Introduction
Periodontal disease frequently occurs in stomatology
and the incidence is rapidly increasing, as it manifests in >90%
of all patients referred to stomatology clinics (1–3).
Typical features of periodontal disease are the progressive
destruction of tissues, gingival inflammation and loss of
supporting tissues (particularly the absorption of alveolar bones),
which leads to loosening or detachment of teeth (4,5). A
number of factors contribute to the occurrence of periodontal
disease. An initiating factor is the existence of pathogens;
however, the most important independent risk factor is smoking,
although genetics, diabetes and autoimmunity are known factors
(6,7). In the clinic, the incidence and
severity of smoking-induced periodontal disease are increased
compared with those in non-smoking patients, and disease is
accompanied by an increased rate of tooth loss (8). Deeper periodontal pockets exist in
patients who smoke, and these patients exhibit more severe alveolar
bone loss and absorption, which compromises the treatment efficacy
(9). Numerous harmful substances
exist in tobacco which severely affect public health. As the
primary component of tobacco, nicotine causes direct damage to
human tissues, and causes indirect injury to humoral/cell immunity
via the release of inflammatory mediators (10,11).
A study suggested that nicotine may cause ischemia and inflammation
in periodontal and gingival tissues, and inhibit the mineralization
of alveolar bones (12).
Catalpol is an effective component that is extracted
from Radix Rehmanniae (figwort family) in traditional
Chinese medicine. It is a benzyl glycoside compound with a small
molecular weight (13,14). Studies have demonstrated that
catalpol exerts a number of biological activities, including
clearing oxygen free radicals, inhibiting the permeability of
microvessels, and antioxidant, anti-tumor, anti-fungal/viral,
anti-Alzheimer's disease, and anti-inflammatory properties
(15). Catalpol may exert its
anti-inflammatory effects via multiple routes, including
suppression of the overproduction of focal inflammatory mediators,
and the protection of tissues by mediating cytokines (16). Therefore, the present study aimed
to investigate the protective effect and underlying mechanism of
catalpol against nicotine-induced alveolar bone injury. This may
aid the clinical treatment and understanding of the pathogenesis of
periodontal disease.
Materials and methods
Animals
A total of 24 healthy male Wistar rats [5 weeks old;
specific pathogen free (SPF) grade; body weight, 120±20 g] were
purchased from the Laboratory Animal Center of Harbin Medical
University (Harbin, China). Animals were kept in an SPF facility
with fixed temperature (21±1°C) and humidity (50–70%) and a 12/12 h
dark-light cycle. All rats were provided with food and water ad
libitum.
Rats were used for all experiments, and all
procedures were approved by the Animal Ethics Committee of the
College of Stomatology, Harbin Medical University (Harbin,
China).
Reagents and instruments
Surgical instruments were purchased from Suzhou
Sunan Zimmered Medical Instrument Co., Ltd. (Jiangsu, China)
(16). Nicotine was purchased from
Yipu Ruisi Technology Co., Ltd. (Beijing, China) (16). TRIzol reagent was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
bone alkaline phosphatase (AP) assay kit was purchased from Roche
Diagnostics (Basel, Switzerland). Polyvinylidene difluoride
membranes were from Pall Life Sciences (Port Washington, NY, USA).
Chemical reagents for western blotting were purchased from Beyotime
Institute of Biotechnology (Haimen, China) (16). Enhanced chemiluminescence reagent
was purchased from GE Healthcare (Chicago, IL, USA). Rabbit
anti-rat tumor necrosis factor (TNF)-α (cat. no. 3707),
cyclooxygenase-2 (COX-2) monoclonal antibodies (cat. no. 12282),
rabbit b-actin monoclonal antibody (cat. no. 4907), and goat
anti-rabbit horseradish peroxidase conjugated immunoglobulin G
antibody (cat. no. 7074) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The RNA extraction kit (TRIzol
reagent) and high-Capacity cDNA Reverse Transcription kit were
purchased from Thermo Fisher Scientific, Inc. Rat-specific
osteocalcin assay kit was purchased from BTI Biotechnology
Institute UK, Ltd. (Colchester, UK). The microplate reader was
produced by BD Biosciences (Franklin Lakes, NJ, USA). The DNA
amplifier (Gene Amp PCR system 2400) was purchased from
PerkinElmer, Inc. (Waltham, MA, USA). The automatic biochemical
analyzer was purchased from Beckman Coulter, Inc. (Brea, CA, USA).
Other chemical reagents were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China) (10).
Animal grouping
A total of 24 rats were randomly divided into three
groups (n=8): Control group, nicotine group and catalpol group.
Nicotine injection was performed to generate the alveolar bone
injury model and rat periodontal disease.
Nicotine-induced periodontal disease
model and treatment with catalpol
Following 1 week of acclimation, the periodontal
disease model was prepared. Prior to surgery, rats were fasted for
12 h. Nicotine (0.7 mg/kg) in sterile saline was intraperitoneally
injected for 30 consecutive days, with an equal volume of saline in
the control group (16). In the
catalpol treatment group, 2 µg/kg catalpol was subcutaneously
injected for 14 days subsequent to generating the nicotine-induced
periodontal disease model.
