distant metastasis
(pM) and pathological tumor-node-metastasis (pTNM)
classification.
SPARCL1 vector construction
The HKC human kidney cells were cultured and
collected, then RNA was extracted using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.). Random primers were used to develop the
first strand of cDNA. The 5′region of the SPARCL1 gene was isolated
using primer pairs cDNA3.1(−)hSPARCL1-NF/cDNA3.1(−)hSPARCL1-SOE-R
following denaturation at 98°C for 2 min, 36 cycles of 98°C for 10
sec, 52°C for 30 sec, 72°C for 90 sec, and post-elongation for 10
min at 72°C. The mixture comprised 1X PCR buffer, 0.2 mM dNTPs, 0.2
µM each primer, 2 units of Primer STAR HS DNA polymerase (cat. no.
DR044A; Takara Bio, Inc., Otsu, Japan) and sterile distilled water.
Similarly, the intact 3′region was isolated using
cDNA3.1(−)hSPARCL1-SOE-F/hSPARCL1-3′UTR-R and
cDNA3.1(−)hSPARCL1-SOE-F/cDNA3.1(−)hSPARCL1-EcoRI-R
successively. The full coding region of SPARCL1 was established by
splicing overlap extension PCR with the
cDNA3.1(−)hSPARCL1-XhoI-F/cDNA3.1(−)hSPARCL1-EcoRI-R
primer pairs. Terminally, the SPARCL1 gene was directly cloned into
the pcDNA3.1 (−) vector via the XhoI and EcoRI sites.
The sequences of the primer pairs are listed in Table I.
 | Table I.Sequences of primers. |
Table I.
Sequences of primers.
| Name | Sequence
(5′-3′) |
|---|
|
cDNA3.1(−)hSPARCL1-NF |
CAACTGAAGGTACATTGGACATA |
|
cDNA3.1(−)hSPARCL1-XhoI-F |
ACGGGCCCTCTAGACTCGAGATGAGTGAGCCTCAGGAGAAA |
|
cDNA3.1(−)hSPARCL1-EcoRI-R |
TAGTCCAGTGTGGTGGAATTCTCAAAACAAGAGATTTTCATCTATG |
|
cDNA3.1(−)hSPARCL1-Soe-F |
AATGAAAATATAGGTACCACTGAGC |
|
cDNA3.1(−)hSPARCL1-Soe-R |
GCTCAGTGGTACCTATATTTTCATT |
|
hSPARCL1-3′UTR-R |
TACAAGTATCACAGCTGCAT |
SPARCL1 transfection
When the confluence of cells reached ~50–70%, the
RCC cells (AHCK, Caki-1 and 769-p), which were cultured in DMEM
supplemented with 10% FCS at 37°C in a 5% CO2 cell
culture incubator, were transfected with the pcDNA3.1 (−)SPARCL1
and control vectors using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. The transfected cells were cultured in
DMEM supplemented with 10% FBS at 37°C in 5% CO2 for 48
h, and then harvested for analysis. The expression of SPARCL1 was
detected using western blot analysis, and in vitro migration
and invasion assays were also performed.
Cell viability assays
The cells were seeded in 96-well plates at a
concentration of 5,000 cells/well, and cell viability were
determined using an MTT assay at 48 or 72 h according to routine
procedures.
Wound-healing assay
Cell migration was assessed using a classic
wound-healing assay. The RCC cells were seeded in 6-well plates at
a concentration of 5×105 cells/well, and transfected
following attachment. Following transfection for 4–6 h, a wound was
introduced via a scratch on the monolayer of cells using a 20-µl
pipette tip, following which the cells were washed with PBS twice
to remove non-adherent cells. Images (magnification, ×100) of the
regions were captured using a photomicroscope at several time
points (0, 24, 36 and 48 h).
