Introduction
Stroke has become a leading cause of mortality and
disability worldwide. In the USA and the UK, the morbidity of heart
disease and cancer is reportedly 0.2% of the population each year.
In China, ~200 million people are affected by stroke every year,
and as a result, 70–80% of these patients will suffer with
life-changing disabilities (1).
Strokes can be ischemic or hemorrhagic in nature; ~80% of strokes
are ischemic stroke (2).
Currently, therapy for ischemic stroke remains limited, and
vascular recanalization and antiplatelet therapy are the only two
therapeutic strategies that have been proven to be effective during
the acute phase of ischemic stroke (3). For acute ischemic stroke, intravenous
thrombolytic therapy within 4.5 h following the acute stroke is the
most widely accepted method for vascular recanalization. but the
narrow time window, hemorrhage complications and the low
recanalization rates in patients with macrovascular occlusion often
limit its use (4). Antiplatelet
therapy is an important strategy for preventing recurrent stroke by
simultaneously blocking different platelet activation pathways.
Aspirin is the most widely used antiplatelet agent in current use,
however, it has been reported that combination antiplatelet therapy
with aspirin and clopidogrel increased the risk of hemorrhage
complications (5,6). Therefore, novel, effective and widely
applicable pharmacological treatments are urgently required.
The pathophysiology of stroke is very complex and
involves a number of factors, including inflammation, oxidative
stress, apoptosis and excitotoxicity (7). Ischemic stroke induces the expression
of high levels of adhesion molecules, cytokines and inflammatory
mediators, including nitric oxide (NO), interleukin-1β (IL-1β) and
tumor necrosis factor-α (TNF-α). These high levels lead to marked
local and systemic inflammatory responses (8,9).
Inflammation itself may exacerbate the spread of damage to the
ischemic penumbra, increase the levels of inflammatory cytokines
and thus, further aggravate brain injury (10). Therefore, the identification of
novel drugs to regulate the inflammatory response is a promising
method for the treatment of cerebral ischemic injury. Brain-derived
neurotrophic factor (BDNF), a member of the neurotrophin family,
serves an important role in the development and plasticity of the
brain. It also protects the brain against injuries induced by
stress conditions such as ischemia and trauma, by promoting nerve
regeneration, preventing neuronal death, and regulating synaptic
plasticity and cell survival (11). Glial cell-derived neurotrophic
factor (GDNF) is another important neurotrophin, which serves an
important role in reducing apoptosis in neurons and promoting
neurological outcomes following hypoxia-ischemia, by inhibiting the
production of caspase-3, TNF-α, NO and induced nitric oxide
synthase (iNOS) (12). Vascular
endothelial growth factor (VEGF) is an angiogenic peptide involved
in nerve vascular remodeling, promoting growth in neurons and glial
cells, and protecting neuronal tissues from cell death induced by
hypoxia or ischemia in order to achieve neuroprotection (13). These observations have indicated
that BDNF, GDNF and VEGF may be potential therapeutic agents for
the prevention of neuronal cell death following cerebral
ischemia.
Traditional Chinese medicine (TCM) is used due to
its rich sources, low costs, few side effects and its
multi-targeting effects (14,15).
Therefore, it is important to investigate the effects and
mechanisms associated with anti-cerebral ischemia drugs used in
TCM. The TCM Mao Dong Qing, the dry roots of Ilex pubescens
Hook. et Arn, is an evergreen shrub that is predominantly found in
the southern regions of China. It has been used primarily for the
treatment of hypercholesterolemia and cardiovascular diseases, such
as stroke and coronary artery disease, in addition to peripheral
vascular disease (16–18). Preliminary studies by our group
identified the beneficial effects of Ilex pubescens total
flavonoids (IPTF) in rat and mice models of cerebral ischemia
(19–21). However, its underlying mechanism
and compound activity with regard to its effects against focal
cerebral ischemia injury remain to be elucidated. In the present
study, the effect of IPTF on a rat model of focal cerebral
ischemia/reperfusion (I/R) injury, induced by middle cerebral
artery occlusion (MCAO), was investigated. The potential mechanisms
underlying the alterations in the levels of inflammation-associated
molecules and the increased secretion of neurotrophic factors were
also examined.
Materials and methods
Plant collection and
identification
Ilex pubescens was purchased from Henan Province
Pharmaceutical Co., Ltd. (Zhengzhou, China) and was identified by
Professor Chengming Dong and Professor Suiqing Chen from the
Department of Medicinal Plants, School of Pharmacy, Henan
University of Chinese Medicine (Zhengzhou, China). The voucher
specimen (no. XX20140916001) was kept in the group's
laboratory.
Preparation of extracts
The total flavonoids were obtained from Ilex
pubescens using the method described previously (22). Briefly, air-dried Ilex
pubescens was treated twice with 70% aqueous ethanol (1:10,
w/v) for 1.5 h, then a further 1 h under reflux conditions at 80°C.
The filtered extracts were concentrated in a vacuum evaporator at
60°C to obtain the crude extracts. The crude extracts were
dissolved in water (adjusted to pH 4.5), and loaded into glass
columns, which were wet-packed with AB-8 macroporous resin.
Following elution with water and 10% aqueous ethanol, the elutriant
with 40% ethanol was collected, dried using a vacuum at 60°C and
then stored at 4°C. The total flavonoid content in the extract was
>50%, as determined by ultraviolet spectrophotometry, performed
as described in a previous study (23).
Animal treatment and
administration
A total of 96 adult male Wistar rats [SPF grade;
age, 8 weeks; weight, 280–300 g; Laboratory Animal Center of Hebei
Province, Shijiazhuang, China (certificate no. 907048)], were
employed in the present study. Rats were housed in an
environmentally controlled breeding room (22–24°C; 50–55%; 12-h
dark/light cycle), with free access to standard laboratory food and
water. The present study was approved by the Ethics Committee of
Henan University of Chinese Medicine (Henan, China). Rats were
divided into the following 4 groups (n=24/group): Sham operation
group [sham; intragastric (ig) administration of saline and surgery
without MCAO], ischemic group (model group; MCAO surgery with ig
administration of saline), and the third and fourth groups were
pretreated with a high (200 mg/kg/day; IPTF-1 group) or low ig dose
of IPTF (100 mg/kg/day; IPTF-2) for 5 days, followed by the
induction of I/R (MCAO surgery + IPTF pretreatment groups).
Induction of focal cerebral I/R via
MCAO surgery
An hour following the last administration of IPTF
pretreatment, all rats were anesthetized with chloral hydrate (300
mg/kg, intraperitoneally; Tianjin Zhiyuan Chemical Reagent Co.,
Ltd., Tianjin, China) and MCAO surgery was then performed according
to Koizumi et al (24) and
Nagasawa and Kogure (25) with a
few modifications. Briefly, the left common carotid artery (CCA)
and internal carotid artery (ICA) were isolated via a cervical
midline incision. A nylon monofilament pre-coated with silicone
rubber (0.26 mm external diameter) was inserted into the CCA and
advanced into the ICA to ~19–20 mm from the left CCA bifurcation to
occlude the middle cerebral artery. Then, 2 h after the induction
of ischemia, the filament was slowly withdrawn and animals were
returned to their cages for 22 h of reperfusion. Rats in the sham
operation group received the same surgical procedures without
filament insertion to induce MCAO. Rat body temperature was
maintained at 37.0°C using an infrared lamp throughout surgical
procedures. After the surgery, 22 rats in the sham group, 20 rats
in the model group, 20 rats in the IPTF-1 group and 22 rats in the
IPTF-2 group survived, respectively.
