Introduction
Articular cartilage displays a limited capacity of
self-renewal when suffered from trauma or degenerative disease.
Consequently, stem cell-based tissue engineering is a promising
method for cartilage repair (1–4).
Mesenchymal stem cells (MSCs) are thought to be a promising cell
source for their self-renewal and multi-lineage differentiation
abilities, but the utility of MSCs has many unfavorable outcomes
including low chondrogenic potential, vascularization and
mineralization (5–8). The growth plate chondrocytes have the
ability of continuous reproduction and differentiation. Thus it is
long thought to be a cell source for cartilage repair strategies.
But the growth plate chondrocytes are complicated, ranging from
resting cells to proliferative, pre-hypertrophic and ultimately
hypertrophic chondrocytes, the research about the growth plate
remains elusive (9).
Growth plate chondrocytes are believed to compose
progenitors of different cell types (10,11).
By investigating the clonal relationship and lineage development of
mesenchymal tissues in bone, Chan et al (12) recently isolated a population of
postnatal skeletal stem cells (SSCs) which are important for
postnatal skeletal development. Unlike mesenchymal stem cells, SSCs
seldom diffrentiate into adipocyte (12). Similar research was also conducted
by Worthley et al (13),
they found that bone morphogenetic protein (BMP) antagonist Gremlin
1 defines a population of osteochondroreticular (OCR) stem cells
which mainly concentrated within the metaphysis of long bone and
were also thought to be a population of SSCs. These cells could
self-renew and generate osteoblasts, chondrocytes and reticular
marrow stromal cells, but not adipocytes. They are important for
bone development, bone remodeling and fracture repair (13). Like hematopoietic stem cells, SSCs
are heterogeneous and contain many lineage-restricted stem cells
which lead to unreliable bone and cartilage formation (14). So it is important to isolate
purified subpopulation of SSCs which could differentiate along
chondrogenic lineage steadily.
Cell surface marker based cell purification is a
simple and efficacious cell sorting method. Two alternative markers
comprising endoglin (CD105) and melanoma cell adhesion molecule
(MCAM; CD146) have been identified on the cell surface of isolated
populations of SSCs. These subpopulations of SSCs exhibited
different biological characteristics. CD105, a type III receptor
for the transforming growth factor β (TGF-β) superfamily, is known
as a relatively specific marker for identifying mesenchymal stem
cells (15–18). Several lines of evidence showed
that CD105 is related to chondrogenic potential of human MSCs or
adipose-derived stem cells (ASCs) (4,19–21).
Chan et al (12) found that
CD105+ subpopulation represented a much more
differentiated population of postnatal mouse SSCs compared with
CD105− cell population. CD105+ subpopulation
is responsible for bone and cartilage regeneration and CD105 is a
candidate marker for SSC isolation (12). CD146, a cell adhesion molecule
(CAM) that was originally identified as a tumor marker for melanoma
(MCAM), has been studied as a putative mesenchymal stem cell marker
in human umbilical cord perivascular cells (HUCPVCs) and bone
marrow mesenchymal stromal cells (BMSCs) (22–24).
Compared with CD146− MSCs, the CD146+ MSCs
exhibited a much stronger multi-lineage differentiation potential
and capacity of maintaining stemness and phenotype after long
cultivation (24,25). There was also evidence showing that
CD146 was a marker of cartilage-derived chondroprogenitor cells and
CD146+ cartilage subpopulation exhibited greater
therapeutic potential in cartilage repair and regeneration
(26,27). However, it remains to be
ascertained whether CD105 and CD146 could offer improved SSCs
isolation in the growth plate.
Based on the aforementioned studies, it was
hypothesized that purified SSCs may represent an improved
alternative cell source compared with unsorted growth plate
chondrocytes and ASCs for cartilage repair and tissue engineering.
In the present study, we identified the existence and distribution
of CD105+ SSCs and CD146+ SSCs in the growth
plate. We then purified SSCs using CD105 and CD146 cell surface
markers via magnetic activated cell sorting (MACS) method. Finally,
we compared the colony-forming efficiency (CFE) and multi-lineage
differentiation capacity of unsorted growth plate chondrocytes,
CD105+ SSCs, CD146+ SSCs and ASCs in
vitro.
Materials and methods
Isolation and culture of growth plate
chondrocytes and ASCs
Growth plate chondrocytes were obtained from
1-week-old Sprague-Dawley rats (Experimental Animal Center of
Tongji Hospital, Huazhong University of Science and Technology,
Wuhan, China). All animal procedures were approved by the Ethics
Committee on Animal Experimentation of Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, China).
Growth plate in distal femurs and proximal tibias of
SD rats were isolated and subtly cut into pieces. After several
rinses in phosphate-buffered saline (PBS), the pieces were
subsequently digested with 0.25% trypsin solution (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 20 min and 0.1% solution of
collagenase type II (Invitrogen; Life Technologies, Carlsbad, CA,
USA) at 37°C over night. The cells were then harvested and cultured
in Dulbecco's modified Eagles medium/Ham's F-12 (DMEM/F-12)
supplemented with 10% fetal bovine serum (FBS) (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and antibiotics
[(100 U/ml penicillin G sodium, 100 µg/ml streptomycin sulfate
(Gibco; Thermo Fisher Scientific, Inc.)].
ASCs were isolated from 8-weeks-old Sprague-Dawley
rats (Experimental Animal Center of Tongji Hospital). Adipose
tissue was digested with collagenase type I (Invitrogen; Life
Technologies) for 1 h at 37°C. The cells were then harvested and
cultured in the DMEM/F-12 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and antibiotics [(100 U/ml penicillin G
sodium, 100 µg/ml streptomycin sulfate (Gibco; Thermo Fisher
Scientific, Inc.)]. When reaching 90% confluence, cells were
passaged to obtain sufficient number of ASCs.
Flow cytometry analysis of stem cell
surface markers on the growth plate chondrocytes
Chondrocytes (1×106) from growth plate
(passage 1) were washed in PBS and incubated with corresponding
primary antibodies for one hour at 4°C. Primary antibodies used
here were conjugated antibodies specific for CD44-FITC (dilution
1:100, #550974; BD Biosciences, San Diego, CA, USA), CD29-PE
(dilution 1:80, #48-0291; eBiosciences; Thermo Fisher Scientific,
Inc.), CD34-PE (dilution 1:100; #sc-74499 PE; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), CD45-PE (dilution 1:80,
#12-0461; eBiosciences; Thermo Fisher Scientific, Inc.) and
unconjugated antibodies for CD105 (dilution 1:100, #ab11414) and
CD146 (dilution 1:80, #ab75769) (both from Abcam, Cambridge, MA,
USA). After washing with PBS, cells stained with CD105 and CD146
were incubated with anti-mouse FITC-conjugated secondary antibody
(dilution 1:500, #A0568; Beyotime Institute of Biotechnology,
Guangzhou, China) or Alexa Fluor 594 donkey anti-rabbit IgG
(dilution 1:500, #A-21207; Invitrogen; Life Technologies) for 30
min at 4°C. The cells were washed twice and then re-suspended in
200 µl PBS for the flow cytometry analysis (FACSort; BD
Biosciences).
Immunohistochemistry (IHC) analysis of
CD146 and CD105 expression in the growth plate
Proximal tibias of 1-week-old SD rats were isolated
and fixed with 4% formaldehyde. The samples were decalcificated
with 12.5% EDTA solution, dehydrated, embedded into paraffin wax
and sectioned at 5 mm thickness. Primary mouse anti-CD105 antibody
(dilution 1:100) and rabbit anti-CD146 antibody (dilution 1:250)
(both from Abcam) were used for immune labeling according to
manufacturer's instructions. Then sections were incubated with
biotinylated goat anti-mouse or goat anti-rabbit secondary
antibodies (#BA1001 or #BA1003, dilution 1:2,000; Boster Biological
Technology, Wuhan, China) at RT for 30 min. Reactivity was detected
with a diaminobenzidine tetrahydrochloride (DAB) substrate kit
(Beyotime Institute of Biotechnology), with hematoxylin as the
counterstain. Images were captured by microscopy (FV500; Olympus
Corporation, Tokyo, Japan).
Cell sorting by MACS
About 5×106 growth plate chondrocytes
were harvested for magnetic activated cell sorting (Miltenyi,
Teterow, Germany) according to manufacturer's instructions.