Transwell invasion assay
For the invasion assay, the upper Transwell chamber
was precoated with Matrigel (BD Biosciences, Franklin Lakes, NJ,
USA) according to the manufacturer's protocol. At 48 h
post-transfection, the cells were harvested, counted and suspended
in serum-free DMEM. Subsequently, 1×105 cells in 100 µl
medium were added to the upper chamber. Medium containing 10% FBS
was added to the lower chamber as a chemoattractant. Following
incubation at 37°C for 24 h, the cells in the chambers were fixed
with 4% paraformaldehyde for 20 min, following which 0.1% crystal
violet solution was added for 15 min, and then immersed in
phosphate-buffered saline (PBS) for 20 min. Finally, the cells in
the lower chamber were counted under an inverted microscope. The
cell numbers in five randomly selected fields of view were counted
(magnification, ×100).
Immunohistochemistry
Tissue microarray sections (5-µm) of the
formalin-fixed and paraffin-embedded specimens were deparaffinized
using xylene and rehydrated in graded ethanol. The samples were
then preincubated with 3% H2O2 to inhibit
endogenous peroxide activity. The sections were then incubated at
4°C overnight with primary antibody against SPARCL1 (cat. no. 2728;
1:50; R&D Systems, Inc., Minneapolis, MN, USA) as described
previously (16,23), and then with horseradish peroxidase
(HRP)-labeled secondary antibodies (cat. no. KIT-5004; 50 µl;
MaxVision, Fuzhou, China) at room temperature for 15 min, followed
by the addition of freshly prepared 3,3′-diaminobenzidine for color
development for 5 min. In the control group, the primary antibody
was replaced with PBS. The immunohistochemical images were obtained
with a Leica microscope (Leica Microsystems GmbH, Wetzlar, Germany)
and immunohistochemical staining was evaluated independently by two
examiners, who were blinded to the clinicopathological information.
The intensity of the immunostaining was assessed and assigned
scores as follows: 0, negative staining; 1, low staining intensity;
2, moderate staining intensity; 3, high staining intensity.
Western blot analysis
For western blot analysis, the cells were harvested
and washed twice with ice-cold PBS, followed by lysis with ice-cold
lysis buffer (4% sodium dodecyl sulfate, 20% glycerol and 0.2M
dithiothreitol). Protein concentrations were determined using the
BCA method (Beyotime Institute of Biotechnology, Haimen, China).
Then the samples were separated by 10% SDS-PAGE, transferred onto
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA), and
hybridized 4°C overnight with anti-ERK antibody (cat. no. 9102;
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-p38 antibody (cat. no. 9212; 1:1,000; Cell Signaling
Technology, Inc.), anti-JNK antibody (cat. no. 9252; 1:1,000; Cell
Signaling Technology, Inc.), anti-p-ERK antibody (cat. no. 9101;
1:1,000; Cell Signaling Technology, Inc.), anti-p-p38 antibody
(cat. no. 9211; 1:1,000; Cell Signaling Technology, Inc.),
anti-p-JNK antibody (cat. no. 4668; 1:1,000; Cell Signaling
Technology, Inc.), anti-SPARCL1 antibody and anti-actin antibody
(cat. no. ab8226; 1:2,000; Abcam, Cambridge, UK). The immune
complexes were detected by incubation with anti-rabbit or
anti-mouse IgG antibodies conjugated to HRP (cat. nos. 111-035-003,
and 115-035-003; 1:3,000; Jackson ImmunoResearch Laboratories Inc.,
West Grove, PA, USA) for 1 h at 37°C, followed by ECL detection
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.00 (GraphPad Software, Inc., San Diego, CA, USA).
Data are expressed as the mean ± standard error of the mean.
