Introduction
Breast cancer is one of the most prevalent types of
malignancy in women worldwide (1).
Despite advances in cancer therapeutic strategies, the clinical
outcomes and prognosis for breast cancer patients remain
particularly poor (2). Numerous
genetic and epigenetic alterations have been proposed to contribute
to breast cancer pathogenesis and progression (3); however, the precise molecular
mechanism remains poorly understood. Therefore, investigating the
molecular mechanisms, and developing novel and effective
therapeutic targets for breast cancer therapy are of great
importance.
MicroRNAs (miRNAs) are non-coding, small RNAs
(length, ~22 nucleotides) which post-transcriptionally regulate
gene expression (4,5). miRNAs induce mRNA degradation or
translational repression by targeting the 3′-untranslated region
(UTR) of the target mRNAs (4).
miRNAs are important in carcinogenesis, through modulating various
biological processes, including cell proliferation, apoptosis,
differentiation, migration and invasion (6). Numerous miRNAs are involved in
regulating breast cancer pathogenesis and progression (7–9).
Furthermore, miRNAs may serve as novel biomarkers for diagnosis,
prognosis, and therapeutic tools in breast cancer (10). However, the precise role of miRNAs
in breast cancer requires further investigation.
The sirtuins (SIRTs) are nicotinamide adenine
dinucleotide oxidized form-dependent deacetylases that contribute
significantly to stress responses, inflammation, metabolism, DNA
repair and senescence (11–14).
To date, seven members, including SIRT1-7 have been characterized
in mammals (15). SIRT1 is the
most evaluated sirtuin that regulates various cellular and
metabolic processes (16). SIRT2
has been reported as an important regulator for neurodegenerative
diseases (17). SIRT3 is the major
mitochondrial deacetylase, which regulates global mitochondrial
lysine acetylation (18,19). SIRT4 and SIRT5 have been
significantly implicated in metabolic processes (20,21).
SIRT6 predominantly regulates DNA damage and genome integrity
(22,23), and SIRT7 is the latest
characterized SIRT and evaluation of its function has just begun
(24). SIRT7 is important in
regulating rDNA transcription and protein synthesis (25,26).
Furthermore, SIRT7 has been reported as a response gene in response
to hypoxia, low glucose stress, genomic stress and endoplasmic
reticulum stress (27–30). It is involved in cardiac health,
hepatic steatosis, ageing and senescence (31,32)
and SIRT7 has been suggested as an oncogene in various cancer
types, including hepatocellular carcinoma (26) and colorectal cancer (33), representing a potential
pharmacologic target for cancer therapy (34). High expression levels have been
observed in breast cancer tissues associated with metastasis and
adverse outcomes (35,36). However, the regulation of SIRT7 in
breast cancer remains poorly understood.
Recent studies reported miR-3666 as a tumor
suppressor miRNA in various cancer types (37–39).
However, the role of miR-3666 in breast cancer remains unknown.
According to the reported features of miR-3666, it was hypothesized
that miR-3666 exerts a tumor suppressor role in breast cancer. The
present study aimed to investigate the role and underlying
mechanism of miR-3666 in regulating the development and progression
of breast cancer.
Materials and methods
Cell lines and culture
Human breast cancer cell lines (MCF-7, BT474,
MDA-MB-231, and MDA-MB-468), normal breast epithelial cell line
MCF-10A and 293T cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). BT474 and MDA-MB-231 cells
were cultured in Hyclone RPMI-1640 medium (GE Healthcare Life
Sciences, Logan, UT, USA) while MCF-7, MDA-MB-468, MCF-10A and 293T
cells were cultured in Hyclone Dulbecco's modified Eagle's medium
(GE Healthcare Life Sciences). All cells were grown in medium
containing 10% fetal bovine serum (Hyclone; GE Healthcare Life
Sciences) supplemented with 1% penicillin-streptomycin (Gibco;
Thermo Fisher Scientific Inc., Waltham, MA, USA) in a humidified
atmosphere of 5% CO2 at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNAs were extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.). To detect miR-3666 expression,
cDNA was synthesized by Moloney murine leukemia virus reverse
transcriptase (Takara Biotechnology, Co., Ltd., Dalian, China). To
detect SIRT7 expression levels, cDNA was synthesized using a
miScript Reverse Transcription kit (Qiagen GmbH, Hilden, Germany).
The RT-qPCR was conducted using a SYBR-Green master mix kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) with an Applied
Biosystems AB7500 Real Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the following procedure: 94°C
for 5 min; 30 cycles of 94°C for 20 sec, 55°C for 25 sec, and 72°C
for 35 sec; and 72°C for 10 min. Small nuclear RNA U6 and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as the
internal controls. The relative gene expression was calculated
using the 2−ΔΔCq method as compared with U6 or GAPDH
(40). The fold-change of gene
expression was obtained by normalization against the control group.
The primers used were as follows: Forward,
5′-ACACTCCAGCTGGGCAGTCAAGTGTAGA-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′ for miR-3666; forward,
5′-CGCTTCGGCAGCACATATACTAA-3′ and reverse,
5′-TATGGAACGCTTCACGAATTTGC-3′ for U6; forward,
5′-GTGGACACTGCTTCAGAAAG-3′ and reverse, 5′-CACAGTTCTGAGACACCACA-3′
for SIRT7; and forward, 5′-CCATGTTCGTCATGGGTGTG-3′ and reverse,
5′-GGTGCTAAGCAGTTGGTGGTG-3′ GAPDH.
Cell transfection
The miR-3666 mimics and negative control (miR-NC)
were obtained from Origene Technologies, Inc. (Beijing, China) and
transfected into cells using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at a final concentration of 50 nM. SIRT7
small interfering RNA (siRNA) and negative control (NC siRNA) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and
transfected into cells according to the manufacturer's
instructions. SIRT7-overexpressing vector was generated by cloning
SIRT7 cDNA without a 3′-UTR into a pcDNA3.1 vector (Invitrogen;
Thermo Fisher Scientific, Inc.). The pcDNA3.1-SIRT7 vector was
transfected into cells using Lipofectamine 2000.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells were seeded into 96-well plates at a density
of 1×104 cells/well and cultured overnight. Cells were
then transfected with miR-3666 mimics and cultivated for 48 h, and
20 µl of MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to each well and incubated at 37°C for 4 h.
