Introduction
Inflammatory bowel disease (IBD), encompassing
Crohn's disease (CD) and ulcerative colitis (UC), is an
inflammatory disorder affecting the gastrointestinal tract.
Clinical symptoms include abdominal pain, diarrhea, rectal
bleeding, anemia, fatigue and malnutrition, all conditions
associated with reduced in quality of life (1–3).
Although the exact etiology is complex and remains to be
elucidated, the available evidence suggests that various factors,
such as heredity, defective epithelial barrier, increased
intestinal permeability, antimicrobial peptide production, innate
microbial sensing and autophagy may be involved in pathogenesis of
IBD (2,4). In particular, an excess of
inflammatory mediators and an inadequate function or number of
components that downregulate mucosal immune response may have an
important role in IBD (5,6). The normal intestinal immune system
has two tasks: To combat pathogens and to maintain tolerance
towards the commensal bacterial flora (2,5,7,8).
However, not achieving these tasks leads to the pathological
inflammation of the gut system.
There are various inflammatory cells in the gut and
along with T helper (Th) 1 and Th2 cells, effector CD4+
T helper 17 (Th17) and regulatory CD4+ T cells (Treg)
are also important in IBD. The loss of homeostasis between Th17 and
Treg cells is believed to lead to an aberrant immune response of
IBD (1,5,9).
Th17 cells have a critical role in clarifying extracellular
pathogens, the inappropriate expression of pro-inflammatory
cytokines by those cells, such as interleukin (IL)-17A, IL-17F,
IL-21, IL-22, is believed to favor the occurrence of inflammatory
diseases (9,10). Previous studies have revealed an
increased number of Th17 cells expressing retinoid-related orphan
receptor-γt (ROR-γt), the master transcription factor guiding Th17
differentiation, in the lamina propria of IBD patients, which
suggests that Th17 cells may contribute to the pathology of IBD
(11–13). Th17 cells facilitate intestinal
inflammation in both forms of IBD; however, Treg cells have
anti-inflammatory properties (7).
Treg cells are necessary for the maintenance of mucosal tolerance
and preserve homeostasis in the gut by suppressing the
proliferation and effector functions of effector T cells (9). Treg cells exhibit their
anti-inflammatory function by producing the effective cytokines,
such as IL-10 and transforming growth factor-β (TNF-β) (2,14).
The transcription factor forkhead box p3 (Foxp3), which is crucial
for their differentiation and function, has been confirmed to be
expressed in intestinal mucosa in order to inhibit abnormal immune
responses toward the dietary antigens or commensal flora. Previous
studies have determined that Treg cells are involved in the
anti-inflammatory action in IBD patients (9,14–16).
Additionally, there is evidence that Treg cells are capable of
controlling experimental colitis in animal models (17,18).
Th17 and Treg cells may control the development of
one another to maintain immune homeostasis and achieve pathogen
clearance. TNF-β has been identified as an anti-inflammatory
cytokine produced by Treg cells and the critical common factor for
the proliferation of Th17 and Treg cells (19,20).
It has been previously reported that TNF-β promotes the
differentiation of Treg cells by inducing Foxp3 expression, whereas
it favors the differentiation of Th17 cells in the concurrent
administration of IL-6 and TNF-β (21–24).
Total glucosides of paeony (TGP), extracted from the
root of Paeonia lactiflora Pall., contains 96.2% of
paloniflorin (PF) and traces of hydroxyl-paconiflorin, abbiflorin,
paeonin, benzoylpaeoniflorin (25). According to the dispensatory of
TGP, paeoniflorin is the monomer which has an effective role and
accounts for 90% of TGP. As a traditional Chinese herbal medicine,
TGP has been identified to exhibit a wide range of pharmacological
activities, including anti-inflammatory, antioxidative,
antihepatic, analgesic activity without evident toxic or side
effects (26–30). TGP has been used for dysmenorrhea,
muscle cramping and spasms, giddiness and fever in China for over
1,500 years. TGP was approved to enter the Chinese market in 1998,
and has been used for the treatment of rheumatoid arthritis (RA),
systemic lupus erythematosus (SLE), ankylosing spondylitis (AS) and
hepatitis (31,32). Previous studies revealed that TGP
may inhibit Th1/Th17 immune response by downregulating the
expression of transcriptional factor T-box expressed in T cells
(T-bet) and RORgt. Meanwhile, TGP may increase the
CD4+CD25+ Treg differentiation by activating
the transcription factor Foxp3 (30,33–36).
A previous study revealed that TGP effectively attenuated
inflammation in 2,4,6-trinitrobezence sulfonic acid
(TNBS)/ethanol-induced colitis in rats (37). This evidence supported the
hypothesis that TGP may be a promising candidate Chinese drug for
treatment of IBD. However, whether TGP reduces inflammation of IBD
via regulating Th17/Treg balance remains to be elucidated.
Therefore, the present study measured the expression of Th17 and
Treg-associated cytokines and transcription factors, and the
proportion of Th17 and Treg cells in the TNBS-induced colitis
model. The purpose of the present study was to further elucidate
the regulatory effects and underlying mechanism of TGP in a rat
model of TNBS-induced colitis via Th17/Treg immune homeostasis.
Materials and methods
Animals
A total of 32 Sprague-Dawley (SD) rats (male,
6–8-weeks old, mean weight, ~200 g) were purchased from Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China). Animals were
kept in standard cages (5 rats/cage) under specific pathogen-free
conditions in the Laboratory Animal Research Center of Wenzhou
Medical University. Rats were given free access to autoclaved tap
water and to a standard diet, maintained under controlled
conditions of light (12-h light-dark cycle), temperature (22–24°C)
and humidity (45–55%). All procedures were ethically approved
according to the Guide for the Care and Use of the Administration
Committee of Experimental Animals of Wenzhou Medical University
(Wenzhou, China).
Ethical considerations
In accordance with the Association for the
Assessment and Accreditation of Laboratory Animal Care
International, rats were maintained under specific pathogen-free
conditions and studied according to protocols approved by the
Wenzhou Medical University Animal Care and Use Committee (approval
no. wydw2013-0071).
Induction of experimental colitis in
rats
Rats were weighed and anesthetized by
intraperitoneal injection of ketamine/xylazine solution (80 ml/10 g
body weight). TNBS (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was dissolved in alcohol (50:50 vol/vol). TNBS solution
(100 mg/kg body weight) was administered intrarectally via 3.5
F-catheter to rats maintained for 60 sec in a vertical position.
The catheter was inserted into the colon 8 cm proximal to the anus.
The normal control group received 0.9% saline intrarectally
(38).
Treatment with TGP
SD rats were randomly assigned to four groups
(n=8/group): Normal control group, TNBS-treated group,
sulfasalazine (SASP)-treated group and TGP-treated group. SASP
(Shanghai Sunve Pharmaceutical Co., Ltd., Shanghai, China) and TGP
(Ningbo Liwah Pharmaceutical Co., Ltd., Ningbo, China) were
respectively administered at the doses of 100 mg/kg/day in the
SASP-treated and TGP-treated group by gastric gavage daily from day
1 (24 h after the induction of colitis) for 7 days prior sacrifice.
Normal control group and TNBS-treated group were treated with an
equal volume of 0.9% saline alone intragastrically.
Clinical analysis of colitis
The clinical progression of colitis was monitored
daily for body weight, diarrhea and hemafecia (3). Loss of body weight was calculated as
the percentage difference relative to initial body weight. Diarrhea
was scored as follows: 0, Normal; 2, loose stools; and 4, diarrhea
that remained adhesive to the anus. Bleeding scores were assessed
as follows: 0, Negative hemoccult; 2, positive hemoccult; and 4,
obvious bleeding (39).
Assessment of colonic damage
Rats were sacrificed 7 days after drug treatment.
The colon was removed and opened longitudinally. The macroscopic
damage was measured by a blinded observer with the following score
system (40,41): 0, Normal; 1, hyperemia, edema, no
ulcer; 2, hyperemia, edema, small linear ulcers or petechiae; 3,
hyperemia, edema, wide ulcers, necrosis, or adhesions; 4,
hyperemia, edema, megacolon, stenosis, or perforation. For
histological analysis, the colonic fragments (0.5 cm) were fixed in
4% paraformaldehyde, dehydrated, embedded in paraffin, sectioned (4
µm thickness) and stained with 10% hematoxylin for 2 min and 0.5%
eosin for 1 min at room temperature. The pathological sections were
observed under a light microscope by two blinded pathologists and
the microscopic damage was scored as follows (42): 0, No evidence of inflammation; 1,
low level of inflammation with scattered infiltrating mononuclear
cells (1–2 foci); 2, moderate inflammation with multiple foci; 3,
high level of inflammation with increased vascular density and
marked wall thickening; 4, maximal severity of inflammation with
transmural leukocyte infiltration and loss of goblet cells.
Immunohistochemistry
The colon specimens were fixed with 4%
paraformaldehyde, embedded with paraffin, and sectioned at 4 µm for
immunohistochemical staining for IL-17, IL-6, ROR-γt, tumor
necrosis factor-α (TNF-α), IL-10, TNF-β and Foxp3. After incubation
with xylene and descending concentrations of ethanol, antigens were
retrieved by citrate buffer for 15 min at 100°C. Then endogenous
peroxidases were removed in 3% hydrogen peroxidase for 15 min at
room temperature followed by 5% goat serum for 1 h at 37°C for
blocking. Subsequently, sections were incubated with polyclonal
rabbit anti-rat antibodies IL-17 (cat. no. ab79056; 1:300), ROR-γt
(cat. no. ab78007; 1:50), TNF-α (cat. no. ab6671; 1:250), IL-10
(cat. no. ab192271; 1:1,000), TNF-β (cat. no. ab92486; 1:100) and
monoclonal mouse anti-rat IL-6 (cat. no. ab9324; 1:250), Foxp3
(cat. no. ab22510; 1:50; Abcam, Cambridge, MA, USA) in an optimum
concentration overnight at 4°C and then incubated with horseradish
peroxidase-conjugated secondary antibody (cat. nos. PV.6001 and
PV.6002; OriGene Technologies, Inc., Beijing, China) for 30 min at
37°C. Antibody bindings were counterstained with hematoxylin,
dehydrated with ascending concentrations of ethanol, cleared in
xylene and mounted. A negative control was performed according to
the same procedure. Images were acquired with a biological imaging
microscope (BX53; Olympus Corporation, Tokyo, Japan).