Sample collection
Following model generation, blood samples were
collected from rat abdominal aortas. Following incubation for 30
min at room temperature, blood samples were centrifuged at 1,500 ×
g at 4°C for 10 min. Supernatants were saved and frozen at −20°C.
At 1 and 2 weeks during catalpol treatment, four rats from each
group were sacrificed. Periodontal tissue and maxilla samples (5
µm) were collected and stored at −80°C.
Monitoring of alveolar bone loss
level
At 1 and 2 weeks during treatment, the loss of
alveolar bone was measured via probing test of first molar on
bilateral maxilla as described previously (17).
ELISA analysis
Supernatants were extracted from blood samples to
measure the expression profile of bone AP (cat. no. MBS722033,
MyBioSource, San Diego, CA, USA) and bone osteocalcin (cat. no.
MBS728975, MyBioSource) using ELISA kits, following the
manufacturers' protocols. A total of 50 µl serially-diluted
standard curve samples were added into the wells of a 96-well
plate. A total of 50 µl test samples were added into wells in
triplicate. Following incubation, washing buffer was added five
times to each well for 30 sec. Enzyme label reagent was added into
each well (50 µl), incubated at 37°C for 30 min and washed.
Chromogenic substrates A and B were sequentially added (50 µl
each). Following 10 min development at 37°C in the dark, 50 µl
stopping buffer was added to each well to quench the reaction.
Optical density values at a wavelength of 450 nm were measured
using a microplate reader within 15 min. Sample concentrations were
determined using a linear regression function.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol reagent was used to extract the mRNA from
periodontal tissues of all rats. cDNA synthesis was performed
according to the manufacturer's protocol of the RT kit. Primers
were designed based on the sequences of target gene fragments and
were synthesized by Yingjun Biotechnology Co., Ltd. (Shanghai,
China) (18,19). Primer sequences (5′-3′) are
presented in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ |
|---|
| GADPH |
ACCAGGTATCTGCTGGTTG |
TAACCATGATGTCAGCGTGGT |
| TNF-α |
GCATGACCTGCTTATGACTG |
TTCGTTCCGCTCAACTCTTA |
| COX |
TGCTTATGCATGATGCCGACT |
CGCTTCTTCGTCAACTCTTATC |
The qPCR was performed on target genes using the
following conditions: 55°C for 1 min, followed by 35 cycles at 92°C
for 30 sec, 58°C for 45 sec and 72°C for 35 sec. Using GAPDH as the
internal reference, fluorescent (SYBR-Green Dye; Thermo Fisher
Scientific, Inc.) quantification was performed to obtain cycle
threshold values of all standards and samples. Using cycle
threshold values of the standards as the reference, linear
functions were plotted, on which quantitative analysis was
performed by 2−ΔΔCq method (20).
Western blotting
Total protein was extracted from periodontal
tissues. Tissues were homogenized in liquid nitrogen, with the
addition of RIPA Lysis and Extraction Buffer (Thermo Fisher
Scientific, Inc.) for 15–30 min. Ultrasound was used to rupture
cells (4 times for 5 sec), which were subsequently centrifuged at
10,000 × g at 4°C for 15 min. Supernatants were saved and
quantified by Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.). Proteins were separated by SDS-PAGE on a 10%
gel, and were transferred to polyvinylidene difluoride membranes
using a semi-dry method. Non-specific binding was blocked using 5%
nonfat milk powder at room temperature for 2 h. Primary antibodies
against TNF-α and COX-2 (1:1,000 dilution) were added at 4°C
overnight. The following day, PBS-Tween-20 was added to wash the
membrane, followed by the addition of a goat anti-rabbit secondary
antibody (1:2,000 dilution) for 30 min in the dark. Chromogenic
substrate (enhanced chemiluminescence) was added for 1 min
development. The membrane was exposed, scanned and the density of
bands was quantified with Quantity One software version 4.6.5
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each experiment
was repeated four times.
Statistical analysis
SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA) was used to perform the statistical analysis. Measurement data
are expressed as the mean ± standard deviation. The comparisons
among multiple groups were performed using one-way analysis of
variance (ANOVA) followed by the least significant difference post
hoc test, whereas, two-way ANOVA was performed for comparison of
difference between groups at different time points. Linear
regression was performed by SPSS software for the quantitative
analysis of samples for the ELISA assay based on curve obtained
from the standard data. P<0.05 was considered to indicate a
statistically significance difference.
Results
Analysis of probing depth in
periodontal tissues
A probing test was performed in periodontal tissues
to analyze the injury to alveolar bone induced by nicotine and the
protective effect of catalpol. The results revealed a significantly
elevated probing depth in the first molar of the bilateral maxilla
in the nicotine intervention group compared with the control
(P<0.05; Table II). Following
catalpol treatment, the probing depth of periodontal tissues became
shallower in the first week and exhibited a significant decrease
compared with the nicotine group in the second week (P<0.05;
Table II).
 | Table II.Probing depth of periodontal tissues
(mm). |
Table II.