Subsequently, the culture media were discarded and 200 µl dimethyl
sulfoxide was added to each well. Optical density values at a
wavelength of 490 nm were detected using an ELISA reader (Bio-Rad
Laboratories, Inc.).
Bromodeoxyuridine (BrdU) assay
The BrdU assay was performed using a BrdU cell
proliferation assay kit (Cell Signaling Technology, Inc., Danvers,
MA, USA) in accordance with the manufacturer's instructions.
Briefly, cells were seeded into 96-well plates (1×104
cells/well) and transfected with miR-3666 mimics for 48 h.
Thereafter, 10 µl BrdU solution was added to each well and cultured
for 2 h. Following removal of the culture media, 150 µl denaturing
solution was added to each well and incubated at room temperature
for 1 h. Subsequently, peroxidase conjugated anti-BrdU was added
and incubated for 1 h at room temperature. The optical density
values at a wavelength of 450 nm were measured by an ELISA reader
(Bio-Rad Laboratories, Inc.).
Caspase-3 activity assay
Caspase-3 activity assay was performed using a
commercial kit (Roche Applied Science, Madison, WI, USA) according
to the manufacturer's instructions. Briefly, following treatment,
cells were lysed and the supernatant was harvested followed by
incubation with DEVD-pNA substrate (Roche Applied Science) at 37°C
for 2 h. Optical density values at a wavelength of 405 nm were
determined using an ELISA reader (Bio-Rad Laboratories, Inc.).
Dual-luciferase reporter assay
miRNA targets were predicted using the algorithms of
TargetSan (https://www.targetscan.org) (41). The 3′-UTR of SIRT7 containing the
wild-type or mutant binding sites of miR-3666 were cloned into
pmirGLO vector (Promega Corporation, Madison, WI, USA) followed by
transfection into 293T cells with miR-3666 mimics using
Lipofectamine 2000. After 48 h of incubation, cells were harvested
and detected using a Dual-Luciferase assay kit (Promega
Corporation). The relative luciferase activity was calculated
according to the following formula: Firefly
luciferase/Renilla luciferase.
Western blot analysis
Total protein was extracted using RIPA buffer
(Sigma-Aldrich; Merck KGaA). Equal quantities (40 µg) of proteins
were loaded onto 10% sodium dodecyl sulfate polyacrylamide gels
(Sangon Biotech Co., Ltd., Shanghai, China) for separation. The
separated proteins were then transferred to a polyvinylidene
fluoride membrane (EMD Millipore, Billerica, MA, USA) followed by
incubation with 3% nonfat milk for 1 h. The membrane was incubated
with primary antibodies at 4°C overnight. Anti-SIRT7 (cat. no.
sc-135055; dilution, 1:500) and anti GAPDH (cat. no. sc-367714;
dilution, 1:800) primary antibodies were both purchased from Santa
Cruz Biotechnology, Inc. Subsequently, the membrane was washed with
Tris-buffered saline containing 0.1% Tween-20 three times and then
blotted with horseradish peroxidase conjugated secondary antibodies
(cat. no. A0208; dilution, 1:1,000, Beyotime Institute of
Biotechnology, Haimen, China) for 1 h at 37°C. The protein bands
were visualized using enhanced chemiluminescence (EMD Millipore).
The intensity of the bands on the membrane was analyzed by
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). Relative protein expression was calculated by
normalization against GAPDH. The fold-change of protein expression
was obtained by normalization with the control group.
Statistical analysis
All values are presented as means ± standard
deviation and the statistical analyses were performed using SPSS
version 18.0 (SPSS Inc., Chicago, IL, USA). Differences were
analyzed by one-way analysis of variance with a Bonferroni
correction. P<0.05 was considered to indicate a statistically
significant difference.
Results
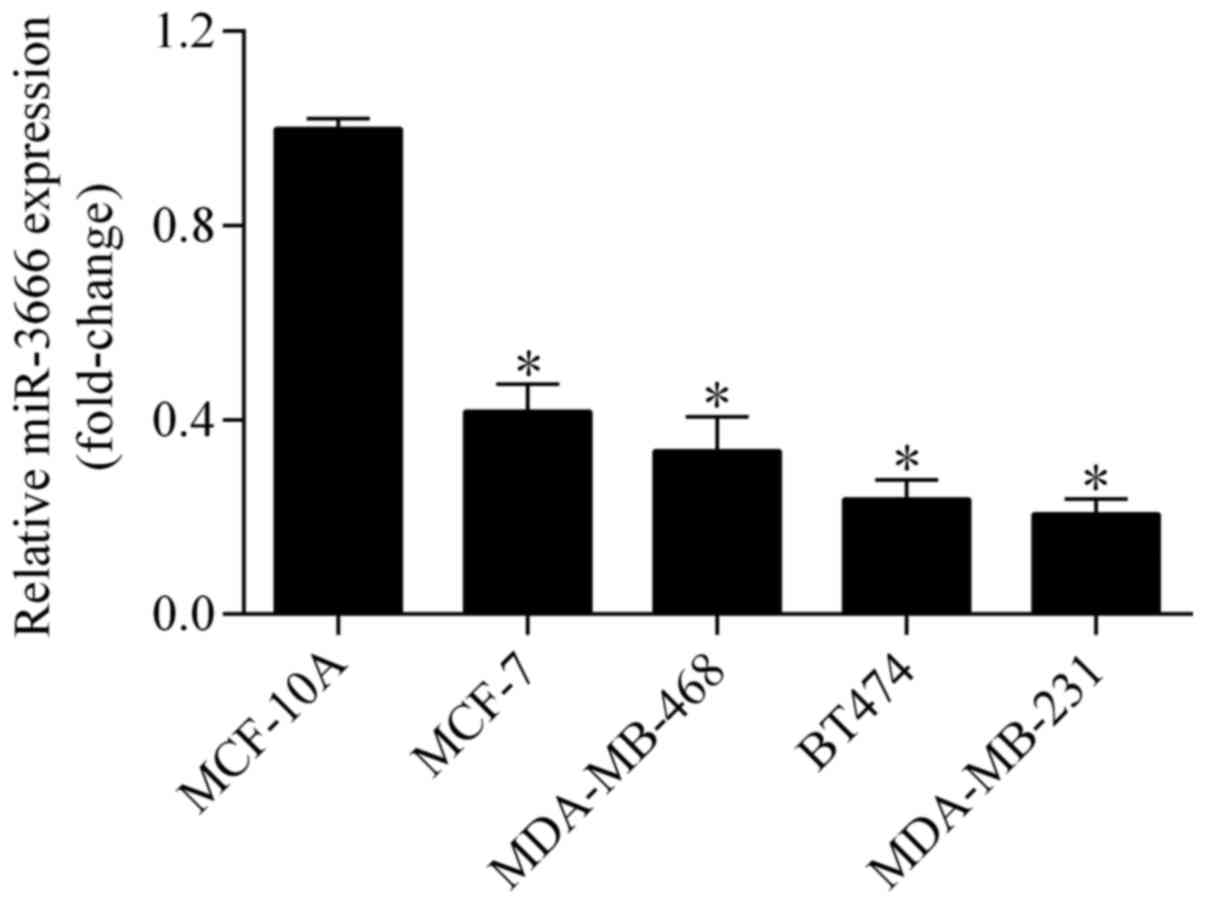
MiR-3666 is downregulated in breast
cancer cell lines
To investigate the potential relevance of miR-3666
in breast cancer, its expression was examined in breast cancer cell
lines using RT-qPCR. The results demonstrated that the expression
level of miR-3666 was significantly downregulated in breast cancer
cell lines (MCF-7, MDA-MB-468, BT474 and MDA-MB-231) compared with
the normal breast epithelial cell line, MCF-10A (Fig. 1), indicating a tumor suppressive
role of miR-3666 in breast cancer.
