Introduction
The liver is the largest organ which detoxifies
various metabolites and produces plasma proteins, and is necessary
for the digestive system. However, numerous factors, including
pathogen infections, alcohol, harmful chemicals and drug abuse, may
lead to liver trauma, such as hepatitis, cirrhosis, fibrosis and
hepatic steatosis (1). These may
eventually lead to the development of hepatocellular carcinoma
(2). Therefore, it is essential to
develop novel approaches to protect the liver from injury.
Numerous researchers have selected natural
phytochemicals to develop hepatotherapeutic agents (3). These phytochemicals can ameliorate
illness and provide a safer way to protect against hepatic injury
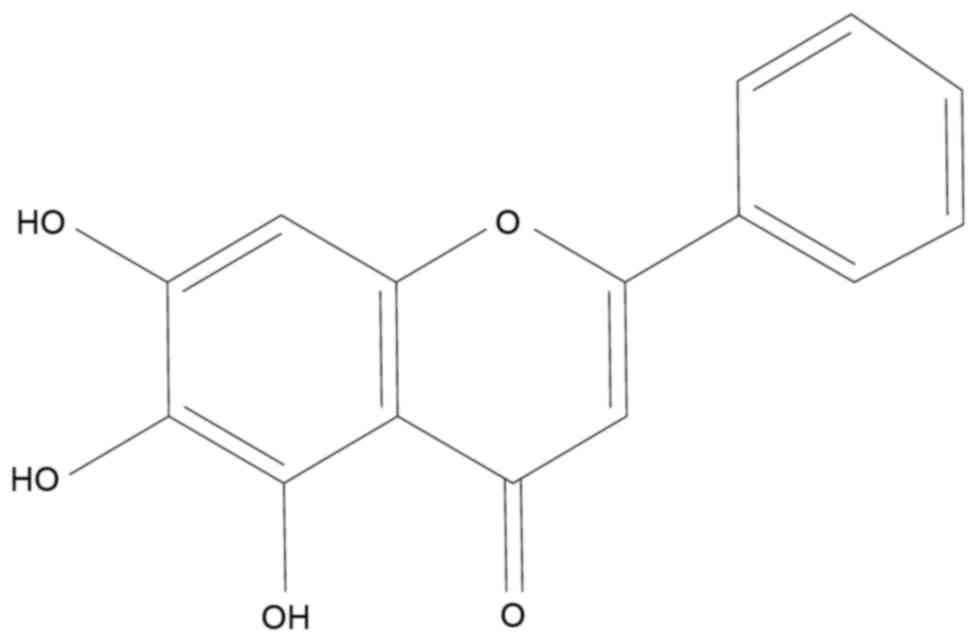
(4). Baicalein (BA, Fig. 1) is one of the major flavonoids
isolated from the dried Scutellariae Radix (5). BA exhibits a variety of biological
activities, including antibacterial (6), antioxidative (7), cardiovascular protective (8,9),
neuroprotective (10) and
anticancer (11). Furthermore,
treatment with BA may protect against liver ischemia/reperfusion
injury (12), polymicrobial
sepsis-induced liver injury (13),
concanavalin A-induced hepatitis (14) and carbon tetrachloride
(CCl4)-induced liver fibrosis (15).
Certain physiological activities in liver such as
detoxification may generate high levels of reactive oxygen species
(ROS), which leads to oxidative stress (16). These free radicals are highly
unstable and alter membrane permeability, which lead to hepatic
tissue injury (17).
Tert-butyl hydroperoxide (t-BHP) is an organic
peroxide which can be metabolized to free radical intermediates
that subsequently affect cell integrity by initiating lipid
peroxidation, resulting in oxidative hepatotoxicity in hepatocytes
(18). Alternatively, t-BHP
as a model compound is often used to investigate toxicity and the
mechanisms of liver injury caused by oxidative stress. It may cause
a variety of liver injuries such as elevating leakage of liver
enzymes, including alanine aminotransferase and aspartate
aminotransferase (19), forming
malondialdehyde and suppressing glutathione depletion (18). These alterations finally lead to
chronic hepatitis and hepatic fibrosis. Therefore, investigation of
t-BHP-induced hepatotoxicity may be critical to develop
hepatoprotective therapies.
Autophagy is a self-digestive process that degrades
cellular organelles and proteins in order to maintain cellular
homeostasis and ensure cell survival under stressful situations
(20). In general, autophagy is an
effective cellular defense system against a variety of pathologic
diseases (21). Previous evidence
indicates that autophagy serves as a cell survival mechanism
against multiple liver injuries. Induction of autophagy is a
defensive mechanism against usnic acid-induced toxicity in hepatic
cells (22). Furthermore,
activation of caspase 1 can protect against hypoxia/reoxygenation
injury by up-regulating beclin1 and mitochondrial autophagy in the
liver (23). In our previous
study, BA induced protective autophagy in HepG2 hepatocellular
carcinoma cells (24). The present
study aimed to detect whether BA triggers autophagy and its role in
t-BHP-induced liver injury in LO2 cells.
Materials and methods
Materials
Baicalein (≥98.5%, Zelang Group, Nanjing, China) was
dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) as a stock solution. Phosphate-buffered saline
(PBS) powder, RPMI 1640 medium, fetal bovine serum (FBS),
penicillin/streptomycin and trypsin-EDTA were obtained from Gibco;
Thermo Fisher Scientific (Waltham, MA, USA).
Tetraethylbenzimi-dazolylcarbocyanine iodide (JC-1) dye was
purchased from Thermo Fisher Scientific, Inc.