Neurological deficit scores
Neurological deficit scores were recorded for each
rat 22 h after ischemic injury (n=10–11 rats/group) in a
blind-controlled manner, according to Longa's five-point scale
method (26). The following
grading system was applied: Grade 0, no deficit; grade 1, failure
to extend right forepaw; grade 2, circling to right; grade 3,
falling to right; grade 4, inability to walk spontaneously and a
reduced level of consciousness.
Cerebral infarct volume
measurement
Following behavioral testing, rats (n=10–11
rats/group) were anesthetized with chloral hydrate (300 mg/kg,
intraperitoneally) and sacrificed, and the brain tissues removed
and separated into 6 coronal slices of 2-mm thickness. Brain slices
were stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC; cat.
no. T8877; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) solution
in the dark at 37°C for 15 min. The cerebral infarct volume was
measured using the method described by Belayev et al
(27). In order to eliminate the
contribution of swelling/edema to the ischemic lesion, the cerebral
infarct volume was calculated with the following formula: Corrected
infarct volume = contralateral hemisphere volume - (ipsilateral
hemisphere volume - measured infarct volume) (28).
Tissue preparation
The remaining rats (n=10–11/group) were anesthetized
with 10% chloral hydrate (300 mg/kg intraperitoneally), which was
followed by rapid removal of the brain. The olfactory bulbs were
removed and the brains were cut coronally into two parts at the
optic nerve intersection. The first part of the tissue beginning at
the rostral end was used to produce a 10% brain homogenate with
saline, followed by centrifugation at 2,300 × g for 15 min at 4°C.
The supernatant was collected and stored at −20°C until assay
experiments. The remaining tissue was post-fixed in 4%
paraformaldehyde (cat. no. AR1068; Wuhan Boster Biological
Technology Co., Ltd., Wuhan, China) in 0.1 mol/l phosphate-buffered
saline (PBS; pH 7.4; cat. no. P1010; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) at 4°C for 1 week for
histological and immunohistochemical examination.
Detection of the levels of IL-1b,
TNF-α and IL-10 in brain homogenate
The supernatant of the brain homogenate was used to
detect the levels of IL-1β (cat. no. SXM026), TNF-α (cat. no.
SX01165) and IL-10 (cat. no. SXB005) using ELISA kits (all from
Shanghai Senxiong Biotech Industry Co., Ltd., Shanghai, China)
according to the manufacturer's protocols.
Detection of the content of NO and the
activities of total NOS (TNOS) and iNOS in brain homogenate
The content of NO in brain tissue homogenate was
assayed using the nitrate reductase method and an NO assay kit
(cat. no. A012; Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer's instructions. The
level was expressed as µmol/g protein. The activities of TNOS and
iNOS were measured using TNOS and iNOS assay kits (NOS kit, cat.
no. A014-1; TP kit, cat. no. A045-2; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's instructions; results are expressed as U/mg
protein.
Hematoxylin and eosin (H&E)
staining
A total of 6 fixed brain tissues were randomly
selected from each group, dehydrated with graded alcohol series
(80, 95 and 100%) and embedded in paraffin. Then, sliced into
coronal 5-µm-thick sections, at least 6 from every sample. Then
standard H&E staining was performed according to H&E
staining kit (cat. no. G1120; Beijing Solarbio Science &
Technology Co., Ltd.). Briefly, the sections were dewaxed with
dimethylbenzene (Yantai Shuangshuang Chemical Co., Ltd., Yantai,
China), and the slices were rehydrated with graded alcohol series
(100, 95, 80 and 70%) for 5 min in each concentration and washed
with running water twice in turn. Then, the slices were immersed in
Harris hematoxylin for 5 min, differentiated for 0.5 min, and
immersed in tap water for 15 min to return the nuclei to blue.
Next, the slices are immersed in 70–80% alcohol for 1 min and in
0.5% eosin solution for 2 min. dehydrated with graded alcohol
series (95 and 100%) for 2 min in each concentration, cleared in
dimethylbenzene and sealed with neutral balsam. All of the steps
above were carried out at room temperature. Neuronal death in the
cortical subfield of the ischemia side was evaluated under a light
microscope in 2 high power microscopic fields (magnification, ×400;
Olympus BX50; Olympus Corporation, Tokyo, Japan). They were then
scored with the following histological grading system (29): I, a few neurons damaged; II,
numerous neurons damaged; III, majority of neurons damaged; IV,
vast majority of neurons damaged.
Immunohistochemical staining
The remaining paraffin-embedded sections were used
to detect the expressions of BDNF, GDNF and VEGF. Briefly, 3
sections were deparaffinized, rehydrated with a graded alcohol
series and washed with PBS. Then the sections were incubated in 3%
H2O2 at room temperature for 10 min to quench
the endogenous peroxidase activity and rinsed in PBS. Following
these procedures, an antigen retrieval procedure was performed by
treating the samples in 0.01 M sodium citrate buffer solution (pH
6.0, cat. no. C1120; Beijing Solarbio Science & Technology Co.,
Ltd.) at 98–100°C for 20 min. Samples were then subjected to
incubation in 5% normal goat serum (Wuhan Boster Biological
Technology Co., Ltd.) in 0.01 M PBS for 30 min at room temperature.
Sections were incubated with rabbit polyclonal anti-BDNF, anti-GDNF
or anti-VEGF antibodies (anti-BDNF; cat. no. BA0565-1; anti-GDNF;
cat. no. BA0890 and anti-VEGF, cat. no. BA0407; all 1:50 and from
Wuhan Boster Biological Technology Co., Ltd.), respectively, in PBS
for 2 h at 37°C. The negative control sections were incubated with
5% normal goat serum for 2 h at 37°C. Sections were then incubated
with biotin-labeled secondary antibody (polyclonal goat anti-rabbit
IgG/streptavidin antibody; 1:50; OriGene Technologies, Inc.,
Beijing, China) at 37°C for 30 min, and then incubated with a
Strept-Avidin-Biotin-Peroxidase complex (SABC-POD; cat. no. SA1025;
Wuhan Boster Biological Technology Co., Ltd.) for 30 min at 37°C.
The results were visualized using a diaminobenzidine kit (DAB, cat.
no. AR1022, Wuhan Boster Biological Technology Co., Ltd.). Finally,
the sections were mounted onto polylysine-coated slides and all
slides were photographed under a light microscope (magnification,
×400; Olympus BX50). The intensity of BDNF, GDNF and VEGF in the
cortex and hippocampus CA1 in each section was evaluated using
Image-Pro Plus, version 5.1 (Media Cybernetics, Inc., Rockville,
MD, USA) in two high power microscopic fields, and expressed as the
positive signal area and the integrated optical density.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). Enumeration
data were analyzed using the Kruskal-Wallis test, and the
Mann-Whitney U-test was conducted for comparisons between groups.