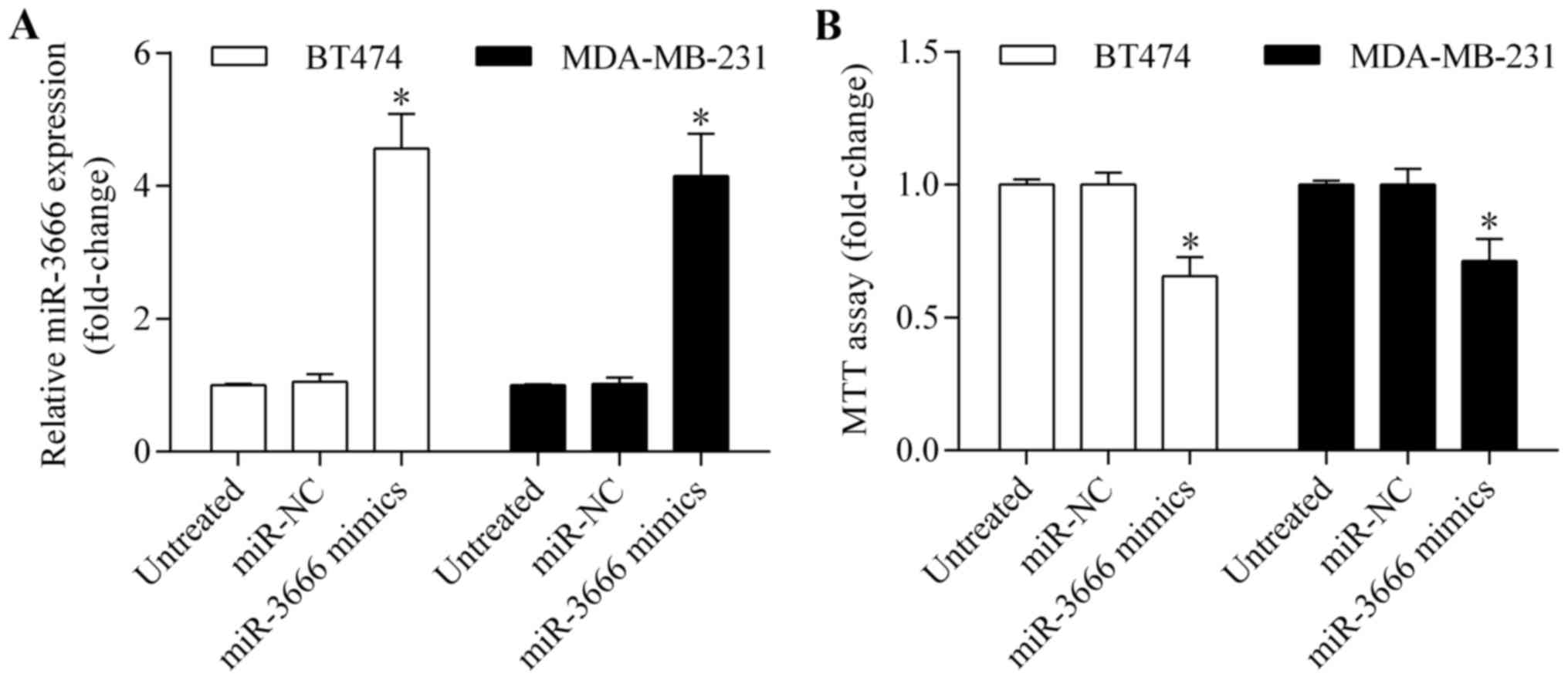
Overexpression of miR-3666 inhibits
proliferation and promotes apoptosis of breast cancer cells
As miR-3666 demonstrated a lower expression level in
BT474 and MDA-MB-231 cells, these two cell lines were selected for
subsequent experiments. To investigate the potential biological
effect of miR-3666 in breast cancer, gain-of-function experiments
were performed by transiently transfecting miR-3666 mimics into
BT474 and MDA-MB-231 cells. The expression level of miR-3666 was
markedly upregulated by miR-3666 transfection, as detected by
RT-qPCR (Fig. 2A). The effect of
miR-3666 overexpression on cell viability and growth was then
examined by MTT assay. It was observed that miR-3666 overexpression
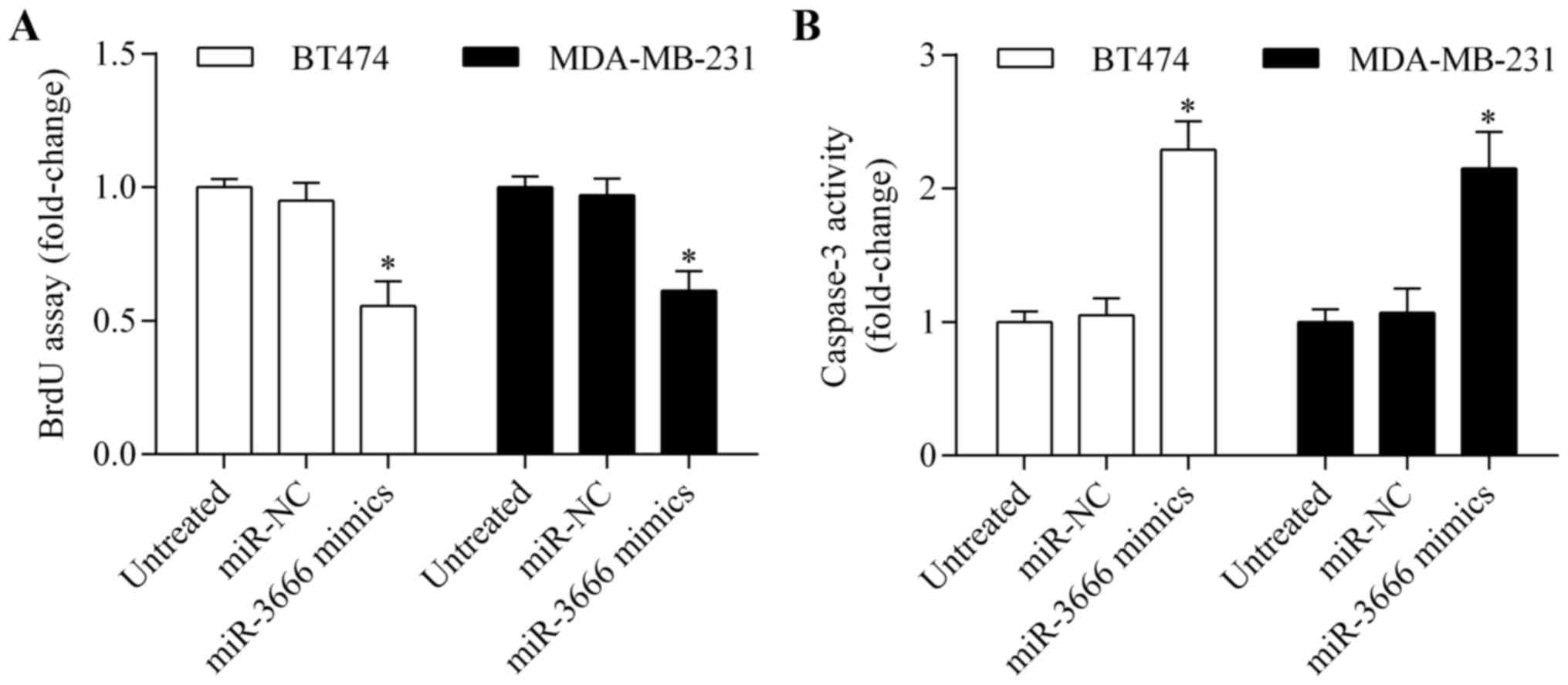
significantly suppressed breast cancer cell growth (Fig. 2B). In addition, BrdU assay