Briefly, single-cell suspensions were incubated with mouse
anti-CD105 antibody (dilution 1:100) or rabbit anti-CD146 antibody
(dilution 1:80) (both from Abcam) for 30 min at 37°C. After
centrifugated at 300 × g for 10 min and washed by PBS twice, the
cells were then incubated with anti-rabbit IgG microbeads or
anti-mouse IgG microbeads for 15 min at 37°C. The cells were then
washed and resuspended with washing buffer and added into the MS
column, the CD146+ SSCs and CD105+ SSCs were
obtained following the instructions. The sorted cells were cultured
in growth medium to obtain sufficient quantities for further
research.
Immunofluorescence assay
To confirm the expression of CD105 and CD146 in
sorted cells, immunofluorescence staining was performed. Cells were
grown to sub confluence on coverslips in 12-wells plate. Cells were
then fixed with 4% paraformaldehyde for 20 min at room temperature
and washed 3 times with PBS. After that, the cells were incubated
with 0.1% Triton X-100 for 15 min at room temperature (RT) and
blocked with 1% bovine serum albumin (Biosharp, Hefei, China) for 1
h at room temperature. Then the cells were incubated with mouse
anti-CD105 antibody (dilution 1:100) or rabbit anti-CD146 antibody
(dilution 1:80) (both from Abcam) overnight at 4°C. After washing
with PBS, cells stained with CD105 or CD146 were incubated with
anti-mouse FITC-conjugated secondary antibody (dilution 1:500,
#A0568; Beyotime Institute of Biotechnology) or Alexa Fluor 594
donkey anti-rabbit IgG (dilusion 1:1,000, #A-21207; Invitrogen;
Life Technologies) for 1 h at room temperature. The nuclei were
then labeled with DAPI (50 µg/ml; Sigma-Aldrich; Merck KGaA) for 5
min. Fluorescence images were captured by fluorescence microscopy
(FV500; Olympus Corporation).
CFE assay
The proliferative induction capability of the cells
was evaluated by CFE analysis. Briefly, 100 cells were seeded and
cultured in 60-mm dishes. Two weeks later, colonies formed from
single cell were fixed in 4% paraformaldehyde for 20 min at RT.
After washing with PBS for three times, Giemsa (#ab150670; Abcam)
staining was performed according to manufacturer's instructions.
Colonies with >50 cells were counted and recorded.
Multi-lineage differentiation
assays
Cells at passage 3 were initially seeded in a 6-well
plate at the density of 3×104 cells/cm2. When
they reached 90% confluence, cells were washed with PBS and
transferred to commercially available adipogenic or osteogenic
medium (#RASMD-90031 or #RASMD-90021; Cyagen Biosciences Inc.,
Santa Clara, CA, USA). After 3 weeks, cells were fixed by 4%
paraformaldehyde and washed 3 times with PBS. For adipogenic assay,
cells were incubated with Oil red O (Cyagen Biosciences Inc.) for
30 min to detect the intra-cellular lipid droplets. For
quantitative evaluation, dye content was extracted using
isopropanol and the absorbance at a wavelength of 540 nm was
detected. For osteogenic assay, cells were stained with Alizarin
Red (Cyagen Biosciences Inc.) for 10 min at room temperature.
A pellet culture system was used for chondrogenic
differentiation. Briefly, 1×106 cells were placed and
centrifugated at 300 × g for 15 min in a 15 ml polypropylene tube
to form a pellet. Then pellets were cultured in the chondrogenic
medium (#RASMD-90041; Cyagen Biosciences Inc.), which was changed
every 3 days. After induction for 3 weeks, the cell pellets were
fixed by 4% paraformaldehyde, dehydrated and then embedded into
paraffin wax. Tissue blocks were cut into 5 mm sections and were
placed on slides and dried overnight at 37°C. Chondrogenic
differentiation was finally assessed by Alician blue staining
(Cyagen Biosciences Inc.) and IHC using antibodies against aggrecan
(dilution 1:50, #ab36861) and type II collagen (dilution 1:100,
#ab185430) (both from Abcam) according to the manufacturer's
protocols.
Real-time quantitative polymerase
chain reaction (RT-qPCR) analysis
Total mRNA was extracted using TRIzol reagent
(Invitrogen; Life Technologies) following the manufacturer's
instructions. Complementary DNA (cDNA) was obtained from total RNA
using ReverTra Ace qPCR RT kit (Toyobo Co., Ltd., Osaka, Japan)
according to the manufacturer's protocols. Then the cDNA was
amplified by SYBR Green Real-Time PCR Master Mix (Toyobo Co., Ltd.)
with following cycling conditions: 30 sec of polymerase activation
at 95°C, followed by 40 cycles of 95°C for 5 sec and 60°C for 30
sec. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as
an internal control. The relative expression levels of each gene
were calculated using the comparative 2−ΔΔCt method
(28). Sequences of primers for
interested genes are listed in Table
I.
 | Table I.Primers used for RT-qPCR. |
Table I.
Primers used for RT-qPCR.
| Gene | Accession no. | Primer sequence
(5′-3′) |
|---|
| PPAR-γ | NM_001145366 | F:
CGGTTGATTTCTCCAGCATT |
|
|
| R:
TCGCACTTTGGTATTCTTGG |
| LPL | NM_012598 | F:
AACATTGGAGAAGCCATTCG |
|
|
| R:
TTCATTCAGCAGGGAGTCAA |
| AP2 | NM_053365 | F:
ATGTGTCATGAAAGGCGTGA |
|
|
| R:
AAACCACCAAATCCCATCAA |
| RUNX2 | NM_001278483 | F:
CTACTCTGCCGAGCTACGAAAT |
|
|
| R:
TCTGTCTGTGCCTTCTTGGTTC |
| ALP | NM_013059 | F:
CCTGGACCTCATCAGCATTT |
|
|
| R:
AGGGAAGGGTCAGTCAGGTT |
| OPN | NM_012881 | F:
CAAGGACCAACTACAACCA |
|
|
| R:
GGAGACAGGAGGCAAGG |
| SOX9 | NM_080403 | F:
GTGGGAGCGACAACTTTACC |
|
|
| R:
GCGAGCACTTAGCAGAGGC |
| Aggrecan | NM_022190 | F:
CAAACAGCAGAAACAGCCAAGT |
|
|
| R:
GAAGGCATAAGCATGTGAAAGTG |
| COL2 | NM_012929 | F:
CACCCAGAGTGGAAGAGCG |
|
|
| R:
TCAGTGGACAGTAGACGGAGGA |
Statistical analyses
All experiments were performed in triplicate, and
one representative set was chosen to be shown. All data are
presented as mean and standard deviation (SD) and analyzed with
GraphPad Prism 6.0. The significance of difference was determined
using the Student's t-test and analysis of variance (ANOVA);
differences with P<0.05 were considered significant.