Comparisons between two conditions were analyzed using two-tailed
unpaired t-tests when the data were normally distributed; otherwise
Mann-Whitney analysis was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of SPARCL1 is downregulated
in RCC cells
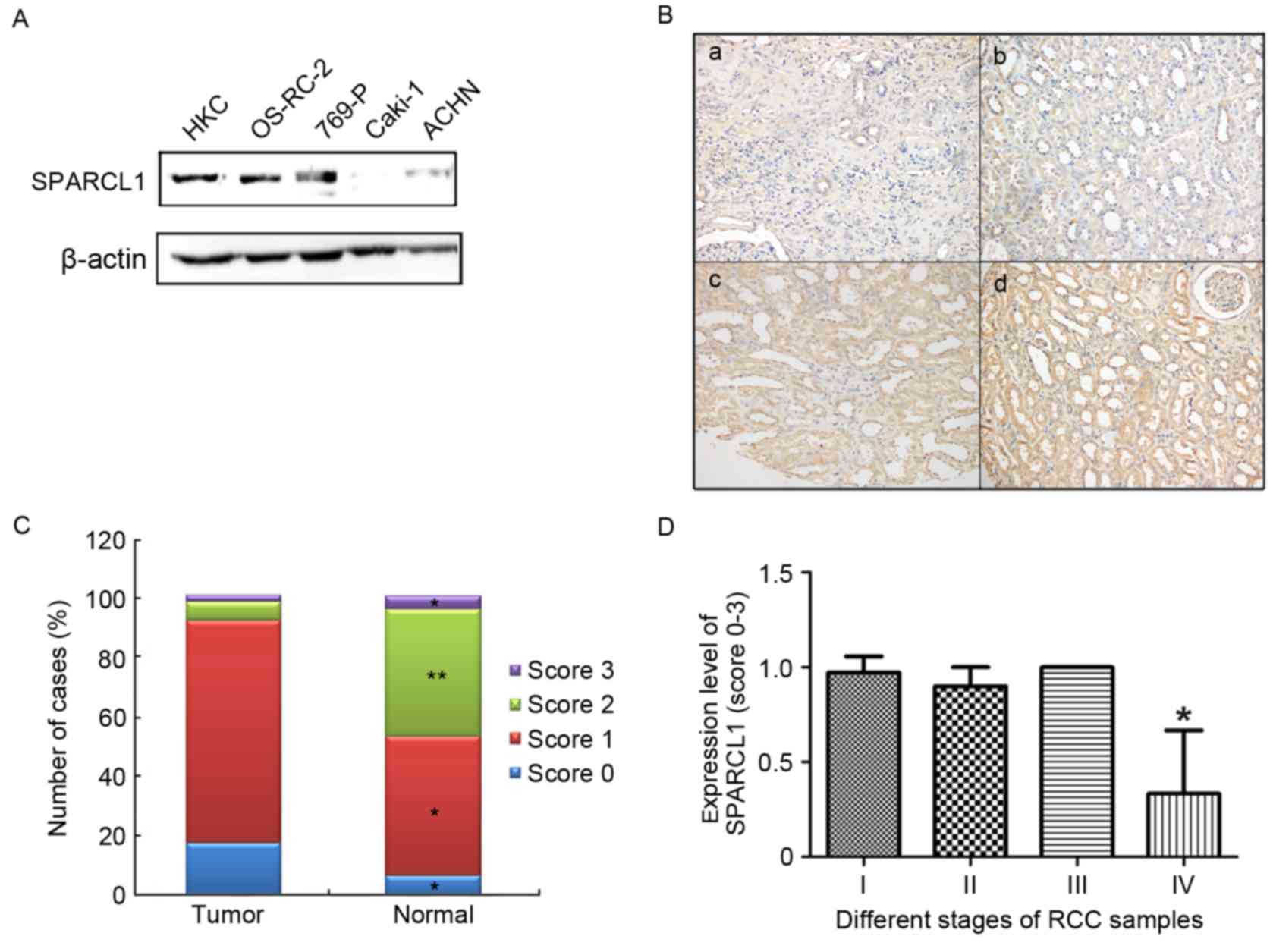
To examine the possible role of SPARCL1 in RCC,
western blot analysis was performed to quantify the protein levels
of SPARCL1. The results showed that the expression of SPARCL1 was
significantly reduced in the RCC cells, compared with that in the
normal HKC cells (Fig. 1A).