indicated that proliferation of BT474 and MDA-MB-231 cells was
markedly inhibited by miR-3666 overexpression (Fig. 3A). Furthermore, overexpression of
miR-3666 significantly promoted apoptosis of BT474 and MDA-MB-231
cells (Fig. 3B). These results
indicate that miR-3666 functions as a tumor suppressor.
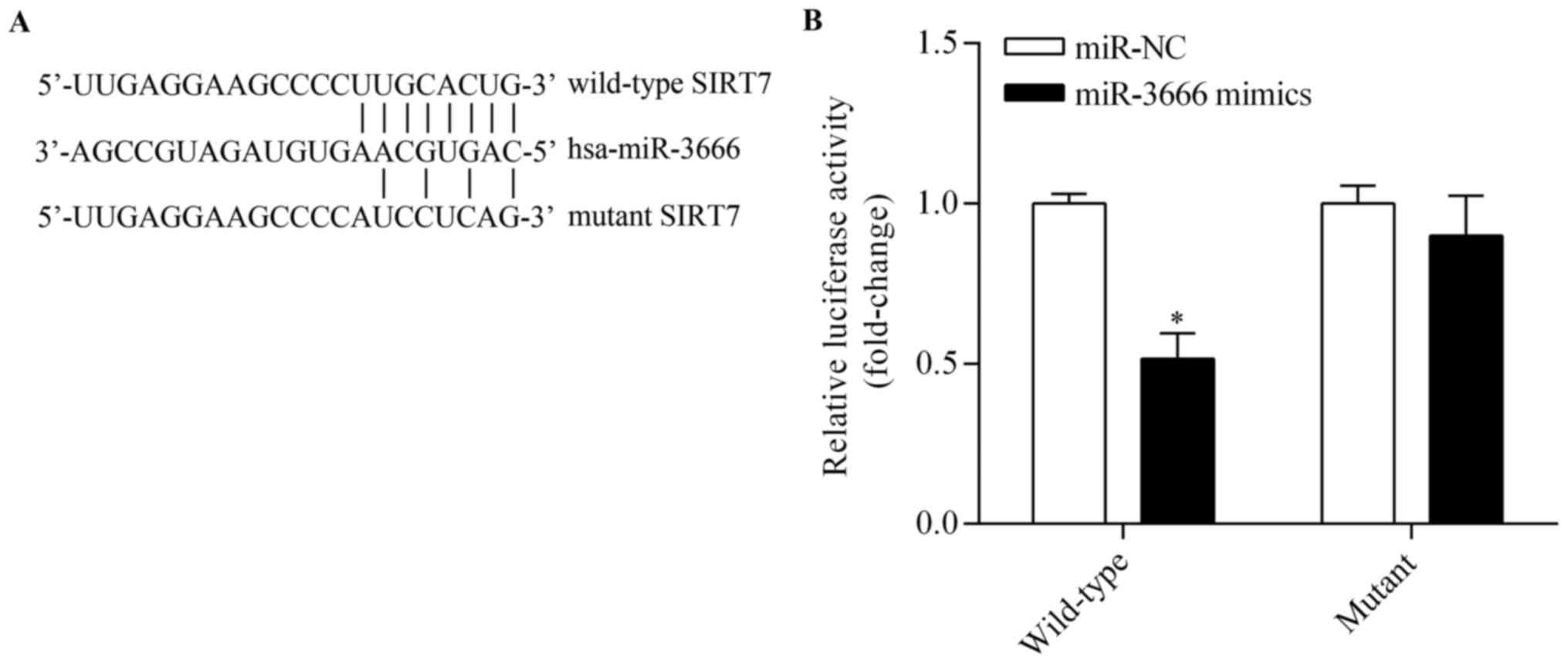
SIRT7 is a direct target of
miR-3666
To elucidate the molecular mechanism by which
miR-3666 regulates breast cancer cell proliferation, bioinformatic
analyses were performed using TargetScan to predict potential
target genes. Among these target genes, SIRT7, which is a novel
oncogene, was notable. The putative binding sites of miR-3666
within the 3′-UTR of SIRT7 are presented in Fig. 4A. To verify whether SIRT is a
direct target of miR-3666, a Dual-Luciferase reporter system
containing either wild-type or mutant 3′-UTR of SIRT7 was used.