Results
Surface antigen profiles
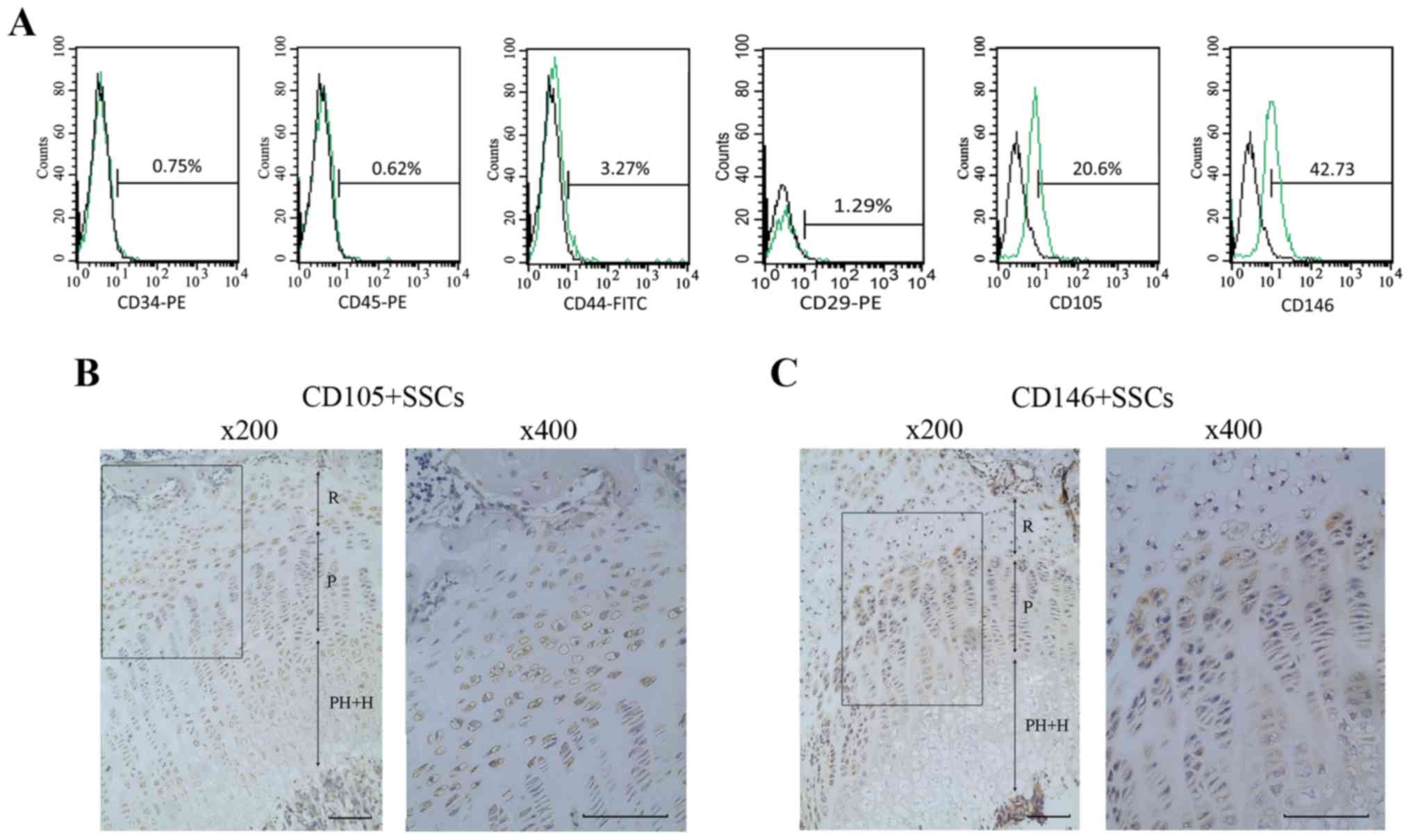
Flow cytometry analyses were performed to assess the
phenotype of the growth plate chondrocytes. Results showed that
42.73% of the cells were positive for CD146 and 20.6% of the cells
were positive for CD105. Meanwhile, growth plate chondrocytes were
negative for the hematopoietic markers (CD34 and CD45) and
MSC-associated surface markers (CD29 and CD44) (Fig. 1A).
Distribution of CD146+ SSCs
and CD105+ SSCs in growth plate
The growth plate was divided into resting zone,
proliferative zone, prehypertrophic and hypertrophic zone. We then
identified the distribution of CD146+ SSCs and
CD105+ SSCs in growth plate using IHC. As shown in
Fig. 1B and C, CD146+
SSCs mainly located at the resting zone, partly located at the
proliferating zone of the growth plate, while the CD105+
SSCs mainly located at the resting zone and the hypertrophic zone
of growth plate.
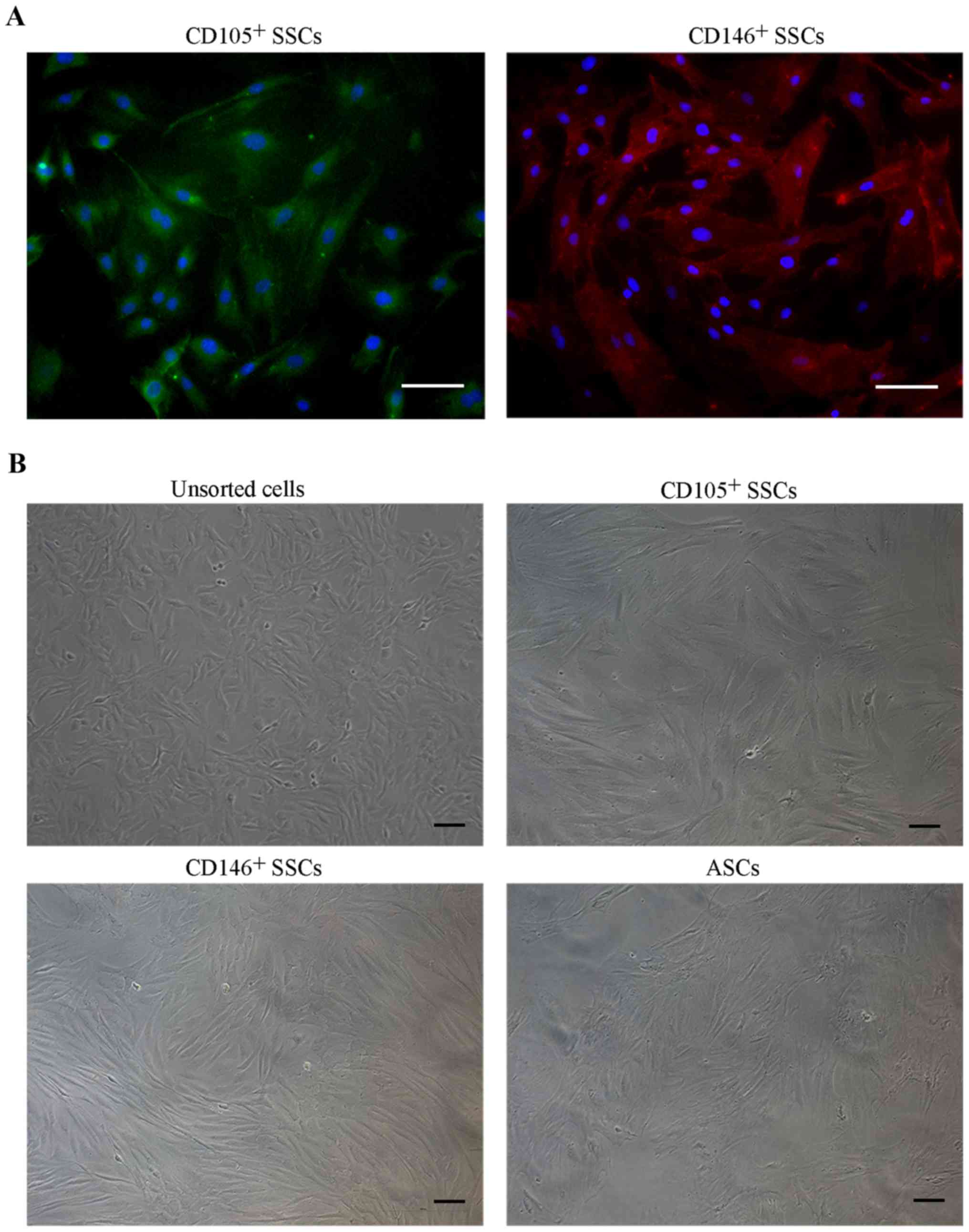
Isolation and cell morphology of
CD146+ SSCs and CD105+ SSCs
We then isolated CD146+ SSCs and
CD105+ SSCs using MACS method. Immunofluorescence
staining of the isolated cells confirmed that the CD146+
SSCs and CD105+ SSCs were successfully isolated and
cultured respectively (Fig. 2A).
For cell morphology (Fig. 2B),
unsorted growth plate chondrocytes generally showed heterogeneous
populations containing triangle and fibroblast-like cells. Isolated
CD146+ SSCs and CD105+ SSCs cells were more
fibroblast-like. Obvious differences in morphology were noted
between unsorted and MACS-sorted CD146+ and
CD105+ subpopulations.
