Introduction
Cell-based therapies are a promising alternative
strategy to liver organ transplantation for the treatment of liver
disease (1,2). Stem or progenitor cells have been
considered as potential sources of cells for hepatocyte replacement
and functional recovery due to their characteristics of
self-renewal, generation of progeny and multiple-differentiation
(3,4). However, recent studies have reported
that stem cell-derived mature liver cells may be a better option
than stem cells for liver cell transplantation therapy (5,6).
Furthermore, the efficiency of hepatic differentiation of stem
cells is low, and although the induced cells express liver-specific
markers, they fail to exhibit adequate hepatocyte functionality
(7–9). Thus, an effective and reliable method
to induce hepatic differentiation of stem cells is increasingly
important and needs to be investigated.
In preliminary work, a relatively valid method to
induce hepatic differentiation and maturation of hepatic progenitor
cells (HPCs) was reported. The combination of 2% horse serum (HS),
0.1 µmol/l dexamethasone (Dex), 10 ng/ml hepatic growth factor
(HGF) and 20 ng/ml fibroblast growth factor 4 (FGF4) effectively
induced the maturation and function of HPCs, but resulted in fewer
periodic acid-Schiff (PAS) stain positive cells compared with the
untreated group (10,11). Therefore, PAS staining may not be a
suitable method for testing the hepatocyte function of induced stem
cells. PAS staining is an important method to mark carbohydrates,
and is commonly used to evaluate glycogen storage and function of
mature hepatocytes (12,13). To allow the use of this important
experimental approach in the detection of functional hepatocytes
following the induction of differentiation, the possible reasons
need to be elucidated.
In the present study, 2% HS was demonstrated to be
an important factor affecting PAS staining results, but did not
affect the differentiation, maturation and function of induced
HPCs. By replacing the induction medium with media containing 10%
fetal bovine serum (FBS) following induction, glycogen synthesis
and accumulation of HPCs may be recovered, which may restore the
ability to perform PAS staining. The present study may, therefore,
aid in the application of the PAS staining method during detection
of induced hepatocytes cultured in 2% HS.
Materials and methods
Cell culture and chemicals
Hepatic progenitor cells were obtained from the
livers of mouse embryos at 14.5 days post coitus and named as
hepatic progenitor 14.5d (HP14.5d) cells, as described by Bi et
al (14). Cells were
maintained in complete Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 units/ml penicillin and 100 µg/ml streptomycin at 37°C in 5%
CO2. HP14.5d cells were cultured with 0.1 µmol/l Dex, 10
ng/ml HGF and 20 ng/ml FGF4 in DMEM containing 2% HS (Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a 5% CO2 atmosphere
for 12 days to induce differentiation, as previously described
(11). To detect the effect of
serum change on the function and PAS staining result of induced
cells, the induction medium was replaced with DMEM supplemented
with 10% FBS, 0.1 µmol/l Dex, 10 ng/ml HGF and 20 ng/ml FGF4.
Unless otherwise indicated, all chemicals were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Gaussia luciferase reporter assay
(Gluc assay)
Prior to induction, HP14.5d cells (8×104)
were seeded in 24-well culture plates at an initial confluence of
30% and transfected with a homemade plasmid containing an albumin
(ALB) promoter-driven luciferase reporter gene (pSEB-ALB-Gluc),
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) as the transfection reagent (15). Briefly, the ALB promoter was
amplified by polymerase chain reaction and inserted into the
multi-cloning site of a pBGLuc vector, as previously described
(14,15). The sequence of the pBGLuc plasmid
sequence can be accessed at: http://www.boneandcancer.org/MOLab%20Vectors%20after%20Nov%201%202005/pBGLuc.pdf.
At the indicated time points, culture medium was collected and GLuc
activity was assayed using the Gaussia Luciferase Assay kit (New
England Biolabs, Inc., Ipswich, MA, USA). All measurements were
performed in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Total RNA (10 mg) was reverse transcribed
into cDNA with hexamer primers using Superscript II reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.). Primers
specific for the genes of interest were designed using Primer3
software version 2.3.7 (source code available at: http://sourceforge.net/projects/primer3/) (16,17)
and are presented in Table I.
SYBR-Green-based quantitative real-time PCR analysis (Bioteke
Corporation, Beijing, China) was carried out under the following
conditions: with 40 cycles of denaturation at 94°C for 20 sec,
annealing at 55°C for 20 sec and extension at 70°C for 20 sec. Gene
expression was quantified using the 2−∆∆Cq method
(18). Data are reported as the
fold change of control, following normalization against GAPDH
expression.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers.
| Gene target | Primer sequence
(5′-3′) |
|---|
| GAPDH |
F-GGCTGCCCAGAACATCAT |
|
|
R-CGGACACATTGGGGGTAG |
| AFP |
F-ACGAGGAAAGCCCCTCAG |
|
|
R-GCCATTCCCTCACCACAG |
| ALB |
F-CCAGACATTCCCCAATGC |
|
|
R-CAAGTTCCGCCCTGTCAT |
| CK18 |
F-CTGGGCTCTGTGCGAACT |
|
|
R-ACAGAGCCACCCCAGACA |
| TAT |
F-ACCTTCAATCCCATCCGA |
|
|
R-TCCCGACTGGATAGGTAG |
Periodic acid-Schiff (PAS)
staining
Cells were seeded in 24-well plates and induced for
12 days, then fixed with 4% paraformaldehyde for 10 min. Following
washing with PBS, cells were incubated with 0.5% periodic acid
solution for 5 min, then stained with Schiff's reagent for 15 min,
followed by counterstaining with hematoxylin solution for 2 min.
All steps were performed at room temperature, and cells were rinsed
with tap water after each step. At least 10 non-overlapping fields
of view in each group were recorded under a microscope and cells
with cytoplasm stained purple-red were counted as positive.