Probing depth of periodontal tissues
(mm).
| Group | First week (n=4) | Second week
(n=4) |
|---|
| Control |
0.19±0.03 |
0.21±0.02 |
| Nicotine |
0.87±0.02a |
1.21±0.03a |
| Catalpol |
0.79±0.04a |
0.81±0.03a,b |
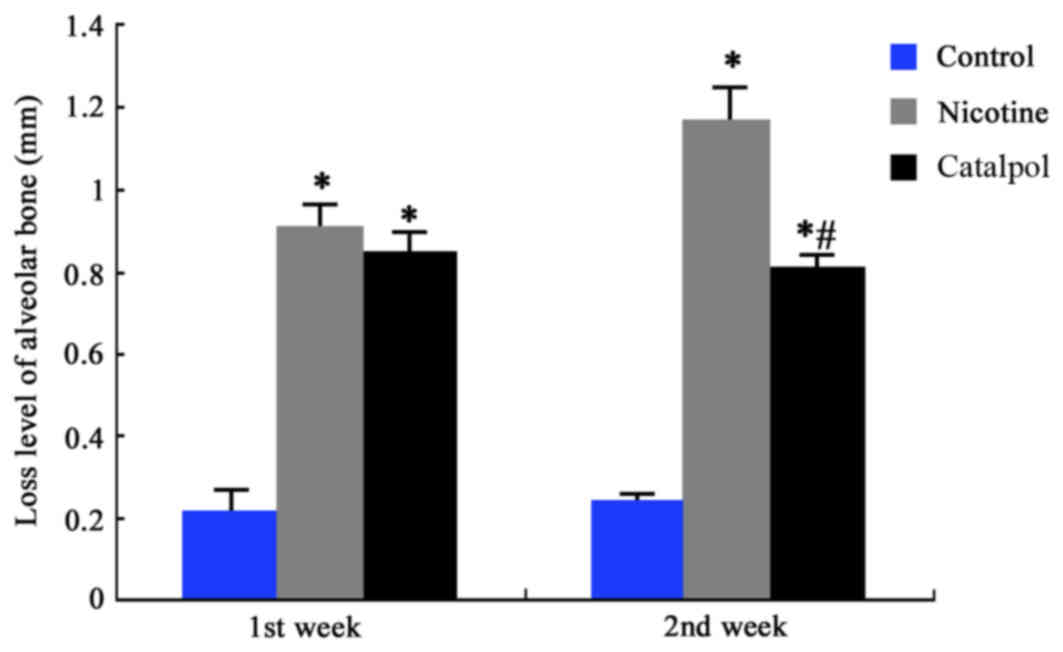
Loss of rat alveolar bones
The degree of alveolar bone loss was measured by the
distance between the top of the alveolar bone ridge and the
cemento-enamel junction. The results demonstrated a significantly
higher alveolar bone loss in nicotine-treated rats compared with
the control group (P<0.05; Fig.
1). Following treatment with catalpol, bone loss improved at
the first week; however, this was not significant. At the second
week, the bone loss was significantly alleviated compared with the
nicotine group (P<0.05; Fig.
1). These results suggested that nicotine may cause significant
injury to alveolar bone. Treatment with catalpol, however, may
significantly improve and reverse this nicotine-induced injury to
alveolar bone.