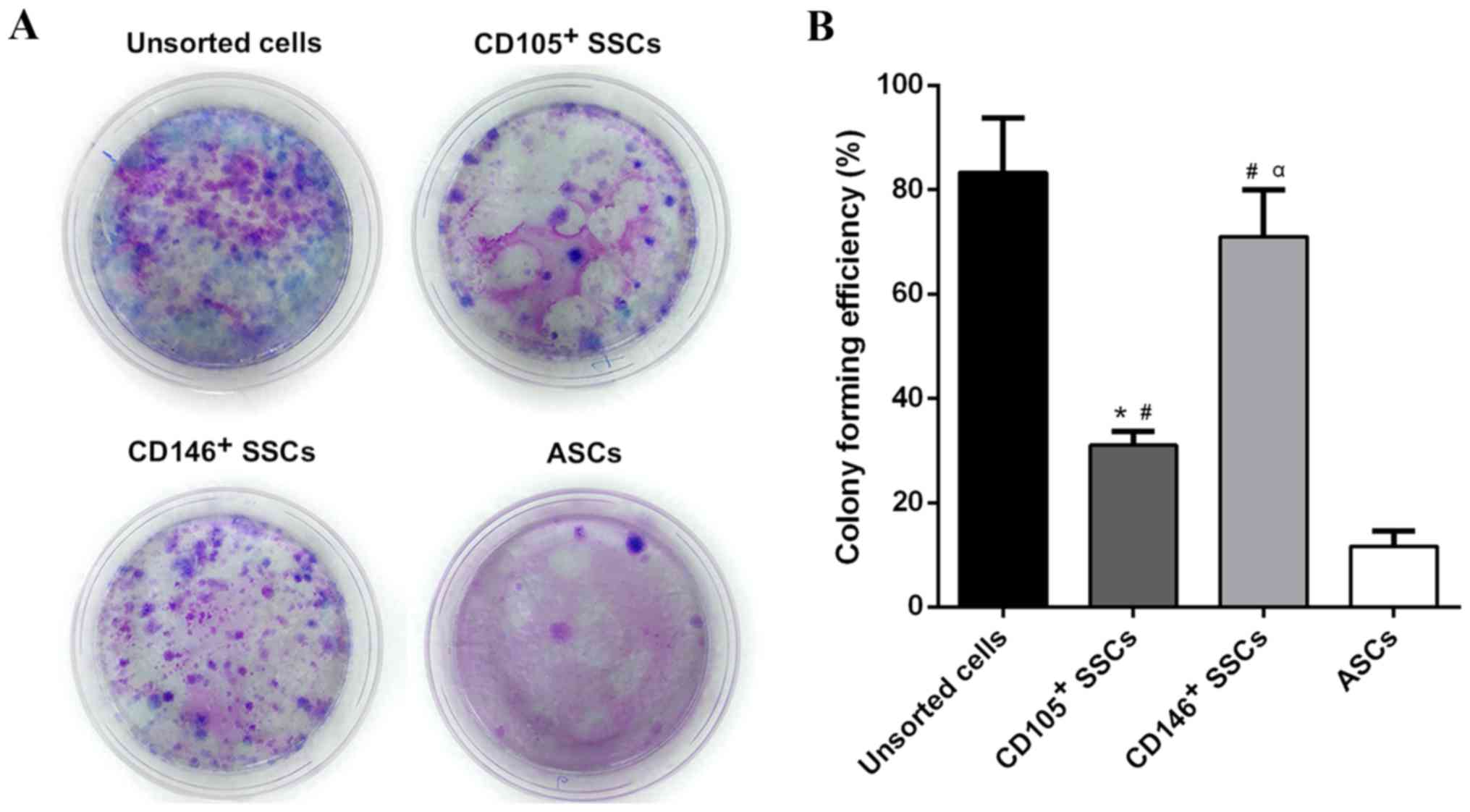
The CFE of CD146+ SSCs and
CD105+ SSCs compared with unsorted cells and ASCs
After 14 days of incubation, colony-forming unites
were observed in all four groups. The unsorted growth plate
chondrocytes yielded the highest number of colonies followed by
CD146+ SSCs and CD105+ SSCs. The CFE of
CD146+ SSCs was 2.2 fold stronger than CD105+
SSCs. The ASCs showed a much weaker CFE compared with the unsorted
growth plate chondrocytes, CD105+ SSCs and
CD146+ SSCs (Fig.
3).
Adipogenic differentiation potential
of CD146+ SSCs and CD105+ SSCs compared with
unsorted cells and ASCs
The adipogenic differentiation capacity of
CD146+ SSCs and CD105+ SSCs was measured and
compared with unsorted cells and ASCs. After 21 days of adipogenic
induction, a large amount of oil-red positive lipid droplets were
found in unsorted cells and ASCs, while little was detected in
CD105+ SSCs or CD146+ SSCs (Fig. 4A and B).
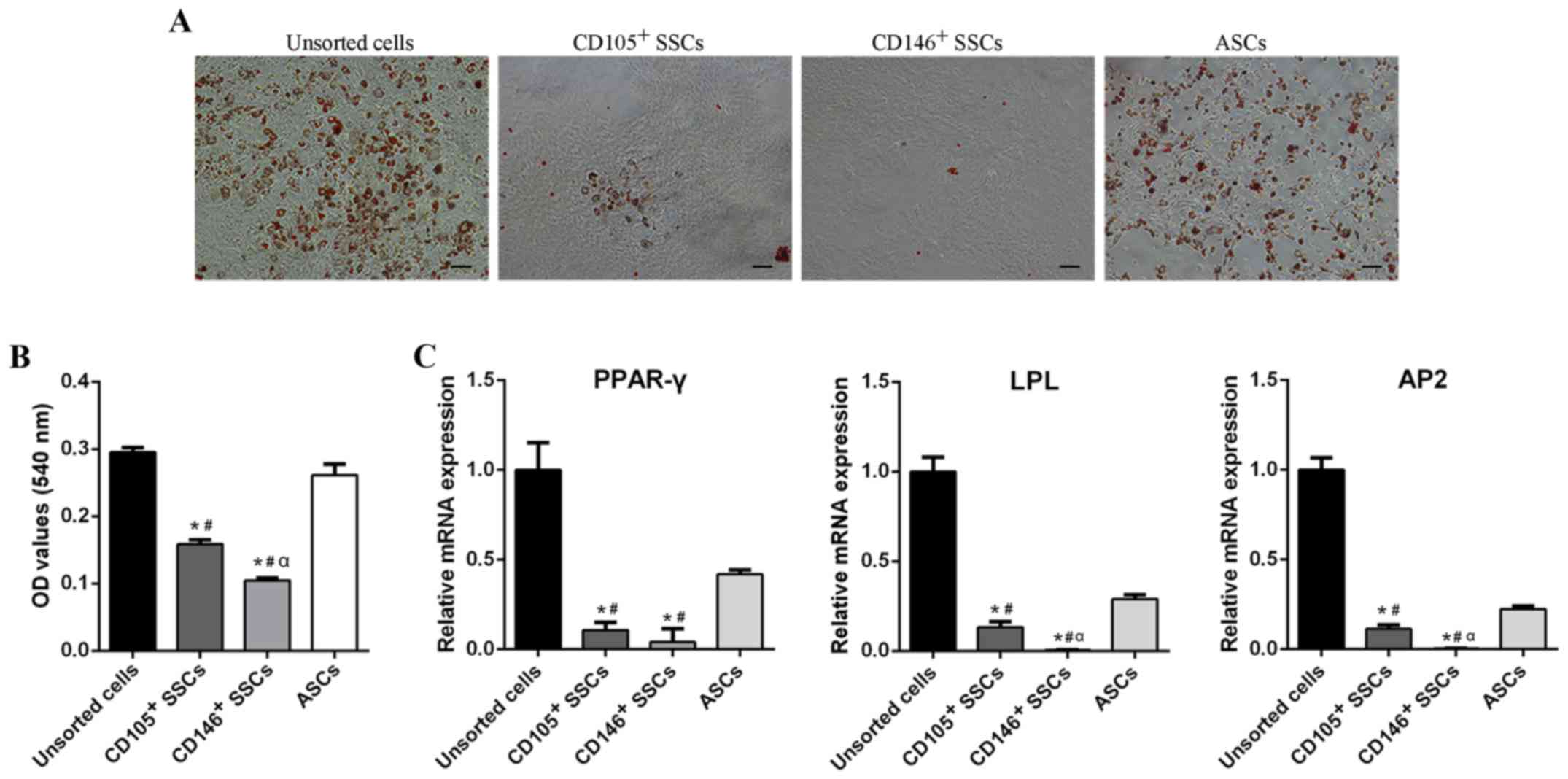 | Figure 4.Comparison of adipogenic
differentiation potential. (A) Oil Red O staining of unsorted
cells, CD105+ SSCs, CD146+ SSCs and ASCs.
Scar bar, 200 µm. (B) Quantitative measurement of lipid droplets in
all four groups. (C) The mRNA levels of PPAR-γ, LPL and AP2 were
detected using reverse transcription-quantitative polymerase chain
reaction. All data are from 3 independent experiments and are
presented as means ± SD. *P<0.05 vs. unsorted cells;
#P<0.05 vs. ASCs; αP<0.05 vs.