Measurement data were analyzed by a one-way analysis of variance
and a least significant difference post hoc test was used for
comparisons between groups. Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
IPTF treatment reduces brain damage in
cerebral ischemic rats induced by MCAO
Neurological deficit scores, cerebral infarct volume
and brain histopathology were investigated to determine the
neuroprotective effects of IPTF in cerebral ischemic rats induced
by MCAO. The results of the neurological deficit scores are
displayed in Table I. The
neurological deficit score of the model group was 2.50±0.71 and
when compared with the sham operation group there was a significant
difference (P<0.01). Also, when compared with the model group,
pretreatment with 200 mg/kg IPTF significantly reduced the
impairment in neurological function induced by I/R (IPTF-1 group;
neurological deficit score, 1.80±0.63; P<0.05). The results
indicated that IPTF pretreatment may improve behavioral injury in
rats following MCAO.
 | Table I.Effect of IPTF on the neurological
deficit scores of rats following MCAO. |
Table I.
Effect of IPTF on the neurological
deficit scores of rats following MCAO.
| Group | n | NDS |
|---|
| Sham | 11 |
0.00±0.00a |
| Model | 10 | 2.50±0.71 |
| IPTF-1 | 10 |
1.80±0.63b |
| IPTF-2 | 11 | 2.00±0.79 |
The cerebral infarct volume was determined by TTC
staining. The infarcted tissue was stained white and normal tissues
were red. When compared with the model group, IPTF pretreatment
(200 and 100 mg/kg) significantly reduced the infarct volume
induced by MCAO (Fig. 1A and
B).
 | Figure 1.Effect of IPTF on cerebral infarction
and the histological grades in the cortex of MCAO model rats. (A)
Representative 2,3,5-triphenyltetrazolium chloride-stained brain
coronal sections obtained from the sham operated, model (MCAO
operated), IPTF-1 (200 mg/kg pretreatment) and IPTF-2 (100 mg/kg
pretreatment) groups. The normal tissue was stained red while the
infarct area was white. (B) v rate identified in the four groups.
Results were quantified using ImageJ software and values are
expressed as the mean ± standard deviation. (C) Neuronal death in
the cortical subfield of ischemia side was evaluated under a light
microscope and scored by histological grade (n=6 random rat brain
tissues/group). Data were analyzed using the Kruskal-Wallis test.
The number of rat tissues exhibiting each histological grade is
presented. The following histological grading system was used: I, a
few neurons damaged; II, numerous neurons damaged; III, majority of
neurons damaged; IV, vast majority of neurons damaged. *P<0.05
and **P<0.01 vs. model group. IPTF, Ilex pubescens total
flavonoids; MCAO, middle cerebral artery occlusion; IPTF-1, rats
pretreated with 200 mg/kg IPTF; IPTF-2, rats pretreated with 100
mg/kg IPTF. |
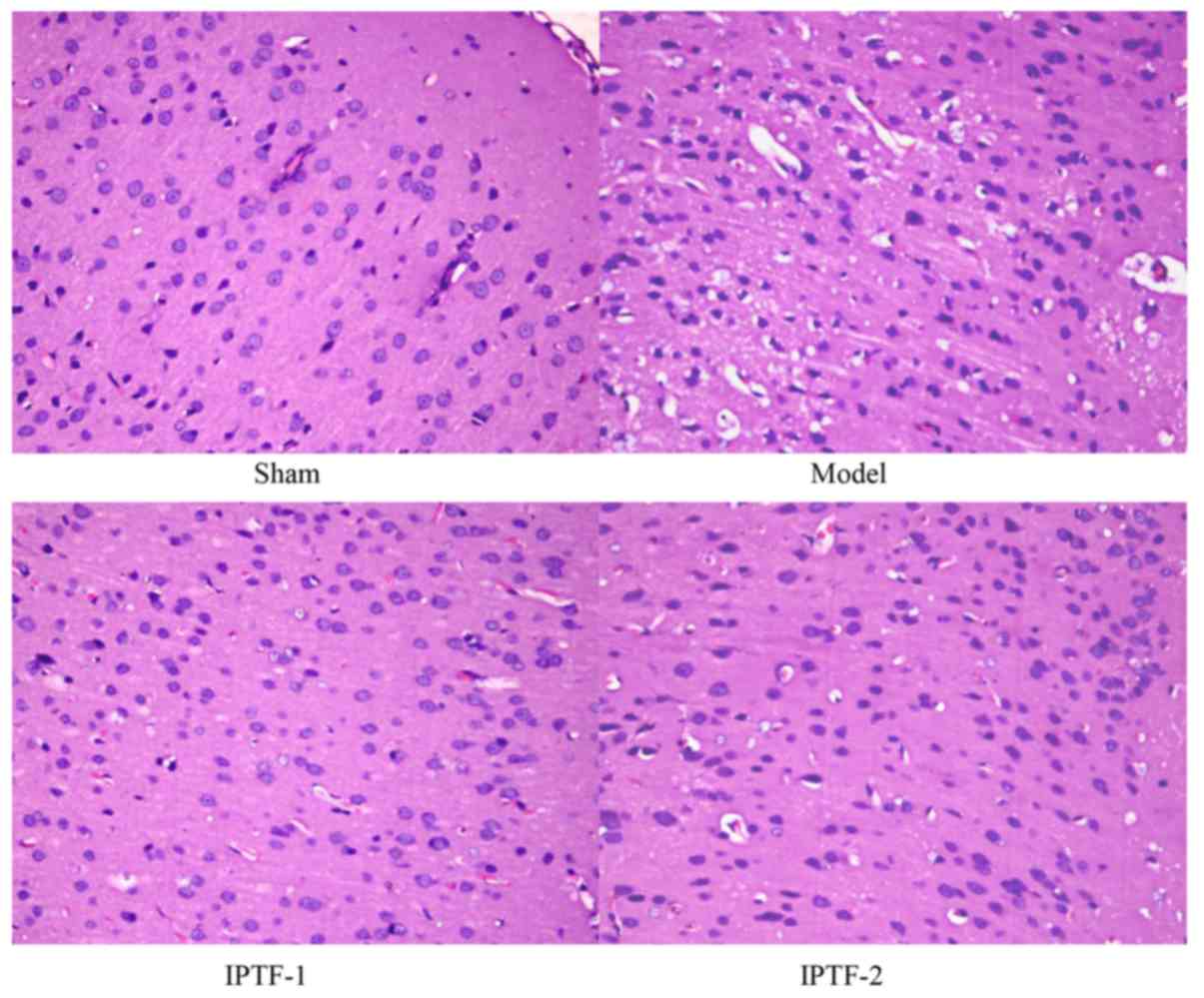
The results of H&E staining revealed that the
cell membrane and nucleus of neurons in the cerebral cortex
subfield were clearly visible in the sham operation group. When
compared with the sham operation group, marked pathological lesions
were visible in the I/R model group. Neurons that were swollen,
atrophic, lightly stained and undergoing necrosis were observed in
the I/R model group. Pathological lesions induced by cerebral
ischemia were markedly reduced when compared with the model group
in the IPTF pretreatment groups. The results of the corresponding
histological grading supported the histological results, the number
of neurons with edema, that were atrophic or necrotic, were reduced
in the IPTF pretreatment groups, particularly in the high-dose IPTF
group (IPTF-1; P<0.05; Figs. 1C
and 2). These results illustrated
that pretreatment with IPTF may reduce brain injury induced by MCAO
in rats.