Indocyanine green (ICG) uptake
Induced cells were washed with PBS and incubated in
complete DMEM medium containing 1 mg/ml freshly prepared ICG
reagent at 37°C for 1 h. At least 10 non-overlapping fields of view
were recorded under the microscope and cells with green nuclear
staining were counted as positive. Cells were then incubated with
ICG-free complete medium at 37°C for 6 h to detect the function of
ICG release.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS software version 19.0 (IBM SPSS,
Armonk, NY, USA). The statistical significance of the differences
between groups was assessed using a two-tailed Student's t-test for
pair-wise comparisons, or a one-way analysis of variance followed
by a post hoc Student-Newman-Keuls test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Successful induction of HPCs
maturation and differentiation in vitro
The differentiation of HP14.5d cells was evaluated
by exploring morphological and hepatic related markers. Uninduced
HP14.5d cells exhibited large, elongated and irregular polygonal
morphologies with one or two nuclei (Fig. 1A, top panel). Following 3 days of
induction, 80% of treated cells fused; all cells were fused on day
6. On induction day 12, the induced cells were arranged closely
like paving stones and the nucleus/cytoplasm (N/C) ratio was
decreased (Fig. 1A, bottom panel).
However, the increase in cell density in the induced group was
smaller compared with the control group (Fig. 1A), potentially due to the low serum
concentration in the induction medium.
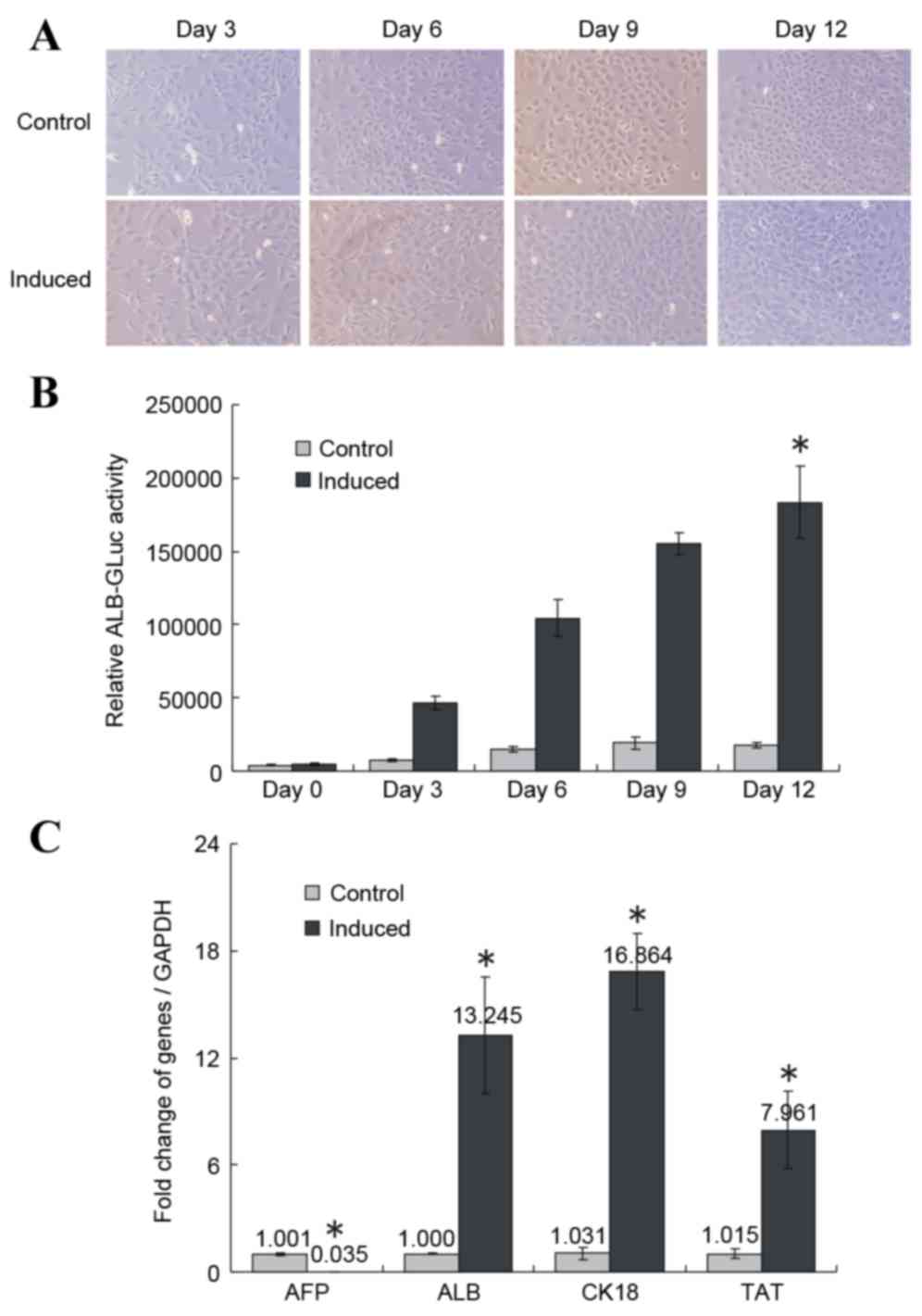 | Figure 1.Induced differentiation of HP14.5d
cells in vitro. (A) Cell morphology of uninduced control and
induced HP14.5d cells following 3,6, 9 and 12 days of induction
(original magnification, ×200). (B) ALB-GLuc activity in HP14.5d
cells following 3, 6, 9 and 12 days of induction. *P<0.05 vs.
control (n=3). (C) Semi-quantitative RT-PCR analysis of mRNA
expression levels of hepatic related genes AFP, ALB, CK18 and TAT.