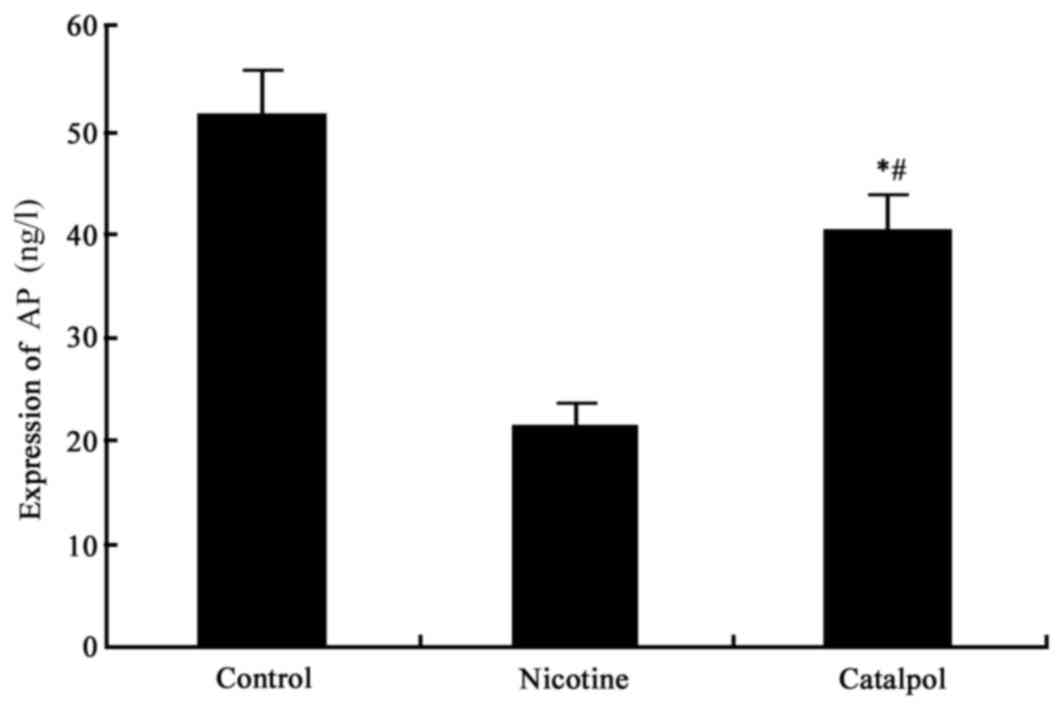
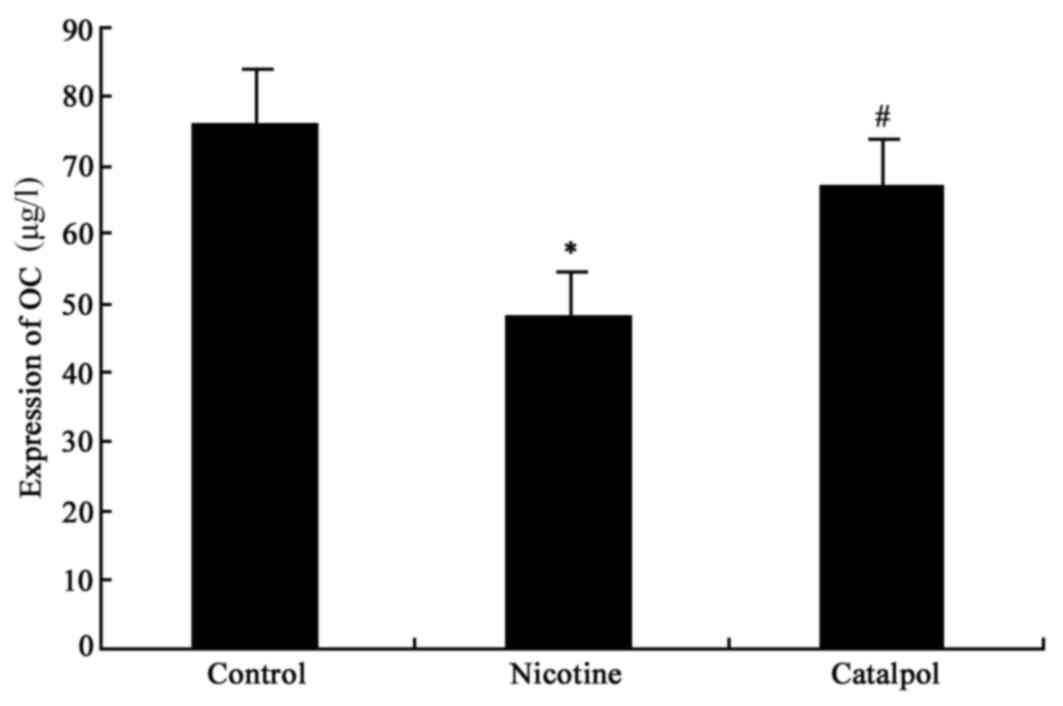
AP and osteocalcin expression
ELISA analysis was used to detect the bone AP and
osteocalcin (OC) level. The results demonstrated significantly
decreased AP and OC levels in nicotine-treated rats compared with
the control group (P<0.05; Figs.
2 and 3). Following treatment
with catalpol, rat AP and OC levels were significantly increased
(P<0.05; Figs. 2 and 3).
