Introduction
Contrast-induced nephropathy (CIN) is a severe
complication that occurs in response to intravascular
administration of radio contrast media (CM), and is the third most
common cause of hospital-acquired acute kidney injury (AKI)
(1). CIN not only prolongs
hospitalization and increases the medical burden, but also
significantly increases the risk of cardiovascular events,
long-term chronic kidney disease and mortality (2). A previous study indicated that
iodinated CM-induced cell apoptosis serves an important role in the
pathogenesis of CIN (3). In
addition, CM induces ischemia, hypoxia, oxidative stress and
toxicity, resulting in HK-2 cell apoptosis and kidney damage
(4). Our previous study
demonstrated that iohexol is able to cause injury and apoptosis of
HK-2 cells in a time- and dose-dependent manner (5). Despite numerous efforts being made to
reduce the risk of CIN, effective treatment strategies are required
and detailed mechanisms regarding the pathophysiology of CIN remain
to be elucidated.
Autophagy is a cellular process that results in the
removal of damaged organelles and the recycling of cellular
proteins (6). In the majority of
cases, autophagy serves an important role in maintaining cell
survival and tissue homeostasis (7,8).
During autophagy, the cytosolic form of microtubule-associated
protein 1A/1B-light chain 3 (LC3), LC3-I, is converted to
LC3-phosphatidylethanolamine conjugate (LC3-II) by the E1-like
enzyme autophagy-related protein 7 (Atg7), which is recruited to
autophagosomal membranes (9).
A previous study demonstrated that there was a close
association between autophagy and apoptosis; autophagy reduces cell
apoptosis and serves a protective role against AKI, including
ischemia/reperfusion- or cisplatin-induced kidney injury (10). However, the role of autophagy in
iohexol-induced HK-2 cell apoptosis remains unclear. Therefore, the
present study was conducted to investigate the role of autophagy in
iohexol-induced HK-2 cell injury and apoptosis, with the aim of
providing a potential therapeutic strategy for CIN.
Materials and methods
Cell culture
HK-2 cells were obtained from the Kidney Disease
Laboratory of Second Xiangya Hospital of Central South University
(Changsha, China). The cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2 in Dulbecco's modified
Eagle's medium (DMEM)/F12 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and antibiotics (100 IU/ml
penicillin and 100 µg/ml streptomycin). Iohexol was purchased from
Nycomed China (Shanghai, China) and 3-methyladenine (3-MA) was
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Cell treatment
HK-2 cells were incubated in FBS-free DMEM/F12 for
12 h. The establishment of a damaged cells model was performed as
previously reported (11). 3-MA
(10 mM) was used to inhibit the formation of autophagosomes. The
cells were divided into the following four groups, all of which
were cultured at 37°C: Control group, in which cells were cultured
in DMEM/F12 supplemented with 1% FBS; CM group, in which cells were
treated with 200 mg iodine/ml iohexol for 6 h; 3-MA group, in which
cells were treated with 10 mM 3-MA for 30 min; and CM + 3-MA group,
in which cells were pretreated with 10 mM 3-MA for 30 min prior to
treatment with 200 mg iodine/ml iohexol for 6 h.
Transmission electron microscopy
(TEM)
HK-2 cells were cultured in 6-well plates at a
density of 1-5×105/ml, and were treated as
aforementioned. The cells were digested and collected routinely.
Subsequently, the cells were suspended and fixed with 2.5%
glutaraldehyde in 0.1 mM PBS (pH 7.4) at 4°C for 2 h. After washing
twice with PBS, cells underwent conventional dehydration, osmosis,
embedding, sectioning and staining, as previously described
(12). Cell ultrastructure was
observed under a Philips 300 electron microscope (Philips
Healthcare, Amsterdam, The Netherlands). TEM was used to detect the
effects of iohexol on HK-2 cell autophagy.
Immunofluorescence assay
Cells were cultured on polylysine-coated glass
slides (Sigma-Aldrich; Merck KGaA) in 6-well plates at a density of
1–5×105/ml, fixed in 4% paraformaldehyde for 15 min,
washed with PBS and permeabilized with 0.1% Triton-X-100
(Sigma-Aldrich) for 5 min, all at room temperature. Subsequently,
the cells were incubated with LC3-II primary antibody for 1 h at
room temperature (1:1,000; cat. no. L8918; Sigma-Aldrich; Merck
KGaA), followed by incubation with a secondary antibody
[fluorescein isothiocyanate (FITC)-conjugated; 1:100; cat. no.
KC-RB-095; KangCheng Biotech, Shanghai, China] at room temperature
for 1 h. Cells were then incubated in the dark with DAPI
(Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min. After
washing with PBS, glass slides were sealed with a seal sheet
containing a fluorescence-quenching agent. A laser-scanning
confocal fluorescence microscope (Olympus BX60; Olympus
Corporation, Tokyo, Japan) was used to capture images.