Co-transfection with miR-3666 mimics markedly inhibited the
luciferase activity of the reporter containing the wild-type 3′-UTR
(Fig. 4B). However, miR-3666
overexpression demonstrated no significant effect on mutant 3′-UTR
of SIRT7 (Fig. 4B). Subsequent
RT-qPCR and western blot analysis indicated that SIRT7 expression
levels were significantly suppressed in BT474 and MDA-MB-231 cells
following transfection with the miR-3666 mimics (Fig. 5A and B). Taken together, these
results indicate that SIRT7 is a direct target of miR-3666.
Knockdown of SIRT7 by siRNA inhibits
proliferation and promotes the apoptosis of breast cancer
cells
To investigate whether SIRT7 is involved in
regulating breast cancer, SIRT7 was silenced by transfecting SIRT7
siRNA (Fig. 6A), and its effect on
cell proliferation and apoptosis was detected. It was found that
silencing SIRT7 using siRNA significantly inhibited proliferation
(Fig. 6B) and promoted apoptosis
(Fig. 6C) of breast cancer cells.
The results indicate that SIRT7 is involved in regulating breast
cancer cell proliferation and apoptosis.
Overexpression of SIRT7 reverses the
miR-3666-induced anti-tumor effects
To investigate whether miR-3666 induced its
anti-tumor effect via SIRT7, a rescue assay was performed using
MDA-MB-231 cells. Recombinant SIRT7 lacking the 3′-UTR sequence
(pcDNA3.1/SIRT7) was exogenously expressed in MDA-MB-231 cells.
Western blotting demonstrated that co-transfection of
pcDNA3.1/SIRT7 and miR-3666 mimics restored the decreased protein
expression level induced by miR-3666 overexpression (Fig. 7A). Overexpression of SIRT7
significantly reversed the inhibitory effect of miR-3666
overexpression on cell proliferation (Fig. 7B and C). Furthermore, the increased
apoptosis induced by miR-3666 overexpression was markedly reversed
by SIRT7 overexpression (Fig. 7D).
Thus, these results indicate that miR-3666 exerts its tumor
suppressive role via SIRT7.
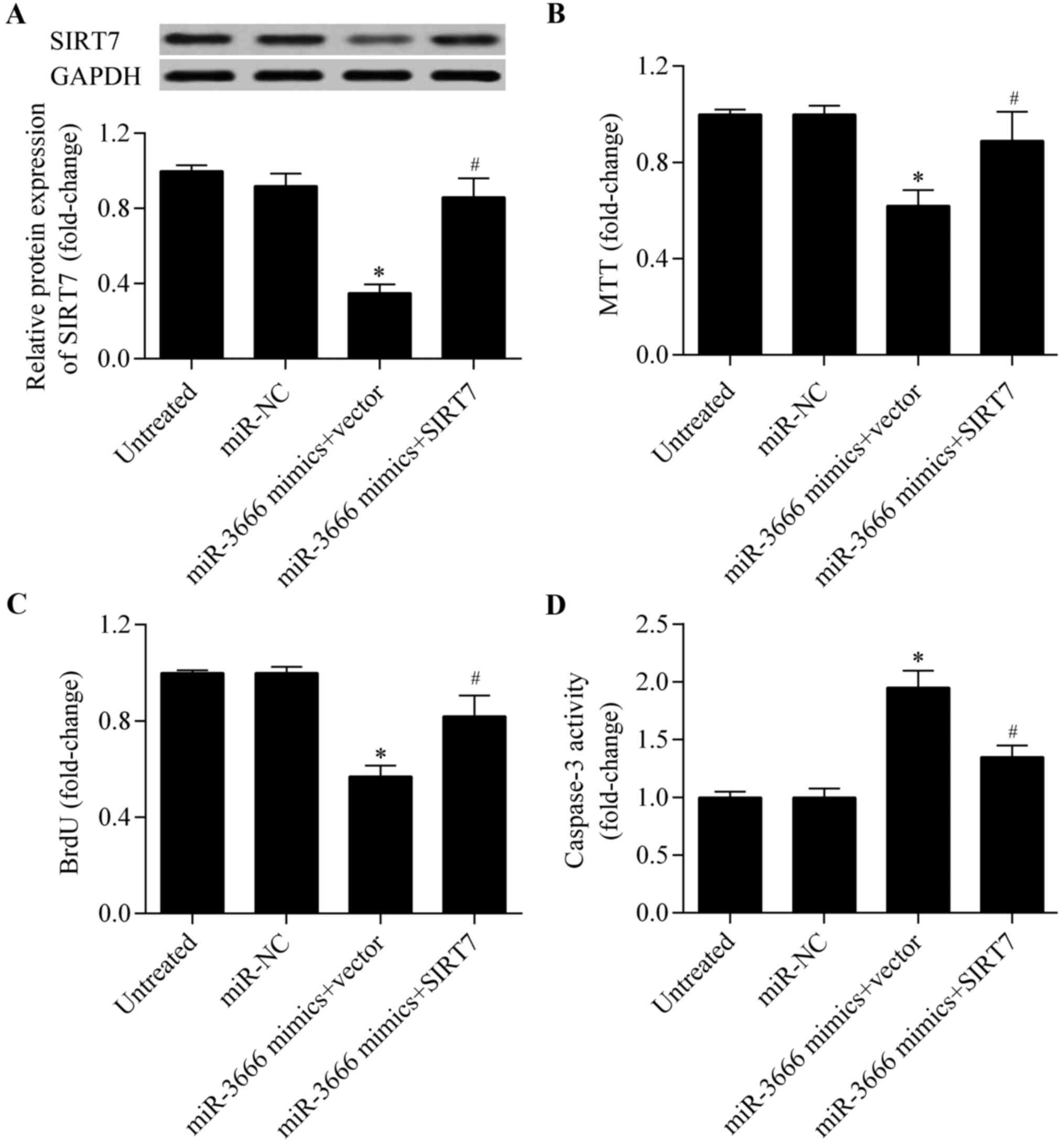 | Figure 7.Overexpression of SIRT7 restores the
miR-3666-induced anti-tumor effects. MDA-MB-231 cells were
co-transfected with pcDNA3.1/SIRT7 and miR-3666 mimics for 48 h.