The expression levels of SPARCL1 were also detected
in 50 primary RCC and paired non-cancerous tissues using the tissue
microarray and immunohistochemical staining techniques. Images
showing the four expression levels of SPARCL1 (0, negative
staining; 1, low staining intensity; 2, moderate staining
intensity; 3, high staining intensity) are shown in Fig. 1Ba-d. The results showed that the
expression levels of SPARCL1 in the RCC tissues differed
substantially from those in the normal non-cancerous tissues. In
the paired normal tissues, 46.7% of the tissues showed moderate or
high expression of SPARCL1, whereas this was only observed in 8.35%
of the RCC tissues (Fig. 1C).
Absent or low expression levels of SPARCL1 were observed in 91.65%
of the tumor samples, however, this was only found in 53% of the
paired normal tissues (Fig. 1C).
Among the 50 RCC samples, 41 samples were stage I, five samples
were stage II, one sample was stage III and three samples were
stage IV. The association between expression and pTNM
classification was analyzed, and it was found that the expression
of SPARCL1 was significantly reduced in stage IV samples (Fig. 1D). This is a preliminary finding
due to the small number of patients in the tissue microarray.
SPARCL1 inhibits migration and
invasion, and promotes the proliferation of human RCC cells in
vitro
Metastasis is a problem central to cancer
therapeutics, therefore, the present study aimed to assess whether
the overexpression of SPARCL1 affected tumor migration and
invasion. The overexpression of SPARCL1 was induced in RCC cells,
following which migration and invasion assays were performed. As
shown in Fig. 2, an inverted
microscope was used to observe the differences in the wounded
regions in the ACHN cell cultures at 24, 36 and 72 h. The
overexpression of SPARCL1 markedly reduced the number of migrated
cells. As shown in Fig. 2A, the
region damaged by the scratch in the control group was infiltrated
with migrated RCC cells. In the cells overexpressing SPARCL1, fewer
cells were found to have migrated into the scratch area (Fig. 2B). The numbers of cells were
counted in the wounded regions of the groups at different time
points (Fig. 2C). The
overexpression of SPARCL1 in the RCC cells was confirmed using
western blot analysis prior to migration and invasion assays
(Fig. 2D). These results suggested
that SPARCL1 had an inhibitory effect on RCC cell migration. The
inhibitory effect of SPARCL1 on migration was also confirmed in the
Caki-1 RCC cell line (Fig.
3A-C).
As shown in Fig. 4A and
B, the overexpression of SPARCL1 markedly reduced the number of
cells migrating to the lower side of the membrane in the Caki-1
cells. Counting of the invasive cells per field was performed, as
shown in Fig. 4C. The effect of
SPARCL1 on the proliferation of RCC cell lines was detected using
MTT assays (Fig. 5A-C). The
results showed that the proliferation rates of the Caki-1, ACHN and
769-p cells were accelerated by the overexpression of SPARCL1 for
48 or 72 h, respectively, suggesting that SPARCL1 promoted RCC cell
proliferation.
 | Figure 5.SPARCL1 promotes the proliferation of
RCC cells and reduces the activation of p38/JNK/ERK
mitogen-activated protein kinases. (A-C) RCC cells were transfected
with NC or SPARCL. After 48 and 72 h, cell numbers were assessed
using an MTT assay. Statistical analysis with Student's t-test
showed a significant increase in cell growth when SPARCL1 was
overexpressed. *P<0.05. (D) Western blot analysis of the protein
expression levels of total p38, JNK and ERK, and p-p38, p-JNK and
p-ERK in RCC cells. RCC, renal cell carcinoma; SPARCL1, SPARC-like
1; NC, negative control; JNK, c-Jun N-terminal kinase; ERK,
extracellular signal-regulated kinase; p-, phosphorylated. |
SPARCL1 inhibits activation of the
p38/JNK/ERK MAPK pathway
MAPK kinases are crucial enzymes at the intersection
of several biological pathways, which regulate cell
differentiation, proliferation and survival. MAPK comprises three
protein kinases: JNK, p38 and ERK. In vitro, the
overexpression of SPARCL1 appeared to significantly reduce the
phosphorylation of JNK, ERK and p38 proteins in the RCC cells
(Fig. 5D). These results showed
that SPARCL1 may contribute to inactivation of the JNK/ERK/p38
signaling pathway, and lead to the inhibition of RCC cell
proliferation and migration.