Western blot analysis
Total protein of colon specimens was extracted using
a radioimmunoprecipitation lysis buffer (Solarbio Science &
Technology Co., Ltd., Beijing, China) and phenylmethylsulfonyl
fluoride. The protein concentration was analyzed using a BCA kit
(Tiangen Biotech Co., Ltd., Beijing, China). Equal amounts of
protein (50 µg per lane) were separated on 12% SDS-polyacrylamide
gels and transferred onto polyvinylidene fluoride (PVDF) membrane
(EMD Millipore, Bedford, MA, USA). After blocking with 5% non-fat
milk for 90 min at room temperature, the membranes were incubated
overnight at 4°C with anti-IL-17 (cat. no. ab79056; polyclonal,
rabbit anti-rat; 1:1,000), anti-IL-6 (cat. no. ab9324; monoclonal,
mouse anti-rat; 1:2,500), anti-ROR-γt (cat. no. ab78007;
polyclonal, rabbit anti-rat; 1:1,000), anti-TNF-α (cat. no. ab6671;
polyclonal, rabbit anti-rat; 1:1,000), anti-IL-10 (cat. no.
ab192271; polyclonal, rabbit anti-rat; 1:1,000), anti-TNF-β (cat.
no. ab92486; polyclonal, rabbit anti-rat; 1:1,000) and anti-Foxp3
(cat. no. ab22510; monoclonal, mouse anti-rat; 1:1,000; all from
Abcam, Cambridge, MA, USA) and anti-glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; cat. no. BS60630; polyclonal, rabbit
anti-rat; 1:1,000; Bioworld Technology, Inc., St. Louis Park, MN,
USA) and washed three times with TBST. Then the membranes were
incubated with secondary antibodies-conjugated to horseradish
peroxidase (cat. no. 7074; 1:5,000; Cell Signaling Technology,
Inc., Danvers, MA, USA) for 1 h at room temperature followed by
washing three times. Immunoreactive bands were visualized by an
enhanced chemiluminescence kit (Bio-Rad Laboratories, Hercules, CA,
USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the colon tissue,
spleen mononuclear cells and mesenteric lymph node (MLN)
mononuclear cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. RNA (1 µg total) was reverse-transcribed into cDNA.
Reverse transcription was carried out by RevertAid First Strand
cDNA Synthesis kit (cat. no. K1622; Thermo Scientific Inc.). The
temperature conditions for the reverse transcription were as
follows: Initial cycle at 25°C for 5 min, followed by 42°C for 60
min and 72°C for 10 min, allowed to cool to 4°C. The mRNA
expression of several genes was quantified with SYBR-Green
Real-time PCR Master Mix Plus (Thermo Fisher Scientific, Inc.) by
an ABI 7500 Sequence-Detection system (Thermo Fisher Scientific,
Inc.). The PCR cycling conditions were as follows: An initial
denaturation and activation at 95°C for10 min, followed by 40
amplification cycles of 95°C for 15 sec and 60°C for 60 sec. The
primer sequences are presented in Table I. GAPDH was used as reference gene.
Relative gene expression levels were calculated by the
2−∆∆Cq method (43).
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
| Gene | Sense (5′-3′) | Antisense
(5′-3′) |
|---|
| GAPDH |
GACATGCCGCCTGGAGAAAC |
AGCCCAGGATGCCCTTTAGT |
| IL-17 |
TACAGTGAAGGCAGCGGTA |
GCTAAGGGAGTTGAGGACTTTC |
| IL-6 |
TGCCTTCCCTACTTCACA |
ACAACTCTTTTCTCATTTCCA |
| TNF-α |
GTCGTAGCAAACCACCAAGC |
GAAGAGAACCTGGGAGTAGATAAGG |
| ROR-γt |
AGGCAAATACGGTGGTGTGG |
ATTGCAGATGCTCCACTCTCC |
| TGF-β |
ATTCCTGGCGTTACCTTG |
CCCTGTATTCCGTCTCCT |
| IL-10 |
GGAGTGAAGACCAGCAAA |
GCAACCCAAGTAACCCTT |
| Foxp3 |
CCATAATATGCGGCCCCCTT |
GCGGGGTGGTTTCTGAAGTA |
Enzyme-linked immunosorbent assay
(ELISA) analysis
Peripheral blood of rats was drawn into
EDTA-anticoagulant tubes, then centrifuged for 15 min at 3,000 × g
at 4°C. The plasma was collected and stored at −80°C until tested.
ELISA kits were used to assess the plasma concentrations of IL-17
(cat. no. 10353-09R), IL-6 (cat. no. 10752-09R), TNF-α (cat. no.
10917-09R), IL-10 (cat. no. 10726-09R) and TNF-β (cat. no.
10973-09R; all from Shanghai Boyun Biotech Co., Ltd., Shanghai
China) in accordance with the manufacturer's protocols.
Flow cytometry analysis
The spleen and MLN were removed from rats 7 days
after drug treatment and isolated mononuclear cells. In order to
analyze Th17 cells, cells were stimulated with phorbol 12-myristate
13-acetate (PMA) (50 ng/ml) and ionomycin (1 µg/ml) in the presence
of Brefeldin A (10 µg/ml) and monensin (1.4 µg/ml) (Hangzhou
MultiSciences Biotech Co., Ltd., Hangzhou China) at 37°C and 5%
CO2 for 4 h. Cells were washed with PBS and
surface-labeled with fluorescein isothiocyanate-(FITC-) conjugated
anti-CD4 (0.5%; cat. no. 85-11-0041-81; eBioscience; Thermo Fisher
Scientific, Inc.) for 40 min at 4°C. The cells were subsequently
fixed and permeabilized by fixation/permeabilization buffer (BD
Biosciences, San Jose, CA, USA) and labeled with
phycoerythrin-(PE-) conjugated anti-IL-17 (1%; cat. no.
85-12-7177-81; eBioscience; Thermo Fisher Scientific, Inc.) for 40
min at 4°C. For analysis of Treg cells, without PMA and ionomycin
stimulation, surface staining was performed with FITC-conjugated
anti-CD4 (0.5%; cat. no. 85-11-0041-81; eBioscience; Thermo Fisher
Scientific, Inc.) and allophycocyanin-conjugated anti-CD25 (1.5%;
cat. no. 85-17-0390-82; eBioscience; Thermo Fisher Scientific,
Inc.) for 40 min at 4°C. Afterwards, cells were fixed and
permeabilized, and intracellular staining was performed with
PE-conjugated anti-Foxp3 (1.25%; cat. no. 85-12-5773-82;
eBioscience; Thermo Fisher Scientific, Inc.) for 40 min at 4°C.
Appropriate isotype controls were used in the experiments. The
stained cells were detected by a FACSCalibur flow cytometer (BD
Biosciences) and the data were analyzed with FlowJo version 7.6.1
(FlowJo LLC, Ashland, OR, USA).
Statistical analysis
Statistical analysis was performed using SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as
mean ± standard error and one-way analysis of variance was used for
multiple comparisons, followed by Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TGP ameliorates acute inflammatory of
TNBS-induced colitis in rats
To evaluate the potential therapeutic effect of TGP
in the TNBS-induced colitis, the present study tested the clinical
symptoms and macroscopic scores in the aforementioned groups. As
expected, the rats acquired severe symptoms characterized by weight
loss, diarrhea and bleeding following TNBS instillation compared
with the normal control group. However, TGP and SASP treatment
rapidly reversed weight loss and decreased bleeding and diarrhea
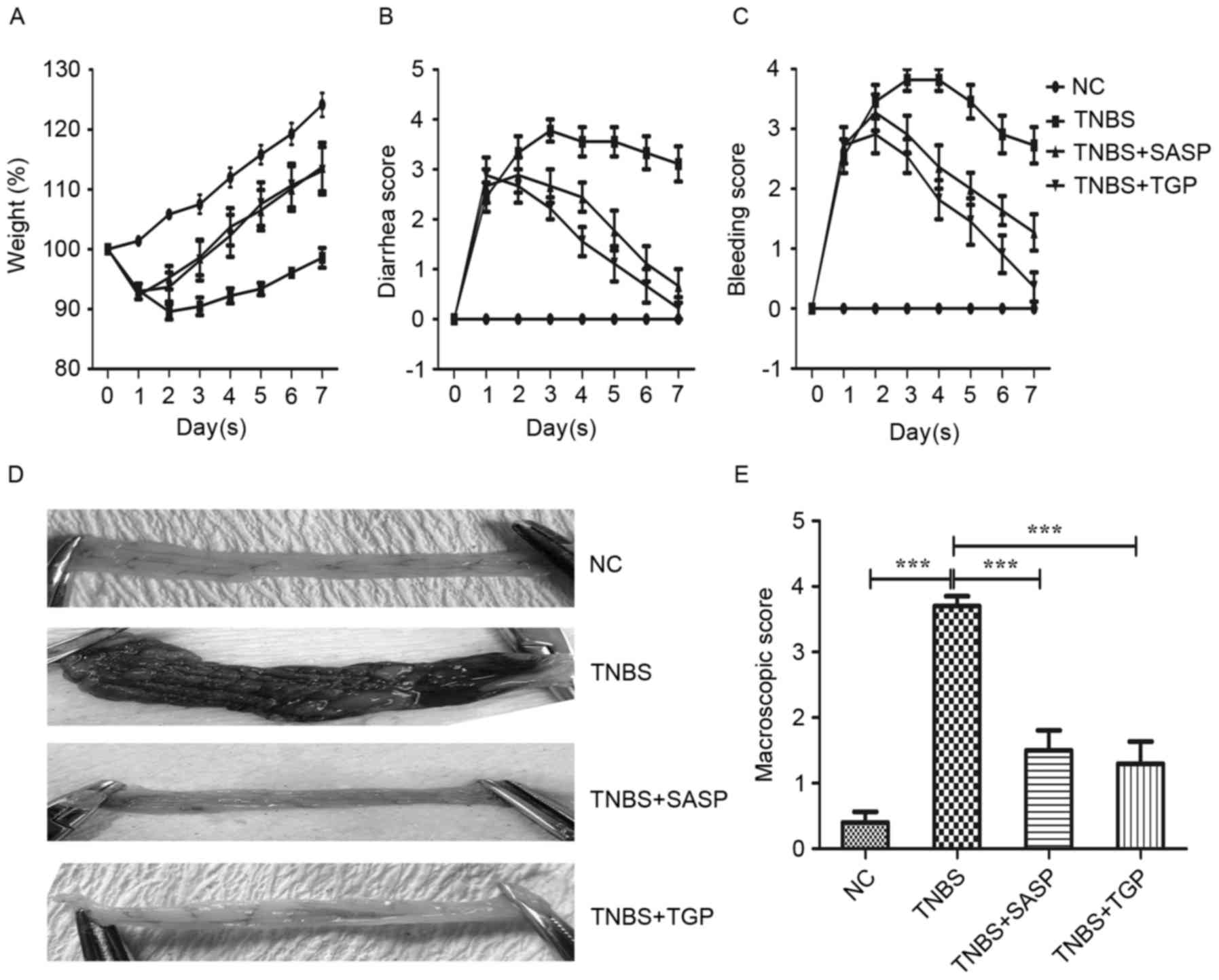
scores compared with the TNBS group (Fig. 1A-C). The present study observed
marked macroscopic change accompanied by hyperemia, edema, ulcers,
necrosis and adhesion in TNBS-induced group, whereas the colons
from TGP or SASP group exhibited no or a slight macroscopic damage
(Fig. 1D). Consistently, the
macroscopic scores of TNBS-induced rats were significantly
increased compared with the control group and the TGP and
SASP-treated groups (Fig. 1E).