2,7′-Dichlorodihydrofluorescein diacetate (H2DCF-DA),
t-BHP and
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-2Htetrazolium bromide
(MTT) were obtained from Sigma-Aldrich; Merck KGaA. The
Cytotoxicity Detection kit (lactate dehydrogenase, LDH) was
obtained from Roche Diagnostics GmbH (Mannheim, Germany). Rabbit
primary antibodies against microtubule-associated protein
1A/1B-light chain 3 (LC3; cat. no. 2775s), cleaved-PARP (c-PARP;
cat. no. 9532), B-cell lymphoma 2 (Bcl-2; cat. no. 4223s), Bcl-2
associated X protein (Bax; cat. no. 5023s), survivin (cat. no.
2808) and GAPDH (cat. no. 2118), and the horseradish
peroxidase-conjugated goat anti-rabbit secondary IgG antibody (cat.
no. 7074) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cell culture
The LO2 immortalized healthy human liver cell line
(25,26) was obtained from Shanghai Institute
of Biochemistry and Cell Biology (Shanghai, China). Cells were
cultured in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were cultured in an
incubator with 5% CO2 at 37°C. During treatments, cells
were cultured in RPMI 1640 medium with 0.5% FBS and 100 U/ml
penicillin and 100 µg/ml streptomycin with or without indicated
treatments.
MTT assay
LO2 cells were seeded into 96-well plates at a
density of 5×103 cells/well and cultured at 37°C for 24
h. LO2 cells were pretreated with 0, 12.5, 25 or 50 µM BA for 12 h
and exposed to 1 mM t-BHP for 4 h. Cell proliferation was
determined by adding 1 mg/ml MTT-containing medium for 4 h,
followed by 100 µl DMSO to solubilize the formazan, and agitation
for 10 min in the dark. The absorbance at a wavelength of 570 nm
using a SpectraMax M5 Microplate Reader (Molecular Devices, LLC,
Sunnyvale, CA, USA). The results were analyzed based on at least
three independent experiments.
Lactate dehydrogenase (LDH) assay
LO2 cells were seeded into 96-well plates at a
density of 8×103 cells/well and cultured at 37°C for 24
h. LO2 cells were pretreated with 0, 12.5, 25 or 50 µM BA for 12 h
and exposed to 1 mM t-BHP for 4 h. Cell injury was
determined by measuring the quantity of LDH released into the
incubation medium. A Cytotoxicity Detection kit was used to detect
released LDH activity, according to the manufacturer's protocol.
The absorbance was measured at a wavelength of 490 nm using a
SpectraMax M5 Microplate Reader.
Measurement of mitochondrial membrane
potential (MMP)
The MMP was determined by fluorescent probe JC-1
staining assay. Briefly, LO2 cells were pretreated with various
concentrations of BA (0, 12.5, 25 or 50 µM) for 12 h, followed by
treating with 1 mM t-BHP for 30 min. Following this, the
cells were stained with JC-1 (1 µg/ml) for a further 30 min. The
fluorescence of JC-1 was observed using an IN Cell Analyzer 2000
(GE Healthcare Life Sciences, Little Chalfont, UK).
Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) staining analysis
An apoptosis detection kit (BioVision, Inc.
Milpitas, CA, USA) containing annexin V-FITC and PI was used to
identify apoptotic cells, according to the manufacturer's protocol.
Briefly, LO2 cells were seeded into 6-well plates at a density of
2×106 cells/well. Following a 2-h incubation with
t-BHP in the presence or absence of BA (25 µM) for 24 h, LO2
cells were harvested, washed and resuspended in 500 µl binding
buffer containing 5 µl annexin V-FITC and 5 µl PI for 30 min at
25°C. A total of 10,000 cells were analyzed by using a flow
cytometer (Becton Dickinson FACS Canto, Franklin Lakes, NJ) and
FlowJo software (version 7.6.1; Tree Star, Inc., Ashland, OR,
USA).
Detection of intracellular ROS
ROS formation was determined using the probe
H2DCF-DA. Briefly, LO2 cells were pretreated with 25 µM
BA for 12 h and incubated with 5 µM CM-H2DCFDA at 37°C for 15 min.
Following this, cells were treated with 1 mM t-BHP for
another 30 min and harvested for analysis by flow cytometry.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
lysis buffer (Beyotime, Shanghai, China) for 30 min at 4°C,
and subsequently centrifuged for 20 min (14,000 × g and 4°C). The
supernatant fraction was obtained as protein and the protein
concentrations were determined using a Bicinchoninic Acid Protein
Assay kit (Pierce, Rockford, IL, USA). Total proteins (25 µg) were
separated by 12% SDS-PAGE, followed by transferring onto PVDF
membranes. After blocking with 5% nonfat milk at room temperature
for 1 h with agitation, the membranes were probed with LC3
(1:2,000), c-PARP (1:2,000), Bcl-2 (1:2,000), Bax (1:2,000),
survivin (1:2,000) and GAPDH (1:2,000) primary antibodies, which
were diluted with PBS-0.1% Tween-20, overnight at 4°C. After
washing with PBS with Tween-20 three times for 15 min each, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody (1:2,000) at room temperature for 1 h. Protein
bands were visualized using an Enhanced Chemiluminescence advanced
western blot detection kit (GE Healthcare Life Sciences).