Western blot analysis
Cells were lysed with cell lysis buffer
(radioimmunoprecipitation assay buffer: phenylmethylsulfonyl
fluoride, 100:1; Beyotime Institutes of Biotechnology, Shanghai,
China) on ice for 30 min and were centrifuged at 8,500 × g for 5
min at 4°C. The protein concentration was determined using the
Bicinchoninic Acid Protein Assay Reagent kit (Beyotime Institutes
of Biotechnology). Proteins (20 µg) were separated by 10% SDS-PAGE
and were transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with
calf serum (Gibco; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature and incubated with anti-LC3-II (1:1,000; cat. no.
L8918; Sigma-Aldrich; Merck KGaA), anti-Atg7 (1:200; cat. no.
A2856; Sigma-Aldrich; Merck KGaA) and anti-β-actin antibodies
(1:2,000; cat. no. sc-7210; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) overnight at 4°C, followed by incubation with a secondary
antibody (1:2,000; cat. no. A0545; Sigma-Aldrich; Merck KGaA) for 1
h at room temperature. Enhanced chemiluminescent reagent (EMD
Millipore) was used for chemiluminescence detection. The Kodak Gel
Logic 200 Imaging System (Kodak, Rochester, NY, USA) was used to
analyze the results.
Cell injury assay
HK-2 cells were cultured in 24-well plates at a
density of 1–5×105/ml. Once they reached 70–80%
confluence, the cells were incubated with serum-free medium for 12
h and treated with iohexol and 3-MA as indicated. Cell injury was
assessed using a lactate dehydrogenase (LDH) assay (Beyotime
Institutes of Biotechnology) and an automatic biochemistry analyzer
(Abbott Laboratories, Chicago, IL, USA).
MTT assay
An MTT assay (Sigma-Aldrich; Merck KGaA) was used to
assess the viability and proliferation of HK-2 cells (4). Briefly, confluent HK-2 monolayers
were incubated for 6 h with control medium, 200 mg/ml iohexol, or
200 mg/ml iohexol + 10 mM 3-MA. Subsequently, 20 µl (5 mg/ml) MTT