CD105+ SSCs. PPAR-γ, peroxisome proliferators-activated
receptor-γ; LPL, lipoprteinlipase; AP2, adipocyte fatty
acid-binding protein 2; ASCs, adipose-derived stem cells; SSCs,
skeletal stem cells; CD, cluster of differentiation. |
RT-qPCR showed similar results. After 2 week of
adipogenic induction, CD105+ and CD146+
enriched populations exhibited decreased peroxisome
proliferators-activated receptor-γ (PPAR-γ), lipoprteinlipase (LPL)
and adipocyte fatty acid-binding protein 2 (AP2) expression
compared with unsorted cells and ASCs. The PPAR-γ expression of
unsorted cells was 9-fold over the CD105+ SSCs, 24-fold
over the CD146+ SSCs, and 2-fold over ASCs. Similar
trends were also observed in the LPL and AP2 expression (Fig. 4C). These results suggested that
CD105+ SSCs and CD146+ SSCs could hardly
differentiate into adipocytes.
Osteogenic differentiation potential
of CD146+ SSCs and CD105+ SSCs compared with
unsorted cells and ASCs
To assess the osteogenic differentiation potential
of CD146+ SSCs and CD105+ SSCs, cells were
cultured in osteogenic medium for 21 days. All four groups showed
formation of red calcium deposits. The CD146+ SSCs and
CD105+ SSCs exhibited similar amounts of
calcium-containing mineralized nodules compared with unsorted cells
and ASCs (Fig. 5A).
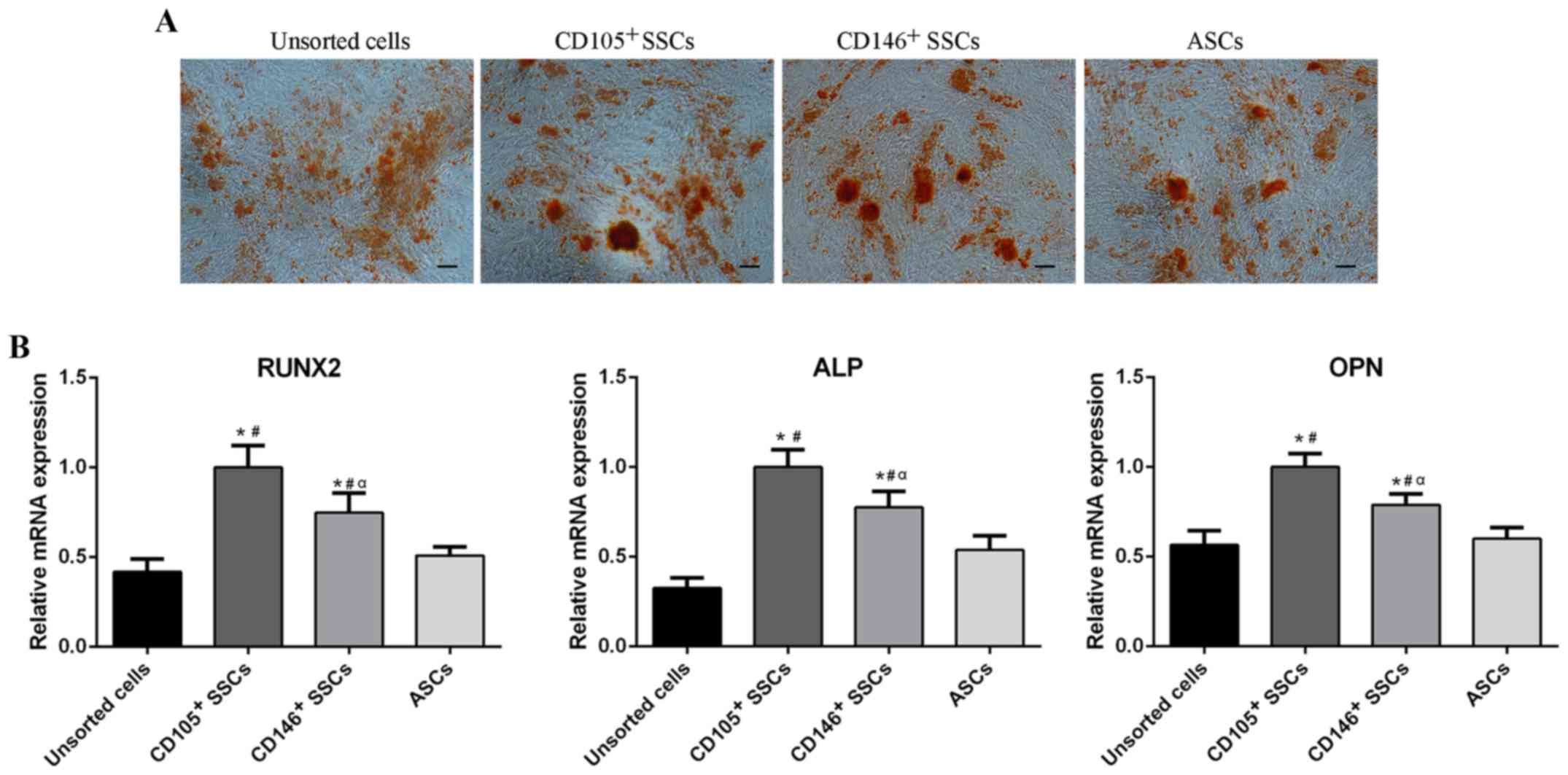 | Figure 5.Comparison of osteogenic
differentiation potential. (A) Alizarin red staining of unsorted
cells, CD105+ SSCs, CD146+ SSCs and ASCs.
Scar bar, 100 µm. (B) The mRNA levels of Runx2, ALP and OPN were
detected using reverse transcription-quantitative polymerase chain
reaction. All data are from 3 independent experiments and are
presented as means ± SD. *P<0.05 vs. unsorted cells;
#P<0.05 vs. ASCs, αP<0.05 vs.
CD105+ SSCs. RUNX2, runt-related transcription factor 2;
ALP, alkaline phosphatase; OPN, osteopontin; ASCs, adipose-derived
stem cells; SSCs, skeletal stem cells; CD, cluster of
differentiation. |
Total RNA was extracted from the cells respectively
after cultivation in osteogenic medium for 2 weeks. RT-qPCR was
then conducted to assess the expression of osteogenic related genes
including runt-related transcription factor 2 (RUNX2), alkaline
phosphatase (ALP) and osteopontin (OPN). After 2 weeks of
osteogenic induction, the CD105+ SSCs showed higher
expression of RUNX2, ALP and OPN than the CD146+ SSCs.
The RUNX2, ALP and OPN levels in CD105+ SSCs and
CD146+ SSCs were higher than unsorted cells and ASCs
(Fig. 5B).