Chloroquine (CQ) and rapamycin (RAPA)
treatment in LO2 cells
LO2 cells were seeded into 96-well plates at a
density of 5×103 cells/well (MTT assay) or
8×103 cells/well (LDH assay) and cultured at 37°C for 24
h. Cells were subsequently pretreated with 10 µM CQ (Sigma-Aldrich;
Merck KGaA) or 100 nM RAPA (Sigma-Aldrich; Merck KGaA) at 37°C for
1 h, followed by treatment with BA (25 µM) at 37°C for an
additional 12 h. Subsequently, the cells were treated with or
without 1 mM t-BHP at 37°C for 4 h. The change in cell
viability and LDH release were subsequently detected as described
above for MTT and LDH release assays, respectively. For western
blot analysis, LO2 cells were seeded into 6-well plates at a
density of 2×106 cells/wells and cultured at 37°C for 24
h. Cells were then pretreated with 10 µM CQ or 100 nM RAPA at 37°C
for 1 h, followed by treatment with BA (25 µM) at 37°C for an
additional 12 h. LC3-I and LC3-II protein expression was detected
as described above for western blot analysis.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance was analyzed by one-way analysis of
variance followed by the Tukey post-hoc test using Graph Pad Prism
version 5 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
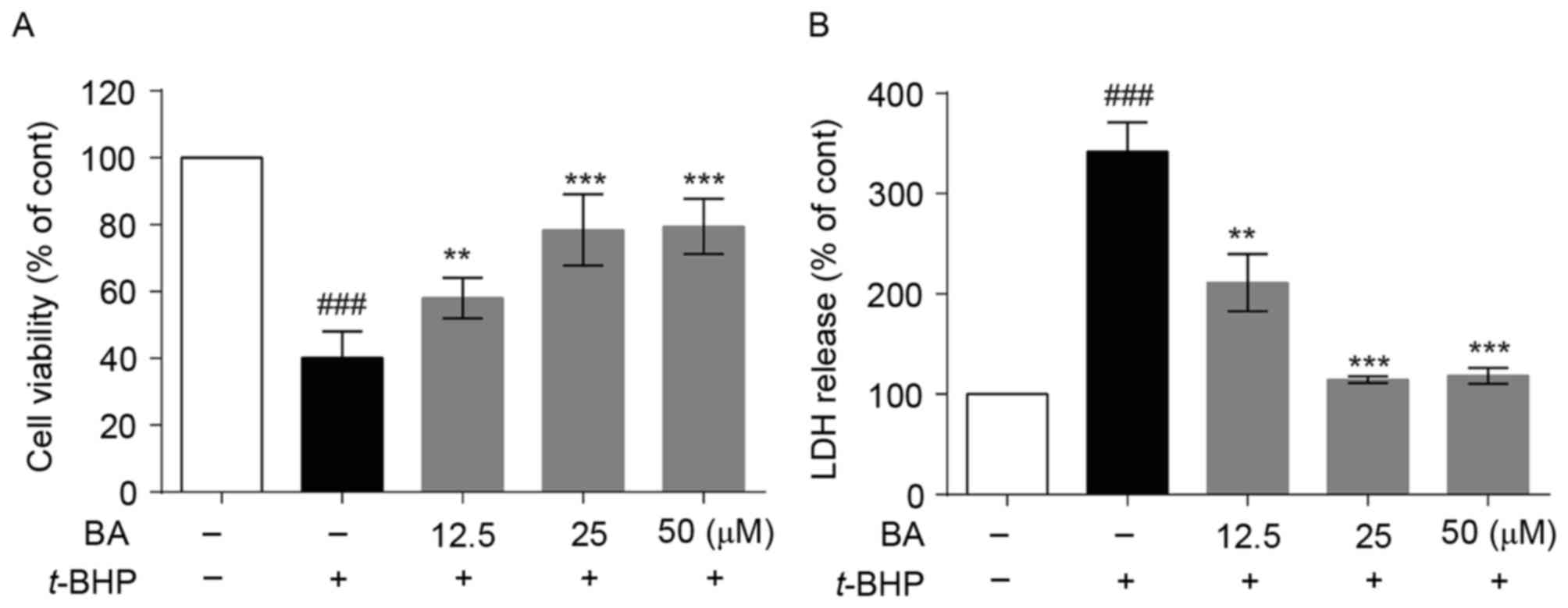
BA protects against t-BHP-induced LO2
cell injury
To investigate whether BA protects cells from
t-BHP-induced cytotoxicity, LO2 cells were pretreated with
BA for 12 h and then exposed to 1 mM t-BHP for another 4 h.
The cell viability was assessed by MTT assay. As presented in
Fig. 2A, the cell viability of
t-BHP-treated group decrease to 40.1±4.6% compared with the
control group. Following pretreatment with 12.5, 25 and 50 µM BA,
cell viability significantly increased to 58.0±3.5, 78.4±6.1 and
79.5±4.8%, respectively. The results of the LDH assay demonstrated
that t-BHP increased the LDH release rate from the basal
level of 100.0 to 341.9±17.0%, which was decreased to 211.3±16.5,
114.4±2.0 and 118.5±4.5% by 12.5, 25 and 50 µM BA pretreatment,
respectively (Fig. 2B). The
results indicated that BA obviously reduced LDH leakage induced by
t-BHP in a dose-dependent manner, suggesting that
pretreatment with BA may block t-BHP-induced cell
injury.
BA attenuates t-BHP-induced
apoptosis
Annexin V and PI staining were used to detect
apoptotic cells. Pretreatment of 25 µM BA significantly decreased
percentage of apoptotic cells from 66.4±4.0 to 18.8±5.8 in LO2
cells (Fig. 3A and B). It is well
known that depolarization of MMP is an early event in the process
of cell apoptosis. To identify the effect of BA on
t-BHP-induced early apoptosis in LO2 cells, the MMP in LO2
cells was evaluated by JC-1 staining assay. Once the MMP is
depolarized, the color of the dye alters reversibly from red to
green fluorescence (27). As
presented in Fig. 3C, exposure to
1 mM t-BHP in LO2 cells resulted in an increase in green
fluorescence intensity. Pretreatment with BA (12.5, 25 and 50 µM)
attenuated MMP disruption, indicating that BA may protect the cells
against t-BHP-induced apoptosis. Additionally, the
expression levels of numerous proteins associated with apoptosis
were detected. Following t-BHP treatment, the antiapoptotic
proteins Bcl-2 and survivin were markedly downregulated, while the
levels of c-PARP and Bax were obviously upregulated. Notably, these
effects were reversed by pre-treatment with 25 µM BA (Fig. 3D).