was added to the wells and was incubated for 4 h at 37°C, after
which 150 µl dimethyl sulfoxide was added and oscillated for 10
min. Cell viability was measured using a Labsystems Wellscan MK2
(Thermo Fisher Scientific, Inc.) at a 490-nm wavelength.
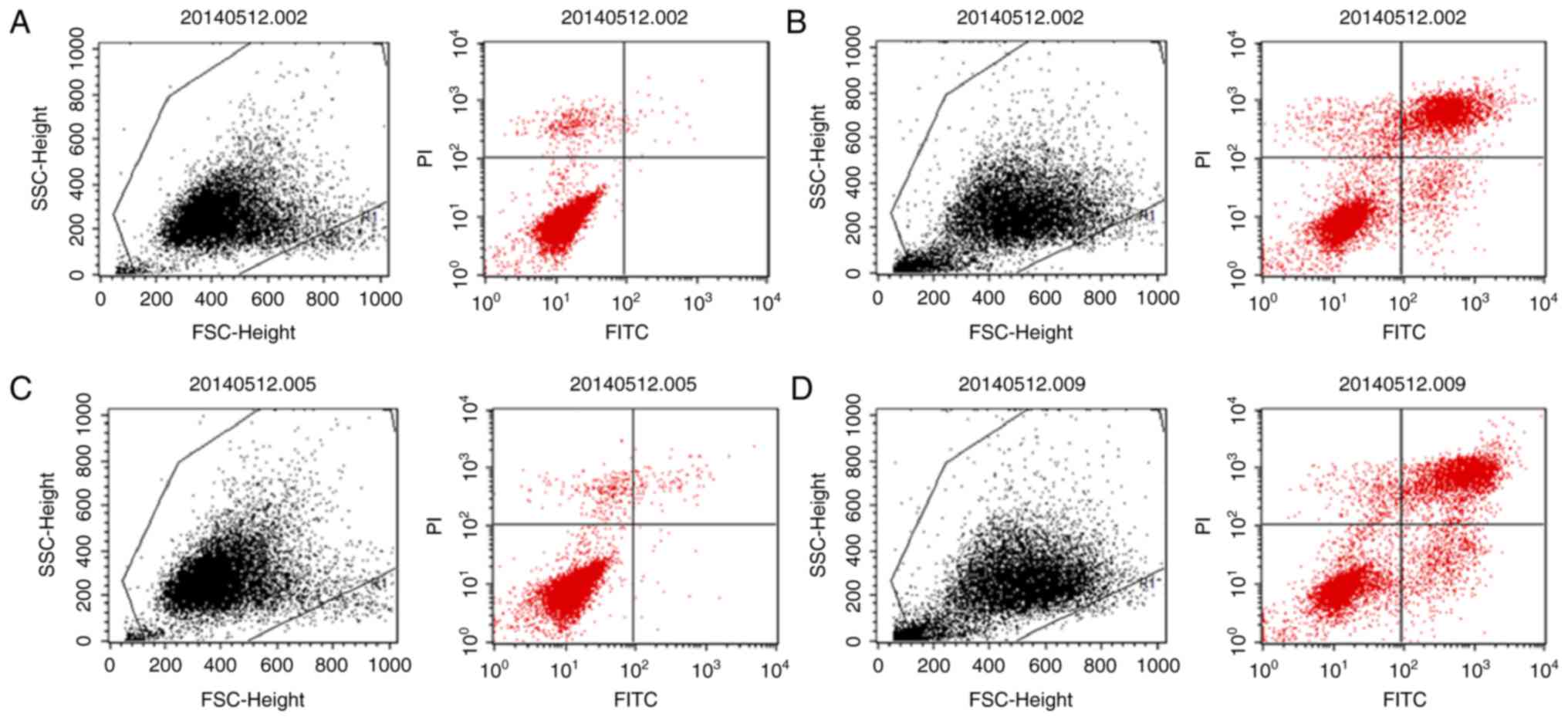
Cell apoptosis assay
Cell apoptosis was detected with an Annexin V
FITC-conjugated/propidium iodide (PI) apoptosis kit (Nanjing Keygen
Biotech Co., Ltd., Nanjing, China) using flow cytometry. Cells were
seeded in a 6-well plate at 2×105 cells/well. Once the
cells reached 70–80% confluence, they were incubated with
serum-free medium for 12 h and treated with iohexol and 3-MA as
aforementioned. Subsequently, the cells were collected and
incubated with 5 µl Annexin V-FITC and 5 µl PI (50 mg/ml) for 15
min in the dark at room temperature. The cells were immediately
analyzed using a flow cytometer (BD Biosciences, San Jose, CA,
USA).
Statistical analysis
SPSS software (version 17.0; SPSS, Inc., Chicago,
IL, USA) was used to conduct statistical analysis. Data are
presented as the mean ± standard deviation from 6 independent
experiments. For comparisons between any two groups, an unpaired
Student's t-test was performed. Comparisons between data from more
than two groups were assessed by one-way analysis of variance
followed by Fisher's least significant difference test for post hoc
comparisons. P<0.05 was considered to indicate statistically
significant difference.
Results
Iohexol promotes autophagy in HK-2
cells
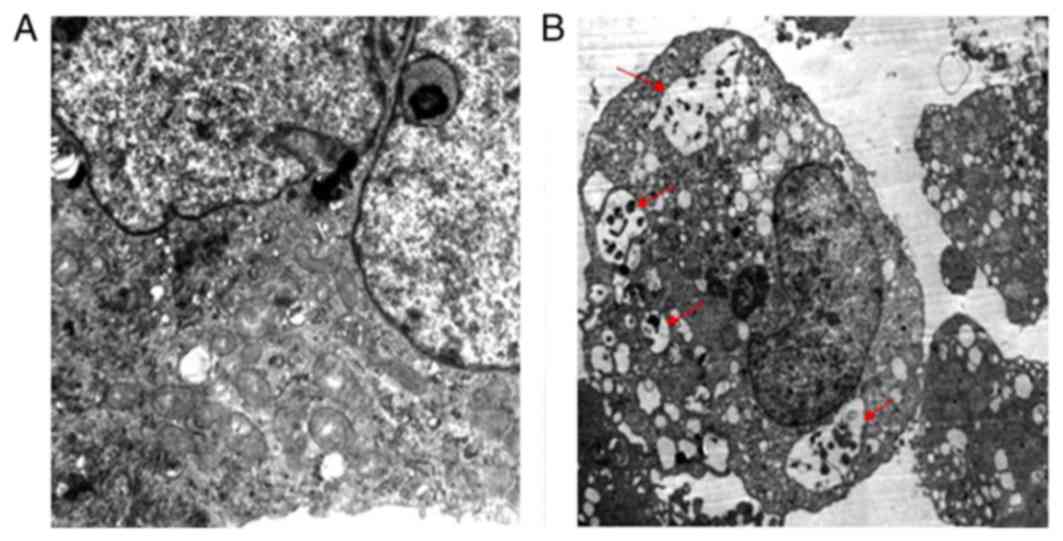
The present study used TEM to detect the effects of
iohexol on autophagy. As presented in Fig. 1A, in the control group, the HK-2
cells exhibited normal cytoplasm with no autophagosomal formation.
However, a marked accumulation of autophagosomes was observed in
the iohexol-treated group; cytoplasmic material and/or membrane
vesicles were encapsulated in vacuoles (red arrows; Fig. 1B).