RT-PCR was performed following 12 days of induction. *P<0.05 vs.
control (n≥3). ALB, albumin; GLuc, Gaussia luciferase;
RT-PCR, reverse transcription-polymerase chain reaction; AFP, α
fetoprotein; CK18, keratin 18; TAT, tyrosine aminotransferase. |
To detect relative ALB expression levels, the
pSEB-ALB-GLuc reporter plasmid was transfected into the HP14.5d
cells prior to induction. Relative ALB-GLuc activity was assessed
on days 0, 3, 6, 9 and 12 of induction with the 2% HS/Dex/HGF/FGF4
induction medium. The GLuc assay evaluates the activity of the ALB
promoter, which indirectly indicates ALB expression levels in cells
(14,15,19).
Compared with the control group, the relative ALB-GLuc activity
began to increase on day 3 of treatment, and continued to grow
until day 12 (P<0.05; Fig. 1B).
RT-qPCR demonstrated that AFP expression decreased significantly
following 12 days of induction compared with the control group
(P<0.05; Fig. 1C), whereas the
expression of the liver-specific markers ALB, CK-18 and TAT was
significantly upregulated compared with the control group
(P<0.05; Fig. 1C).
Induction in medium with 2% HS
promotes ICG uptake, but does not increase the number of positive
PAS stained cells
ICG uptake and PAS staining are methods commonly
used to detect the metabolism and synthesis function of liver cells
(14,20,21).
ICG uptake and PAS staining of HP14.5d cells were examined
following 12 days of induction (Fig.
2A). Uninduced control HP14.5d cells exhibited low levels of
ICG uptake and glycogen storage (Fig.
2A, left panel). In the induced group, the number of
ICG-positive stained cells was markedly increased compared with the
control group, as expected (Fig.
2A). Therefore, as indicated by the cellular morphology
(Fig. 1A), the expression of
hepatic stem cell markers (Fig. 1B and
C) and ICG uptake (Fig. 2A,
bottom panel), the induction of HP14.5d cells led to hepatic
differentiation. However, fewer PAS-positive cells were identified
in the induced HP14.5d cells compared with in the uninduced control
group (Fig. 2A, top panel).
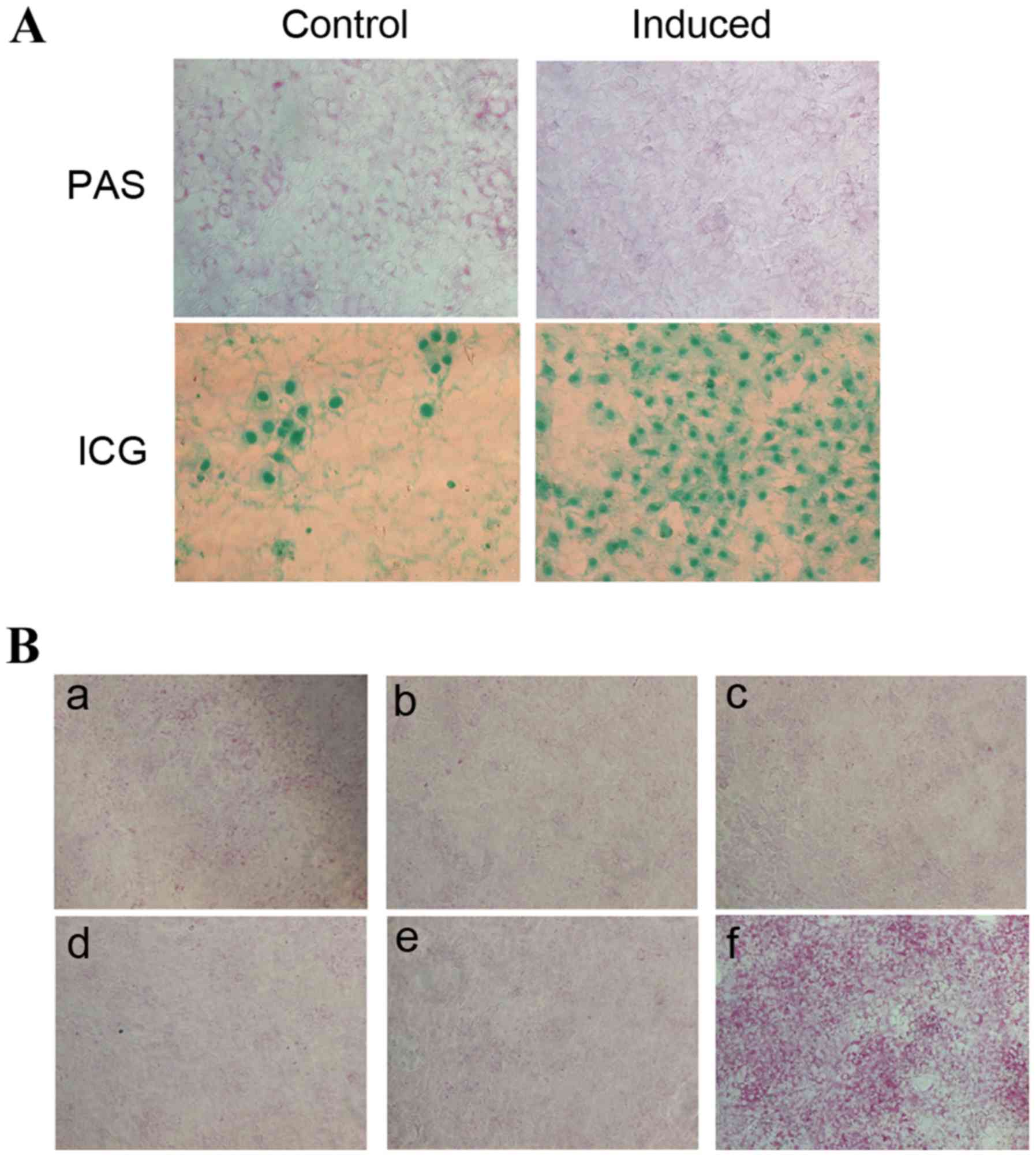 | Figure 2.HS influences PAS staining in induced
HP14.5d cells. (A) PAS staining and ICG uptake demonstrated the
glycogen storage and transport-metabolism function, respectively,
of HP14.5d cells induced for 12 days. Uninduced HP14.5d cells were
set up as a control. Photomicrographs were captured under ×200
magnification. (B) PAS staining of HP14.5d cells following
induction for 12 days in: (a) complete DMEM containing 10% FBS
(uninduced); (b) induction medium (DMEM containing 0.1 µmol/l Dex,
10 ng/ml HGF, 20 ng/ml FGF4 and 2% HS); (c) induction medium
without Dex; (d) induction medium without HGF; (e) induction medium
without FGF4; (f) induction medium with 2% HS replaced with 10%
FBS. Photomicrographs were captured under ×100 magnification.