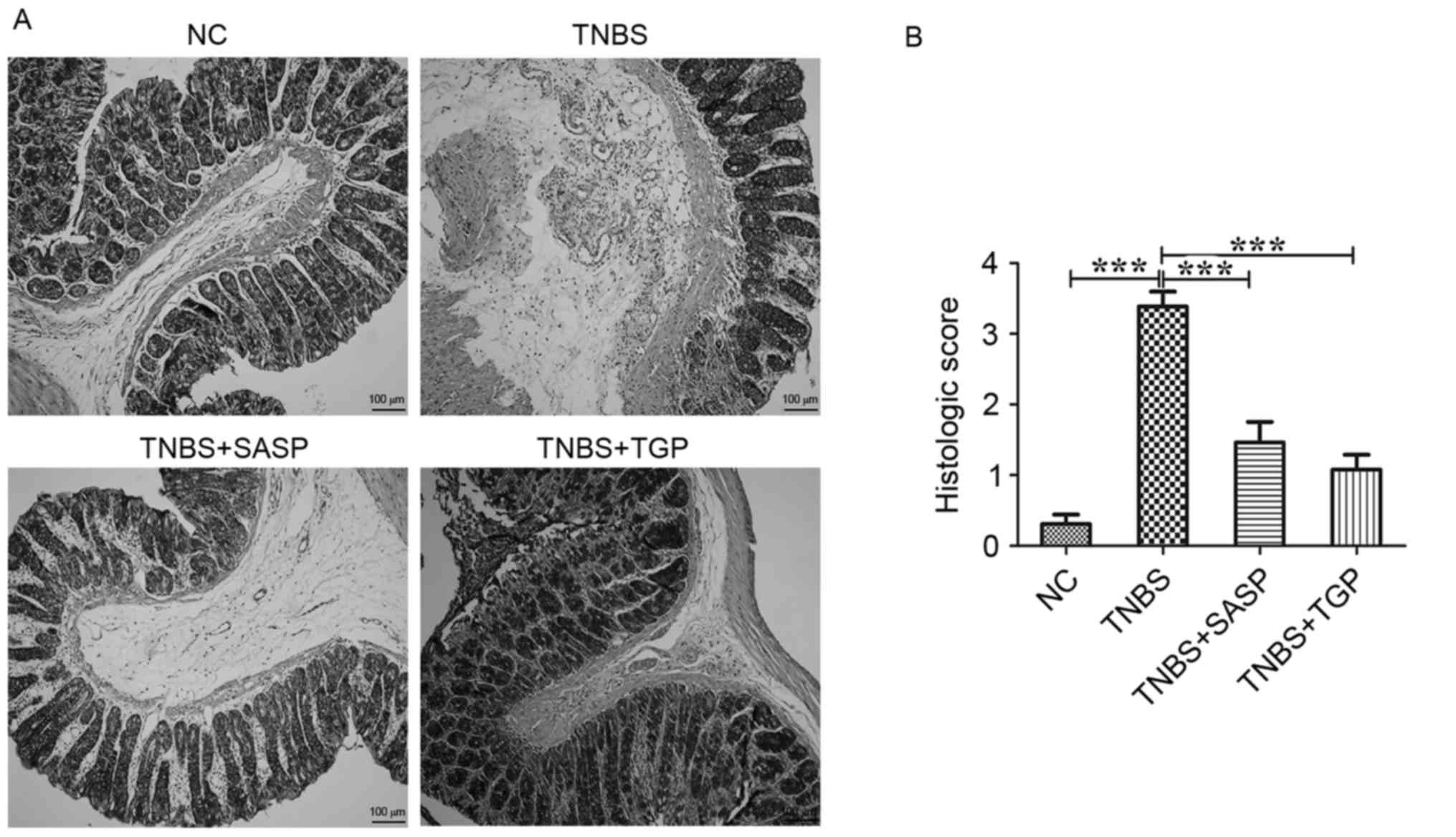
Histopathologically, the colonic tissues of TNBS-induced rats
exhibited a reduced number of goblet cells, loss of crypts, damage
of crypts, infiltration by inflammatory cells and extensive
destruction of the mucosal layer compared with the control group
(Fig. 2A). However, histological
signs of inflammatory activity were distinctly reduced following
TGP administration in TNBS-rats, similar to the SASP-treated group
(Fig. 2). Therefore, treatment of
TGP is effective in modulating the development of acute
TNBS-induced colitis and the efficacy of TGP was similar to
SASP.
TGP regulates the production of Th17-
and Treg-associated cytokines and transcription factors in plasma
and colonic tissues of TNBS-induced colitis model
In order to investigate the effects of TGP on acute
inflammation, plasma and colonic tissue samples were collected at 7
days and the production of signature cytokines and transcription
factors was measured by ELISA, western blotting and
immunohistochemistry. The current results revealed that
Th17-associated pro-inflammatory cytokines and transcription
factors in colonic tissues of TNBS-induced rats, such as IL-17,
IL-6, TNF-α and ROR-γt, were significantly reduced following
administration of TGP and SASP treatment (Figs. 3 and 4). The TGP and SASP-treated groups also
had higher levels of IL-10, TNF-β and Foxp3 in colonic tissues
compared with the untreated TNBS and control groups (Figs. 3 and 4). Similarly, TGP administration
downregulated the expression of IL-17, TNF-α, IL-6 and upregulated
the level of IL-10 and TNF-β in plasma of TNBS-induced rats
(Fig. 5). These findings indicate
that TGP inhibits Th17 responses; however, may promote Treg
responses in TNBS-induced colitis and there was no significant
difference between treatment with TGP or SASP.