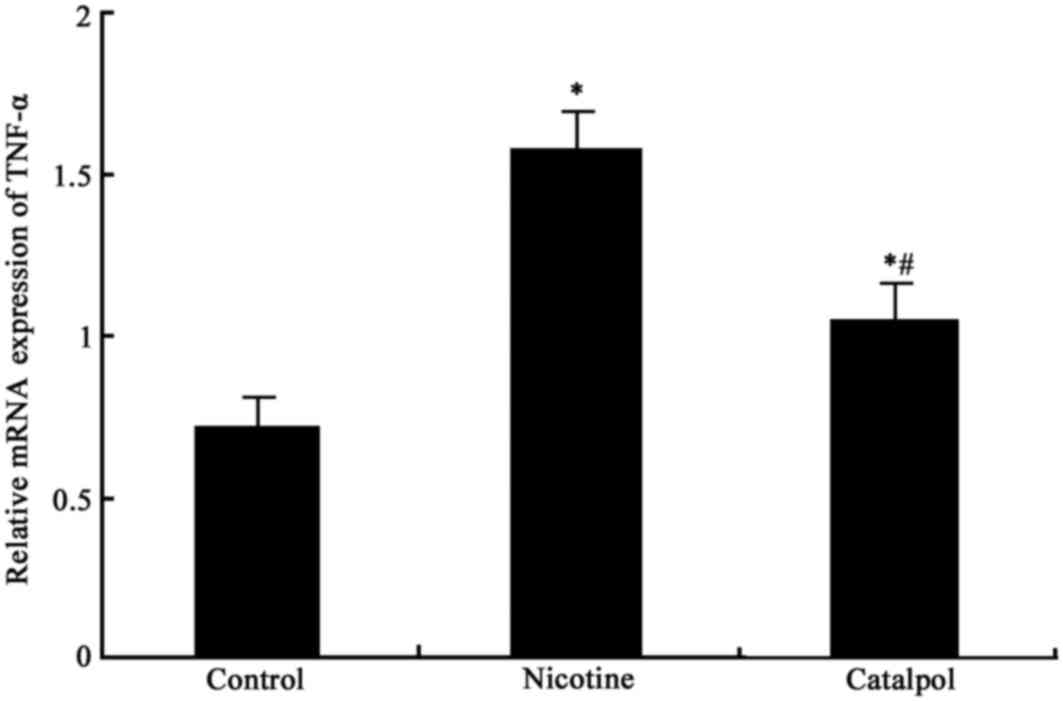
Expression of TNF-α mRNA in
periodontal tissues
The mRNA expression levels of TNF-α in periodontal
tissues in rats from all groups was measured. There was
significantly elevated TNF-α expression in periodontal tissues in
nicotine-treated rats compared with control rats (P<0.05;
Fig. 4). Following treatment with
catalpol, the TNF-α mRNA expression level was significantly reduced
compared with the nicotine group (P<0.05; Fig. 4).
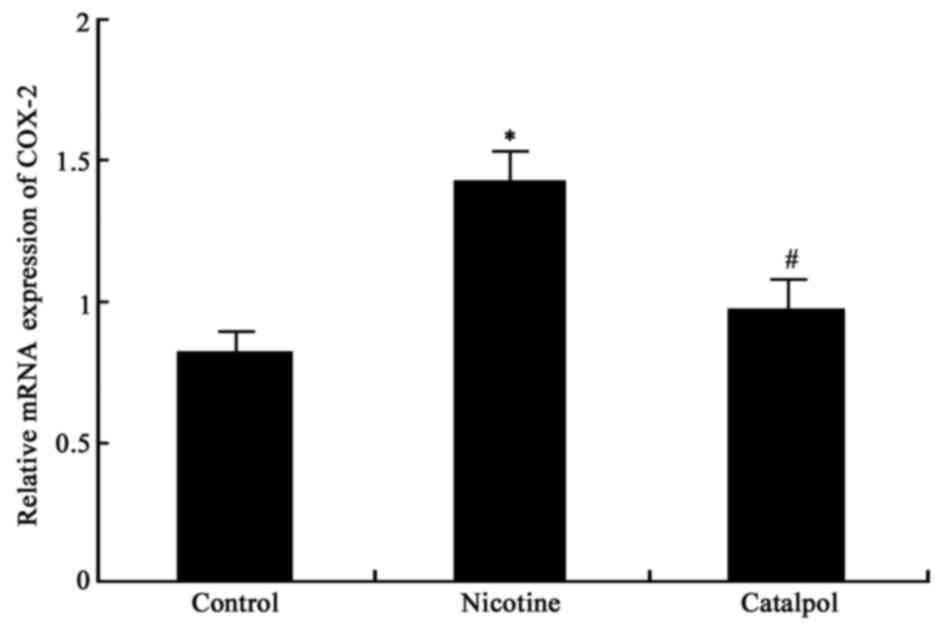
Expression of COX-2 mRNA in
periodontal tissues
The mRNA expression levels of COX-2 in periodontal
tissues were measured. There were significantly elevated COX-2 mRNA
expression levels in nicotine-treated rat periodontal tissues
compared with the control group (P<0.05; Fig. 5). Treatment with catalpol
significantly reduced COX-2 mRNA expression levels compared with
the nicotine group (P<0.05; Fig.
5).