Effects of IPTF on the inflammatory
response in cerebral ischemic injury
The levels of IL-1β, IL-10 NO and TNF-α in rat
brains were detected in order to determine the influence of IPTF on
the inflammatory response in cerebral I/R injury. The results are
presented in Table II. The levels
of IL-1β and TNF-α in the ischemic hemispheres of the model group
were significantly increased when compared with the sham operation
group (P<0.05 and P<0.01, respectively). The level of IL-1β
in the IPTF-1 treatment group (200 mg/kg IPTF) was significantly
decreased when compared with the model group (P<0.05). In
addition, the levels of TNF-α were significantly decreased in the
two IPTF pretreatment groups (P<0.05) when compared with the
model group. By contrast, the expression of IL-10 in the model
group exhibited no significant change when compared with the sham
operation group. The 100 mg/kg IPTF pretreatment group had a
tendency to increased expression of IL-10, but no significant
difference (P>0.05) was observed when compared with the model
group. However, it was significantly increased in the 200 mg/kg
IPTF treatment group (IPTF-1; P<0.05; Table II).
 | Table II.Effect of IPTF on the content of
IL-1β, TNF-α and IL-10 in the brain tissue of rats with middle
cerebral artery occlusion. |
Table II.
Effect of IPTF on the content of
IL-1β, TNF-α and IL-10 in the brain tissue of rats with middle
cerebral artery occlusion.
| Group | n | IL-1β (ng/g
prot) | TNF-α (ng/g
prot) | IL-10 (ng/g
prot) |
|---|
| Sham | 10 |
9.83±2.76a |
2.00±0.95b |
9.34±4.06 |
| Model | 9 |
13.01±2.86 |
4.24±1.61 |
12.00±3.24 |
| IPTF-1 | 9 |
9.28±3.89a |
2.85±1.19a |
19.75±10.07a |
| IPTF-2 | 9 |
11.85±3.34 |
2.84±1.54a |
18.08±10.38 |
NO, as a pre-inflammatory mediator, is derived from
NOS and serves an important role in cerebral ischemia and the
effects of an ischemic insult (30). Table
III indicates that the levels of TNOS, iNOS, constitutive NOS
(cNOS) and NO in the ischemic hemispheres were significantly
increased in model group when compared with the sham operation
group. In addition, IPTF pretreatment (100 and 200 mg/kg)
significantly downregulated the levels of TNOS, iNOS, cNOS and NO
in the ischemic hemispheres of rats when compared with the model
group (P<0.05 and P<0.01; Table III).
 | Table III.Effect of IPTF on the activities of
total-, induced- and constitutive nitric oxide synthase, and the
content of nitric oxide in brain tissues of rats with middle
cerebral artery occlusion. |
Table III.
Effect of IPTF on the activities of
total-, induced- and constitutive nitric oxide synthase, and the
content of nitric oxide in brain tissues of rats with middle
cerebral artery occlusion.
| Group | n | TNOS (U/mg
prot) | iNOS (U/mg
prot) | cNOS (U/mg
prot) | NO (µmol/g
prot) |
|---|
| Sham | 11 |
1.86±0.79a |
0.49±0.23a |
1.02±0.32a |
5.64±6.11a |
| Model | 10 |
2.85±0.53 |
0.89±0.26 |
2.04±0.59 |
14.96±5.26 |
| IPTF-1 | 10 |
1.58±0.70a |
0.50±0.17a |
0.90±0.48a |
10.27±4.00b |
| IPTF-2 | 11 |
1.48±0.40a |
0.41±0.19a |
1.08±0.43a |
9.57±4.32 |
In conclusion, the results demonstrated that IPTF
reduced the inflammatory response by regulating the levels of
IL-1β, IL-10, TNF-α and NO and the activity NOS against ischemic
injury.
Effects of IPTF on the expression of
BDNF, GDNF and VEGF in the cortex and hippocampus CA1 subfields of
MCAO rats
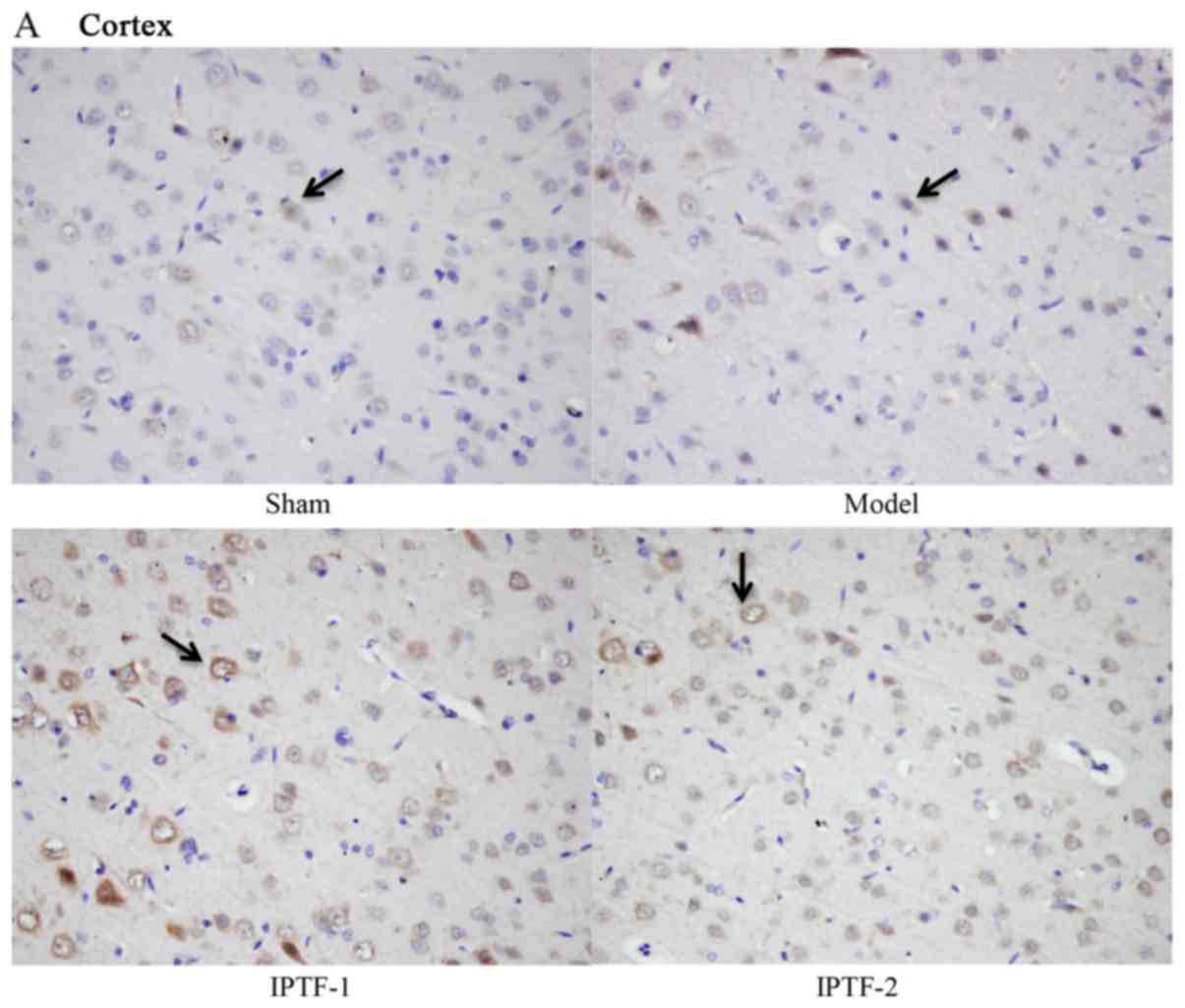
The expressions of BDNF, GDNF and VEGF in the cortex
and hippocampus CA1 subfields were detected by immunohistochemical
staining. Immunohistochemistry demonstrated that IPTF pretreatment
significantly increased the expression of BDNF, GDNF and VEGF in
cortex and hippocampus CA1 subfields of ischemic hemispheres when
compared with the model group (P<0.05 and P<0.01; Figs. 3–5).