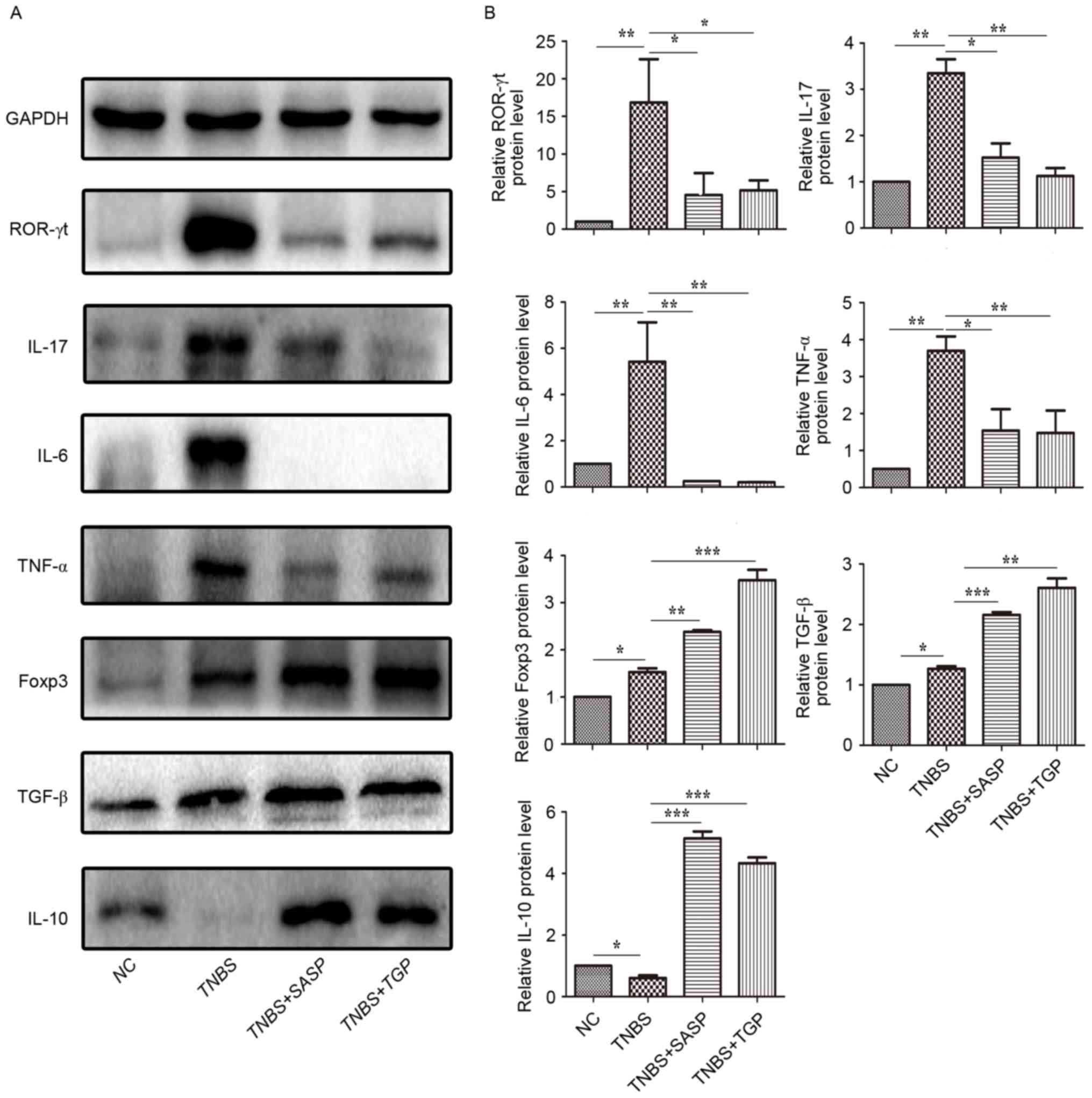 | Figure 3.TGP regulates the production of Th17-
and Treg-associated cytokines and transcription factors in colonic
tissues of TNBS-induced colitis in a similar manner to SASP. (A)
Expression of proteins ROR-γt, IL-17, IL-6, TNF-α, Foxp3, TNF-β,
IL-10 were determined by western blotting. (B) Data are presented
as the mean ± standard error of the mean. *P<0.05, **P<0.01,
***P<0.001, n≥6 per group. NC, normal control group; TGP, total
glycosides of paeony; SASP, sulfasalazine; Th17, effector
CD4+ T helper 17; Treg, regulatory CD4+ T
cells; TNBS, trinitrobenzene sulfonic acid-induced group;
TNBS+SASP, TNBS-induced rats treated with SASP; TNBS+TGP,
TNBS-induced rats treated with TGP; IL, interleukin; TNF-α, tumor
necrosis factor-α; ROR-γt, retinoic acid related orphan
receptor-γt; TNF-β, transforming growth factor-β; Foxp3, forkhead
boxp3. |
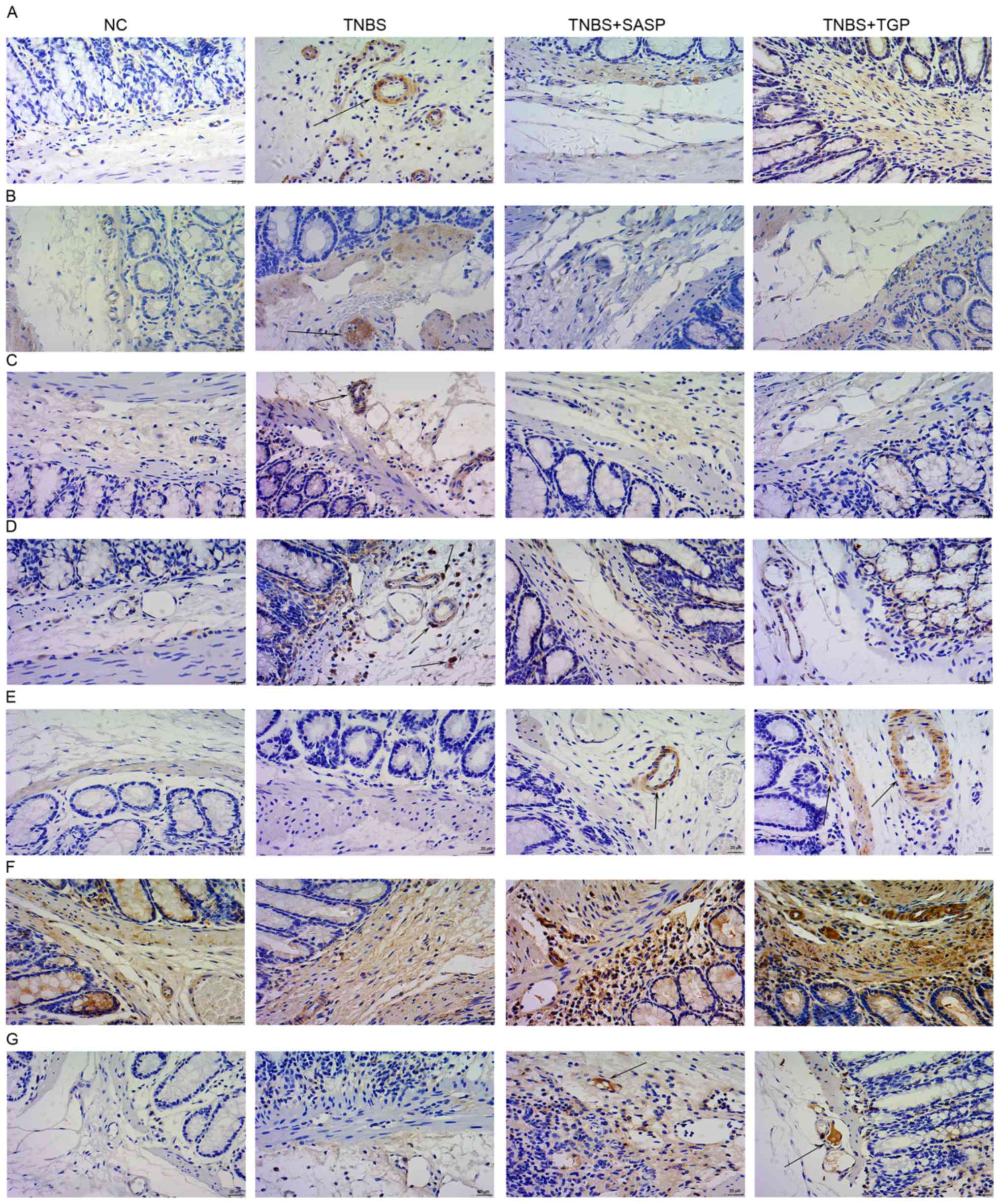 | Figure 4.TGP regulates the production of Th17-
and Treg-associated cytokines and transcription factors in colonic
tissues of TNBS-induced colitis in a similar manner to SASP.
Immunohistochemical detection of (A) IL-17, (B) IL-6, (C) TNF-α,
(D) ROR-γ, (E) IL-10, (F) TNF-β, (G) Foxp3 in the colonic tissues.
All tissue sections were counterstained with hematoxylin.
Magnification, ×400. n≥6 per group. NC, normal control group; TGP,
total glycosides of paeony; SASP, sulfasalazine; Th17, effector
CD4+ T helper 17; Treg, regulatory CD4+ T
cells; TNBS, trinitrobenzene sulfonic acid-induced group;
TNBS+SASP, TNBS-induced rats treated with TNBS-induced rats treated
with SASP; IL, interleukin; TNF-α, tumor necrosis factor-α; ROR-γt,
retinoic acid related orphan receptor-γt; TNF-β, transforming
growth factor-β; Foxp3, forkhead boxp3. |
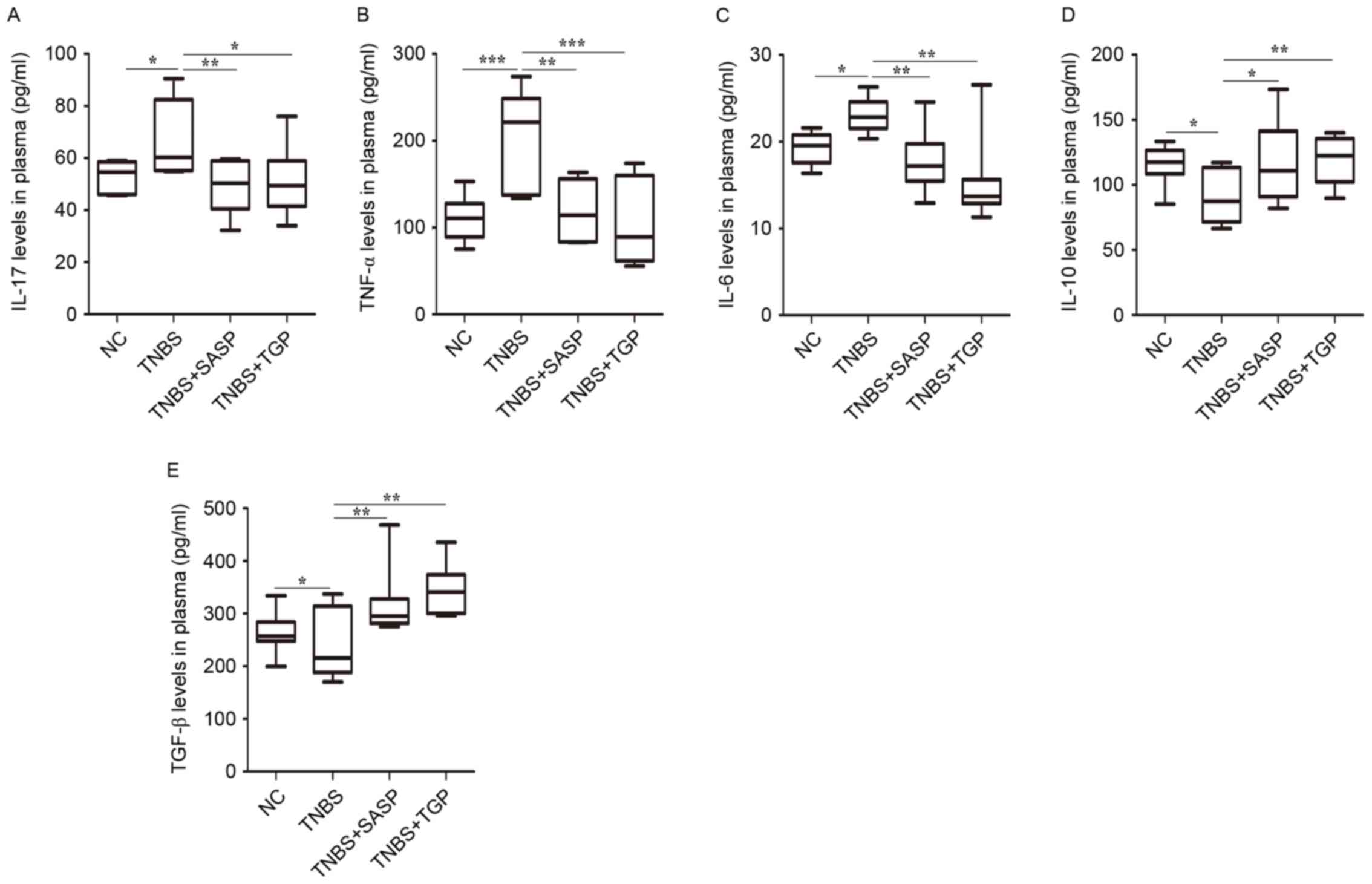 | Figure 5.TGP regulates the levels of Th17- and
Treg-associated cytokines in plasma in a similar manner to SASP.