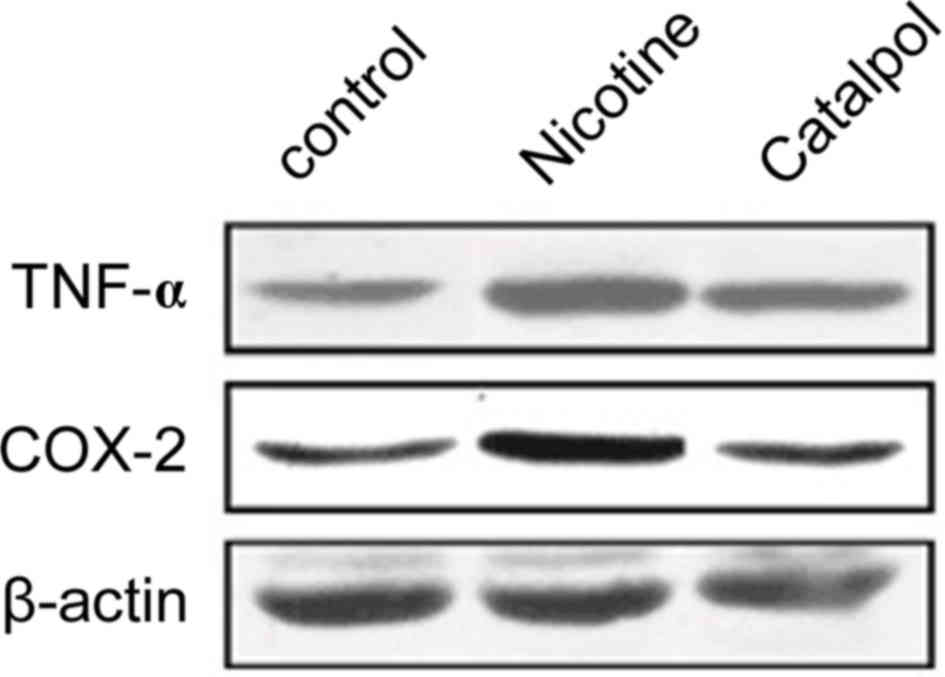
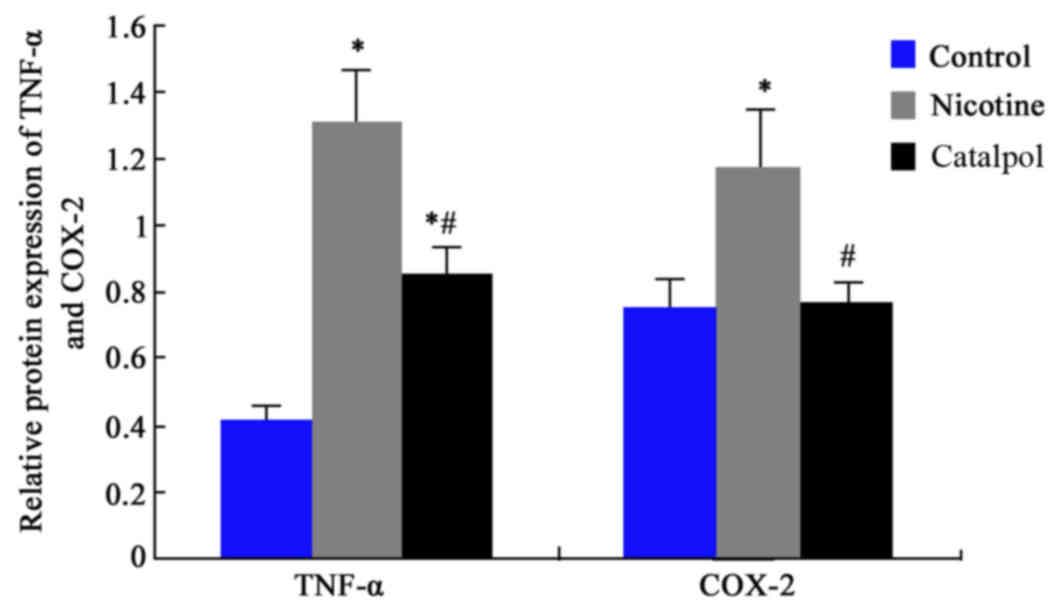
TNF-α and COX-2 protein
expression
Western blotting was further employed to analyze the
expression levels of TNF-α and COX-2 proteins in rat periodontal
tissues. Results revealed significantly elevated protein levels of
TNF-α and COX-2 after nicotine treatment compared with the control
group (P<0.05; Figs. 6 and
7). Treatment with catalpol
significantly decreased the protein levels in periodontal tissues
compared with the nicotine group (P<0.05; Figs. 6 and 7).
Discussion
Smoking is a risk factor for periodontal disease as
it may elevate the presence of pathogens in periodontal tissues,
and increase the incidence of hemorrhage. Excessive smoking for
long periods of time leads to destruction of periodontal tissues.
Periodontal disease in smokers is often associated with severe
alveolar injury (10). As an
important component of tobacco, nicotine may cause body tissue
damage accompanied by an inflammatory response. It may induce
severe injury to alveolar bones via inhibition of mineralization
(19,21). The present study used a periodontal
disease model and induced alveolar bone injury via nicotine
infusion, and demonstrated that nicotine caused the loss of
alveolar bone. Extracted from a traditional Chinese medicine,
catalpol exhibits multiple pharmaceutical activities and serves
important roles as an anti-inflammatory agent and mediator of
oxidation-reduction homeostasis (22). The present study demonstrated that
catalpol may alleviate the loss of alveolar bone in a
nicotine-induced rat alveolar bone injury model.