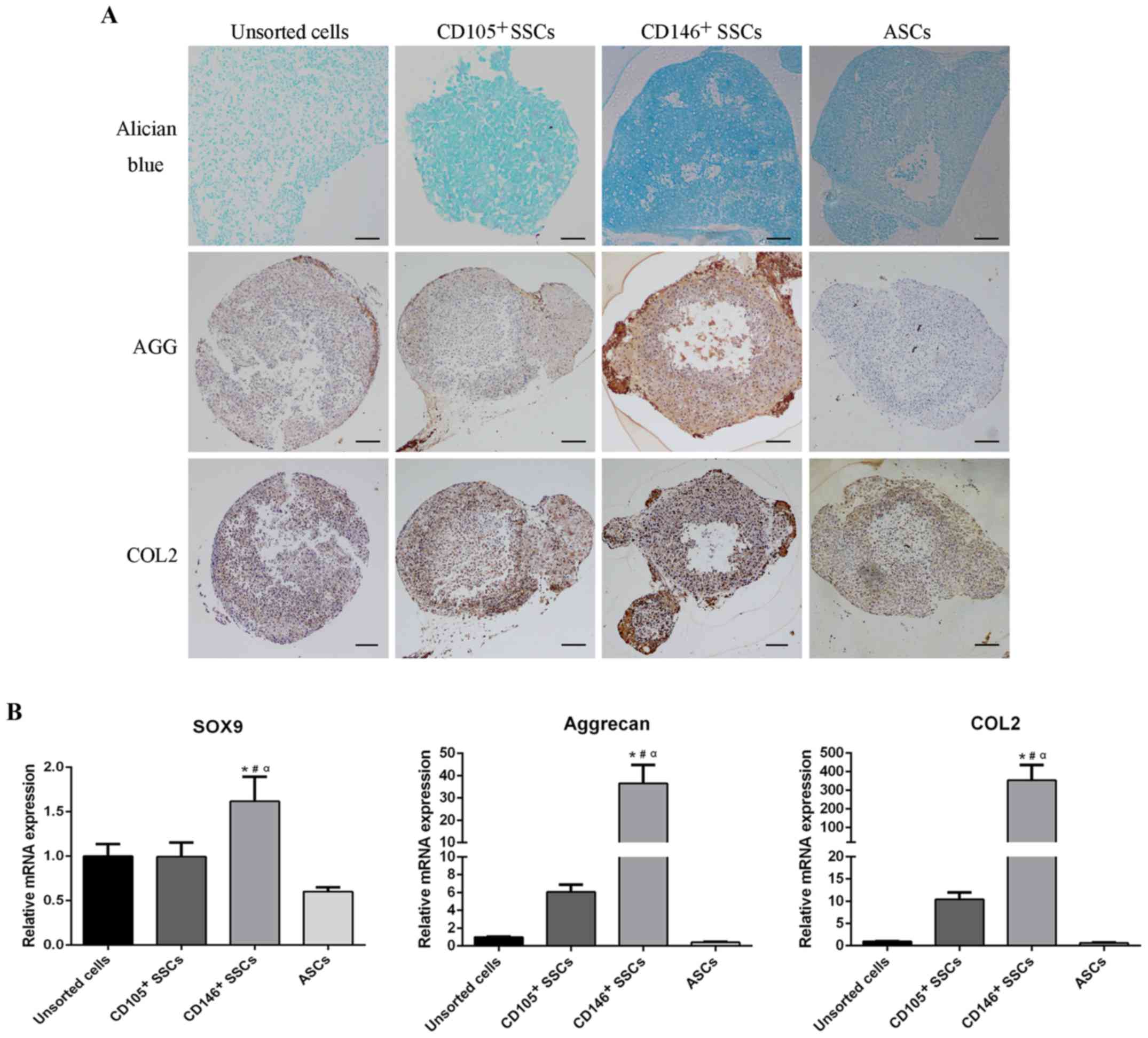
Chondrogenic differentiation potential
of CD146+ and CD105+ SSCs compared with
unsorted cells and ASCs
To assess the chondrogenic differentiation potential
of CD146+ SSCs and CD105+ SSCs, micromass
pellets were cultured in chondrogenic medium for 21 days. Alician
blue staining was performed to characterize the proteoglycan
formation. Differentiated chondrocytes surface markers, aggrecan
and type II collagen, were assessed using IHC. Alician blue
staining revealed a higher content of proteoglycan in
CD146+ pellets. Consistently, CD146+ pellets
showed much higher contents of aggrecan and type II collagen
compared with pellets in other three groups (Fig. 6A).
After cultivation in chondrogenic medium for 2
weeks, total RNA was extracted and subjected to RT-qPCR. The mRNA
levels of chondrogenic markers including SRY-box containing gene 9
(SOX9), type II collagen and aggrecan were evaluated. As shown in
Fig. 6B, CD146+ SSCs
exhibited higher mRNA level of SOX9 compaired with unsorted cells,
CD105+ SSCs and ASCs. Furthermore, extraordinarily high
levels of aggrecan and type II collagen were observed in the
CD146+ SSCs. The aggrecan expression level in
CD146+ SSCs was 36-fold over unsorted cells, 6-fold over
CD105+ SSCs, and 91.4-fold over ASCs. Consistently, the
expression level of type II collegen in CD146+ SSCs was
353-fold over unsorted cells, 34-fold over CD105+ SSCs
and 548-fold over ASCs. These results suggested that the
CD146+ SSCs exhibited a strong and steady chondrogenic
differentiation capacity in vitro.
Discussion
The mesenchymal stem cells represent a powerful tool
for cartilage tissue regeneration, but their utility is limited by
low chondrogenic potential, vascularization and mineralization
(29). So it is important to
isolate a stem cell which could differentiate strictly along
chondrogenic lineage. The regenerative capacity of bone indicates
the presence of skeletal stem cell in bone. However, while this
regenerative ability has long been recognized, the identity of the
responsible cell population in vivo has only recently been
confirmed. Recent researches demonstrated the existence of SSCs at
the end of long bones. SSCs could self-renew and generate bone and
cartilage, but not adipocytes (12). Like hematopoietic stem cells, SSCs
are diverse, with distinct cell-surface marker profiles and
distinct fates (14).
Theoretically, SSCs with appropriate cell surface markers could
offer an ideal cell source for cartilage tissue engineering. In the
present study, we successfully isolated SSCs from growth plate
using cell surface markers CD105 and CD146. These subpopulations of
SSCs could self-renew and differentiate into osteoblasts and
chondrocytes but not adipocytes.
The growth plate contains three zones that include
chondrocytes at different stages of differentiation (30–32).
The zone closest to the epiphysis is termed the resting zone. The
resting zone is thought to contain chondrocytes that serve as
progenitor cells, which can generate new clones of rapidly
proliferating chondrocytes (9,33).
In the present study, flow cytometry showed that the growth plate
chondrocytes are positive for CD105 and CD146. IHC revealed that
the CD105+ SSCs mainly located in the resting zone and
hypertrophic zone while the CD146+ SSCs mainly located
in the resting zone and proliferating zone. Many researches
demonstrated the existence of CD146+ subpopulation in
osteoarthritis cartilage chondrocytes and indicated that CD146 is a
chondroprogenitor-associated marker (34,35).
CD146 was reported to be involved in cell-cell, cell-matrix
interactions and cell migration (36–38).
There are evidences identifying that CD146+
subpopulation has a high migration abilitiy and is responsible for
tumor metastasis as well as tissue repair (39,40).
A previous study suggested that CD146 was also expressed in bone
marrow derived mesenchymal stem cells and had been used for the
isolation of this cell population (41). Thus we postulated that SSCs mainly
located in the resting zone of the growth plate and could reproduce
and migrate to the place where needed.
SSCs were thought to contribute to the postnatal
growth of the long bone and bone fracture repair (42–44).
SSCs contain different types of progenitors, which are responsible
for bone and cartilage formation, but not for adipose tissue or
muscle tissue formation. The CD105 was demonstrated as a marker of
SSCs (12). In this study, we
found that the CD146+ cells exhibited high osteogenic,
chondrogenic differentiation capacity and extremely low adipogeneic
capacity in vitro, suggesting that CD146+ cells
are also a subset of SSCs. Furthermore, results of RT-qPCR showed
that the expression of chondrogenic related genes, such as aggrecan
and type II collagen, in the CD146+ SSCs were
extraordinarily higher than CD105+ SSCs and ASCs. These
results were corroborated by IHC. Taken together, our data
implicated that the CD146+ subpopulation represents a
subset of SSCs which is much differentiated and prone to
differentiate into chondrocytes. The CD146+
subpopulation is a chondrogenic lineage restricted stem cells, or
precartilaginous stem cell. It has the ability of efficiently
differentiating into chondrocytes with extensive extracellular
matrix (ECM) formation.
Both CD105+ and CD146+ SSCs
were discussed in the present study. But there are also evidences
showing that CD105+ subpopulation is responsible for
endochondral ossification and has lost the chondrogenesis potential
(6,45). Consistently, we found that
CD105+ subpopulation expressed higher levels of
osteogenetic genes. Taking into account that CD146+ MSCs
derived from bone marrow were prone to differentiate into
chondrocytes (46) and that
CD146+ chondroprogenitor cells with multi-lineage
differentiation abilities were found in articular cartilage
(35), CD146 might be regarded as
a marker for chondroprogenitor subpopulation. Our data also
revealed that CD146+ SSCs isolated from the growth plate
exhibited superior chondrogenic differentiation capacity compared
CD105+ SSCs. Taken together, these data suggest that
CD105+ subpopulations and CD146+
subpopulations may have different cell fate and that
CD105+ SSCs may be used to enhance endochondral
ossification while CD146+ SSCs are more appropriate as a
stem cell source for cartilage repair.