Untreated, cells without treatment; miR-NC, cells treated with
miR-NC; miR-3666 mimics + vector, cells treated with miR-3666
mimics and pcDNA3.1 empty vector; miR-3666 mimics + SIRT7, cells
treated with miR-3666 mimics and pcDNA3.1/SIRT7 vector. (A) SIRT7
protein expression levels were detected by western blot analysis.
Cell proliferation was detected by (B) MTT and (C) BrdU assays. (D)
Cell apoptosis was detected by caspase-3 activity assay. *P<0.05
vs. Untreated and miR-NC; #P<0.05 vs. miR-3666 mimics
+ vector. SIRT7, sirtuin 7; miR, mircroRNA; NC, negative control;
MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
BrdU, bromodeoxyuridine. |
Discussion
Dysregulation of miRNAs is involved in the
initiation and progression of breast cancer, and miRNA-based
therapeutic strategies present as a potential therapeutic strategy
for breast cancer (10). In the
current study, it was demonstrated that miR-3666 is a novel miRNA
involved in regulating breast cancer progression. miR-3666
expression was observed to be downregulated in breast cancer cell
lines. The overexpression of miR-3666 significantly inhibited
proliferation and promoted apoptosis of breast cancer cells. SIRT7
was identified as the target gene of miR-3666, which contributed to
the miR-3666-mediated anti-tumor effect. Thus, these findings
revealed a novel microRNA-based mechanism for breast cancer
pathogenesis.
Various miRNAs have been identified to be
dysregulated in breast cancer, which function as oncogenes or tumor
suppressors, and are involved in the development of breast cancer
(42–45). Recent studies have reported that
miR-3666 functions as a tumor suppressor (37–39).
MiR-3666 is reportedly decreased in cervical cancer and inhibits
cervical cancer cell metastasis (37). Wang et al (38) reported that low miR-3666 expression
levels were observed in thyroid carcinoma and associated with poor
survival rate. Furthermore, in vitro experiments revealed
that miR-3666 inhibited thyroid carcinoma cell proliferation
(38). A more recent study reports
that miR-3666 inhibits the growth of non-small cell lung cancer
cells (39). Consistent with these
findings, the present study supported a tumor suppressor role of
miR-3666 in breast cancer. miR-3666 expression levels were
decreased in breast cancer cell lines in the present study, and
overexpression of miR-3666 inhibited breast cancer cell
proliferation. However, the underlying molecular mechanism requires
further elucidation.
To elucidate the molecular mechanism by which
miR-3666 inhibits breast cancer cell proliferation, bioinformatic
analyses were conducted and SIRT7 was identified as the functional
target gene of miR-3666. SIRT7 is a lysine deacetylase that
selectively catalyzes the deacetylation of lysine 18 on histone H3
that maintains oncogenic transformation (46). Increasing evidence indicates SIRT7
as an oncogene in various types of cancer (34). SIRT7 is overexpressed in
hepatocellular carcinoma, cervical, ovarian, colorectal, gastric
and lung cancer, and is involved in regulating cancer cell
proliferation, apoptosis and metastasis (25,33,39,47–49).
Ashraf et al (35)
demonstrated that SIRT7 was significantly increased in breast
cancer associated with node-positive breast cancer. Aljada et
al (50) reported that high
expression levels of SIRT7 were associated with early stage breast
cancer (50). Furthermore, a high
level of SIRT7 expression has been suggested as a predictor of
adverse outcomes in breast cancer (36). These findings indicate an oncogenic
role of SIRT7 in breast cancer. However, the precise biological
role of SIRT7 in breast cancer remains unclear. In the present
study, knockdown of SIRT7 inhibited proliferation and promoted
apoptosis of breast cancer. In addition, SIRT7 was observed to be
regulated by miR-3666. The decreased miR-3666 expression level may
contribute to the high expression levels of SIRT7 in breast cancer.
It has been reported that miR-3666 inhibits tumor progression by
targeting zinc finger E-box binding homeobox 1 (37) or met proto-oncogene (38). In the current study, SIRT7 was
identified to be a functional target gene of miR-3666. These
findings are consistent with a recent study, which demonstrated
that miR-3666 inhibits lung cancer cell growth by targeting SIRT7
(39).
The regulation of SIRT7 by miRNAs has been widely
reported (51,52). miR-93 regulates adiposity by
targeting SIRT7 (51), miR-152
induces human dental pulp stem cell senescence by targeting SIRT7
(53), and miR-125b is reported to
inhibit tumor development by targeting SIRT7 in hepatocellular
carcinoma (26,54) and bladder cancer (55). These studies indicate that SIRT7
undergoes epigenetic regulation via miRNAs, which is important for
pathological processes.