AP primarily exists in liver, kidney and bone
tissues. It is an extracellular enzyme secreted by osteoblasts, and
may additionally be produced from periodontal membrane cells and
alveolar bone cells. It may affect the synthesis of osteoblasts
during maturation, and is an important marker for bone
mineralization and activity of osteoblasts (23). Osteocalcin is secreted by
osteoblasts during the matrix mineralization stage, and primarily
facilitates bone mineralization (24). The present study demonstrated that
nicotine inhibited AP and osteocalcin expression, thereby leading
to injury to alveolar bone. Following treatment with catalpol, the
secretion levels of AP and osteocalcin were somewhat restored,
suggesting that catalpol may exert a protective effect on alveolar
bone.
Following entry into the body, nicotine directly
initiates the secretion and release of large quantities of
inflammatory factors into the serum. This leads to an imbalance of
oxygen free radicals in periodontal tissues, production of
inflammatory mediators including TNF-α and prostaglandin,
periodontal inflammation, destruction of periodontal tissues and
injury to alveolar bone (25).
COX-2 is an important enzyme that catalyzes the synthesis of
prostaglandin from arachidonic acid. The present study, therefore,
analyzed the effect of nicotine on the expression of TNF-α and
COX-2 in periodontal tissues. The results revealed that nicotine
may facilitate the mRNA and protein expression of TNF-α and COX-2
in periodontal tissues, which may aggravate inflammation and cause
further damage to alveolar bone. Further treatment using catalpol,
which has notable anti-inflammatory and antioxidant effects,
inhibited the expression of TNF-α and COX-2 (at the mRNA and
protein levels). This suggested that catalpol may alleviate tissue
injury to the periodontium, protect alveolar bone and slow the
progression of periodontal disease via inhibition of cytokines and
mediation of the homeostasis of oxygen free radicals (26,27).
In conclusion, catalpol may facilitate the
mineralization of alveolar bone via inhibition of inflammatory
factors, and thus improve nicotine-induced injury to alveolar bone.
The present study may provide a reference to the pathogenesis of
periodontal disease, and may increase treatment choices for
alveolar bone injury.
Acknowledgements
The authors would like to thank Dr Su Ma and Dr
Peihong Liu (Harbin Medical University) for their support and
assistance with this manuscript.
References
|
1
|
Trubiani O, Giacoppo S, Ballerini P,
Diomede F, Piattelli A, Bramanti P and Mazzon E: Alternative source
of stem cells derived from human periodontal ligament: A new
treatment for experimental autoimmune encephalomyelitis. Stem Cell
Res Ther. 7:12016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toregeani JF, Nassar CA, Nassar PO,
Toregeani KM, Gonzatto GK, Vendrame R, Castilhos JS, Rotta LS,
Reinheimer AC, Longoni A and Barcella MW: Evaluation of
periodontitis treatment effects on carotid intima-media thickness
and expression of laboratory markers related to atherosclerosis.
Gen Dent. 64:55–62. 2016.PubMed/NCBI
|
|
3
|
Yang J, Zhang Q, Chen M, Wu WZ, Wang R,
Liu CJ, Li B, Shi XL, Du HS and Tan HB: Association between
Helicobacter pylori infection and risk of periodontal diseases in
Han Chinese: A case-control study. Med Sci Monit. 22:121–126. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pullishery F, Panchmal GS and Siddique S:
Salivary thiocyanate, uric acid and pH as biomarkers of periodontal
disease in tobacco users and non-users- an in-vitro study. J Clin
Diagn Res. 9:ZC47–ZC50. 2015.PubMed/NCBI
|
|
5
|
Wyganowska-Swiatkowska M and Nohawica MM:
Effect of tobacco smoking on human gingival and periodontal
fibroblasts. A systematic review of literature. Przegl Lek.
72:158–160. 2015.PubMed/NCBI
|
|
6
|
Warad S, Kalburgi NB, Manak M, Kalburgi
VC, Koregol AC, Patanashetti J, Rao S and Kokatnur MV: Determining
the effect of Gutkha on serum levels of vitamin B12 and folic acid
as compared to smoking among chronic periodontitis subjects: A
cross-sectional study. J Clin Diagn Res. 8:ZC85–ZC89.
2014.PubMed/NCBI
|
|
7
|
Ng TK, Huang L, Cao D, Yip YW, Tsang WM,
Yam GH, Pang CP and Cheung HS: Cigarette smoking hinders human
periodontal ligament-derived stem cell proliferation, migration and
differentiation potentials. Sci Rep. 5:78282015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Archana MS, Bagewadi A and Keluskar V:
Assessment and comparison of phagocytic function and viability of
polymorphonuclear leukocytes in saliva of smokers and non-smokers.
Arch Oral Biol. 60:229–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang R, Li M, Ye M, Yang K, Xu X and
Gregory RL: Effects of Nicotine on Streptococcus gordonii growth,
biofilm formation, and cell aggregation. Appl Environ Microbiol.