The proliferative capacity of stem cells is
important with regard to their application in cell therapy. In the
present study, the proliferative capacity of the CD105+
and the CD146+ subpopulations were compared with that of
unsorted cells and ASCs using CFE assay. Of note, it was identified
that the unsorted growth plate chondrocytes exhibited the highest
CFE in vitro. Chan et al (12) suggested that the SSCs are diverse,
similar to the diverse hematopoietic progenitor cells that generate
various differentiated blood cells. This may imply that the
unsorted growth plate chondrocytes contain subpopulations which are
more primitive and have higher capacity of self-renewal. However,
the oil-red staining results showed that they were prone to
differentiate into adipocytes. The results of RT-qPCR identified
that the unsorted growth plate chondrocytes expressed much higher
level of adipogenic differentiation related genes, including
PPAR-γ, LPL and AP2. PPAR-γ is a key transcriptional regulator of
adipogenesis. Wang et al (47) found that PPAR-γ is expressed in
growth plate chondrocytes and PPAR-γ is able to promote adipogenic
differentiation in growth plate chondrocytes, while negatively
regulate chondrogenic differentiation and terminal
differentiation.
In the present study, we isolated purified
subpopulations of SSCs using MACS method. We found that both
CD105+ and CD146+ subpopulations had a higher
CFE than ASCs. Moreover, compared with the CD105+
subpopulation, the CFE of the CD146+ subpopulation was
much higher. Consistently, CD146+ subpopulations mainly
located in the resting zone and proliferative zone of the growth
plate, which suggested that CD146+ subpopulations are
progenitor cells which can generate new clones of rapidly
proliferating chondrocytes.
ASCs are attractive stem cell source for tissue
engineering (48–50). These cells can be obtained by
simple liposuction and had ability of multi-lineage
differentiation. Under appropriate culture conditions, they can
differentiate along osteogenic lineage (50–52),
chondrogenic lineage (53), and
adipogenic lineage. Our results showed that CD146+ SSCs
exhibited higher colony forming capacity compared compared with
ASCs. Most importantly, the CD146+ SSCs showed a steady
and high chondrogenic related gene expression and ECM formation
in vitro after a long period of cultivation. These results
also implied that compared with ASCs the CD146+ SSCs
could be a much more appropriate stem cell source for cartilage
tissue engineering.
In summary, we identified the existence of SSCs in
the growth plate and the CD105+ and the
CD146+ subpopulations represented subsets of SSCs which
could generate chondrocytes and osteocytes, but not adipocytes.
Compared with CD105+ subpopulations and ASCs, the
CD146+ subpopulation exhibited higher colony forming
capacity and continuous high chondrogenic differentiation capacity
in vitro. Thus we propose that CD146+
subpopulation is chondrogenic lineage restricted SSCs and it could
provide cell candidates for cell-based cartilage regeneration.
Acknowledgements
This work was supported by grants from the National
Natural Science Foundation of China (no. 81371915 and
81572094).
References
|
1
|
Blunk T, Sieminski AL, Gooch KJ, Courter
DL, Hollander AP, Nahir AM, Langer R, Vunjak-Novakovic G and Freed
LE: Differential effects of growth factors on tissue-engineered
cartilage. Tissue Eng. 8:73–84. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishimura D, Yamamoto N, Tajima K, Ohno A,
Yamamoto Y, Washimi O and Yamada H: Differentiation of
adipose-derived stromal vascular fraction culture cells into
chondrocytes using the method of cell sorting with a mesenchymal
stem cell marker. Tohoku J Exp Med. 216:149–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jo CH, Lee YG, Shin WH, Kim H, Chai JW,
Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, et al: Intra-articular
injection of mesenchymal stem cells for the treatment of
osteoarthritis of the knee: A proof-of-concept clinical trial. Stem
cells. 32:1254–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang T, Liu W, Lv X, Sun H, Zhang L, Liu
Y, Zhang WJ, Cao Y and Zhou G: Potent in vitro chondrogenesis of
CD105 enriched human adipose-derived stem cells. Biomaterials.
31:3564–3571. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi S, Takebe T, Inui M, Iwai S, Kan
H, Zheng YW, Maegawa J and Taniguchi H: Reconstruction of human
elastic cartilage by a CD44+ CD90+ stem cell
in the ear perichondrium. Proc Natl Acad Sci USA. 108:pp.
14479–14484. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crisan M, Yap S, Casteilla L, Chen CW,
Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al: A
perivascular origin for mesenchymal stem cells in multiple human
organs. Cell Stem Cell. 3:301–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhumiratana S, Eton RE, Oungoulian SR, Wan
LQ, Ateshian GA and Vunjak-Novakovic G: Large, stratified, and
mechanically functional human cartilage grown in vitro by
mesenchymal condensation. Proc Natl Acad Sci USA. 111:pp.
6940–6945. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oldershaw RA: Cell sources for the
regeneration of articular cartilage: The past, the horizon and the
future. Int J Exp Pathol. 93:389–400. 2012.PubMed/NCBI
|
|
9
|
Belluoccio D, Bernardo BC, Rowley L and
Bateman JF: A microarray approach for comparative expression
profiling of the discrete maturation zones of mouse growth plate
cartilage. Biochim Biophys Acta. 1779:330–340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pichler K, Schmidt B, Fischerauer EE,
Rinner B, Dohr G, Leithner A and Weinberg AM: Behaviour of human
physeal chondro-progenitorcells in early growth plate injury
response in vitro. Int Orthop. 36:1961–1966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gothard D, Cheung K, Kanczler JM, Wilson
DI and Oreffo RO: Regionally-derived cell populations and skeletal
stem cells from human foetal femora exhibit specific osteochondral
and multi-lineage differentiation capacity in vitro and ex vivo.
Stem Cell Res Ther. 6:2512015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan CK, Seo EY, Chen JY, Lo D, McArdle A,
Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, et al:
Identification and specification of the mouse skeletal stem cell.
Cell. 160:285–298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Worthley DL, Churchill M, Compton JT,
Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y,
et al: Gremlin 1 identifies a skeletal stem cell with bone,
cartilage and reticular stromal potential. Cell. 160:269–284. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan CK, Lindau P, Jiang W, Chen JY, Zhang
LF, Chen CC, Seita J, Sahoo D, Kim JB, Lee A, et al: Clonal
precursor of bone, cartilage, and hematopoietic niche stromal
cells. Proc Natl Acad Sci USA. 110:pp. 12643–12648. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiba H, Ishii G, Ito TK, Aoyagi K, Sasaki
H, Nagai K and Ochiai A: CD105-positive cells in pulmonary arterial
blood of adult human lung cancer patients include mesenchymal
progenitors. Stem cells. 26:2523–2530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salamon A, Jonitz-Heincke A, Adam S,
Rychly J, Müller-Hilke B, Bader R, Lochner K and Peters K:
Articular cartilage-derived cells hold a strong osteogenic
differentiation potential in comparison to mesenchymal stem cells
in vitro. Exp Cell Res. 319:2856–2865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amiri F, Halabian R, Harati M Dehgan,
Bahadori M, Mehdipour A, Roushandeh A Mohammadi and Roudkenar M
Habibi: Positive selection of Wharton's jelly-derived CD105(+)
cells by MACS technique and their subsequent cultivation under
suspension culture condition: A simple, versatile culturing method
to enhance the multipotentiality of mesenchymal stem cells.
Hematology. 20:208–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Odabas S, Sayar F, Güven G,
Yanikkaya-Demirel G and Pişkin E: Separation of mesenchymal stem
cells with magnetic nanosorbents carrying CD105 and CD73 antibodies
in flow-through and batch systems. J Chromatogr B Analyt Technol
Biomed Life Sci. 861:74–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi J, Chen A, You H, Li K, Zhang D and Guo
F: Proliferation and chondrogenic differentiation of CD105-positive
enriched rat synovium-derived mesenchymal stem cells in
three-dimensional porous scaffolds. Biomed Mater. 6:0150062011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan CK, Chen CC, Luppen CA, Kim JB,
DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL and Weissman IL:
Endochondral ossification is required for haematopoietic stem-cell
niche formation. Nature. 457:490–494. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gothard D, Greenhough J, Ralph E and
Oreffo RO: Prospective isolation of human bone marrow stromal cell
subsets: A comparative study between Stro-1-, CD146- and
CD105-enriched populations. J Tissue Eng. 5:20417314145517632014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bakopoulou A, Leyhausen G, Volk J, Koidis
P and Geurtsen W: Comparative characterization of
STRO-1(neg)/CD146(pos) and STRO-1(pos)/CD146(pos) apical papilla
stem cells enriched with flow cytometry. Arch Oral Biol.