In conclusion, the data presented by the present
study indicates that miR-3666 is an important regulator of breast
cancer development. The overexpression of miR-3666 inhibits breast
cancer cell proliferation by inhibiting SIRT7. These findings
indicate that miR-3666 may serve as a potential candidate for the
development of miRNA-based anti-cancer therapeutic strategies.
Acknowledgements
This study was supported by Science Foundation of
Inner Mongolia University for the Nationalities (NMDYB1454).
Glossary
Abbreviations
Abbreviations:
|
miRNAs
|
microRNAs
|
|
SIRT7
|
sirtuin7
|
|
UTR
|
untranslated region
|
|
FBS
|
fetal bovine serum
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
BrdU
|
Bromodeoxyuridine
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jovanovic J, Rønneberg JA, Tost J and
Kristensen V: The epigenetics of breast cancer. Mol Oncol.
4:242–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calmon MF, Jeschke J, Zhang W, Dhir M,
Siebenkäs C, Herrera A, Tsai HC, O'Hagan HM, Pappou EP, Hooker CM,
et al: Epigenetic silencing of neurofilament genes promotes an
aggressive phenotype in breast cancer. Epigenetics. 10:622–632.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: MicroRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manikandan J, Aarthi JJ, Kumar SD and
Pushparaj PN: Oncomirs: The potential role of non-coding microRNAs
in understanding cancer. Bioinformation. 2:330–334. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu M, Yang R, Urrehman U, Ye C, Yan X,
Cui S, Hong Y, Gu Y, Liu Y, Zhao C, et al: MiR-19b suppresses PTPRG
to promote breast tumorigenesis. Oncotarget. 7:64100–64108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mutlu M, Saatci Ö, Ansari SA, Yurdusev E,
Shehwana H, Konu Ö, Raza U and Şahin Ö: miR-564 acts as a dual
inhibitor of PI3 K and MAPK signaling networks and inhibits
proliferation and invasion in breast cancer. Sci Rep. 6:325412016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Z, Chen D, Nie J, Zhou S, Wang J,
Tang Q and Yang X: MicroRNA-143 targets CD44 to inhibit breast
cancer progression and stem cell-like properties. Mol Med Rep.
13:5193–5199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Houtkooper RH, Pirinen E and Auwerx J:
Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol
Cell Biol. 13:225–238. 2012.PubMed/NCBI
|
|
12
|
Bosch-Presegué L and Vaquero A: The dual
role of sirtuins in cancer. Genes Cancer. 2:648–662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
North BJ and Verdin E: Sirtuins:
Sir2-related NAD-dependent protein deacetylases. Genome Biol.
5:2242004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsushima S and Sadoshima J: The role of
sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol.
309:H1375–H1389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng X, Tan J, Li M, Song S, Miao Y and
Zhang Q: Sirt1: Role under the condition of ischemia/hypoxia. Cell
Mol Neurobiol. 37:17–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Donmez G and Outeiro TF: SIRT1 and SIRT2:
Emerging targets in neurodegeneration. EMBO Mol Med. 5:344–352.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lombard DB, Alt FW, Cheng HL, Bunkenborg
J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D,
Murphy A, et al: Mammalian Sir2 homolog SIRT3 regulates global
mitochondrial lysine acetylation. Mol Cell Biol. 27:8807–8814.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Onyango P, Celic I, McCaffery JM, Boeke JD
and Feinberg AP: SIRT3, a human SIR2 homologue, is an NAD-dependent
deacetylase localized to mitochondria. Proc Natl Acad Sci USA.
99:pp. 13653–13658. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakagawa T, Lomb DJ, Haigis MC and
Guarente L: SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and
regulates the urea cycle. Cell. 137:560–570. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haigis MC, Mostoslavsky R, Haigis KM,
Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos
GD, Karow M, Blander G, et al: SIRT4 inhibits glutamate
dehydrogenase and opposes the effects of calorie restriction in
pancreatic beta cells. Cell. 126:941–954. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mao Z, Hine C, Tian X, Van Meter M, Au M,
Vaidya A, Seluanov A and Gorbunova V: SIRT6 promotes DNA repair
under stress by activating PARP1. Science. 332:1443–1446. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McCord RA, Michishita E, Hong T, Berber E,
Boxer LD, Kusumoto R, Guan S, Shi X, Gozani O, Burlingame AL, et
al: SIRT6 stabilizes DNA-dependent protein kinase at chromatin for
DNA double-strand break repair. Aging (Albany NY). 1:109–121. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kiran S, Anwar T, Kiran M and Ramakrishna
G: Sirtuin 7 in cell proliferation, stress and disease: Rise of the
Seventh Sirtuin! Cell Signal. 27:1–682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ford E, Voit R, Liszt G, Magin C, Grummt I
and Guarente L: Mammalian Sir2 homolog SIRT7 is an activator of RNA
polymerase I transcription. Genes Dev. 20:1075–1080. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ,
Kim MG, Chang YG, Shen Q, Park WS, Lee JY, et al: Sirtuin7
oncogenic potential in human hepatocellular carcinoma and its
regulation by the tumor suppressors MiR-125a-5p and MiR-125b.