Representative images of ≥3 independent experiments are presented.
HS, horse serum; PAS, periodic acid-Schiff; ICG, indocyanine green;
DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum;
Dex, dexamethasone; HGF, hepatic growth factor; FGF4, fibroblast
growth factor 4. |
In order to establish which component of the
induction medium may impact the PAS staining result, the medium was
altered by omission of each component individually (Fig. 2B). Similarly, fewer PAS-positive
cells were identified among induced HP14.5d cells compared with in
the uninduced control group (Fig. 2B-a
and b). Omission of Dex (Fig.
2B-c), HGF (Fig. 2B-d), and
FGF4 (Fig. 2B-e) from the
induction medium did not alter PAS staining compared with the
normal induction medium (Fig.
2B-b). However, replacement of 2% HS with 10% FBS (Fig. 2B-f), resulted in a visible increase
in the number of PAS-positive cells compared with cells in the
complete induction medium (Fig.
2B-f). Therefore, these results suggested that the 2% HS in the
induction medium is the factor affecting the results of PAS
staining.
Replacing the induction medium with
media containing 10% FBS affects the results of PAS staining
Investigations were then performed to evaluate
whether 2% HS affected glycogen storage in induced liver cells or
just affected the results of the PAS staining method. On induction
day 12, the induction medium was replaced with medium containing
10% FBS + 0.1 µmol/l Dex + 10 ng/ml HGF + 20 ng/ml FGF4. PAS
staining was then performed 12, 24, 48 and 72 h following
incubation (Fig. 3A).
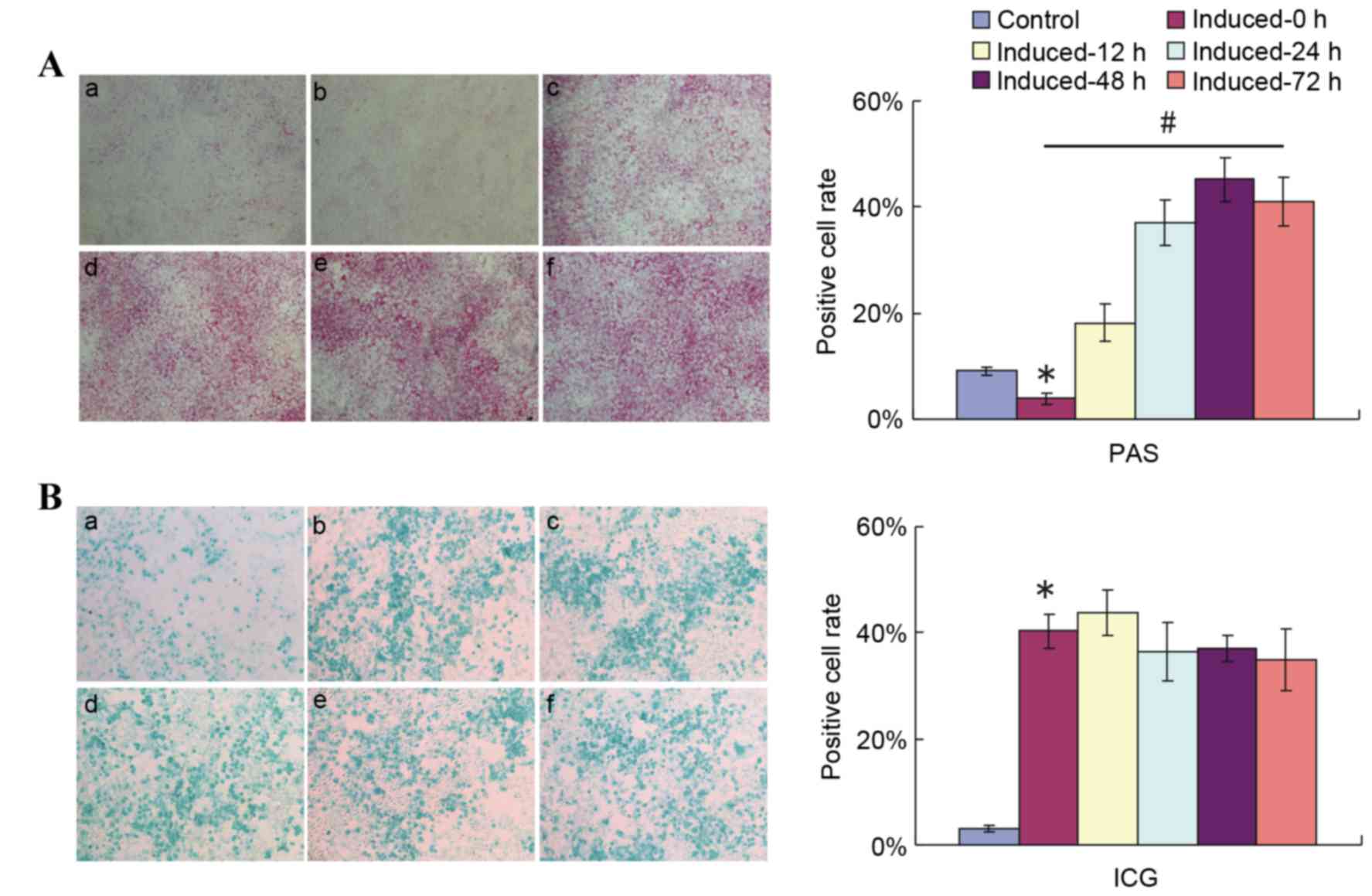 | Figure 3.Replacement of 2% HS with 10% FBS
ahead of PAS staining detection reflects the glycogen storage and
accumulation function of induced HP14.5d cells. HP14.5d cells were
treated with DMEM containing 2% HS + 0.1 µmol/l Dex + 10 ng/ml HGF
+ 20 ng/ml FGF4 for 12 days, then the induction medium was replaced
with complete DMEM with 10% FBS + 0.1 µmol/l Dex + 10 ng/ml HGF +
20 ng/ml FGF4 for different time periods. (A) PAS staining and (B)
ICG uptake were assessed at 12, 24, 48 and 72 h after the induction
medium was changed. (a) Uninduced HP14.5d cells as control; (b)
HP14.5d cells induced for 12 days with no media shift; (c) HP14.5d
cells induced for 12 days and induction medium changed for 12 h;
(d) HP14.5d cells induced for 12 days and induction medium changed
for 24 h; (e) HP14.5d cells induced for 12 days and induction
medium changed for 48 h; (f) HP14.5d cells induced for 12 days and
induction medium changed for 72 h. Photomicrographs were captured
under ×100 magnification. Representative images and data from ≥3
independent experiments are presented. *P<0.05 vs. control;
#P<0.05 among different induced groups. HS, horse
serum; FBS, fetal bovine serum; PAS, periodic acid-Schiff; DMEM,