Discussion
Mao Dong Qing is derived from the dry roots of
Ilex pubescens Hook et Arn. The extracts prepared from the
Ilex pubescens roots have a number of biological activities
including analgesic, dilating blood vessels, reducing plasma
viscosity, inhibiting platelet aggregation, thrombus and enhancing
hypoxia-resistance ability (17,31–34).
The authors' previous studies (19–21)
demonstrated that flavonoids are the prominent active compound in
Ilex pubescens and they had neuroprotective effects against
cerebral ischemia, which, to the best of our knowledge, has not
been reported previously. However, the specific mechanisms
underlying the neuroprotective effects of Ilex pubescens
remain unknown. In the present study, the potential
anti-inflammatory and neuroprotective effects of IPTF were
examined.
It was observed that IPTF pretreatment effectively
improved neurological deficits, reduced cerebral infarct volume and
attenuated brain pathological lesions in a dose-dependent manner in
rats with cerebral I/R injury (Figs.
1 and 2; Tables I and II). Therefore, these results
observations indicate that IPTF has neuroprotective effects on the
cerebral ischemic injury.
The inflammatory response is partly attributable to
the pathogenic progression of cerebral ischemic injury, and the
associated inflammatory cells and mediators (35). It is well known that cytokines are
a group of small glycoproteins (~25 kDa) that serve important roles
in the immune response. Cytokines are produced in response to a
variety of stimuli including pathogens, inflammation or injury, and
exert their effects by binding to their receptors. The major
cytokines associated with inflammation in cerebral ischemic injury
are IL-1β, TNF-α and IL-10 (36).
Under normal conditions, there is a balance between the
pro-inflammatory cytokines IL-1β and TNF-α, and the
anti-inflammatory cytokine IL-10. Inflammatory cells secrete
proinflammatory and anti-inflammatory cytokines during cerebral
ischemic injury; their interactions determine the progression of
inflammation. When excessive levels of pro-inflammatory cytokines
are released as opposed to anti-inflammatory cytokines,
inflammation is promoted, thus inducing tissue damage. The levels
of IL-1β and TNF-α were upregulated following MCAO or global brain
ischemia, however, the level of IL-10 was downregulated (37). A previous study demonstrated that
the proinflammatory cytokines IL-1β and TNF-α were important
mediators involved in brain injury, and IL-10 was observed to
inhibit the production of pre-inflammatory cytokines, thereby
exhibiting neuroprotective properties (38). The present study confirmed that
IL-1β and TNF-α were induced by ischemia; IL-1β was downregulated
in the brain tissues of the IPTF-1 group (200 mg/kg IPTF
pretreatment) and TNF-α was downregulated in the brain tissues of
both IPTF pretreatment groups (200 and 100 mg/kg; IPTF-1 and −2,
respectively). The level of IL-10 in the brain tissue increased in
response to cerebral ischemic injury when comparing the model and
sham operation groups, however there was no significant difference,
while the levels significantly increased when pretreated with 200
mg/kg IPTF (IPTF-1 vs. model group).
NO is a pre-inflammatory mediator derived from NOS
that is involved in inflammation during cerebral ischemic injury.
There are at least two isoforms of NOS: cNOS and iNOS (39–41).
The cNOS class is comprised of endothelial constitutive NOS (eNOS),
which is present in vascular endothelial cells, and nervous
constitutive NOS (nNOS), which is present in the central and
peripheral nervous system (42,43).
eNOS and nNOS are predominantly expressed in the early phase of the
ischemia, whereas iNOS is primarily expressed in the late phase. In
the early phase of cerebral ischemia, NO is predominantly produced
by nNOS to cause brain damage, and in the late phase, NO is
predominantly produced by iNOS (44). In addition, iNOS mediates cerebral
ischemic injury, potentially via the action of NO on the
mitochondrial respiratory chain, resulting in energy depletion
(30). A previous study has
indicated that the expression levels of iNOS and nNOS increased,
however, eNOS expression decreased in MCAO rats (45). A nNOS blocker, 7-NI, has been
observed to attenuate the disruption in the blood-brain barrier
following transient focal cerebral ischemia; it also improved
neurological deficits and reduced the area of cerebral infarction
(46,47). In the present study, the level of
NO, and the activities of TNOS, iNOS and cNOS were detected 24 h
following the induction of cerebral ischemia. The levels of NO,
TNOS, iNOS and cNOS significantly increased in the MCAO model
group, which is consistent with the results of previous studies
(8–10). The levels of NO, TNOS, iNOS and
cNOS significantly decreased in the two IPTF pretreatment groups
when compared to the model group. These results indicated that the
neuroprotective effects of IPTF might be achieved via a decrease in
the levels of inflammation-associated molecules in brain
tissues.
Neurotrophins are a family of peptide growth factors
that include BDNF, GDNF and VEGF. These growth factors have been
demonstrated to protect neurons from damage, and are therefore very
important in treatments for neurological disorders including
Parkinson's disease, ischemic stroke and Alzheimer's disease
(11,13,48–53).
Neurotrophins have also been reported to improve angiogenesis,
neurogenesis and neurite outgrowth in the brain, and overexpression
of these factors has demonstrated their therapeutic actions in
cerebral ischemia (54). BDNF was
first identified in cerebral ischemia, promoting neurological
function recovery and improving neurogenesis (55).