Effect of drugs treatment on the levels of (A) IL-17, (B) TNF-α,
(C) IL-6, (D) IL-10, (E) TNF-β in plasma are presented. *P<0.05,
**P<0.01, ***P<0.001, n≥6 per group. NC, normal control
group; TGP, total glycosides of paeony; SASP, sulfasalazine; Th17,
effector CD4+ T helper 17; Treg, regulatory
CD4+ T cells; TNBS, trinitrobenzene sulfonic
acid-induced group; TNBS+SASP, TNBS-induced rats treated with SASP;
TNBS+TGP, TNBS-induced rats treated with TGP; IL, interleukin;
TNF-α, tumor necrosis factor-α; ROR-γt, retinoic acid related
orphan receptor-γt; TNF-β, transforming growth factor-β; Foxp3,
forkhead boxp3. |
TGP modulates the mRNA levels of Th17-
and Treg-associated cytokines and transcription factors in the
TNBS-induced colitis
To determine whether TGP may adjust the mRNA
expression of transcription factors and cytokines in Th17 and Treg
cells, we tested the expression levels using RT-qPCR. TGP
administration in TNBS-rats led to lower mRNA expression levels of
TNF-α, IL-6, IL-17 and ROR-γt in the colonic tissues, MLN and
splenic lymphocytes (Figs. 6A-D,
7A-D and 8A-D). In contrast, the mRNA expression of
IL-10, TNF-β and Foxp3 increased in TNBS-rats treated with TGP
(Figs. 6E-G, 7E-G and 8E-G).
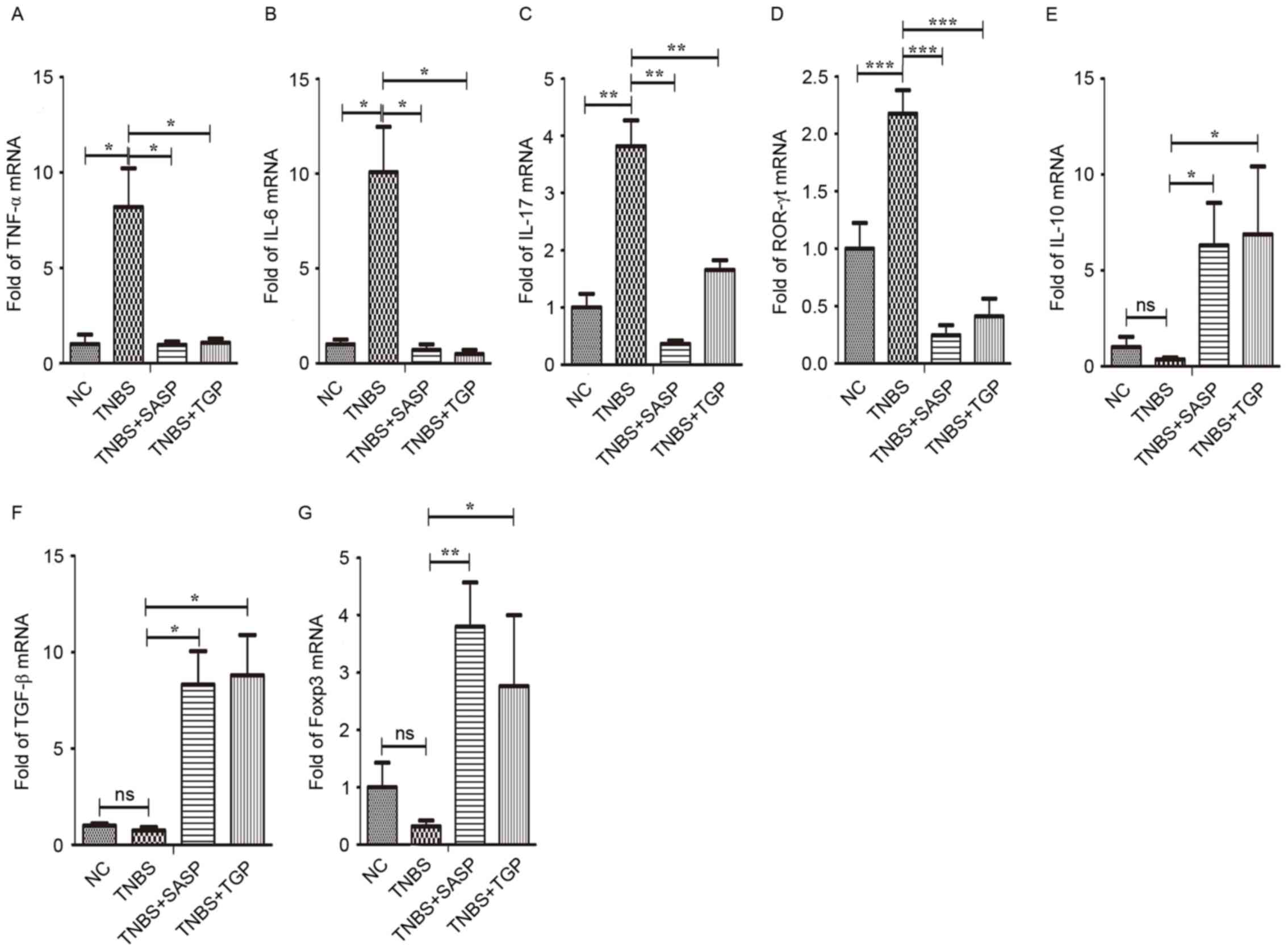 | Figure 6.TGP modulates the mRNA levels of
Th17- and Treg-associated cytokines and transcription factors in
the colonic tissue of TNBS-induced colitis in a similar manner to
SASP. Total mRNA was extracted from colonic tissue to analyze the
expression of (A) TNF-α, (B) IL-6, (C) IL-17, (D) ROR-γt, (E)
IL-10, (F) TNF-β, (G) Foxp3 by quantitative polymerase chain
reaction. *P<0.05, **P<0.01, ***P<0.001, n≥6 per group.