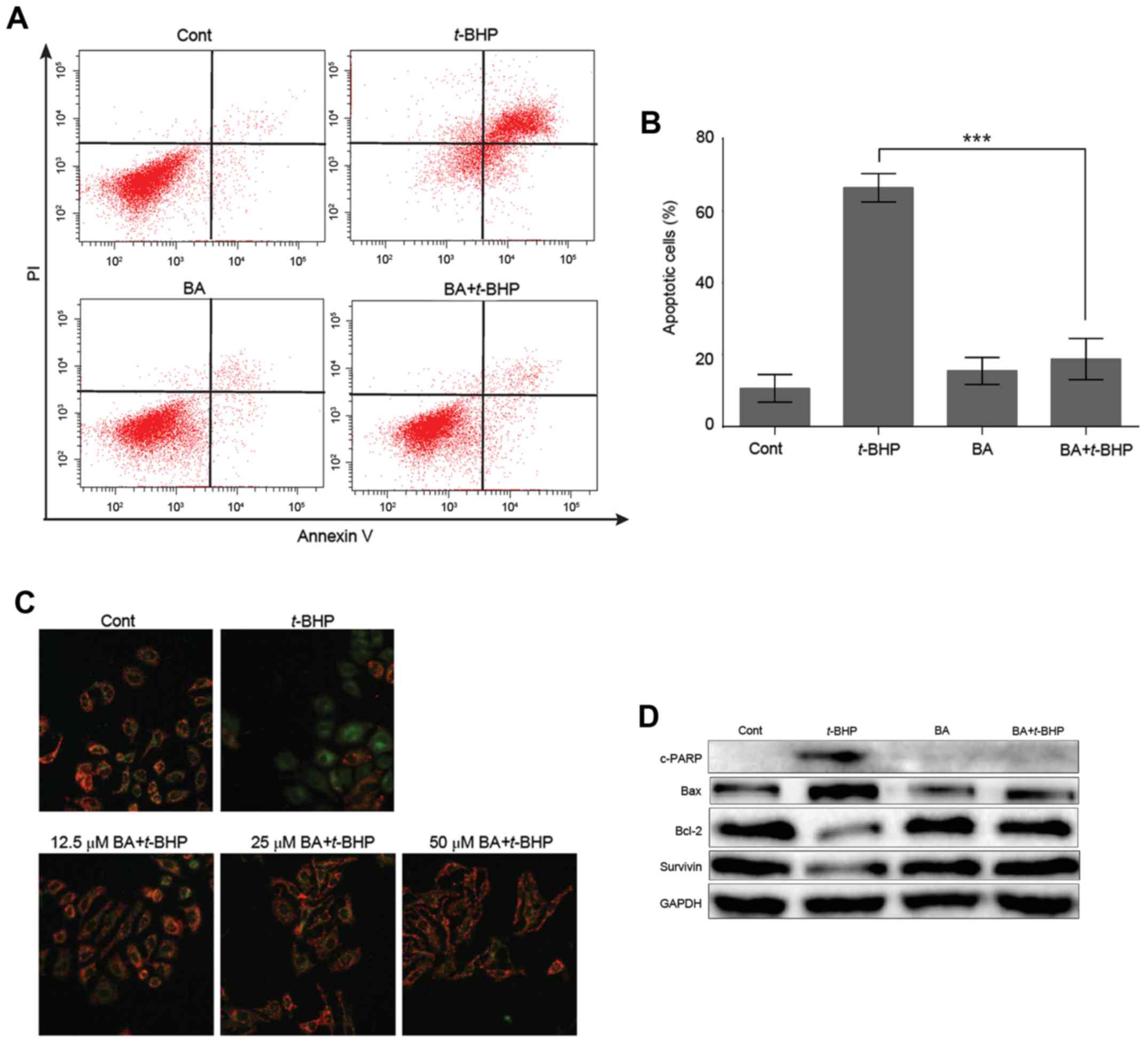 | Figure 3.BA attenuates t-BHP-induced
apoptosis in LO2 cells. (A) After pretreatment with vehicle control
or 25 µM BA for 12 h, LO2 cells were treated with 1 mM t-BHP
for another 2 h and then stained with Annexin V-fluorescein
isothiocyanate and PI. Apoptotic cells were quantified by flow
cytometry. (B) Quantification of three independent tests. Data are
presented as mean ± SD of at least three independent experiments.