Previous research has demonstrated that the
mechanism underlying the neuroprotective effect of GDNF is
associated with antiapoptosis and antioxidative effects, and also
the induction of progenitor cell proliferation in rats with stroke
(56–58). A previous report indicated that
BDNF and GDNF may be potential therapeutic target proteins for
ischemic stroke (59). Therefore,
the present study investigated the changes in BDNF and GDNF
expression in the cortex and hippocampus CA1 of MCAO rats
pretreated with IPTF. The results indicated that the expression of
BDNF and GDNF in the cortex and hippocampus CA1 subfields were
upregulated in the two IPTF groups following cerebral I/R. Taking
the results of the aforementioned previous studies into account, it
was proposed that the effect of IPTF on reducing neuronal necrosis
may be associated with the upregulation of BDNF and GDNF
expression.
A previous study indicated that the expression of
VEGF increases following cerebral I/R (60). VEGF is a factor associated with
angiogenesis and vascular permeability. Hypoxic conditions can
induce the transcriptional and post-transcriptional regulation of
VEGF expression in the brain tissue, which is associated with an
increase in angiogenesis (61–64).
The expression of VEGF is also increased follow cerebral ischemia,
which accelerates the formation of novel blood vessels in the brain
(65,66). It has been reported that the
neuroprotective effects of VEGF are primarily focused on activating
the phosphoinositide 3-kinase/protein kinase B pathway, inhibiting
the activation of caspase-3, the specific potassium currents, the
extracellular signal-regulated kinase signaling pathway and the
endoplasmic reticulum stress pathway, and increasing the
proliferation, migration and differentiation of neuronal
progenitors simultaneously (13).
In the present study, the expression of VEGF was observed in the
hippocampus CA1 and cortex and the findings were consistent with
those of previous reports (67,68).
The results demonstrated that the expression of VEGF in the
hippocampus CA1 and cortex were upregulated in the MCAO model
group, particularly in the cortex, which was significantly
different to the expression observed in the sham operation group.
In addition, pretreatment with IPTF significantly upregulated the
expression of VEGF in comparison with the model group. These
results indicated that the neuroprotective effects of IPTF may be
achieved via the upregulated expression of VEGF in the hippocampus
CA1 and cortex.
The recovery of nervous function is a complex
process, which is the result of interactions between a number of
factors (69–74). The behavioral tests used in the
present study were designed to evaluate the level of brain damage
in rats, and neurological deficit scores were significantly
decreased in rats pretreated with a high dose of IPTF. In addition,
IPTF ameliorated the histological injury in brain tissues. In
conclusion, the results of the present study suggested that IPTF
pretreatment may have neuroprotective effects in cerebral ischemic
injury, which may be closely associated with the decreased
production of certain proinflammatory cytokines including NO, IL-1β
and TNF-α, the increased production of the anti-inflammatory
cytokine IL-10, the inhibition of TNOS, iNOS and cNOS activities,
and the upregulation of BDNF, GDNF and VEGF expression. With
further elucidation of its underlying mechanisms of action and
clinical verification, IPTF may be a potential neuroprotective
treatment for cerebral ischemic injuries.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. U1204827 and
81503206) and the Fundamental Scientific Research Funds for the
Henan Universities (grant no. 2014KYYWF-QN27). The authors would
like to thank Professor Chengming Dong and Professor Suiqing Chen
(Department of Medicinal Plants, School of Pharmacy, Henan
University of Chinese Medicine, Zhengzhou, China) for identifying
the plant material.
Glossary
Abbreviations
Abbreviations:
|
I/R
|
ischemia/reperfusion
|
|
IPTF
|
Ilex pubescens total
flavonoids
|
|
MCAO
|
middle cerebral artery occlusion
|
|
CCA
|
common carotid artery
|
|
ICA
|
internal carotid artery
|
|
TTC
|
2,3,5-triphenyltetrazolium
chloride
|
|
NOS
|
nitric oxide synthase
|
|
TNOS
|
total NOS
|
|
iNOS
|
induced NOS
|
|
cNOS
|
constitutive NOS
|
|
eNOS
|
endothelial constitutive NOS
|
|
nNOS
|
nervous constitutive NOS
|
|
NO
|
nitric oxide
|
|
IL-1β
|
interleukin-1β
|
|
TNF-α
|
tumor necrosis factor-α
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
VEGF
|
vascular endothelial growth factor
|
|
GDNF
|
glial cell-derived neurotrophic
factor
|
|
TCM
|
traditional Chinese medicine
|
References
|
1
|
Zhang Y, Zhang S, Li H, Huang M, Xu W, Chu
K, Chen L and Chen X: Ameliorative effects of Gualou Guizhi
decoction on inflammation in focal cerebral ischemic-reperfusion
injury. Mol Med Rep. 12:988–994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duong TT, Chami B, McMahon AC, Fong GM,
Dennis JM, Freedman SB and Witting PK: Pre-treatment with the
synthetic antioxidant T-butyl bisphenol protects cerebral tissues
from experimental ischemia reperfusion injury. J Neurochem.
130:733–747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu LP, Xu AD, Wong LK, Wang DZ and Wang
YJ: and Expert consensus group of the evaluation & intervention
of collateral circulation for ischemic stroke: Chinese consensus
statement on the evaluation and intervention of collateral
circulation for ischemic stroke. CNS Neurosci Ther. 20:202–208.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marmagkiolis K, Hakeem A, Cilingiroglu M,
Gundogdu B, Iliescu C, Tsitlakidou D and Katramados A: Safety and
efficacy of stent retrievers for the management of acute ischemic
stroke: Comprehensive review and meta-analysis. JACC Cardiovasc
Interv. 8:1758–1765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asdaghi N and Romano JG: Dual antiplatelet
therapy in acute ischemic stroke. Curr Atheroscler Rep. 17:372015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Niu PP, Guo ZN, Jin H, Xing YQ and Yang Y:
Antiplatelet regimens in the long-term secondary prevention of
transient ischaemic attack and ischaemic stroke: An updated network
meta-analysis. BMJ Open. 6:e0090132016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brouns R and De Deyn PP: The complexity of
neurobiological processes in acute ischemic stroke. Clin Neurol
Neurosurg. 111:483–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu XM, Zhou MM, Hu XM and Zeng FD:
Neuroprotective effects of scutellarin on rat neuronal damage
induced by cerebral ischemia/reperfusion. Acta Pharmacol Sin.
26:1454–1459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung YS, Park JH, Kim H, Kim SY, Hwang JY,
Hong KW, Bae SS, Choi BT, Lee SW and Shin HK: Probucol inhibits
LPS-induced microglia activation and ameliorates brain ischemic
injury in normal and hyperlipidemic mice. Acta Pharmacol Sin.
37:1031–1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang F, Li N, Jiang L, Chen L and Huang
M: Neuroprotective effects of (−)-epigallocatechin-3-gallate
against focal cerebral ischemia/reperfusion injury in rats through
attenuation of inflammation. Neurochem Res. 40:1691–1698. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mattson MP, Maudsley S and Martin B: BDNF
and 5-HT: A dynamic duo in age-related neuronal plasticity and
neurodegenerative disorders. Trends Neurosci. 27:589–594. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Lan R, Wang J, Li XY, Zhu DN, Ma
YZ, Wu JT and Liu ZH: Acupuncture reduced apoptosis and
up-regulated BDNF and GDNF expression in hippocampus following
hypoxia-ischemia in neonatal rats. J Ethnopharmacol. 172:124–132.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Guo L, Liu R and Liu H:
Neuroprotective effects of VEGF administration after focal cerebral
ischemia/reperfusion: Dose response and time window. Neurochem Int.