Discussion
The present study analyzed the results of SPARCL1
immunohistochemical staining, and found that almost 92% of the RCC
samples were SPARCL1-negative or low intensity, and only 8% of the
RCC samples were SPARCL1-moderate or high intensity. It was found
that ~54% of the normal tissues were SPARCL1-negative or showed low
intensity positive staining, and almost 46% of the normal tissues
were SPARCL1-positive at a moderate or high intensity. These
results showed that the expression of SPARCL1 was significantly
reduced in RCC tissues, comparison with that in normal tissues. It
was also found that the expression of SPARCL1 was significantly
reduced at stage IV. This suggested that a high expression of
SPARCL1 offers potential as a prognostic factor. Future
investigations aim to increase the sample size to investigate the
association between the expression of SPARCL1 and kidney
staging.
SPARCL1 is downregulated in several types of cancer,
which inhibits cancer cell growth, migration or invasion,
suggesting that SPARCL1 may function as a tumor suppressor
(2,11–13,16).
The present study found that the expression levels of SPARCL1 were
significantly reduced in RCC cells. Using traditional in
vitro approaches to examine the function of SPARCL1, the
present study on RCC cell lines demonstrated that SPARCL1 decreased
the migratory and invasive properties of RCC cells but did not
restrict the growth of the RCC cells. SPARCL1 may be a clinically
useful biomarker with diagnostic, prognostic and therapeutic
value.
MAPK pathways are crucial in regulating cell
differentiation (24–26), proliferation (27–30)
and survival (31–33). Mutations of the MAPK pathway
proteins have been reported to be associated with several types of
human cancer (34–38). MAPK signaling has been shown to be
crucial in tumor genesis and tumor metastasis (39–41).
The overexpression of MAPK was also found in 52% of human RCC cases
in a previous study (42). In the
present study, it was found that SPARCL1 downregulated the
expression levels of p-p38, p-JNK and p-ERK in the RCC cells,
suggesting that SPARCL1 may regulate the progression of RCC through
the p38/JNK/ERK MAPK pathway. Further investigations are required
to examine the effect and mechanism of SPARCL1 in treating RCC.
References
|
1
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lopez-Beltran A, Carrasco JC, Cheng L,
Scarpelli M, Kirkali Z and Montironi R: 2009 update on the
classification of renal epithelial tumors in adults. Int J Urol.
16:432–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopez-Beltran A, Scarpelli M, Montironi R
and Kirkali Z: 2004 WHO classification of the renal tumors of the
adults. Eur Urol. 49:798–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brekken RA and Sage EH: SPARC, a
matricellular protein: At the crossroads of cell-matrix
communication. Matrix Biol. 19:816–827. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vannahme C, Smyth N, Miosge N, Gösling S,
Frie C, Paulsson M, Maurer P and Hartmann U: Characterization of
SMOC-1, a novel modular calcium-binding protein in basement
membranes. J Biol Chem. 277:37977–37986. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vannahme C, Gösling S, Paulsson M, Maurer
P and Hartmann U: Characterization of SMOC-2, a modular
extracellular calcium-binding protein. Biochem J. 373:805–814.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan Q and Sage EH: SPARC, a matricellular
glycoprotein with important biological functions. J Histochem
Cytochem. 47:1495–1506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu SJ, Yu JK, Ge WT, Hu HG, Yuan Y and
Zheng S: SPARCL1, Shp2, MSH2, E-cadherin, p53, ADCY-2 and MAPK are
prognosis-related in colorectal cancer. World J Gastroenterol.
17:2028–2036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylor BS, Schultz N, Hieronymus H, et al:
Integrative genomic profiling of human prostate cancer. Cancer
Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandran UR, Ma C, Dhir R, Bisceglia M,
Lyons-Weiler M, Liang W, Michalopoulos G, Becich M and Monzon FA:
Gene expression profiles of prostate cancer reveal involvement of
multiple molecular pathways in the metastatic process. BMC Cancer.