Iohexol upregulates LC3-II and Atg7
expression levels
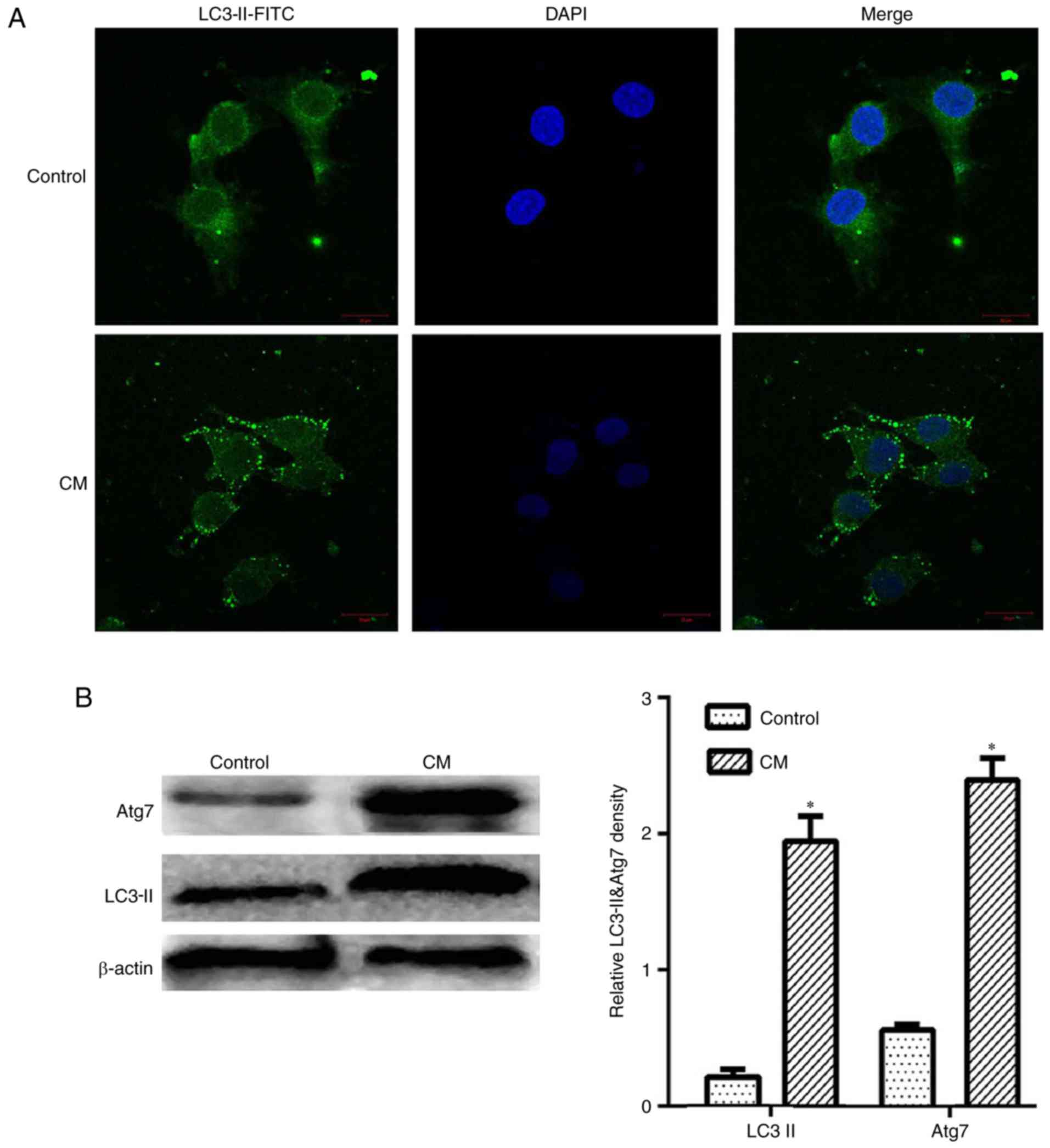
LC3 is a representative autophagosome marker, which
is essential for the formation of autophagosomes. During autophagy,
the cytosolic form of LC3 (LC3-I) is converted to LC3-II by Atg7
(E1-like enzyme) and Atg3 (E2-like enzyme) (8). In the present study, the results of
an immunofluorescence assay demonstrated that LC3-II expression was
evidently upregulated in the cytoplasm by iohexol (Fig. 2A). Furthermore, these observations
were confirmed by western blot analysis. As shown in Fig. 2B, LC3-II and Atg7 expression were
markedly upregulated by iohexol (200 mg iodine/ml) compared with
the control group (P<0.05). These results indicated that iohexol
may increase the levels of autophagy in HK-2 cells.