Dulbecco's modified Eagle's medium; Dex, dexamethasone; HGF,
hepatic growth factor; FGF4, fibroblast growth factor 4; ICG,
indocyanine green. |
While limited purple staining was observed in the
uninduced control in medium contaning 10% FBS (Fig. 3A-a), the number of purple stained
cells in the induced group in 2% HS-containing medium without a
shift to 10% FBS-containing induction medium (Fig. 3A-b, induced-0 h group) was
significantly smaller compared with in the uninduced control group.
Increasing numbers of PAS-positive cells were observed in the
induced groups in which the medium was shifted to medium containing
10% FBS instead of 2% HS at different time points prior to PAS
staining (P<0.05; Fig. 3A).
Compared with cells that were not shifted to media with 10% FBS
(Fig. 3A-b), the number of
PAS-positive cells increased slightly upon media shift and
incubation for 12 h (Fig. 3A-c).
The number of purple stained cells increased further following 24
(Fig. 3A-d) and 48 h (Fig. 3A-e) of incubation with 10% FBS
induction medium, but no further increase was observed following 72
h of incubation with 10% FBS induction medium compared with 48 h of
incubation (Fig. 3A-f). A highly
significant difference was detected in PAS-positive cell numbers
between the induced-0 h and induced-48 h groups. At the same time
points following induction and media shift, ICG uptake was
demonstrated not to be influenced by the shift to incubation in 10%
FBS medium (Fig. 3B). These
results indicated that the serum condition did not affect the
hepatic function of induced cells, but PAS staining method.
Replacing cell induction medium with
media containing 10% FBS following 12 days of induction does not
affect the differentiation of HP14.5d cells
Investigations into whether changing the induction
medium affects the differentiation of induced HP14.5d cells were
then performed. As demonstrated in Fig. 4A, the cell morphology of induced
HP14.5d cells was not changed by shifting into media containing 10%
FBS. However, the cell numbers in the group incubated for 72 h in
the replacement media were higher than in the unshifted group
(Fig. 4A), which may be due to
better nutrition and increased cell proliferation in the condition
with 10% FBS. Replacing the induction media with media containing
10% FBS had no significant effect on the relative ALB-GLuc activity
of induced HP14.5d cells, regardless of time of incubation,
compared with the unshifted cells (Fig. 4B). In addition, the medium change
had no effect on expression of hepatic specific markers of induced
HP14.5d cells, compared with the unshifted cells (Fig. 4C). These results, therefore,
demonstrated that shifting cells into media with 10% FBS following
normal induction for 12 days did not affect the differentiation of
HPCs.