7:642007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hurley PJ, Marchionni L, Simons BW, Ross
AE, Peskoe SB, Miller RM, Erho N, Vergara IA, Ghadessi M, Huang Z,
et al: Secreted protein, acidic and rich in cysteine-like 1
(SPARCL1) is down regulated in aggressive prostate cancers and is
prognostic for poor clinical outcome. Proc Natl Acad Sci USA.
109:14977–14982. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bendik I, Schraml P and Ludwig CU:
Characterization of MAST9/Hevin, a SPARC-like protein, that is
down-regulated in non-small cell lung cancer. Cancer Res.
58:626–629. 1998.PubMed/NCBI
|
|
14
|
Nelson PS, Plymate SR, Wang K, True LD,
Ware JL, Gan L, Liu AY and Hood L: Hevin, an antiadhesive
extracellular matrix protein, is down-regulated in metastatic
prostate adenocarcinoma. Cancer Res. 58:232–236. 1998.PubMed/NCBI
|
|
15
|
Zaravinos A, Lambrou GI, Boulalas I,
Delakas D and Spandidos DA: Identification of common differentially
expressed genes in urinary bladder cancer. PLoS One. 6:e181352011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esposito I, Kayed H, Keleg S, Giese T,
Sage EH, Schirmacher P, Friess H and Kleeff J: Tumor-suppressor
function of SPARC-like protein 1/Hevin in pancreatic cancer.
Neoplasia. 9:8–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang Y, Qiu Q, Jiang M, Jin R, Lehmann
BD, Strand DW, Jovanovic B, DeGraff DJ, Zheng Y, Yousif DA, et al:
SPARCL1 suppresses metastasis in prostate cancer. Mol Oncol.
7:1019–1030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hurley PJ, Hughes RM, Simons BW, et al:
Androgen-Regulated SPARCL1 in the tumor microenvironment inhibits
metastatic progression. Cancer Res. 75:4322–4334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sivaraman VS, Wang H, Nuovo GJ and Malbon
CC: Hyperexpression of mitogen-activated protein kinase in human
breast cancer. J Clin Invest. 99:1478–1483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoshino R, Chatani Y, Yamori T, Tsuruo T,
Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J and Kohno
M: Constitutive activation of the 41-/43-kDa mitogen-activated
protein kinase signaling pathway in human tumors. Oncogene.
18:813–822. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eliceiri BP, Klemke R, Strömblad S and
Cheresh DA: Integrin alphavbeta3 requirement for sustained
mitogen-activated protein kinase activity during angiogenesis. J
Cell Bio. 140:1255–1263. 1998. View Article : Google Scholar
|
|
22
|
Mandell JW, Hussaini IM, Zecevic M, Weber
MJ and VandenBerg SR: In situ visualization of intratumor growth
factor signaling: Immunohistochemical localization of activated
ERK/MAP kinase in glial neoplasms. Am J Pathol. 153:1411–1423.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Røe OD, Szulkin A, Anderssen E, Flatberg
A, Sandeck H, Amundsen T, Erlandsen SE, Dobra K and Sundstrøm SH:
Molecular resistance fingerprint of pemetrexed and platinum in a
long-term survivor of mesothelioma. PLoS One. 7:e405212012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang RL, Yuan Y, Tu J, Zou GM and Li Q:
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways
converge on Runx2 to regulate BMP-2-induced osteoblastic
differentiation. Cell Death Dis. 5:e11872014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo C, Yang XG, Wang F and Ma XY: IL-1α
induces apoptosis and inhibits the osteoblast differentiation of
MC3T3-E1 cells through the JNK and p38 MAPK pathways. Int J Mol
Med. 38:319–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kyosseva SV: Targeting MAPK signaling in
Age-related macular degeneration. Ophthalmol Eye Dis. 8:23–30.