58:1556–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Middleton J, Americh L, Gayon R, Julien D,
Mansat M, Mansat P, Anract P, Cantagrel A, Cattan P, Reimund JM, et
al: A comparative study of endothelial cell markers expressed in
chronically inflamed human tissues: MECA-79, Duffy antigen receptor
for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146.
J Pathol. 206:260–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schugar RC, Chirieleison SM, Wescoe KE,
Schmidt BT, Askew Y, Nance JJ, Evron JM, Peault B and Deasy BM:
High harvest yield, high expansion, and phenotype stability of
CD146 mesenchymal stromal cells from whole primitive human
umbilical cord tissue. J Biomed Biotechnol. 2009:7895262009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsang WP, Shu Y, Kwok PL, Zhang F, Lee KK,
Tang MK, Li G, Chan KM, Chan WY and Wan C: CD146+ human
umbilical cord perivascular cells maintain stemness under hypoxia
and as a cell source for skeletal regeneration. PLoS One.
8:e761532013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu CC, Liu FL, Sytwu HK, Tsai CY and Chang
DM: CD146+ mesenchymal stem cells display greater
therapeutic potential than CD146-cells for treating
collagen-induced arthritis in mice. Stem Cell Res Ther. 7:232016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang Y, Cai Y, Zhang W, Yin Z, Hu C, Tong
T, Lu P, Zhang S, Neculai D, Tuan RS and Ouyang HW: Human
cartilage-derived progenitor cells from committed chondrocytes for
efficient cartilage repair and regeneration. Stem Cells Transl Med.
5:733–744. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong HL, Siu WS, Fung CH, Zhang C, Shum
WT, Zhou XL, Lau CB, Zhang JF, Leung PC, Fu WM and Ko CH:
Characteristics of stem cells derived from rat fascia: In vitro
proliferative and multilineage potential assessment. Mol Med Rep.
11:1982–1990. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi Y, Du Y, Li W, Dai X, Zhao T and Yan W:
Cartilage repair using mesenchymal stem cell (MSC) sheet and
MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg
Sports Traumatol Arthrosc. 22:1424–1433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Villemure I and Stokes IA: Growth plate
mechanics and mechanobiology. A survey of present understanding. J
Biomech. 42:1793–1803. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lui JC, Nilsson O and Baron J: Recent
research on the growth plate: Recent insights into the regulation
of the growth plate. J Mol Endocrinol. 53:T1–T9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chagin AS and Kronenberg HM: Role of
G-proteins in the differentiation of epiphyseal chondrocytes. J Mol
Endocrinol. 53:R39–R45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Belluoccio D, Etich J, Rosenbaum S, Frie
C, Grskovic I, Stermann J, Ehlen H, Vogel S, Zaucke F, von der Mark
K, et al: Sorting of growth plate chondrocytes allows the isolation
and characterization of cells of a defined differentiation status.
J Bone Miner Res. 25:1267–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu J, Wang W, Kapila Y, Lotz J and Kapila
S: Multiple differentiation capacity of
STRO-1+/CD146+ PDL mesenchymal progenitor
cells. Stem Cells Dev. 18:487–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Su X, Zuo W, Wu Z, Chen J, Wu N, Ma P, Xia
Z, Jiang C, Ye Z, Liu S, et al: CD146 as a new marker for an
increased chondroprogenitor cell sub-population in the later stages
of osteoarthritis. J Orthop Res. 33:84–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Z and Yan X: CD146, a
multi-functional molecule beyond adhesion. Cancer Lett.
330:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guezguez B, Vigneron P, Lamerant N, Kieda
C, Jaffredo T and Dunon D: Dual role of melanoma cell adhesion
molecule (MCAM)/CD146 in lymphocyte endothelium interaction:
MCAM/CD146 promotes rolling via microvilli induction in lymphocyte
and is an endothelial adhesion receptor. J Immunol. 179:6673–6685.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin HJ, Kwon JH, Kim M, Bae YK, Choi SJ,
Oh W, Yang YS and Jeon HB: Downregulation of melanoma cell adhesion
molecule (MCAM/CD146) accelerates cellular senescence in human
umbilical cord blood-derived mesenchymal stem cells. Stem Cells
Transl Med. 5:427–439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Qian YN, Luo YT, Duan HX, Feng LQ, Bi Q,
Wang YJ and Yan XY: Adhesion molecule CD146 and its soluble form
correlate well with carotid atherosclerosis and plaque instability.
CNS Neurosci Ther. 20:438–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bu P, Zhuang J, Feng J, Yang D, Shen X and
Yan X: Visualization of CD146 dimerization and its regulation in
living cells. Biochim Biophys Acta. 1773:513–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sorrentino A, Ferracin M, Castelli G,
Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C
and Valtieri M: Isolation and characterization of CD146+
multipotent mesenchymal stromal cells. Exp Hematol. 36:1035–1046.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Balakumaran A, Mishra PJ, Pawelczyk E,
Yoshizawa S, Sworder BJ, Cherman N, Kuznetsov SA, Bianco P, Giri N,
Savage SA, et al: Bone marrow skeletal stem/progenitor cell defects
in dyskeratosis congenita and telomere biology disorders. Blood.
125:793–802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dawson JI, Kanczler J, Tare R, Kassem M
and Oreffo RO: Concise review: Bridging the gap: Bone regeneration
using skeletal stem cell-based strategies-where are we now? Stem
cells. 32:35–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Agarwal S, Loder SJ, Sorkin M, Li S,
Shrestha S, Zhao B, Mishina Y, James AW and Levi B: Analysis of
bone-cartilage-stromal progenitor populations in trauma induced and
genetic models of heterotopic ossification. Stem cells.
34:1692–1701. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aslan H, Zilberman Y, Kandel L, Liebergall
M, Oskouian RJ, Gazit D and Gazit Z: Osteogenic differentiation of
noncultured immunoisolated bone marrow-derived CD105+
cells. Stem cells. 24:1728–1737. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Aicher WK, Bühring HJ, Hart M, Rolauffs B,
Badke A and Klein G: Regeneration of cartilage and bone by defined
subsets of mesenchymal stromal cells-potential and pitfalls. Adv
Drug Deliv Rev. 63:342–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang L, Shao YY and Ballock RT: Peroxisome
proliferator-activated receptor-gamma promotes adipogenic changes
in growth plate chondrocytes in vitro. PPAR Res. 2006:672972006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferrer-Lorente R, Bejar MT, Tous M,
Vilahur G and Badimon L: Systems biology approach to identify
alterations in the stem cell reservoir of subcutaneous adipose
tissue in a rat model of diabetes: Effects on differentiation
potential and function. Diabetologia. 57:246–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ma T, Liu H, Chen W, Xia X, Bai X, Liang
L, Zhang Y and Liang T: Implanted adipose-derived stem cells
attenuate small-for-size liver graft injury by secretion of VEGF in
rats. Am J Transplant. 12:620–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Erdman CP, Dosier CR, Olivares-Navarrete
R, Baile C, Guldberg RE, Schwartz Z and Boyan BD: Effects of
resveratrol on enrichment of adipose-derived stem cells and their
differentiation to osteoblasts in two-and three-dimensional
cultures. J Tissue Eng Regen Med. 6 Suppl 3:S34–S46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ye Y, Du Y, Guo F, Gong C, Yang K and Qin
L: Comparative study of the osteogenic differentiation capacity of
human bone marrow- and human adipose-derived stem cells under
cyclic tensile stretch using quantitative analysis. Int J Mol Med.
30:1327–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Quarto N, Senarath-Yapa K, Renda A and
Longaker MT: TWIST1 silencing enhances in vitro and in vivo
osteogenic differentiation of human adipose-derived stem cells by
triggering activation of BMP-ERK/FGF signaling and TAZ
upregulation. Stem cells. 33:833–847. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hamid AA, Idrus RB, Saim AB, Sathappan S
and Chua KH: Characterization of human adipose-derived stem cells
and expression of chondrogenic genes during induction of cartilage
differentiation. Clinics (Sao Paulo). 67:99–106. 2012. View Article : Google Scholar : PubMed/NCBI
|