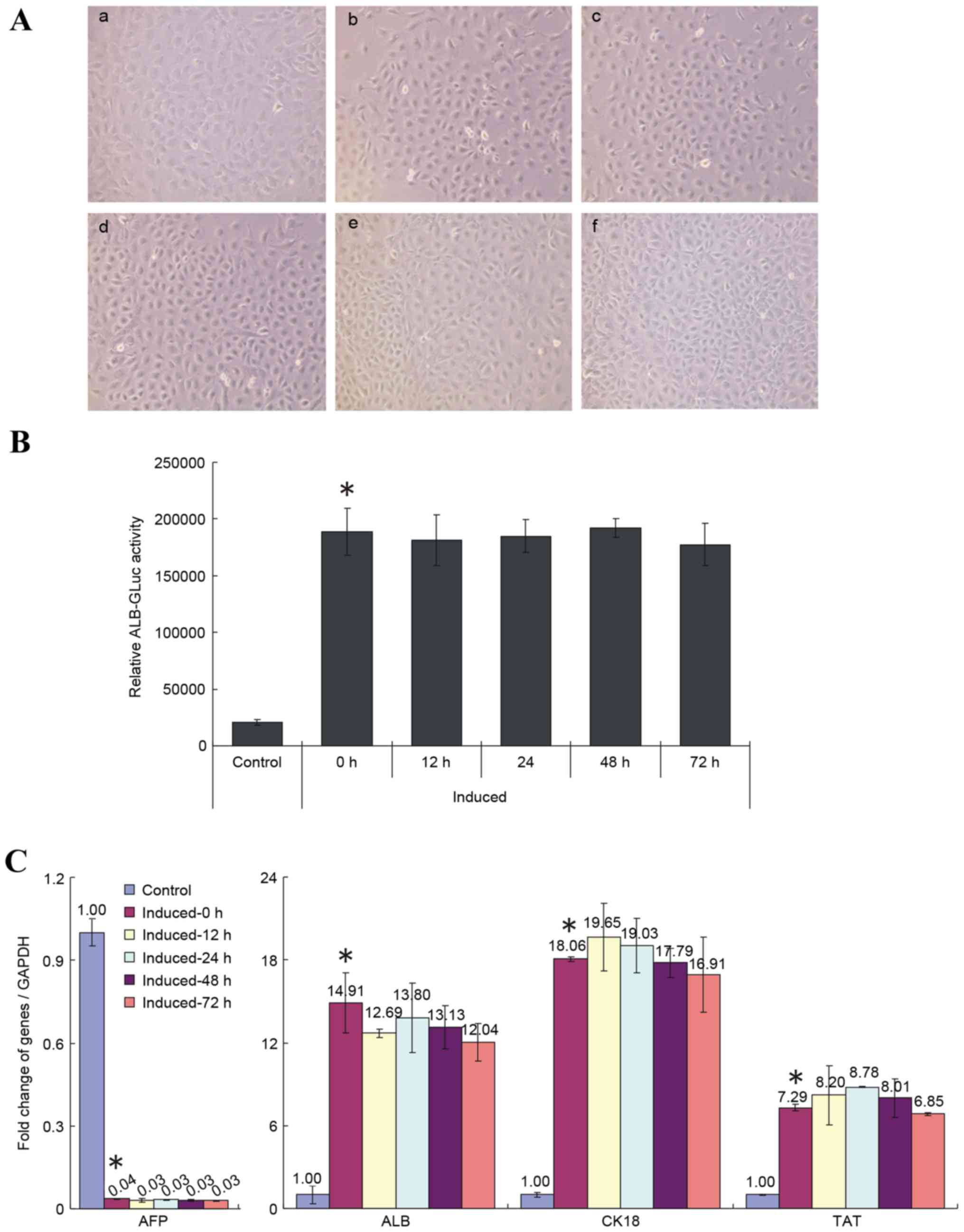 | Figure 4.Replacement of 2% HS with 10% FBS
following 12 days of induction does not influence the induced
differentiation of HP14.5d cells. HP14.5d cells were treated with
DMEM containing 2% HS + 0.1 µmol/l Dex + 10 ng/ml HGF + 20 ng/ml
FGF4 for 12 days, then the induction medium was replaced with
complete DMEM with 10% FBS + 0.1 µmol/l Dex + 10 ng/ml HGF + 20
ng/ml FGF4 for different time periods: (a) Uninduced HP14.5d cells
as control; (b) HP14.5d cells induced for 12 days with no media
shift; (c) HP14.5d cells induced for 12 days and induction medium
changed for 12 h; (d) HP14.5d cells induced for 12 days and
induction medium changed for 24 h; (e) HP14.5d cells induced for 12
days and induction medium changed for 48 h; (f) HP14.5d cells
induced for 12 days and induction medium changed for 72 h. (A)
HP14.5d cell morphologies. Photomicrographs were captured under
×200 magnification. (B) ALB-GLuc activity in HP14.5d cells.
*P<0.05 vs. control (n=3). (C) Semi-quantitative reverse
transcription-polymerase chain reaction analysis of mRNA expression
levels of hepatic related genes AFP, ALB, CK18 and TAT. *P<0.05
vs. control (n≥3). HS, horse serum; FBS, fetal bovine serum; DMEM,
Dulbecco's modified Eagle's medium; Dex, dexamethasone; HGF,
hepatic growth factor; FGF4, fibroblast growth factor 4; ALB,
albumin; GLuc, Gaussia luciferase; AFP, α fetoprotein; CK18,
keratin 18; TAT, tyrosine aminotransferase. |
Discussion
Liver stem cell transplantation technology is based
on the differentiation potential and proliferation ability of liver
stem cells (22,23). Compared with undifferentiated stem
cells, induced cells that undergo maturation and differentiation
in vitro prior to transplantation exhibit greater abilities
to compensate or rebuild liver function (24–26).
In order to make the liver stem cells more efficiently
differentiate into mature and functional liver cells, recent
studies have attempted to induce liver stem cells by providing a
series of stimulation factors (27,28).
As a prospective seed cells for transplantation, HPCs derived from
liver tissues at an early stage of embryonic development can be
stably expanded in vitro and possess the potential of
bidirectional differentiation into both hepatocytic and
cholangiocytic lineages (29,30).
In previous studies, this laboratory reported a relatively
effective method to induce hepatic differentiation and maturation
of HPCs (10,11); however, the results of PAS
staining, a method typically used to evaluate the glycogen storage
function of mature liver cells, was inconsistent with the
expression of hepatic marker genes and metabolic detoxification of
ICG uptake. In the present study, factors that may affect the
outcome of PAS staining in measurements of liver function were
investigated.
As previously, this study demonstrated that this
induction method effectively increased the ALB-Gluc activity,
hepatic marker expression and ICG uptake of HPCs, indicating their
maturation and differentiation. However, PAS staining in induced
cells was less than in the uninduced control. The induction medium
was primarily composed of four added factors: 2% HS, 0.1 µmol/l
Dex, 10 ng/ml HGF and 20 ng/ml FGF4. Therefore, removal of single
inducing factors one by one was necessary. In previous studies,
Dex, HGF and/or FGF4 have been reportedly used to induce bone
marrow mesenchymal stem cells, hematopoietic cells, or mouse
embryonic stem cells differentiation to hepatic cell lines, and PAS
staining was available (31–33).
In the present study, removal of Dex, HGF or FGF4 did not change
the number of positive PAS stained cells. However, when 2% HS was
replaced with 10% FBS, the number of purple stained cells
significantly increased. Therefore, it was proposed that the low
concentration of horse serum in the induction media was an
important factor affecting the PAS staining results.