NC, normal control group; TGP, total glycosides of paeony; SASP,
sulfasalazine; Th17, effector CD4+ T helper 17; Treg,
regulatory CD4+ T cells; TNBS, trinitrobenzene sulfonic
acid-induced group; TNBS+SASP, TNBS-induced rats treated with SASP;
TNBS+TGP, TNBS-induced rats treated with TGP; IL, interleukin;
TNF-α, tumor necrosis factor-α; ROR-γt, retinoic acid related
orphan receptor-γt; TNF-β, transforming growth factor-β; Foxp3,
forkhead boxp3. |
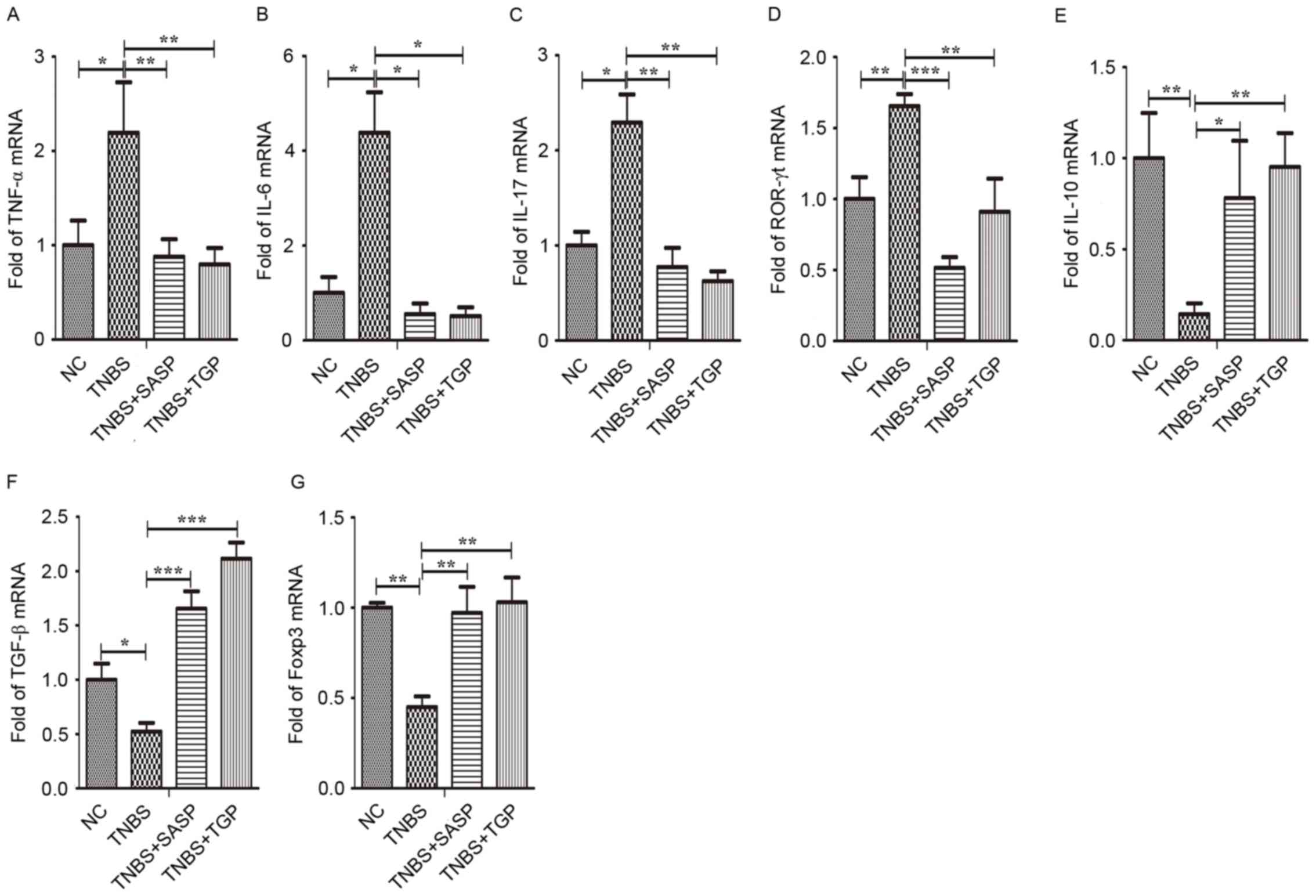 | Figure 7.TGP modulates the mRNA levels of
Th17- and Treg-associated cytokines and transcription factors in
the mesenteric lymph nodes of TNBS-induced colitis in a similar
manner to SASP. Total mRNA was extracted from mononuclear cells in
the MLN to analyze the expression of (A) TNF-α, (B) IL-6, (C)
IL-17, (D) ROR-γt, (E) IL-10, (F) TGF-β, (G) Foxp3 by quantitative
polymerase chain reaction. *P<0.05, **P<0.01, ***P<0.001,
n≥6 per group. NC, normal control group; TGP, total glycosides of
paeony; SASP, sulfasalazine; Th17, effector CD4+ T
helper 17; Treg, regulatory CD4+ T cells; TNBS,
trinitrobenzene sulfonic acid-induced group; TNBS+SASP,
TNBS-induced rats treated with SASP; TNBS+TGP, TNBS-induced rats
treated with TGP; IL, interleukin; TNF-α, tumor necrosis factor-α;
ROR-γt, retinoic acid related orphan receptor-γt; TNF-β,
transforming growth factor-β; Foxp3, forkhead boxp3. |
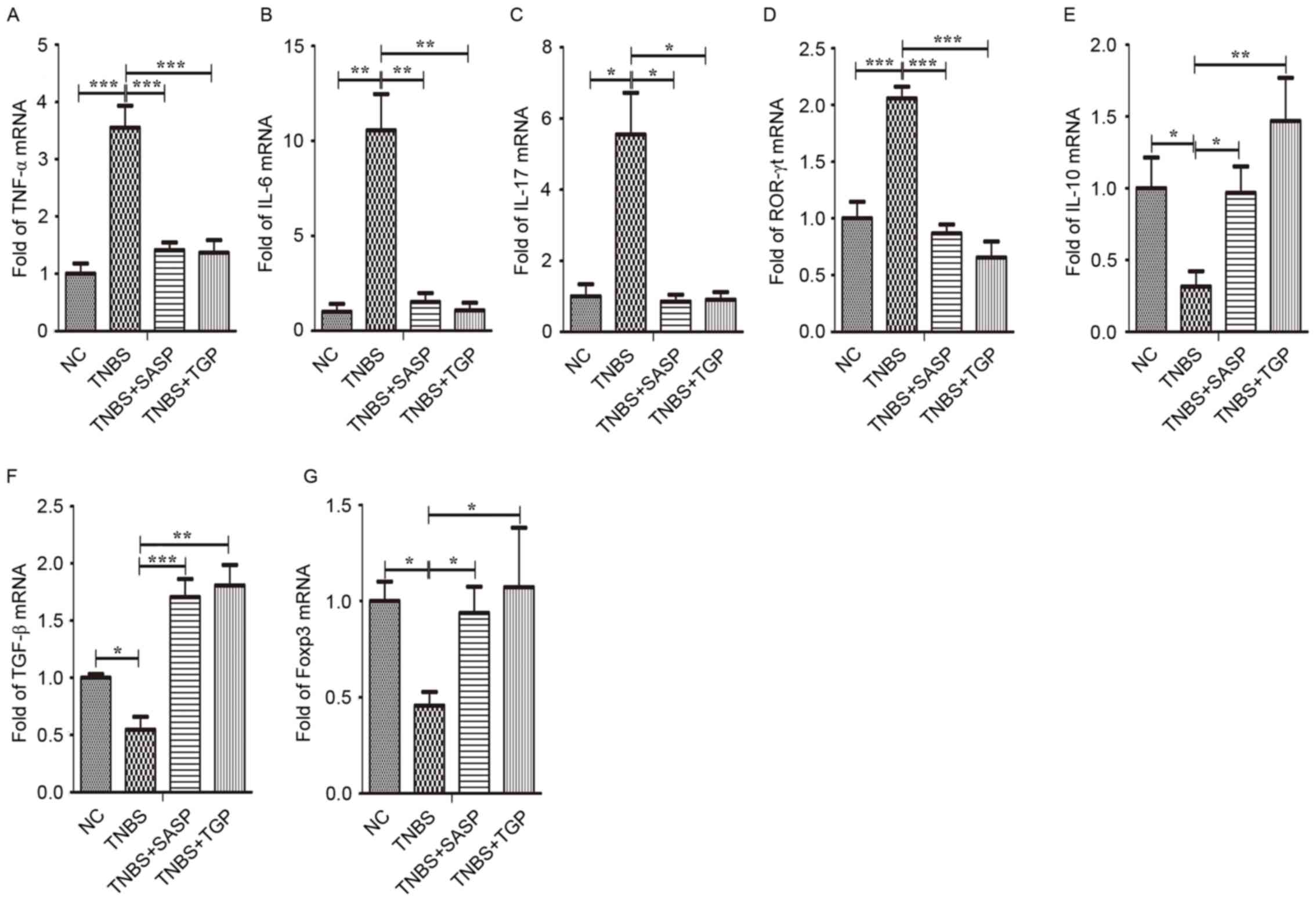 | Figure 8.TGP modulates the mRNA levels of
Th17- and Treg-associated cytokines and transcription factors in
the spleen of TNBS-induced colitis in a similar manner to SASP.
Total mRNA was extracted from mononuclear cells in the spleen to
analyze the expression of (A) TNF-α, (B) IL-6, (C) IL-17, (D)
ROR-γt, (E) IL-10, (F) TNF-β, (G) Foxp3 by quantitative polymerase
chain reaction. *P<0.05, **P<0.01, ***P<0.001, n≥6 per
group. NC, normal control group; TGP, total glycosides of paeony;
SASP, sulfasalazine; Th17, effector CD4+ T helper 17;
Treg, regulatory CD4+ T cells; TNBS, trinitrobenzene
sulfonic acid-induced group; TNBS+SASP, TNBS-induced rats treated
with SASP; TNBS+TGP, TNBS-induced rats treated with TGP; IL,
interleukin; TNF-α, tumor necrosis factor-α; ROR-γt, retinoic acid
related orphan receptor-γt; TNF-β, transforming growth factor-β;
Foxp3, forkhead boxp3. |
TGP affects the differentiation of
Th17 and Treg cells in the TNBS-induced colitis
Previous studies have revealed that Th17 and Treg
cells play a distinct role in the control and development of IBD
and the balance between Th17 and Treg cells proliferation levels is
a critical factor in designing therapies for IBD (44,45).
Therefore, the present study hypothesized that TGP may
differentially contribute to the development of Th17 and Treg
cells. In order to determine this, flow cytometry was used to
quantify the frequencies of Th17 and Treg cells separated from
spleen and MLNs of all groups. As presented in Fig. 9C and D, the percentage of
CD4+IL17+(Th17) cells was markedly increased
in the TNBS-induced rats, whereas the administration of TGP
markedly reduced the percentage, as well as the treatment with
SASP. Additionally, the present study revealed that TGP treatment
increased the level of
CD4+CD25+Foxp3+ (Treg) cells
(Fig. 9A and B). Therefore, these
findings indicated that TGP may be able to ameliorate colitis,
which was associated with an increased number of Treg cells and a
reduction of Th17 cells among spleen and MLNs.