2016.PubMed/NCBI
|
|
27
|
Lim W, Jeong M, Bazer FW and Song G:
Coumestrol inhibits proliferation and migration of prostate cancer
cells by regulating AKT ERK1/2, and JNK MAPK cell signaling
cascades. J Cell Physiol. 232:862–871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Gao C, Zhang Y, Gao J, Teng F,
Tian W, Yang W, Yan Y and Xue F: Visfatin stimulates endometrial
cancer cell proliferation via activation of PI3K/Akt and
MAPK/ERK1/2 signalling pathways. Gynecol Oncol. 143:168–178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang A, Fan H, Zhao Y, Zha X, Zhang H, Hu
Z and Tu P: Huaier aqueous extract inhibits proliferation and
metastasis of tuberous sclerosis complex cell models through
downregulation of JAK2/STAT3 and MAPK signaling pathways. Oncol
Rep. 36:1491–1498. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yue X, Wu M, Jiang H, Hao J, Zhao Q, Zhu
Q, Saren G, Zhang Y and Zhang X: Endothelial lipase is upregulated
by interleukin-6 partly via the p38 MAPK and p65 NF-kB signaling
pathways. Mol Med Rep. 14:1979–1985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koyani CN, Kitz K, Rossmann C, Bernhart E,
Huber E, Trummer C, Windischhofer W, Sattler W and Malle E:
Activation of the MAPK/Akt/Nrf2-Egr1/HO-1-GCLc axis protects MG-63
osteosarcoma cells against 15d-PGJ2-mediated cell death. Biochem
Pharmacol. 104:29–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lien LM, Wang MJ, Chen RJ, Chiu HC, Wu JL,
Shen MY, Chou DS, Sheu JR, Lin KH and Lu WJ: Nobiletin, a
polymethoxylated flavone, inhibits glioma cell growth and migration
via arresting cell cycle and suppressing MAPK and Akt pathways.
Phytother Res. 30:214–221. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Safia Kamil M, Jadiya P, Sheikh S, Haque
E, Nazir A, Lakshmi V and Mir SS: The chromone alkaloid,
rohitukine, affords anti-cancer activity via modulating apoptosis
pathways in A549 cell line and yeast mitogen activated protein
kinase (MAPK) pathway. PLoS One. 10:e01379912015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Atiq R, Hertz R, Eldad S, Smeir E and
Bar-Tana J: Suppression of B-Raf(V600E) cancers by MAPK
hyper-activation. Oncotarget. 7:18694–18704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chakraborty C, Sharma AR, Patra BC,
Bhattacharya M, Sharma G and Lee SS: MicroRNAs mediated regulation
of MAPK signaling pathways in chronic myeloid leukemia. Oncotarget.
7:42683–42697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Wen XZ, Bu ZD, Cheng XJ, Xing XF,
Wang XH, Zhang LH, Guo T, Du H, Hu Y, et al: Paclitaxel enhances
tumoricidal potential of TRAIL via inhibition of MAPK in resistant
gastric cancer cells. Oncol Rep. 35:3009–3017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li QC, Liang Y, Tian Y and Hu GR:
Arctigenin induces apoptosis in colon cancer cells through
ROS/p38MAPK pathway. J BUON. 21:87–94. 2016.PubMed/NCBI
|
|
38
|
Lin L and Bivona TG: The Hippo effector
YAP regulates the response of cancer cells to MAPK pathway
inhibitors. Mol Cell Oncol. 3:e10214412015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dunn KL, Espino PS, Drobic B, He S and
Davie JR: The Ras-MAPK signal transduction pathway, cancer and
chromatin remodeling. Biochem Cell Biol. 83:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oka H, Chatani Y, Hoshino R, Ogawa O,
Kakehi Y, Terachi T, Okada Y, Kawaichi M, Kohno M and Yoshida O:
Constitutive activation of mitogen-activated protein (MAP) kinases
in human renal cell carcinoma. Cancer Res. 55:4182–4187.
1995.PubMed/NCBI
|