***P<0.001 vs. t-BHP treatment group. (C) Representative
immunofluorescence images of cells stained with JC-1. Once the MMP
is depolarized, the color of the dye alters reversibly from red to
green fluorescence. (D) Representative western blot images of
c-PARP, Bax, Bcl-2 and survivin protein expression levels. GAPDH
served as an internal control. Cont, control; BA, baicalein;
t-BHP, tert-butyl hydroperoxide; LC3,
microtubule-associated protein 1A/1B-light chain 3; c-PARP, cleaved
PARP; Bcl-2, B-cell lymphoma 2; Bax, B-cell lymphoma 2 associated X
protein; PI, propidium iodide. |
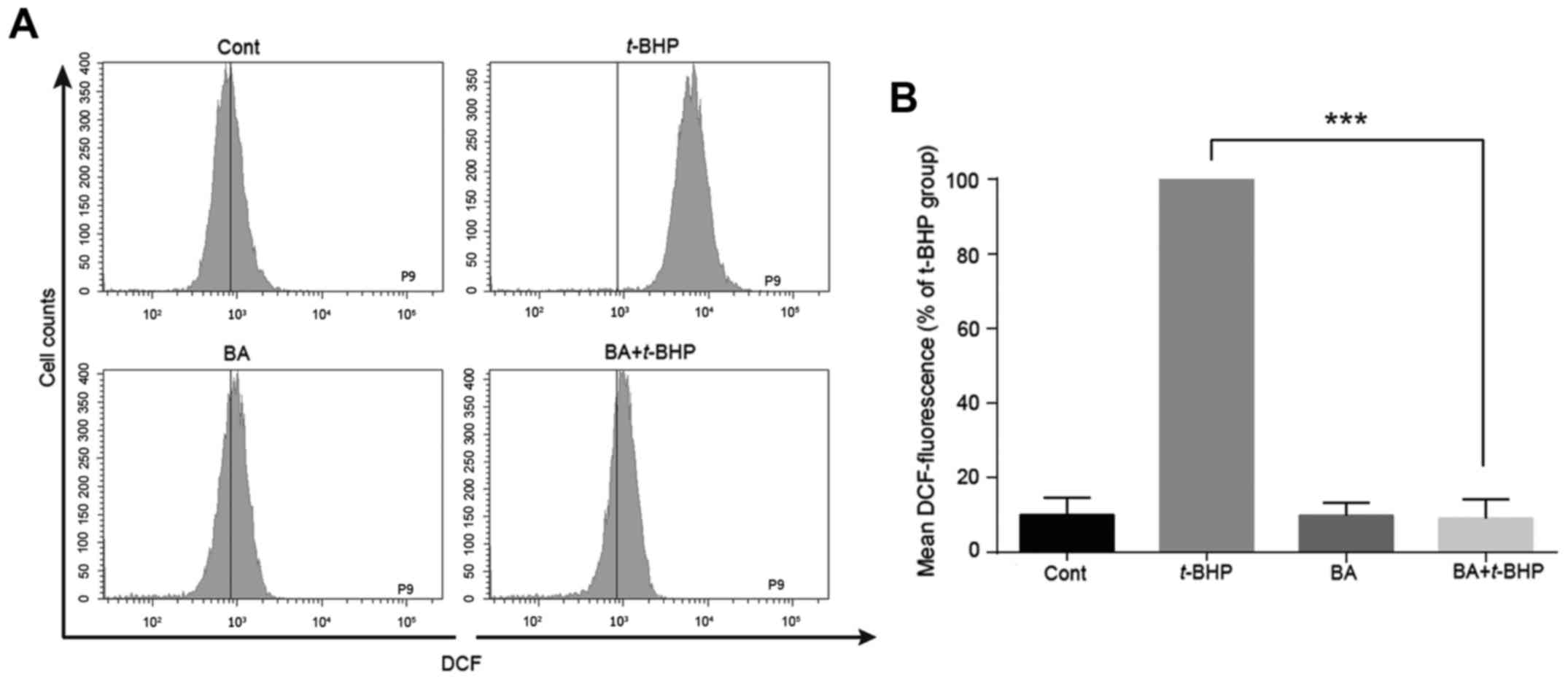
BA decreases ROS generation in LO2
cells
It has been reported that BA has a strong free
radical scavenging activity (28).
To determine whether the protective effect of BA was attributed to
reducing the intracellular ROS levels caused by t-BHP, a ROS
dye (H2DCF-DA) was used to detect alterations in
intracellular peroxide levels. As presented in Fig. 4A and B, when LO2 cells were exposed
to 1 mM t-BHP for 30 min, the intracellular ROS level
significantly increased compared with the control group.
Pretreatment with BA significantly attenuated the increased ROS
level induced by t-BHP from 100 to 10.0±2.0% in LO2 cells,
indicating that BA protected LO2 cells from t-BHP-induced
injury by reducing intracellular production of ROS.
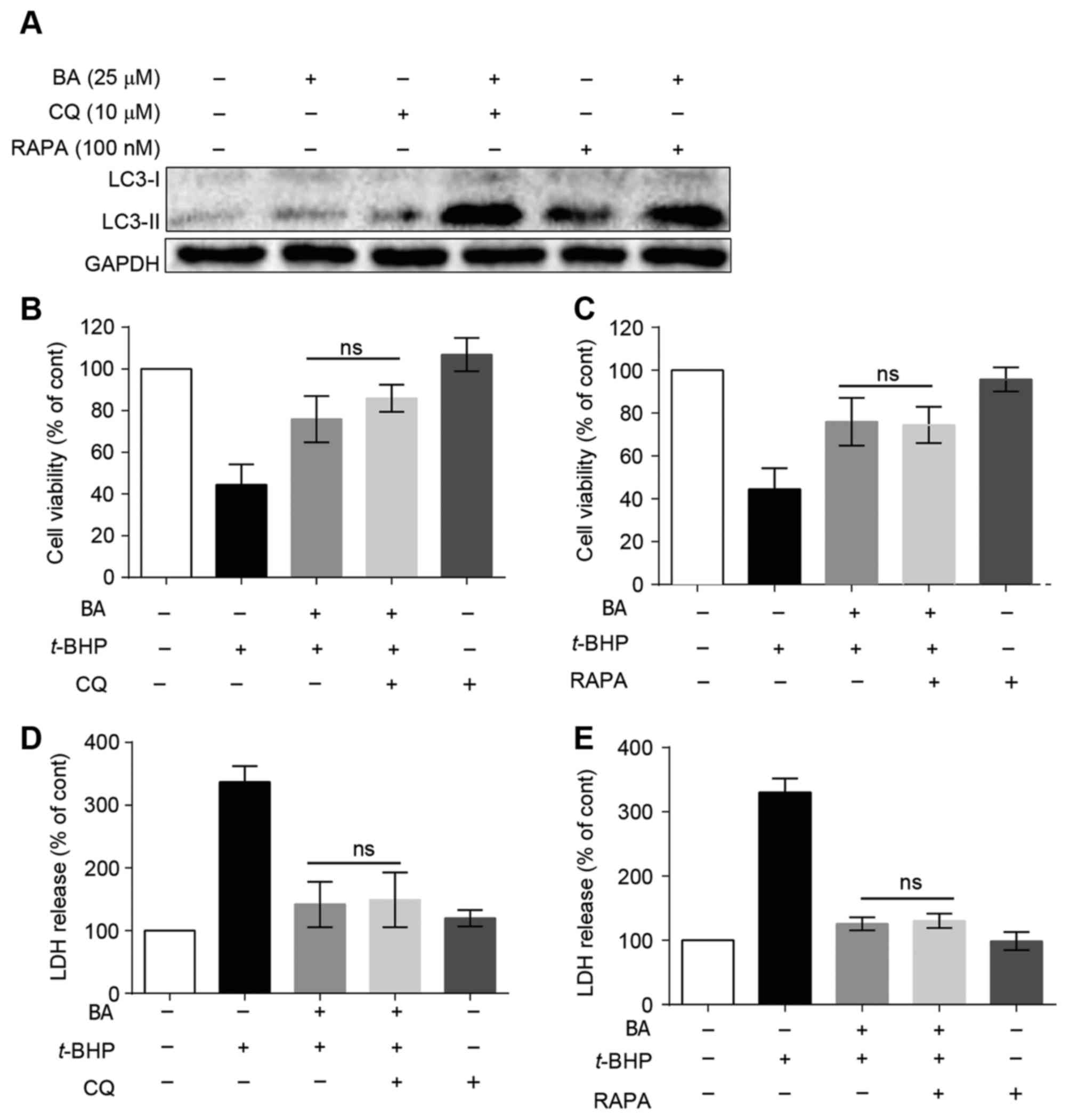
Autophagy may not be involved in the
protective effect of BA against t-BHP-induced LO2 cell injury
To confirm whether autophagy is triggered in
BA-treated LO2 cells, protein expression levels of LC3-II protein,
a marker of autophagy (29), were
determined by western blot analysis. As presented in Fig. 5A, 25 µM BA slightly increased the
expression of LC3-II. To further investigate BA-induced autophagic
flux in LO2 cells, CQ, an autophagy inhibitor that blocks
autophagosome fusion with the lysosome, causing upregulation of
LC3-II protein (30), was used.
LO2 cells were treated with 10 µM CQ for 1 h prior to BA treatment.
The expression of LC3-II was markedly upregulated following CQ and
BA combined treatment. Furthermore, rapamycin, which activates
autophagy by inhibition of the mechanistic target of rapamycin
signaling pathway (31) was used
in these experiments. Following pretreatment with rapamycin, the
expression of LC3-II was also increased (Fig. 5A), indicating that BA potentially
induces autophagic flux in LO2 cells.
Our previous study indicated that BA induces
protective autophagy in HepG2 hepatocellular carcinoma cells
(24). In addition, accumulating
evidence has indicated that autophagy may be a defense mechanism
against liver injury. Therefore, BA-induced autophagy may be