60:592–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miao M, Zhang X and Wang L: Persimmon leaf
flavonoid induces brain ischemic tolerance in mice. Neural Regen
Res. 8:1376–1382. 2013.PubMed/NCBI
|
|
15
|
Hsu SC and Chung JG: Anticancer potential
of emodin. BioMedicine. 2:108–116. 2012. View Article : Google Scholar
|
|
16
|
Hao JX and Yang CZ: Research progress on
pharmacological action and clinical application of Ilex pubescens.
Heilongjiang Med J. 23:592–593. 2010.(In Chinese).
|
|
17
|
Xiong H, Zhao F, Bi J, Zhang Y, Zhao G,
Chen X, Li Y, Yan R, Zhao Q, Qiao H and Zhang G: Two new compounds
from the roots of Ilex pubescens and their cytotoxic activity. J
Nat Med. 70:673–678. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhonghuabencao SAoTCMotPsRoCECo:
Zhonghuabencao. Shanghai Science and Technology Press; Shanghai:
1997
|
|
19
|
Zhang F, Zhang XL and Miao MS: Effect of
flvonoids of Ilex pubescens on blood stasis with cerebral ischemia.
Chin J Exp Tradit Med Form. 18:187–191. 2012.(In Chinese).
|
|
20
|
Cheng X, Zhang XL and Miao MS: Effect of
total flvonoids from radix Ilecis pubescens on rat cerebral
ischemia models. Tradit Chin Drug Res Pharmacol. 23:640–643.
2012.(In Chinese).
|
|
21
|
Zhang F, Zhang XL and Miao MS: Effect on
total flvonoids of radix Ilicis pubescentis in mice models of
cerebral ischemia. Tradit Chin Drug Res Pharmacol. 23:409–412.
2012.(In Chinese).
|
|
22
|
Feng SX, Miao MS, Miao JX, Xu P, Shao P
and Hou CJ: Simultaneous separation and purification technology of
total flavonoids and total saponins from Ilex pubescens by AB-8
macroporous resin. Chin J Exper Trad Med Form. 1–8. 2012.
|
|
23
|
Xu YH, Miao MS, Xu P and Feng SX:
Determination on content of total flavoniods in the effective
fraction of Ilex pubescens Hook. Chin J Chin Med. 1–827. 2011.
|
|
24
|
Koizumi J, Yoshida Y, Nakazawa T and
Ooneda G: Experimental studies of ischemic brain edema: I. A new
experiment model of cerebral embolism in rats in which
recirculation can be introduced in the ischemic area. Jpn J Stroke.
8:1–8. 1986. View Article : Google Scholar
|
|
25
|
Nagasawa H and Kogure K: Correlation
between cerebral blood flow and histologic changes in a new rat
model of middle cerebral artery occlusion. Stroke. 20:1037–1043.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Belayev L, Alonso OF, Busto R, Zhao W and
Ginsberg MD: Middle cerebral artery occlusion in the rat by
intraluminal suture. Neurological and pathological evaluation of an
improved model. Stroke. 27:1616–1622. 1996.discussion 1623.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Shuaib A and Li Q: Quantification
of infarct size on focal cerebral ischemia model of rats using a
simple and economical method. J Neurosci Methods. 84:9–16. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kato H, Liu Y, Araki T and Kogure K:
Temporal profile of the effects of pretreatment with brief cerebral
ischemia on the neuronal damage following secondary ischemic insult
in the gerbil: Cumulative damage and protective effects. Brain Res.
553:238–242. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodrigo J, Fernández AP, Serrano J,
Peinado MA and Martínez A: The role of free radicals in cerebral
hypoxia and ischemia. Free Radic Biol Med. 39:26–50. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang JR, Zhou H, Jiang ZH, Wong YF and Liu
L: In vivo anti-inflammatory and analgesic activities of a purified
saponin fraction derived from the root of Ilex pubescens. Biol
Pharm Bull. 31:643–650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujimoto T, Fujimura K and Kuramoto A:
Electrophysiological evidence that glycoprotein IIb-IIIa complex is
involved in calcium channel activation on human platelet plasma
membrane. J Biol Chem. 266:16370–16375. 1991.PubMed/NCBI
|
|
33
|
Xing XD, Zhang Q, Feng F and Liu WY:
Chemical constituents from stems of Ilex pubescens. Zhong Yao Cai.
35:1429–1431. 2012.PubMed/NCBI
|
|
34
|
Yang ML and Pang PK: The vascular effects
of Ilex pubescens. Planta Med. 4:262–265. 1986. View Article : Google Scholar
|
|
35
|
Muir KW, Tyrrell P, Sattar N and Warburton
E: Inflammation and ischaemic stroke. Current opinion in neurology.
20:334–342. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han HS and Yenari MA: Cellular targets of
brain inflammation in stroke. Curr Opin Investig Drugs. 4:522–529.
2003.PubMed/NCBI
|
|
37
|
Hansel G, Tonon AC, Guella FL, Pettenuzzo
LF, Duarte T, Duarte MMMF, Oses JP, Achaval M and Souza DO:
Guanosine protects against cortical focal ischemia. Mol Neurobiol.
52:1791–1803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhu Y, Chen X, Liu Z, Peng YP and Qiu YH:
Interleukin-10 protection against lipopolysaccharide-induced
neuro-inflammation and neurotoxicity in ventral mesencephalic
cultures. Int J Mol Sci. 17(pii): E252015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moncada S, Palmer RM and Higgs EA: Nitric
oxide: Physiology, pathophysiology, and pharmacology. Pharmacol
Rev. 43:109–142. 1991.PubMed/NCBI
|
|
40
|
Hibbs JB Jr, Taintor RR, Vavrin Z and
Rachlin EM: Nitric oxide: A cytotoxic activated macrophage effector
molecule. Biochem Biophys Res Commun. 157:87–94. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Förstermann U, Schmidt HH, Pollock JS,
Sheng H, Mitchell JA, Warner TD, Nakane M and Murad F: Isoforms of
nitric oxide synthase. Characterization and purification from
different cell types. Biochem Pharmacol. 42:1849–1857. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bredt DS and Snyder SH: Isolation of
nitric oxide synthase, a calmodulin requiring enzyme. Proc Natl
Acad Sci USA. 87:682–685. 1990; View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Knowles RG, Palacios M, Palmer RM and
Moncada S: Kinetic characteristics of nitric oxide synthase from
rat brain. Biochem J. 269:207–210. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hashiguchi A, Yano S, Morioka M, Hamada J,
Ushio Y, Takeuchi Y and Fukunaga K: Up-regulation of endothelial
nitric oxide synthase via phosphatidylinositol 3-kinase pathway
contributes to ischemic tolerance in the CA1 subfield of gerbil
hippocampus. J Cereb Blood Flow Metab. 24:271–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koh PO: Ferulic acid modulates nitric
oxide synthase expression in focal cerebral ischemia. Lab Anim Res.