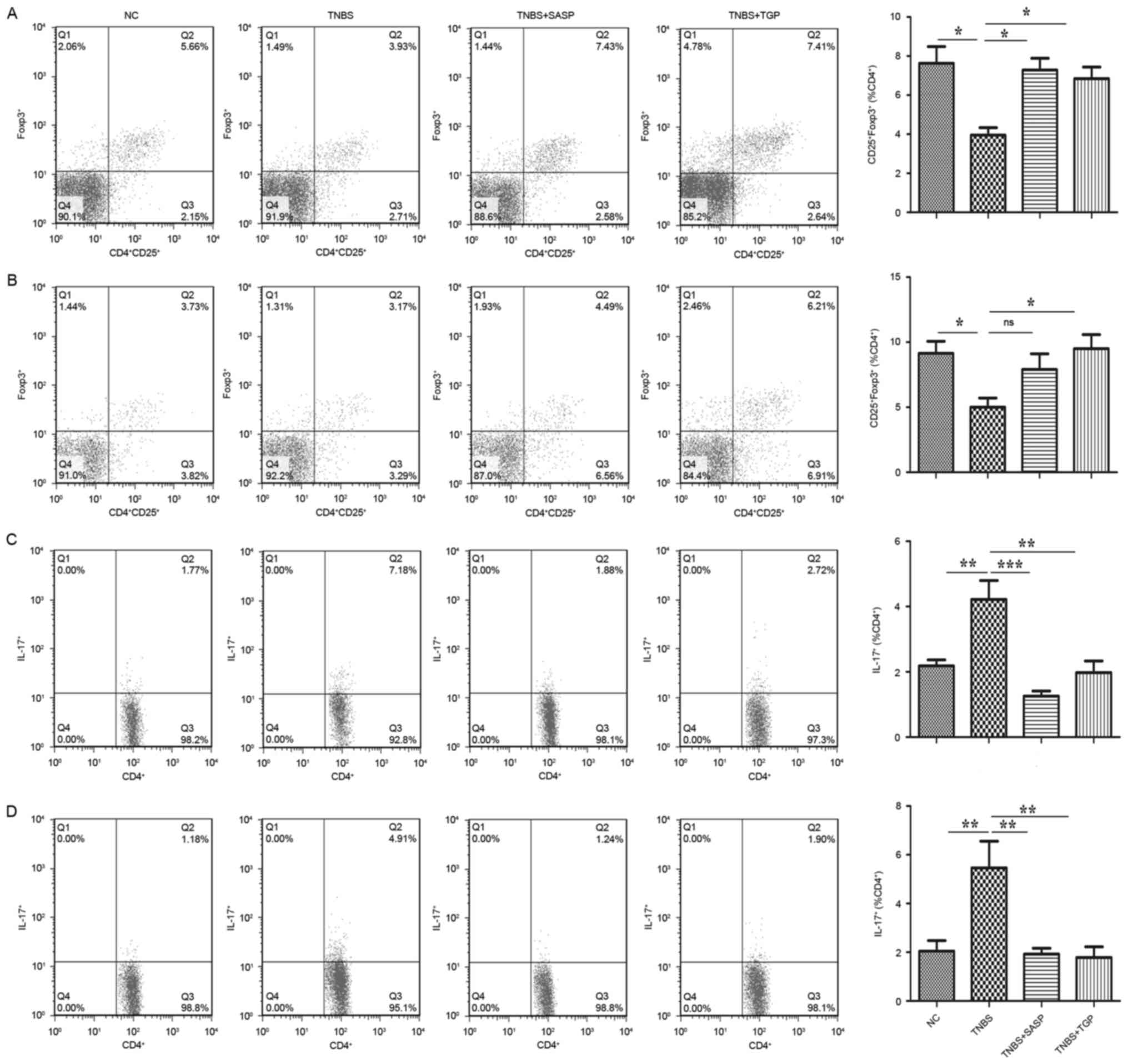 | Figure 9.TGP regulates frequencies of Th17 and
Treg cells in the TNBS-induced colitis in a similar manner to SASP.
Mononuclear cells of spleen and MLN were isolated from each group
and subjected to intracellular IL-17 and Foxp3 staining. The
frequency of Treg
(CD4+CD25+Foxp3+) in the (A) MLN
and (B) spleen and Th17 (CD4+IL-17+) in the
(C) MLN and (D) spleen were determined by flow cytometry.
Quantitative analysis of the frequency was performed
simultaneously. NC, normal control group; TGP, total glycosides of
paeony; SASP, sulfasalazine; Th17, effector CD4+ T
helper 17; Treg, regulatory CD4+ T cells; TNBS,
trinitrobenzene sulfonic acid-induced group; TNBS+SASP,
TNBS-induced rats treated with SASP; TNBS+TGP, TNBS-induced rats
treated with TGP; IL-17, interleukin-17; Foxp3, forkhead boxp3;
MLN, mesenteric lymph nodes. |
Discussion
The medical therapeutic strategies for patients with
IBD include corticosteroids, biological agents and
immunosuppressant drugs. However, efficiency of aforementioned
therapies is limited and side effects (including serious infection,
myelosuppression and hepatic injury) are too serious to ignore
(46). Therefore, more effective
and alternative treatments are urgently needed. Although, TGP has
been used as a prescription immune-regulatory drug for treating
autoimmune diseases including RA, AS and SLE and has exhibited
significant effects in the treatment of several animal models, such
as experimental autoimmune encephalomyelitis, collagen induced
arthritis and TNBS-induced colitis (26,30,33,36),
it remains to be elucidated how TGP exerts its therapeutic
effects.
Previous studies have revealed that the potential
mechanism of TGP in TNBS-induced colitis may be associated with the
adjustment of Th1/Th2 cytokine polarization (37,47).
However, TGP has also been reported to impair Th17 differentiation
by reducing the production of IL-6 and increase the proportion of
Treg cells (30,34). Additionally, perturbation in the
balance between Th17 and Treg cells may lead to aberrant
inflammation responses having a role in IBD (2). Therefore, the present study
investigated the role for TGP in attenuating TNBS-induced colitis
via regulation of the Th17/Treg balance.
Following previous studies (38,48),
the present study selected the well-established model of
TNBS-induced experimental colitis to evaluate the effect of TGP,
which is characterized by intense weight loss, diarrhea, bleeding,
colonic injury and invasion of inflammatory cells infiltration. In
the present study, TGP-treated rats had attenuated weight loss,
clinical signs and a reduction in histological markers of
inflammation.
Additionally, the present study demonstrated that
TGP significantly reduced levels of Th17-associated cytokines
(IL-17) and TNF-α, whereas increased levels of Treg-associated
cytokines (TNF-β and IL-10) were observed in the colon. Similar to
colonic tissue, a decreased secretion of IL-17 and TNF-α and
increased secretion of TNF-β and IL-10 was observed in peripheral
blood, MLNs and spleen. The changes of TNF-α and IL-10 were in line
with a previous study (37). It is
evident that the inappropriate production of IL-17 contributes to
the pathology of IBD and the anti-inflammatory cytokines IL-10 and
TNF-β may prevent the established colitis in animal models of IBD
(6,9,12).
These data indicated that TGP was able to shift the
pro-inflammatory environment to anti-inflammatory status leading to
ameliorate colitis. Previous studies have highlighted that IBD is
associated with imbalance between Th17 and Treg cells and TNF-β
drives the differentiation of Treg cells, whereas Th17 cells are
induced by a combination of IL-6 and TNF-β (1,9,21,24,49).
Therefore, TNF-β is an effector molecule of Treg cells and has a
dual role in the differentiation of Th17 and Treg cells. TNF-β
promotes the production of Treg by inducing Foxp3 expression.
However, in the presence of IL-6, it induces the expression of
ROR-γt and IL-23R to promote the Th17 phenotype (19). In the TGP-treated TNBS-induced rat
group, the present study identified downregulation of IL-6 and
upregulation of TNF-β in the colon, plasma, MLNs and spleen.
Concordant with these results, the present study revealed that TGP
treatment significantly reduced percentages of Th17 cells and
increased percentages of Treg cells in the MLNs and spleen of
TNBS-induced rats. Overall, these data supported the hypothesis
that TGP-regulated Th17 and Treg subset differentiation to control
inflammation of experimental colitis. Additionally, transcription
factors have a crucial role in T-cell differentiation; therefore,
the present study tested lineage-specific transcriptional
regulators ROR-γt and Foxp3. ROR-γt dominated Th17 differentiation
and favored the occurrence of immune response by producing IL-17
cytokines (50). Foxp3 directed
Treg formation and confer their regulatory activity by production
of TNF-β and IL-10 cytokines (15,51).
Therefore, the marked reduction of ROR-γt mRNA and protein levels
and the increased Foxp3 expression in the colon, MLNs and spleen
were regarded as another mechanism that TGP contributed towards
establishing the homeostasis of Th17 and Treg cells in the response
to TNBS-induced damage.
SASP is a commonly prescribed drug with proven
efficacy in the treatment of IBD (52). The present study compared the
effects of TGP and SASP in experimental colitis. Subsequently,
treatment with TGP daily resulted in a therapeutic effect equal to
that of SASP.
In summary, the present study indicated that TGP may
improve symptoms of TNBS-induced colitis by regulating cytokine
production, inhibiting effector phenotype of Th17 cells and
facilitating Treg responses. These anti-inflammatory properties of
TGP may offer an opportunity to use it as a novel candidate for the
treatment of IBD. However, further mechanism studies are required
to prove its immunomodulatory effect in other models of
colitis.
Acknowledgements
The present study was supported by the Public
Technology Research Project of Zhejiang Province (grant no.
2015C33120) and the Natural Science Foundation of China (grant no.
81570495).
Glossary
Abbreviations
Abbreviations:
|
IBD
|
inflammatory bowel disease
|
|
Treg
|
regulatory CD4+ T cells
|
|
IL-17
|
interleukin-17
|
|
Th17
|
effector CD4+ T helper
17
|
|
TNBS
|
trinitrobenzene sulfonic acid
|
|
CD
|
Crohn's disease
|
|
UC
|
ulcerative colitis
|
|
TNF-α
|
tumor necrosis factor-α
|
|
PVDF
|
polyvinylidene fluoride
|
|
PF
|
paloniflorin
|
|
Foxp3
|
forkhead boxp3
|
|
TGP
|
total glycosides of paeony
|
|
RA
|
rheumatoid arthritis
|
|
SLE
|
systemic lupus erythematosus
|
|
AS
|
ankylosing spondylitis
|
|
T-bet
|
T-box expressed in T cells
|
|
SD
|
Sprague-Dawley
|
|
SASP
|
sulfasalazine
|
|
MLN
|
mesenteric lymph nodes
|
|
TNF-β
|
transforming growth factor-β
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
ROR-γt
|
retinoic acid related orphan
receptor-γt
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
References
|
1
|
Cătană CS, Neagoe I Berindan, Cozma V,
Magdaş C, Tăbăran F and Dumitraşcu DL: Contribution of the
IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease.