Investigations were then performed into whether
induction in 2% HS serum affected the glycogen storage function of
induced liver cells or just affected the PAS staining. Two possible
causes of negative PAS staining were considered: i) Induced HP14.5d
cells lost the function of glycogen synthesis or ii) cells were
functional but no glycogen was present in the cells because of the
2% HS in the culture medium. To examine the possible causes,
following treatment of HP14.5d cells with induction medium for 12
days, the 2% HS was removed and replaced with 10% FBS for different
time periods prior to PAS staining assay. When induced HP14.5d
cells were cultured in 10% FBS complete medium for only 12 h, a
large number of PAS positive cells appeared. Since 2% HS was
present during the induction process for 12 days, the serum
condition could only be related to some factors of glycogen
synthesis but did not affect the hepatic function of induced
HP14.5d cells. The purpose of serum is to supply glucose, vitamins,
amino acids and other essential nutrients for cell culture
(34,35). Low levels of glycogen synthesis
following culture in 2% HS may be due to: i) Low serum
concentrations of serum contain low level of glucose, therefore,
the shortage of raw materials limits the product of glycogen; ii)
since glycogen synthesis is a series of biochemical reactions
catalyzed by specific enzymes (36,37),
2% HS may not supply enough amino acids to produce glycogen
synthase kinase, thereby reducing the amount of intracellular
glycogen synthesis, thus affecting the results of PAS staining.
The morphology of cells did not vary according to
the time incubated in induction medium containing 10% FBS, however,
the cell density increased with longer culture times in 10% FBS
induction medium. Proliferation and differentiation are generally
considered to be mutually exclusive, since differentiation is
suppressed during the proliferative phase, and the proliferative
abilities of cells in specific differentiation stages are weakened
(38–40). In the present study, 2% HS was
observed to favor differentiation of HP14.5d cells, while
inhibiting their proliferative abilities. Replacement of 2% HS with
10% FBS induction medium did not affect the expression of
differentiation related markers and ICG uptake, indicating that
HP14.5d cells were matured and differentiated following treatment
for 12 days in 2% HS induction medium. Therefore, the PAS staining
method did not accurately reflect the maturity and synthesis
function of hepatic cells in 2% HS culture conditions.
The present study investigated the role of 2% HS in
the differentiation of HP14.5d cells and verified the negative
effect of 2% HS on glycogen synthesis. Therefore, it may be
hypothesized that PAS staining is not a reliable method for
evaluating hepatic cell function following stem cell induction in
an environment containing 2% HS. In order to use PAS staining to
determine glycogen synthesis, 2% HS should be replaced with 10% FBS
in the culture medium following induction. The results of the
present study suggested that induction media including 2% HS may
have potential as an effective method to induce hepatic
differentiation, and that PAS staining may be used to evaluate the
functionality of 2% HS-induced hepatocytes.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81100309).
References
|
1
|
Kadyk LC, Collins LR, Littman NJ and
Millan MT: Proceedings: Moving toward cell-based therapies for
liver disease. Stem Cells Transl Med. 4:207–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huebert RC and Rakela J: Cellular therapy
for liver disease. Mayo Clin Proc. 89:pp. 414–424. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dalgetty DM, Medine CN, Iredale JP and Hay
DC: Progress and future challenges in stem cell-derived liver
technologies. Am J Physiol Gastrointest Liver Physiol.
297:G241–G248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haridass D, Narain N and Ott M: Hepatocyte
transplantation: Waiting for stem cells. Curr Opin Organ
Transplant. 13:627–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ichinohe N, Kon J, Sasaki K, Nakamura Y,
Ooe H, Tanimizu N and Mitaka T: Growth ability and repopulation
efficiency of transplanted hepatic stem cells, progenitor cells,
and maturehepatocytes in retrorsine-treated rat livers. Cell
Transplant. 21:11–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwartz RE, Fleming HE, Khetani SR and
Bhatia SN: Pluripotent stem cell-derived hepatocyte-like cells.
Biotechnol Adv. 32:504–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pournasr B, Asghari-Vostikolaee MH and
Baharvand H: Transcription factor-mediated reprograming of
fibroblasts to hepatocyte-like cells. Eur J Cell Biol. 94:603–610.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mu N, Liu HB, Meng QH, Du DW, Jiang Y and
Hu HZ: The differentiation of human multipotent adult progenitor
cells into hepatocyte-like cells inducedby coculture with human
hepatocyte line L02. Ann Surg Treat Res. 88:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herrero A, Prigent J, Lombard C, Rosseels
V, Daujat-Chavanieu M, Breckpot K, Najimi M, Deblandre G and Sokal
EM: Adult-derived human liver stem/progenitor cells infused 3 days
postsurgery improve liver regeneration in a mouse model of extended
hepatectomy. Cell Transplant. 26:351–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Y, Zhang WY, Gong M, Huang JY, Tang N,
Feng T, Wei GH, He TC and Bi Y: Low serum concentration facilitates
the differentiation of hepatic progenitor cells. Saudi Med J.
32:128–134. 2011.PubMed/NCBI
|
|
11
|
Bi Y, He Y, Huang JY, Xu L, Tang N, He TC
and Feng T: Induced maturation of hepatic progenitor cells in
vitro. Braz J Med Biol Res. 46:559–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho YA, Noh K, Jue SS, Lee SY and Kim EC:
Melatonin promotes hepatic differentiation of human dental pulp
stem cells: Clinical implications for the prevention of liver
fibrosis. J Pineal Res. 58:127–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pal R, Mamidi MK, Das AK and Bhonde R:
Diverse effects of dimethyl sulfoxide (DMSO) on the differentiation
potential of human embryonic stem cells. Arch Toxicol. 86:651–661.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bi Y, He Y, Huang J, Su Y, Zhu GH, Wang Y,
Qiao M, Zhang BQ, Zhang H, Wang Z..et al: Functional
characteristics of reversibly immortalized hepatic progenitor cells
derived from mouse embryonic liver. Cell Physiol Biochem.