28:273–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jiang Z, Li C, Arrick DM, Yang S, Baluna
AE and Sun H: Role of nitric oxide synthases in early blood-brain
barrier disruption following transient focal cerebral ischemia.
PLoS One. 9:e931342014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iadecola C, Zhang F, Casey R, Nagayama M
and Ross ME: Delayed reduction of ischemic brain injury and
neurological deficits in mice lacking the inducible nitric oxide
synthase gene. J Neurosci. 17:9157–9164. 1997.PubMed/NCBI
|
|
48
|
Kitagawa H, Hayashi T, Mitsumoto Y, Koga
N, Itoyama Y and Abe K: Reduction of ischemic brain injury by
topical application of glial cell line-derived neurotrophic factor
after permanent middle cerebral artery occlusion in rats. Stroke.
29:1417–1422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sheikh MA, Malik YS, Xing Z, Guo Z, Tian
H, Zhu X and Chen X: Polylysine-modified polyethylenimine (PEI-PLL)
mediated VEGF gene delivery protects dopaminergic neurons in cell
culture and in rat models of Parkinson's Disease (PD). Acta
Biomater. 54:58–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sarkar S, Raymick J and Imam S:
Neuroprotective and therapeutic strategies against parkinson's
disease: Recent perspectives. Int J Mol Sci. 17(pii): E9042016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Echeverria V, Barreto GE, Ávila-Rodriguez
M, Tarasov VV and Aliev G: Is VEGF a key target of cotinine and
other potential therapies against Alzheimer disease? Curr Alzheimer
Res. Mar 29–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nigam SM, Xu S, Kritikou JS, Marosi K,
Brodin L and Mattson MP: Exercise and BDNF reduce Aβ production by
enhancing α-secretase processing of APP. J Neurochem. 142:286–296.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wei S: Potential therapeutic action of
natural products from traditional Chinese medicine on Alzheimer's
disease animal models targeting neurotrophic factors. Fundam Clin
Pharmacol. 30:490–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shimamura M, Sato N and Morishita R:
Experimental and clinical application of plasmid DNA in the field
of central nervous diseases. Curr Gene Ther. 11:491–500. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choi Y, Kang SG and Kam KY: Changes in the
BDNF-immunopositive cell population of neocortical layers I and
II/III after focal cerebral ischemia in rats. Brain Res.
1605:76–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nicole O, Ali C, Docagne F, Plawinski L,
MacKenzie ET, Vivien D and Buisson A: Neuroprotection mediated by
glial cell line-derived neurotrophic factor: Involvement of a
reduction of NMDA-induced calcium influx by the mitogen-activated
protein kinase pathway. J Neurosci. 21:3024–3033. 2001.PubMed/NCBI
|
|
57
|
Deng HL and Zhang JT: Anti-lipid
peroxidative effect of ginsenoside Rb1 and Rg1. Chin Med J (Engl).
104:395–398. 1991.PubMed/NCBI
|
|
58
|
Kobayashi T, Ahlenius H, Thored P,
Kobayashi R, Kokaia Z and Lindvall O: Intracerebral infusion of
glial cell line-derived neurotrophic factor promotes striatal
neurogenesis after stroke in adult rats. Stroke. 37:2361–2367.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu Y, Wang S, Luo S, Li Z, Liang F, Zhu
Y, Pei Z and Huang R: Intravenous PEP-1-GDNF is protective after
focal cerebral ischemia in rats. Neurosci Lett. 617:150–155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin TN, Te J, Lee M, Sun GY and Hsu CY:
Induction of basic fibroblast growth factor (bFGF) expression
following focal cerebral ischemia. Brain Res Mol Brain Res.
49:255–265. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Raber J, Fan Y, Matsumori Y, Liu Z,
Weinstein PR, Fike JR and Liu J: Irradiation attenuates
neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol.
55:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang Z, Huang PL, Ma J, Meng W, Ayata C,
Fishman MC and Moskowitz MA: Enlarged infarcts in endothelial
nitric oxide synthase knockout mice are attenuated by
nitro-l-arginine. J Cereb Blood Flow Metab. 16:981–987. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nagayama M, Aber T, Nagayama T, Ross ME
and Iadecola C: Age-dependent increase in ischemic brain injury in
wild-type mice and in mice lacking the inducible nitric oxide
synthase gene. J Cereb Blood Flow Metab. 19:661–666. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhu DY, Liu SH, Sun HS and Lu YM:
Expression of inducible nitric oxide synthase after focal cerebral
ischemia stimulates neurogenesis in the adult rodent dentate gyrus.
J Neurosci. 23:223–229. 2003.PubMed/NCBI
|
|
65
|
Zhang ZG, Zhang L, Tsang W,
Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T
and Chopp M: Correlation of VEGF and angiopoietin expression with
disruption of blood-brain barrier and angiogenesis after focal
cerebral ischemia. J Cereb Blood Flow Metab. 22:379–392. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ogunshola OO, Stewart WB, Mihalcik V,
Solli T, Madri JA and Ment LR: Neuronal VEGF expression correlates
with angiogenesis in postnatal developing rat brain. Brain Res Dev
Brain Res. 119:139–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Krum JM, Mani N and Rosenstein JM:
Angiogenic and astroglial responses to vascular endothelial growth
factor administration in adult rat brain. Neuroscience.
110:589–604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang ZG, Zhang L, Jiang Q, Zhang R,
Davies K, Powers C, Bruggen Nv and Chopp M: VEGF enhances
angiogenesis and promotes blood-brain barrier leakage in the
ischemic brain. J Clin Invest. 106:829–838. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ishii T, Ueyama T, Shigyo M, Kohta M,
Kondoh T, Kuboyama T, Uebi T, Hamada T, Gutmann DH, Aiba A, et al:
A novel Rac1-GSPT1 signaling pathway controls astrogliosis
following central nervous system injury. J Biol Chem.
292:1240–1250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sulaiman WA: Transforming growth factor-β
promotes axonal regeneration after chronic nerve injury. Spine
(Phila Pa 1976). 41 Suppl 7:S292016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lin Q, Wong HL, Tian FR, Huang YD, Xu J,
Yang JJ, Chen PP, Fan ZL, Lu CT and Zhao YZ: Enhanced
neuroprotection with decellularized brain extracellular matrix
containing bFGF after intracerebral transplantation in Parkinson's
disease rat model. Int J Pharm. 517:383–394. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lei C, Wu B, Cao T, Liu M and Hao Z: Brain
recovery mediated by toll-like receptor 4 in rats after
intracerebral hemorrhage. Brain Res. 1632:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Furukawa S: Basic research on neurotrophic
factors and its application to medical uses. Yakugaku Zasshi.
135:1213–1226. 2015.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Stetler RA, Gao Y, Leak RK, Weng Z, Shi Y,
Zhang L, Pu H, Zhang F, Hu X, Hassan S, et al: APE1/Ref-1
facilitates recovery of gray and white matter and neurological
function after mild stroke injury. Proc Natl Acad Sci USA.
113:E3558–E3567. 2016; View Article : Google Scholar : PubMed/NCBI
|