3-MA suppresses iohexol-induced
autophagy
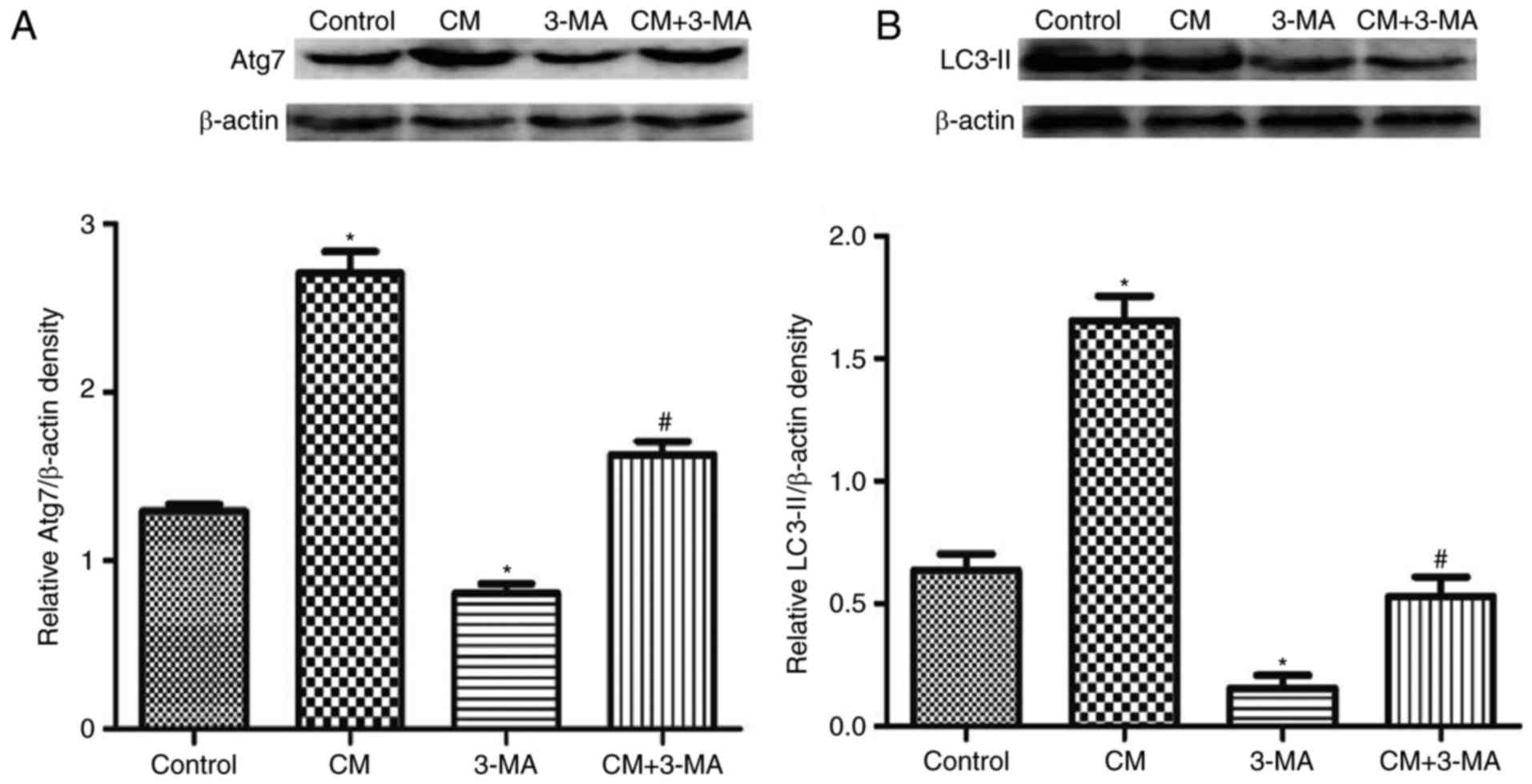
3-MA, which is an inhibitor of phosphatidylinositol
3-kinase, was used to inhibit the initiation of autophagosome
formation. As presented in Fig. 3,
3-MA not only affected the expression levels of LC3-II and Atg7 in
HK-2 cells compared with the control group, but also reversed
iohexol-induced upregulation of LC3-II and Atg7 expression
(Fig. 3).