34:1318–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC,
Luo J, Wang Y, Kang Q, Luo Q, et al: Wnt antagonist SFRP3 inhibits
the differentiation of mouse hepatic progenitor cells. J Cell
Biochem. 108:295–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koressaar T and Remm M: Enhancements and
modifications of primer design program Primer3. Bioinformatics.
23:1289–1291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Untergasser A, Cutcutache I, Koressaar T,
Ye J, Faircloth BC, Remm M and Rozen SG: Primer3-new capabilities
and interfaces. Nucleic Acids Res. 40:e1152012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wille T, Blank K, Schmidt C, Vogt V and
Gerlach RG: Gaussia princeps luciferase as a reporter for
transcriptional activity, protein secretion, and protein-protein
interactions in Salmonella enterica serovar typhimurium. Appl
Environ Microbiol. 78:250–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Y, Cui J, He T and Bi Y: 5-azacytidine
promotes terminal differentiation of hepatic progenitor cells. Mol
Med Rep. 12:2872–2878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He Y, Zhou JW, Xu L, Gong MJ, He TC and Bi
Y: Comparison of proliferation and differentiation potential
between mouse primary hepatocytes and embryonic hepatic progenitor
cells in vitro. Int J Mol Med. 32:476–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yarygin KN, Lupatov AY and Kholodenko IV:
Cell-based therapies of liver diseases: Age-related challenges.
Clin Interv Aging. 10:1909–1924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Christ B, Brückner S and Winkler S: The
therapeutic promise of mesenchymal stem cells for liver
restoration. Trends Mol Med. 21:673–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hindley CJ, Mastrogiovanni G and Huch M:
The plastic liver: Differentiated cells, stem cells, every cell? J
Clin Invest. 124:5099–5102. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tolosa L, Caron J, Hannoun Z, Antoni M,
López S, Burks D, Castell JV, Weber A, Gomez-Lechon MJ and
Dubart-Kupperschmitt A: Transplantation of hESC-derived hepatocytes
protects mice from liver injury. Stem Cell Res Ther. 6:2462015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu C and Li L: In vitro and in vivo
hepatic differentiation of adult somatic stem cells and
extraembryonic stem cells for treating end stage liver diseases.
Stem Cells Int. 2015:8719722015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamiya A: Regulation of the survival and
differentiation of hepatic stem/progenitor cells by acyclic
retinoid. Stem Cell Res Ther. 6:1092015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu WH, Ren LN, Chen T, You N, Liu LY,
Wang T, Yan HT, Luo H and Tang LJ: Unbalanced distribution of
materials: The art of giving rise to hepatocytes from liver
stem/progenitor cells. J Cell Mol Med. 18:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsuchiya A, Heike T, Fujino H, Shiota M,
Umeda K, Yoshimoto M, Matsuda Y, Ichida T, Aoyagi Y and Nakahata T:
Long-term extensive expansion of mouse hepatic stem/progenitor
cells in a novel serum-free culture system. Gastroenterology.
128:2089–2104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Q, Khoury M, Limmon G, Choolani M,
Chan JK and Chen J: Human fetal hepatic progenitor cells are
distinct from, but closely related to, hematopoietic
stem/progenitor cells. Stem Cells. 31:1160–1169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun GY, Dong LY and An W: Involvement of
hepatic stimulator substance in the regulation of hepatoblast
maturation into hepatocytes in vitro. Stem Cells Dev. 23:1675–1687.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Waclawczyk S, Buchheiser A, Flögel U,
Radke TF and Kögler G: In vitro differentiation of unrestricted
somatic stem cells into functional hepatic-like cells displaying a
hepatocyte-like glucose metabolism. J Cell Physiol. 225:545–554.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang XQ, Zang WJ, Bao LJ, Li DL, Song TS,
Xu XL and Yu XJ: Fibroblast growth factor-4 and hepatocyte growth
factor induce differentiation of human umbilical cord blood-derived
mesenchymal stem cells into hepatocytes. World J Gastroenterol.
11:7461–7465. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W, Liu J, Tabata Y, Meng J and Xu H:
The effect of serum in culture on RNAi efficacy through modulation
of polyplexes size. Biomaterials. 35:567–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dahl C, Saito H, Nielsen HV and Schiøtz
PO: The establishment of a combined serum-free and
serum-supplemented culture method of obtaining functional cord
blood-derived human mast cells. J Immunol Methods. 262:137–143.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delgado TC, Silva C, Fernandes I, Caldeira
M, Bastos M, Baptista C, Carvalheiro M, Geraldes CF and Jones JG:
Sources of hepatic glycogen synthesis during an oral glucose
tolerance test: Effect of transaldolase exchange onflux estimates.
Magn Reson Med. 62:1120–1128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Soares AF, Carvalho RA, Veiga FJ and Jones
JG: Effects of galactose on direct and indirect pathway estimates
of hepatic glycogen synthesis. Metab Eng. 12:552–560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yu T, Luo G, Zhang L, Wu J, Zhang H and
Yang G: Leptin promotes proliferation and inhibits differentiation
in porcine skeletal myoblasts. Biosci Biotechnol Biochem. 72:13–21.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang J, Wang B, Xiao Z, Zhao Y, Chen B,
Han J, Gao Y, Ding W, Zhang H and Dai J: Olfactory ensheathing
cells promote proliferation and inhibit neuronal differentiation of
neural progenitor cells through activation of Notch signaling.
Neuroscience. 153:406–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen P, Rossi N, Priddy S, Pierson CR,
Studebaker AW and Johnson RA: EphB2 activation is required for
ependymoma development as well as inhibits differentiation and
promotes proliferation of the transformed cell. Sci Rep.
5:92482015. View Article : Google Scholar : PubMed/NCBI
|


