involved in the protective effects of BA against LO2 cell injury
caused by t-BHP. After inhibiting or activating autophagy by
CQ or rapamycin, cell viabilities detected by MTT were 86.0±3.8
(Fig. 5B) and 74.5±4.9% (Fig. 5C), respectively, which was not
significantly different compared with the BA-treated group in
t-BHP-injured LO2 cells. Similar results were observed in
LDH release assay (Fig. 5D and E),
suggesting that autophagy may be not involved in the protective
effect of BA against t-BHP-induced LO2 cell injury.
Discussion
BA exhibits a variety of biological activities,
including anti-inflammation (32),
antioxidative (33) and hepatic
protective effects (12).
Furthermore, BA is contained in a traditional Chinese medicine
formula named Xiao-Chai-Hu-Tang (34), which has been used to treat chronic
hepatitis (35), liver fibrosis
and cirrhosis (36) for many
years. Thus, much attention has been focused on its protective
function on hepatic injury (13).
Chronic administration of BA may prevent liver fibrosis induced by
CCl4 in rats (15). BA
may also inhibit apoptosis on acute liver failure induced by
d-galactosamine (d-GalN)/lipopolysaccharides (37). The present study further elucidated
the protective role of BA in LO2 liver cells by suppressing
t-BHP-induced hepatic damage. Pretreatment with BA
significantly protected cell injury caused by t-BHP,
demonstrated by increased cell viability and reduced leakage of
LDH, and reduced apoptosis induced by t-BHP, indicating that
BA serves a protective role in liver injury in LO2 cells.
Based on its polyphenolic structure, BA has a strong
antioxidant and free radical scavenging activity (28). Treatment with BA may protect HT22
neuronal cells by inhibiting production of ROS (7). Through reducing ROS production and
calcium overload, BA may also prevent
lysophosphatidylcholine-induced cardiac injury (38). Furthermore, t-BHP may induce
hepatic oxidative damage by inducing cellular oxidative stress,
including lipid peroxidation and glutathione levels (39). In the present study, treatment with
t-BHP increased the production of ROS compared with
untreated controls, whereas pretreatment with BA greatly decreased
ROS levels, indicating that the protective effect of BA was
attributed to reducing intracellular ROS levels caused by
t-BHP. A recent study demonstrated that BA protects against
polymicrobial sepsis-induced liver injury via inhibition of
apoptosis (13). In addition,
overproduction of ROS may lead to apoptotic cell death (40). The results of the present study
also demonstrated that pretreatment with BA attenuates apoptotic
cell death and MMP disruption during LO2 cell injury caused by
t-BHP.