Autophagy serves a protective role in
iohexol-induced cytotoxicity
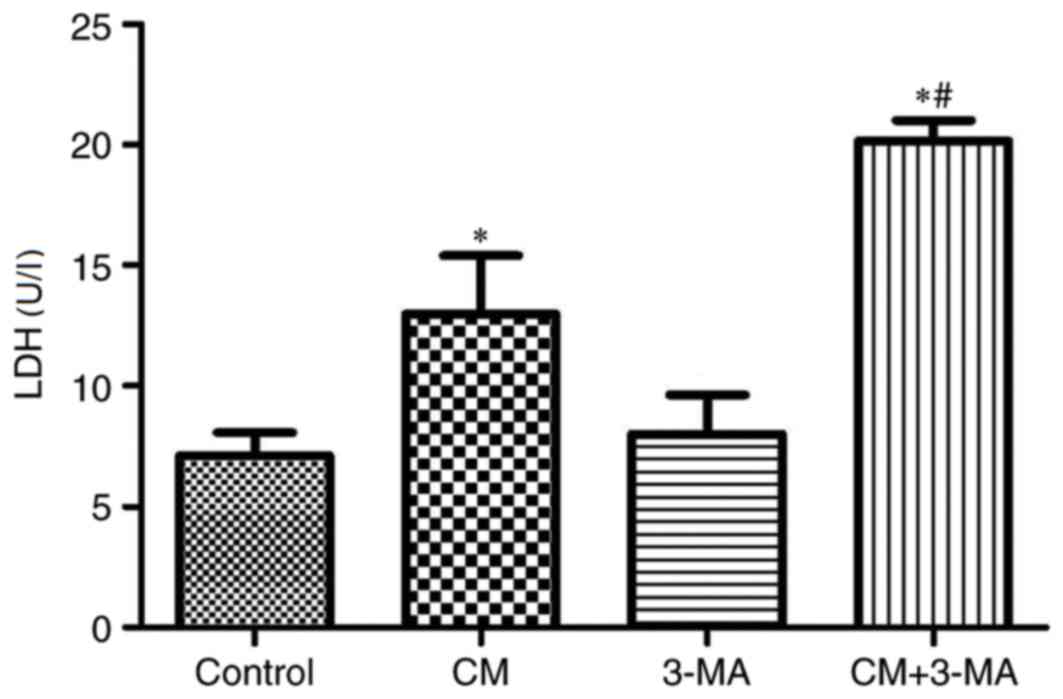
As presented in Fig.
4 and Table I, the
cytotoxicity of iohexol was evaluated by MTT assay and LDH release.
LDH levels were significantly increased (P<0.05) and cell
viability was significantly decreased in the iohexol-treated group
compared with the control group, which was further enhanced by
3-MA.
 | Table I.Effects of 3-MA on cell proliferation
and viability in iohexol-treated HK-2 cells (mean ± standard
deviation, n=6). |
Table I.
Effects of 3-MA on cell proliferation
and viability in iohexol-treated HK-2 cells (mean ± standard
deviation, n=6).
| Group | MTT assay result (OD
value) |
|---|
| Control | 0.585±0.003 |
| CM |
0.345±0.001a |
| 3-MA | 0.534±0.002 |
| CM + 3-MA |
0.216±0.004b |
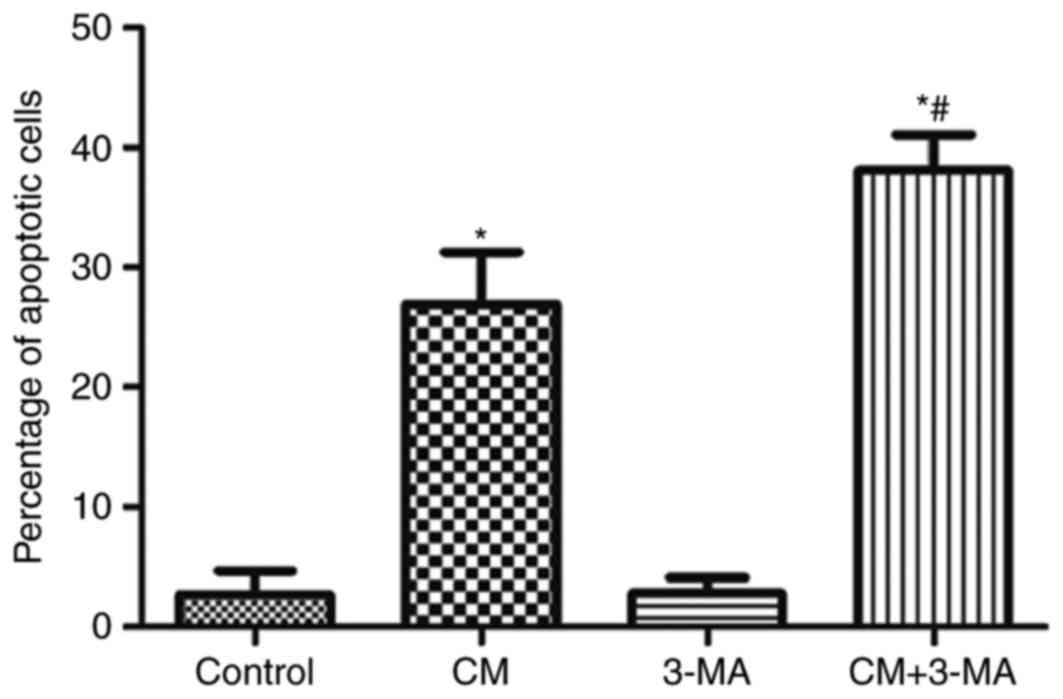
Autophagy serves a protective role in
iohexol-induced apoptosis
The role of autophagy in iohexol-induced HK-2 cell
apoptosis was analyzed (Figs. 5
and 6). As shown in Figs. 5B and 6, iohexol significantly increased
apoptosis of HK-2 cells compared with the control group. Treatment
with 3-MA alone did not affect cell apoptosis (Figs. 5C and 6). However, cell apoptosis was
significantly increased in the iohexol + 3-MA group compared with
the iohexol group (Figs. 5D and
6).