Hepatology. 57:1055–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Seiler J, Santiago-Reichelt M,
Felbel K, Grummt I and Voit R: Repression of RNA polymerase I upon
stress is caused by inhibition of RNA-dependent deacetylation of
PAF53 by SIRT7. Mol Cell. 52:303–313. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hubbi ME, Hu H, Kshitiz, Gilkes DM and
Semenza GL: Sirtuin-7 inhibits the activity of hypoxia-inducible
factors. J Biol Chem. 288:20768–20775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin J, He M, Liu Y, Paredes S, Villanova
L, Brown K, Qiu X, Nabavi N, Mohrin M, Wojnoonski K, et al: SIRT7
represses Myc activity to suppress ER stress and prevent fatty
liver disease. Cell Rep. 5:654–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiran S, Oddi V and Ramakrishna G: Sirtuin
7 promotes cellular survival following genomic stress by
attenuation of DNA damage, SAPK activation and p53 response. Exp
Cell Res. 331:123–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vakhrusheva O, Smolka C, Gajawada P,
Kostin S, Boettger T, Kubin T, Braun T and Bober E: Sirt7 increases
stress resistance of cardiomyocytes and prevents apoptosis and
inflammatory cardiomyopathy in mice. Circ Res. 102:703–710. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ryu D, Jo YS, Lo Sasso G, Stein S, Zhang
H, Perino A, Lee JU, Zeviani M, Romand R, Hottiger MO, et al: A
SIRT7-dependent acetylation switch of GABPβ1 controls mitochondrial
function. Cell Metab. 20:856–869. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu H, Ye W, Wu J, Meng X, Liu RY, Ying X,
Zhou Y, Wang H, Pan C and Huang W: Overexpression of sirt7 exhibits
oncogenic property and serves as a prognostic factor in colorectal
cancer. Clin Cancer Res. 20:3434–3445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paredes S, Villanova L and Chua KF:
Molecular pathways: Emerging roles of mammalian Sirtuin SIRT7 in
cancer. Clin Cancer Res. 20:1741–1746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ashraf N, Zino S, Macintyre A, Kingsmore
D, Payne AP, George WD and Shiels PG: Altered sirtuin expression is
associated with node-positive breast cancer. Br J Cancer.
95:1056–1061. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geng Q, Peng H, Chen F, Luo R and Li R:
High expression of Sirt7 served as a predictor of adverse outcome
in breast cancer. Int J Clin Exp Pathol. 8:1938–1945.
2015.PubMed/NCBI
|
|
37
|
Li L, Han LY, Yu M, Zhou Q, Xu JC and Li
P: Pituitary tumor-transforming gene 1 enhances metastases of
cervical cancer cells through miR-3666-regulated ZEB1. Tumour Biol.
2015.
|
|
38
|
Wang G, Cai C and Chen L: MicroRNA-3666
regulates thyroid carcinoma cell proliferation via MET. Cell
Physiol Biochem. 38:1030–1039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi H, Ji Y, Zhang D, Liu Y and Fang P:
MicroRNA-3666-induced suppression of SIRT7 inhibits the growth of
non-small cell lung cancer cells. Oncol Rep. 36:3051–3057. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Coronnello C and Benos PV: ComiR:
Combinatorial microRNA target prediction tool. Nucleic Acids Res.
41:W159–W164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tao WY, Wang CY, Sun YH, Su YH, Pang D and
Zhang GQ: MicroRNA-34c suppresses breast cancer migration and
invasion by targeting GIT1. J Cancer. 7:1653–1662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hua K, Jin J, Zhang H, Zhao B, Wu C, Xu H
and Fang L: MicroRNA-7 inhibits proliferation, migration and
invasion of thyroid papillary cancer cells via targeting CKS2. Int
J Oncol. 49:1531–1540. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan Y, Jiao G, Wang C, Yang J and Yang W:
MicroRNA-421 inhibits breast cancer metastasis by targeting
metastasis associated 1. Biomed Pharmacother. 83:1398–1406. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guo L, Yuan J, Xie N, Wu H, Chen W, Song S
and Wang X: miRNA-411 acts as a potential tumor suppressor miRNA
via the downregulation of specificity protein 1 in breast cancer.
Mol Med Rep. 14:2975–2982. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barber MF, Michishita-Kioi E, Xi Y,
Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL,
Chen K, et al: SIRT7 links H3K18 deacetylation to maintenance of
oncogenic transformation. Nature. 487:114–118. 2012.PubMed/NCBI
|
|
47
|
Singh S, Kumar PU, Thakur S, Kiran S, Sen
B, Sharma S, Rao VV, Poongothai AR and Ramakrishna G:
Expression/localization patterns of sirtuins (SIRT1, SIRT2 and
SIRT7) during progression of cervical cancer and effects of sirtuin
inhibitors on growth of cervical cancer cells. Tumour Biol.
36:6159–6171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang S, Chen P, Huang Z, Hu X, Chen M, Hu
S, Hu Y and Cai T: Sirt7 promotes gastric cancer growth and
inhibits apoptosis by epigenetically inhibiting miR-34a. Sci Rep.
5:97872015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang HL, Lu RQ, Xie SH, Zheng H, Wen XM,
Gao X and Guo L: SIRT7 exhibits oncogenic potential in human
ovarian cancer cells. Asian Pac J Cancer Prev. 16:3573–3577. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Aljada A, Saleh AM, Alkathiri M, Shamsa
HB, Al-Bawab A and Nasr A: Altered Sirtuin 7 expression is
associated with early stage breast cancer. Breast Cancer (Auckl).
9:3–8. 2015.PubMed/NCBI
|
|
51
|
Cioffi M, Vallespinos-Serrano M, Trabulo
SM, Fernandez-Marcos PJ, Firment AN, Vazquez BN, Vieira CR, Mulero
F, Camara JA, Cronin UP, et al: MiR-93 controls adiposity via
inhibition of Sirt7 and Tbx3. Cell Rep. 12:1594–1605. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kurylowicz A, Owczarz M, Polosak J, Jonas
MI, Lisik W, Jonas M, Chmura A and Puzianowska-Kuznicka M: SIRT1
and SIRT7 expression in adipose tissues of obese and normal-weight
individuals is regulated by microRNAs but not by methylation
status. Int J Obes (Lond). 40:1635–1642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gu S, Ran S, Liu B and Liang J: miR-152
induces human dental pulp stem cell senescence by inhibiting SIRT7
expression. FEBS Lett. 590:1123–1131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao L and Wang W: miR-125b suppresses the
proliferation of hepatocellular carcinoma cells by targeting
Sirtuin7. Int J Clin Exp Med. 8:18469–18475. 2015.PubMed/NCBI
|
|
55
|
Han Y, Liu Y, Zhang H, Wang T, Diao R,
Jiang Z, Gui Y and Cai Z: Hsa-miR-125b suppresses bladder cancer
development by down-regulating oncogene SIRT7 and oncogenic long
non-coding RNA MALAT1. FEBS Lett. 587:3875–3882. 2013. View Article : Google Scholar : PubMed/NCBI
|