Autophagy is actively involved in liver physiology
and pathogenesis (41), and
constitutes an effective defense mechanism against multiple
pathological insults (42). It has
been reported that tonsil-derived mesenchymal stem cells can
differentiate into hepatocyte-like cells and exert a protective
effect against liver fibrosis through autophagy activation
(43). Autophagy can prevent
dasatinib-induced hepatotoxicity both in vitro and in
vivo (44). Regulation of
autophagy can also alleviate fatty liver conditions and liver
injury in mice (45). In our
previous study, BA was demonstrated to induce protective autophagy
in HepG2 hepatocellular carcinoma cells (25). In the present study, autophagy was
triggered in BA-treated LO2 cells, whereas inhibition or activation
of autophagy did not affect the protective effect against
t-BHP induced by BA, suggesting that autophagy may be not
involved in the protective effect of BA in LO2 cells. However,
other biomarkers, such as p62, require further study to confirm
BA-triggered autophagic flux in cells.
In conclusion, BA exerted a strong hepatoprotective
effect against t-BHP-induced liver cell damage via
scavenging ROS generation and inhibiting apoptotic cell death,
suggesting that BA holds great potential as a drug candidate in
protecting hepatotoxicity.
Acknowledgements
The present study was supported by the Science and
Technology Development Fund, Macao SAR (FDCT) (grant no.
074/2012/A3) and the Research Fund of University of Macau (grant
nos. CPG2014-00012-ICMS, MRG013/WYT/2013/ICMS,
MYRG2015-00091-ICMS-QRCM, MYRG2015-00098-ICMS-QRCM and
MYRG2015-00101-ICMS-QRCM).
References
|
1
|
Bernal W, Auzinger G, Dhawan A and Wendon
J: Acute liver failure. Lancet. 376:190–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang A, Sun H and Wang X: Recent advances
in natural products from plants for treatment of liver diseases.
Eur J Med Chem. 63:570–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paterson I and Anderson EA: Chemistry. The
renaissance of natural products as drug candidates. Science.
310:451–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Girish C and Pradhan SC: Drug development
for liver diseases: Focus on picroliv, ellagic acid and curcumin.
Fundam Clin Pharmacol. 22:623–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li C, Lin G and Zuo Z: Pharmacological
effects and pharmacokinetics properties of Radix Scutellariae and
its bioactive flavones. Biopharm Drug Dispos. 32:427–445. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang EJ, Cha SM, Choi SM and Cha JD:
Combination effects of baicalein with antibiotics against oral
pathogens. Arch Oral Biol. 59:1233–1241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi JH, Choi AY, Yoon H, Choe W, Yoon KS,
Ha J, Yeo EJ and Kang I: Baicalein protects HT22 murine hippocampal
neuronal cells against endoplasmic reticulum stress-induced
apoptosis through inhibition of reactive oxygen species production
and CHOP induction. Exp Mol Med. 42:811–822. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cui G, Luk SC, Li RA, Chan KK, Lei SW,
Wang L, Shen H, Leung GP and Lee SM: Cytoprotection of baicalein
against oxidative stress-induced cardiomyocytes injury through the
Nrf2/Keap1 pathway. J Cardiovasc Pharmacol. 65:39–46. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong EK, Yu S, Sanderson JE, Chen KB,
Huang Y and Yu CM: A novel anti-fibrotic agent, baicalein, for the
treatment of myocardial fibrosis in spontaneously hypertensive
rats. Eur J Pharmacol. 658:175–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao L, Li C, Yang RY, Lian WW, Fang JS,
Pang XC, Qin XM, Liu AL and Du GH: Ameliorative effects of
baicalein in MPTP-induced mouse model of Parkinson's disease: A
microarray study. Pharmacol Biochem Behav. 133:155–163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
down-regulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu A, Huang L, Fan H, Fang H, Yang Y, Liu
S, Hu J, Hu Q, Dirsch O and Dahmen U: Baicalein pretreatment
protects against liver ischemia/reperfusion injury via inhibition
of NF-kB pathway in mice. Int Immunopharmacol. 24:72–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu A, Wang W, Fang H, Yang Y, Jiang X,
Liu S, Hu J, Hu Q, Dahmen U and Dirsch O: Baicalein protects
against polymicrobial sepsis-induced liver injury via inhibition of
inflammation and apoptosis in mice. Eur J Pharmacol. 748:45–53.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Shan L, Hua Y, Wang D, Zeng H,
Liu R, Zhang W and Hu Z: Baicalein selectively induces apoptosis in
activated lymphocytes and ameliorates concanavalin a-induced
hepatitis in mice. PLoS One. 8:e695922013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun H, Che QM, Zhao X and Pu XP:
Antifibrotic effects of chronic baicalein administration in a CCl4
liver fibrosis model in rats. Eur J Pharmacol. 631:53–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaeschke H, McGill MR and Ramachandran A:
Oxidant stress, mitochondria, and cell death mechanisms in
drug-induced liver injury: Lessons learned from acetaminophen
hepatotoxicity. Drug Metab Rev. 44:88–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kohen R and Nyska A: Oxidation of
biological systems: Oxidative stress phenomena, antioxidants, redox
reactions, and methods of their quantification. Toxicol Pathol.