Discussion
CIN is a serious complication resulting from the use
of iodinated CM; however, the mechanism underlying CIN remains to
be elucidated. A previous study has indicated that the pathogenesis
of CIN is predominantly associated with renal ischemia, direct
nephrotoxicity and oxidative stress (4). Autophagy is a cellular stress
response that serves important roles in the pathogenesis of various
diseases. Numerous studies have demonstrated that autophagy is
closely associated with apoptosis (7,13,14).
In some cases, cell apoptosis induced by cellular stress may be
reduced by autophagy; however, excessive autophagy can also
contribute to cell death (13,15).
A previous study reported that CM induced organelle damage, which
may result in the activation of autophagy (10). When CM was injected intravenously,
it was filtered by glomerular filtration and directly damaged renal
tubular epithelial cells. An in vitro study suggested that
the number of lysosomes was increased in the cells of the proximal
convoluted tubule following CM injection (16). In addition, CM has been reported to
reduce cell proliferation, alter mitochondrial function and
increase apoptosis of proximal tubule cells (17). Buyuklu et al (18) suggested that oxidative stress,
inflammation, autophagy and apoptosis are increased in rats with
CIN. Consistent with these results, our previous study demonstrated
that CM increased cell apoptosis and reduced cell viability in a
time- and dose-dependent manner (5). The present study detected a marked
accumulation of autophagosomes in iohexol-treated cells. LC3-II and
Atg7 expression were markedly upregulated by iohexol (200 mg
iodine/ml) compared with the control cells. These results indicated
that iohexol markedly increased the levels of autophagy in HK-2
cells, which further supported the hypothesis that CM induces
activation of cell autophagy.
Autophagy is a fundamental cellular homeostatic
process that is used to degrade and recycle cellular proteins, and
remove damaged organelles. Previous studies have demonstrated that
autophagy serves a protective role in AKI (10,19).
Inhibition of autophagy by autophagy inhibitors has been reported
to evidently downregulate cisplatin-induced apoptosis in proximal
tubular cells (20). Jiang et
al (10) reported that
proximal tubule-specific autophagy-deficient mice developed more
severe AKI and increased cisplatin-induced apoptosis. In another
experimental model of cisplatin-induced nephropathy, autophagy was
reported to have a role in cell death, and a precise balance
between apoptosis and autophagy was detected (21). Autophagy contributes to
renoprotection via the modulation of apoptosis and mitochondrial
injury in an animal model of CIN; in vitro studies also
demonstrated that decreased cell viability by iohexol (100 mg
iodine/ml) was aggravated with 3-MA pretreatment (19). However, the effects of autophagy on
iohexol-induced apoptosis of HK-2 cells remain unclear. The present
study demonstrated that the protein expression levels of LC3-II and
Atg7 were upregulated in HK-2 cells by iohexol. When HK-2 cells
were treated with iohexol for 6 h, autophagosomes were accumulated
in the cytoplasm, thus suggesting that iohexol induced autophagy in
HK-2 cells. Furthermore, inhibition of autophagy by 3-MA evidently
enhanced iohexol-induced cell injury and apoptosis of HK-2 cells.
These results indicated that activation of autophagy may attenuate
iohexol-induced cytotoxicity, thus suggesting that iohexol-induced
activation of autophagy may serve a protective role in
iohexol-induced cytotoxicity of renal tubular epithelial cells. The
potential mechanisms were hypothesized as follows: Autophagy may
provide adenosine triphosphate for energy-deficient cells by
consuming aged and damaged organelles or proteins; autophagy may
serve a critical role in removing protein aggregates and damaged
organelles, and may promote cell survival and tissue homeostasis;
autophagy may reduce apoptosis of cells by intervening with
apoptotic signaling pathways.
In conclusion, the present study revealed that
autophagy was upregulated by iohexol in HK-2 cells, and confirmed
that iohexol-induced autophagy may attenuate cell injury and the
apoptosis of HK-2 cells. These findings suggested that autophagy
serves a protective role in the CM-induced injury of renal tubular
epithelial cells. Further studies are required to explore the
specific mechanisms underlying the effects of autophagy on
iohexol-induced cytotoxicity in HK-2 cells.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81570618) and the
Scientific Foundation of Hunan Province, China (grant nos.
2010FJ6008 and 2008JT3005). The authors would like to thank Dr Yiya
Zhang (The Key Laboratory of Protein Chemistry and Developmental
Biology of Ministry of Education, College of Life Sciences, Hunan
Normal University, Changsha, China) for reviewing the
manuscript.