World J Gastroenterol. 21:5823–5830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geremia A, Biancheri P, Allan P, Corazza
GR and Di Sabatino A: Innate and adaptive immunity in inflammatory
bowel disease. Autoimmun Rev. 13:3–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glauben R, Batra A, Stroh T, Erben U,
Fedke I, Lehr HA, Leoni F, Mascagni P, Dinarello CA, Zeitz M and
Siegmund B: Histone deacetylases: Novel targets for prevention of
colitis-associated cancer in mice. Gut. 57:613–622. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salim SY and Söderholm JD: Importance of
disrupted intestinal barrier in inflammatory bowel diseases.
Inflamm Bowel Dis. 17:362–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veltkamp C, Anstaett M, Wahl K, Möller S,
Gangl S, Bachmann O, Hardtke-Wolenski M, Länger F, Stremmel W,
Manns MP, et al: Apoptosis of regulatory T lymphocytes is increased
in chronic inflammatory bowel disease and reversed by anti-TNFα
treatment. Gut. 60:1345–1353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maul J, Loddenkemper C, Mundt P, Berg E,
Giese T, Stallmach A, Zeitz M and Duchmann R: Peripheral and
intestinal regulatory CD4+ CD25(high) T cells in
inflammatory bowel disease. Gastroenterology. 128:1868–1878. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brand S: Crohn's disease: Th1, Th17 or
both? The change of a paradigm: New immunological and genetic
insights implicate Th17 cells in the pathogenesis of Crohn's
disease. Gut. 58:1152–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaser A, Zeissig S and Blumberg RS:
Inflammatory bowel disease. Annu Rev Immunol. 28:573–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eastaff-Leung N, Mabarrack N, Barbour A,
Cummins A and Barry S: Foxp3+ regulatory T cells, Th17
effector cells, and cytokine environment in inflammatory bowel
disease. J Clin Immunol. 30:80–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaffen SL: An overview of IL-17 function
and signaling. Cytokine. 43:402–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dambacher J, Beigel F, Zitzmann K, De Toni
EN, Göke B, Diepolder HM, Auernhammer CJ and Brand S: The role of
the novel Th17 cytokine IL-26 in intestinal inflammation. Gut.
58:1207–1217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujino S, Andoh A, Bamba S, Ogawa A, Hata
K, Araki Y, Bamba T and Fujiyama Y: Increased expression of
interleukin 17 in inflammatory bowel disease. Gut. 52:65–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nielsen OH, Kirman I, Rüdiger N, Hendel J
and Vainer B: Upregulation of interleukin-12 and −17 in active
inflammatory bowel disease. Scand J Gastroenterol. 38:180–185.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valencia X, Stephens G, Goldbach-Mansky R,
Wilson M, Shevach EM and Lipsky PE: TNF downmodulates the function
of human CD4+CD25hi T-regulatory cells.
Blood. 108:253–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Garra A and Vieira P: Regulatory T cells
and mechanisms of immune system control. Nat Med. 10:801–805. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh B, Read S, Asseman C, Malmstrom V,
Mottet C, Stephens LA, Stepankova R, Tlaskalova H and Powrie F:
Control of intestinal inflammation by regulatory T cells. Immunol
Rev. 182:190–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mottet C, Uhlig HH and Powrie F: Cutting
edge: Cure of colitis by CD4+CD25+ regulatory
T cells. J Immunol. 170:3939–3943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galvez J: Role of Th17 cells in the
pathogenesis of Human IBD. ISRN Inflamm. 2014:9284612014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hansen R, Thomson JM, El-Omar EM and Hold
GL: The role of infection in the aetiology of inflammatory bowel
disease. J Gastroenterol. 45:266–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mangan PR, Harrington LE, O'Quinn DB,
Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR and
Weaver CT: Transforming growth factor-beta induces development of
the T(H)17 lineage. Nature. 441:231–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu J and Paul WE: Heterogeneity and
plasticity of T helper cells. Cell Res. 20:4–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu CF: A review on the pharmacology of
Paeonia lactiflora and its chemical components. Zhong Yao Tong Bao.
10:43–45. 1985.(In Chinese). PubMed/NCBI
|
|
26
|
Zhang LL, Wei W, Wang NP, Wang QT, Chen
JY, Chen Y, Wu H and Hu XY: Paeoniflorin suppresses inflammatory
mediator production and regulates G protein-coupled signaling in
fibroblast-like synoviocytes of collagen induced arthritic rats.
Inflamm Res. 57:388–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He DY and Dai SM: Anti-inflammatory and
immunomodulatory effects of Paeonia lactiflora Pall., a traditional
chinese herbal medicine. Front Pharmacol. 2:102011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee B, Shin YW, Bae EA, Han SJ, Kim JS,
Kang SS and Kim DH: Antiallergic effect of the root of Paeonia
lactiflora and its constituents paeoniflorin and paeonol. Arch
Pharm Res. 31:445–450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim ID and Ha BJ: The effects of
paeoniflorin on LPS-induced liver inflammatory reactions. Arch
Pharm Res. 33:959–966. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Xiao L, Ouyang G, Shen Y, Huo R,
Zhou Z, Sun Y, Zhu X, Zhang J, Shen B and Li N: Total glucosides of
paeony inhibits Th1/Th17 cells via decreasing dendritic cells
activation in rheumatoid arthritis. Cell Immunol. 280:156–163.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu L, Wei W, Zheng YQ and Jia XY: Effects
and mechanisms of total glucosides of paeony on joint damage in rat
collagen-induced arthritis. Inflamm Res. 54:211–220. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu Y, Ren K, Liang C, Yuan L, Qi X, Dong
J, Shen J and Lin S: Renoprotective effect of total glucosides of
paeony (TGP) and its mechanism in experimental diabetes. J
Pharmacol Sci. 109:78–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Q, Ma X, Zhu DL, Chen L, Jiang Y,
Zhou L, Cen L, Pi R and Chen X: Total glucosides of peony
attenuates experimental autoimmune encephalomyelitis in C57BL/6
mice. J Neuroimmunol. 284:67–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao M, Liang GP, Tang MN, Luo SY, Zhang
J, Cheng WJ, Chan TM and Lu QJ: Total glucosides of paeony induces
regulatory CD4(+)CD25(+) T cells by increasing Foxp3 demethylation
in lupus CD4(+) T cells. Clin Immunol. 143:180–187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao W, Zhang W, Liu J, Wang Y, Peng X, Lu
D, Qi R, Wang Y and Wang H: Paeoniflorin improves survival in
LPS-challenged mice through the suppression of TNF-α and IL-1β
release and augmentation of IL-10 production. Int Immunopharmacol.
11:172–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu GL, Li YC and Shen YJ: Inhibitory
effect of total glucosides of paeonia on the NF-κB/p65 protein
expression in paws of RA rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
26:1082–1084. 2010.(In Chinese). PubMed/NCBI
|
|
37
|
Zhang Y, Zhou R, Zhou F, Cheng H and Xia
B: Total glucosides of peony attenuates 2,4,6-trinitrobenzene
sulfonic acid/ethanol-induced colitis in rats through adjustment of
TH1/TH2 cytokines polarization. Cell Biochem Biophys. 68:83–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Alex P, Zachos NC, Nguyen T, Gonzales L,
Chen TE, Conklin LS, Centola M and Li X: Distinct cytokine patterns
identified from multiplex profiles of murine DSS and TNBS-induced
colitis. Inflamm Bowel Dis. 15:341–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zou Y, Li WY, Wan Z, Zhao B, He ZW, Wu ZG,
Huang GL, Wang J, Li BB, Lu YJ, et al: Huangqin-Tang ameliorates
TNBS-induced colitis by regulating effector and regulatory CD4(+) T
cells. Biomed Res Int. 2015:1020212015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bell CJ, Gall DG and Wallace JL:
Disruption of colonic electrolyte transport in experimental
colitis. Am J Physiol. 268:G622–G630. 1995.PubMed/NCBI
|
|
42
|
Scheiffele F and Fuss IJ: Induction of
TNBS colitis in mice. Curr Protoc Immunol Chapter. 15:Unit
152002.
|
|
43
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Himmel ME, Yao Y, Orban PC, Steiner TS and
Levings MK: Regulatory T-cell therapy for inflammatory bowel
disease: More questions than answers. Immunology. 136:115–122.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baumgart DC and Sandborn WJ: Inflammatory
bowel disease: Clinical aspects and established and evolving
therapies. Lancet. 369:1641–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Strober W, Fuss IJ and Blumberg RS: The
immunology of mucosal models of inflammation. Annu Rev Immunol.
20:495–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou L, Ivanov II, Spolski R, Min R,
Shenderov K, Egawa T, Levy DE, Leonard WJ and Littman DR: IL-6
programs T(H)-17 cell differentiation by promoting sequential
engagement of the IL-21 and IL-23 pathways. Nat Immunol. 8:967–974.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of
CD4+CD25+ regulatory T cells. Nat Immunol.
4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tamboli CP: Current medical therapy for
chronic inflammatory bowel diseases. Surg Clin North Am.
87:697–725. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou J, Wu ZX, Yang JH, et al: Effect of
total glucosides of Paeony on TNBS-in duced experimental Colitis in
rats. Chin J Gastroenterol. 4:154–158. 2009.
|