30:620–650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joyeux M, Rolland A, Fleurentin J, Mortier
F and Dorfman P: tert-Butyl hydroperoxide-induced injury in
isolated rat hepatocytes: A model for studying anti-hepatotoxic
crude drugs. Planta Med. 56:171–174. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen CH, Tung SY, Huang WS, Lu CC, Lee KC,
Hsieh YY, Chang PJ, Liang HF, Chen JH, Lin TH, et al: Exploring the
effects of tert-butylhydroperoxide induced liver injury using
proteomic approach. Toxicology. 316:61–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang K: Autophagy and apoptosis in liver
injury. Cell Cycle. 14:1631–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen S, Dobrovolsky VN, Liu F, Wu Y, Zhang
Z, Mei N and Guo L: The role of autophagy in usnic acid-induced
toxicity in hepatic cells. Toxicol Sci. 142:33–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun Q, Gao W, Loughran P, Shapiro R, Fan
J, Billiar TR and Scott MJ: Caspase 1 activation is protective
against hepatocyte cell death by up-regulating beclin 1 protein and
mitochondrial autophagy in the setting of redox stress. J Biol
Chem. 288:15947–15958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang YF, Li T, Tang ZH, Chang LL, Zhu H,
Chen XP, Wang YT and Lu JJ: Baicalein triggers autophagy and
inhibits the protein kinase B/Mammalian target of rapamycin pathway
in hepatocellular carcinoma HepG2 cells. Phytother Res. 29:674–679.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen L, Zhang F, Kong D, Zhu X, Chen W,
Wang A and Zheng S: Saikosaponin D disrupts platelet-derived growth
factor-β receptor/p38 pathway leading to mitochondrial apoptosis in
human LO2 hepatocyte cells: A potential mechanism of
hepatotoxicity. Chem Biol Interact. 206:76–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan X, Jiang Z, Bi L, Yang Y and Chen W:
Salvianolic acid A attenuates TNF-α-and d-GalN-induced ER
stress-mediated and mitochondrial-dependent apoptosis by modulating
Bax/Bcl-2 ratio and calcium release in hepatocyte LO2 cells. Naunyn
Schmiedebergs Arch Pharmacol. 388:817–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cossarizza A, Baccarani-Contri M,
Kalashnikova G and Franceschi C: A new method for the
cytofluorometric analysis of mitochondrial membrane potential using
the J-aggregate forming lipophilic cation
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide (JC-1). Biochem Biophys Res Commun. 197:40–45. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woźniak D, Dryś A and Matkowski A:
Antiradical and antioxidant activity of flavones from Scutellariae
baicalensis radix. Nat Prod Res. 29:1567–1570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He J, Yu JJ, Xu Q, Wang L, Zheng JZ, Liu
LZ and Jiang BH: Downregulation of ATG14 by EGR1-MIR152 sensitizes
ovarian cancer cells to cisplatin-induced apoptosis by inhibiting
cyto-protective autophagy. Autophagy. 11:373–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kimura T, Takabatake Y, Takahashi A and
Isaka Y: Chloroquine in cancer therapy: A double-edged sword of
autophagy. Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jung CH, Ro SH, Cao J, Otto NM and Kim DH:
mTOR regulation of autophagy. FEBS Lett. 584:1287–1295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan GW, Zhang Y, Jiang X, Zhu Y, Wang B,
Su L, Cao W, Zhang H and Gao X: Anti-inflammatory activity of
baicalein in LPS-stimulated RAW264.7 macrophages via estrogen
receptor and NF-kB-dependent pathways. Inflammation. 36:1584–1591.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Z, Huang K and Xu H: Protective
effects of flavonoids in the roots of Scutellaria baicalensis
Georgi against hydrogen peroxide-induced oxidative stress in
HS-SY5Y cells. Pharmacol Res. 43:173–178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohtake N, Nakai Y, Yamamoto M, Sakakibara
I, Takeda S, Amagaya S and Aburada M: Separation and isolation
methods for analysis of the active principles of Sho-saiko-to (SST)
oriental medicine. J Chromatogr B Analyt Technol Biomed Life Sci.
812:135–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hirayama C, Okumura M, Tanikawa K, Yano M,
Mizuta M and Ogawa N: A multicenter randomized controlled clinical
trial of Shosaiko-to in chronic active hepatitis. Gastroenterol
Jpn. 24:715–719. 1989.PubMed/NCBI
|
|
36
|
Oka H, Yamamoto S, Kuroki T, Harihara S,
Marumo T, Kim SR, Monna T, Kobayashi K and Tango T: Prospective
study of chemoprevention of hepatocellular carcinoma with
Sho-saiko-to (TJ-9). Cancer. 76:743–749. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu YL, Lian LH, Wan Y and Nan JX:
Baicalein inhibits nuclear factor-kB and apoptosis via c-FLIP and
MAPK in D-GalN/LPS induced acute liver failure in murine models.
Chem Biol Interact. 188:526–534. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen HM, Hsu JH, Liou SF, Chen TJ, Chen
LY, Chiu CC and Yeh JL: Baicalein, an active component of
Scutellaria baicalensis Georgi, prevents
lysophosphatidylcholine-induced cardiac injury by reducing reactive
oxygen species production, calcium overload and apoptosis via MAPK
pathways. BMC Complement Altern Med. 14:2332014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vidyashankar S, K Mitra S and Nandakumar
KS: Liv.52 protects HepG2 cells from oxidative damage induced by
tert-butyl hydroperoxide. Mol Cell Biochem. 333:41–48. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yin XM, Ding WX and Gao W: Autophagy in
the liver. Hepatology. 47:1773–1785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim
HS, Park YS, Jo I, Park JW, Jung SC, et al: Tonsil-derived
mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in
mice via autophagy activation. Sci Rep. 5:86162015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang X, Wang J, Dai J, Shao J, Ma J, Chen
C, Ma S, He Q, Luo P and Yang B: Autophagy protects against
dasatinib-induced hepatotoxicity via p38 signaling. Oncotarget.
6:6203–6217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin CW, Zhang H, Li M, Xiong X, Chen X,
Chen X, Dong XC and Yin XM: Pharmacological promotion of autophagy
alleviates steatosis and injury in alcoholic and non-alcoholic
fatty liver conditions in mice. J Hepatol. 58:993–999. 2013.
View Article : Google Scholar : PubMed/NCBI
|