References
|
1
|
Sun S, Zhang T, Nie P, Hu L, Yu Y, Cui M,
Cai Z, Shen L and He B: A novel rat model of contrast-induced acute
kidney injury. Int J Cardiol. 172:e48–e50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Solomon RJ, Mehran R, Natarajan MK, Doucet
S, Katholi RE, Staniloae CS, Sharma SK, Labinaz M, Gelormini JL and
Barrett BJ: Contrast-induced nephropathy and long-term adverse
events: Cause and effect? Clin J Am Soc Nephrol. 4:1162–1169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nazıroğlu M, Yoldaş N, Uzgur EN and Kayan
M: Role of contrast media on oxidative stress, Ca(2+) signaling and
apoptosis in kidney. J Membr Biol. 246:91–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quintavalle C, Brenca M, De Micco F, Fiore
D, Romano S, Romano MF, Apone F, Bianco A, Zabatta MA, Troncone G,
et al: In vivo and in vitro assessment of pathways involved in
contrast media-induced renal cells apoptosis. Cell Death Dis.
2:e1552011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duan S, Zhou X, Liu F, Peng Y, Chen Y, Pei
Y, Ling G, Zhou L, Li Y, Pi Y, et al: Comparative cytotoxicity of
high-osmolar and low-osmolar contrast media on HKCs in vitro. J
Nephrol. 19:717–724. 2006.PubMed/NCBI
|
|
6
|
Lamb CA, Yoshimori T and Tooze SA: The
autophagosome: Origins unknown, biogenesis complex. Nat Rev Mol
Cell Biol. 14:759–774. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang C, Kaushal V, Shah SV and Kaushal GP:
Autophagy is associated with apoptosis in cisplatin injury to renal
tubular epithelial cells. Am J Physiol Renal Physiol.
294:F777–F787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Howell GM, Gomez H, Collage RD, Loughran
P, Zhang X, Escobar DA, Billiar TR, Zuckerbraun BS and Rosengart
MR: Augmenting autophagy to treat acute kidney injury during
endotoxemia in mice. PLoS One. 8:e695202013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z and Choi ME: Autophagy in kidney
health and disease. Antioxid Redox Signal. 20:519–537. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang M, Wei Q, Dong G, Komatsu M, Su Y
and Dong Z: Autophagy in proximal tubules protects against acute
kidney injury. Kidney Int. 82:1271–1283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu GL, Lei R, Duan SB, Tang MM, Luo M and
Xu Q: Atorvastatin alleviates iodinated contrast media-induced
cytotoxicity in human proximal renal tubular epithelial cells. Exp
Ther Med. August 1–2017.(Epub ahead of print). doi:
10.3892/etm.2017.4859.
|
|
12
|
Winey M, Meehl JB, O'Toole ET and Giddings
TH Jr: Conventional transmission electron microscopy. Mol Biol
Cell. 25:319–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ding Y and Choi ME: Autophagy in diabetic
nephropathy. J Endocrinol. 224:R15–R30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rubinstein AD and Kimchi A: Life in the
balance-a mechanistic view of the crosstalk between autophagy and
apoptosis. J Cell Sci. 125:5259–5268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tervahartiala P, Kivisaari L, Kivisaari R,
Vehmas T and Virtanen I: Structural changes in the renal proximal
tubular cells induced by iodinated contrast media. Nephron.
76:96–102. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hardiek K, Katholi RE, Ramkumar V and
Deitrick C: Proximal tubule cell response to radiographic contrast
media. Am J Physiol Renal Physiol. 280:F61–F70. 2001.PubMed/NCBI
|
|
18
|
Buyuklu M, Kandemir FM, Ozkaraca M, Set T,
Bakirci EM, Topal E, lleriturk M and Turkmen K: Benefical effects
of lycopene against contrast medium-induced oxidative stress,
inflammation, autophagy, and apoptosis in rat kidney. Hum Exp
Toxicol. 34:487–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ko GJ, Bae SY, Hong YA, Pyo HJ and Kwon
YJ: Radiocontrast-induced nephropathy is attenuated by autophagy
through regulation of apoptosis and inflammation. Hum Exp Toxicol.
35:724–736. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Periyasamy-Thandavan S, Jiang M, Wei Q,
Smith R, Yin XM and Dong Z: Autophagy is cytoprotective during
cisplatin injury of renal proximal tubular cells. Kidney Int.
74:631–640. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Domitrović R, Cvijanović O, Pernjak-Pugel
E, Skoda M, Mikelić L and Crnčević-Orlić Z: Berberine exerts
nephroprotective effect against cisplatin-induced kidney damage
through inhibition of oxidative/nitrosative stress, inflammation,
autophagy and apoptosis. Food Chem Toxicol. 62:397–406. 2013.
View Article : Google Scholar : PubMed/NCBI
|