80:7212–7218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lallier TE, Maturin E, Brady M, Stoute D
and Ward T: Resistance to cigarette smoke is increased in
periodontal ligament cells by attachment to collagen and
fibronectin. J Periodontol. 86:91–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imamura K, Kokubu E, Kita D, Ota K,
Ishihara K and Saito A: Cigarette smoke condensate modulates
migration of human gingival epithelial cells and their interactions
with Porphyromonas gingivalis. J Periodontal Res. 50:411–421. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hedna VS, Ansari S, Shahjouei S, Cai PY,
Ahmad AS, Mocco J and Qureshi AI: Validity of laser doppler
flowmetry in predicting outcome in murine intraluminal middle
cerebral artery occlusion stroke. J Vasc Interv Neurol. 8:74–82.
2015.PubMed/NCBI
|
|
13
|
Xue B, Ma B, Zhang Q, Li X, Zhu J, Liu M,
Wu X, Wang C and Wu Z: Pharmacokinetics and tissue distribution of
Aucubin, Ajugol and Catalpol in rats using a validated simultaneous
LC-ESI-MS/MS assay. J Chromatogr B Analyt Technol Biomed Life Sci.
1002:245–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JM, Yang LH, Zhang YY, Niu CL, Cui Y,
Feng WS and Wang GF: BDNF and COX-2 participate in anti-depressive
mechanisms of catalpol in rats undergoing chronic unpredictable
mild stress. Physiol Behav. 151:360–368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Z, Zhang L, Li X, Jiang Z, Sun L, Zhao
G, Zhou G, Zhang H, Shang J and Wang T: Mitochondrial
fusion/fission process involved in the improvement of catalpol on
high glucose-induced hepatic mitochondrial dysfunction. Acta
Biochim Biophys Sin (Shanghai). 47:730–740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tewari D, Khan MP, Sagar N, China SP,
Singh AK, Kheruka SC, Barai S, Tewari MC, Nagar GK, Vishwakarma AL,
et al: Ovariectomized rats with established Osteopenia have
Diminished Mesenchymal stem cells in the bone marrow and Impaired
Homing, Osteoinduction and Bone Regeneration at the fracture site.
Stem Cell Rev. 11:309–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Souza DM, Ricardo LH, Kantoski KZ and
Rocha RF: Influence of alcohol consumption on alveolar bone level
associated with ligature-induced periodontitis in rats. Braz Oral
Res. 23:326–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Javed F, Ahmed H Bashir and Romanos GE:
Association between environmental tobacco smoke and periodontal
disease: a systematic review. Environ Res. 133:117–122. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tao JH, Zhao M, Wang DG, Yang C, Du LY,
Qiu WQ and Jiang S: Biotransformation and metabolic profile of
catalpol with human intestinal microflora by ultra-performance
liquid chromatography coupled with quadrupole time-of-flight mass
spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci:
1009-1010. 1–169. 2016.
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fernandes BS, Hodge JM, Pasco JA, Berk M
and Williams LJ: Effects of depression and serotonergic
antidepressants on bone: Mechanisms and implications for the
treatment of depression. Drugs Aging. 33:21–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernandes G, Wang C, Yuan X, Liu Z, Dziak
R and Yang S: Combination of controlled release platelet-rich
plasma alginate beads and bone morphogenetic Protein-2 genetically
modified mesenchymal stem cells for bone regeneration. J
Periodontol. 87:470–480. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gołąbek K, Ostrowska Z, Ziora K,
Oświęcimska J, Świętochowska E, Marek B, Kajdaniuk D, Strzelczyk J
and Kos-Kudła B: Association between omentin-1, bone metabolism
markers, and cytokines of the RANKL/RANK/OPG system in girls with
anorexia nervosa. Endokrynol Pol. 66:514–520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wadhwa D, Bey A, Hasija M, Moin S, Kumar
A, Aman S and Sharma VK: Determination of levels of nitric oxide in
smoker and nonsmoker patients with chronic periodontitis. J
Periodontal Implant Sci. 43:215–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lazăr L, Loghin A, Bud ES, Cerghizan D,
Horváth E and Nagy EE: Cyclooxygenase-2 and matrix
metalloproteinase-9 expressions correlate with tissue inflammation
degree in periodontal disease. Rom J Morphol Embryol. 56:1441–1446.
2015.PubMed/NCBI
|
|
26
|
Liu JY, Zheng CZ, Hao XP, Zhang DJ, Mao AW
and Yuan P: Catalpol ameliorates diabetic atherosclerosis in
diabetic rabbits. Am J Transl Res. 8:4278–4288. 2016.PubMed/NCBI
|
|
27
|
Tian YY, An LJ, Jiang L, Duan YL, Chen J
and Jiang B: Catalpol protects dopaminergic neurons from
LPS-induced neurotoxicity in mesencephalic neuron-glia cultures.
Life Sci. 80:193–199. 2006. View Article : Google Scholar : PubMed/NCBI
|