Introduction
Osteoarthritis (OA) is the most common form of joint
disease in the world and constitutes a major cause of disability in
the aging population (1). The
disease was previously considered to be a typical non-inflammatory
arthropathy, but currently it is generally accepted that it is an
inflammatory disease (2). Previous
studies have reported that inflammation contributes to the symptoms
and the progression of OA (3,4).
Furthermore, certain researchers have observed that synovial
inflammation is present in the earlier phases of OA prior to
visible cartilage degeneration (5–7).
These studies suggest that disease-modifying interventions
targeting inflammatory processes may be effective for the
prevention and treatment of OA.
The mitogen-activated protein kinase (MAPK)
signaling pathway is present in all eukaryotes and has a major role
in various inflammatory diseases. The members of this signaling
pathway group include p38 MAPK, c-Jun NH2 terminal kinase (JNK) and
extracellular signal-regulated kinase. They are activated via
phosphorylation of specific tyrosine and threonine residues by
upstream factors. MAPKs regulate various physiological processes,
including cell proliferation, differentiation, apoptosis and stress
responses, and p38 and JNK are associated with the regulation of
inflammatory and immune responses (8–11).
The activation of MAPK is involved in the expression of several
inflammatory genes, such as tumor necrosis factor (TNF),
interleukin (IL)-1, IL-6, cyclooxygenase-2 (COX-2) and certain
enzymes such as inducible nitric oxide synthase and matrix
metalloproteinase (MMP)-13 (12–15).
Dual specificity phosphatase 1 (DUSP1; also termed
MAPK phosphatase 1) is one of an 11-member family that inhibits the
activity of MAPKs by dephosphorylating tyrosine and threonine
residues at the MAPK Thr-Xaa-Tyr activation motif. It is a
particularly effective inhibitor of JNK and p38 MAPK signaling
pathways (16). DUSP1 is a nuclear
phosphatase widely expressed in various tissues, and its expression
is regulated by a wide variety of different stimuli, including
cellular stress, cytokines, lipopolysaccharide and glucocorticoids
(17–20). Several studies have demonstrated
that DUSP1 is an important negative regulator of inflammatory
responses (21–26), and the induction of DUSP1 gene
expression is potentially a novel anti-inflammatory strategy.
However, to date, the role of DUSP1 in human OA synovial
inflammation and its molecular mechanisms remain unclear.
The current study investigated the expression of
DUSP1 in cultured human normal fibroblast-like synoviocytes (FLSs)
and OA FLSs, and the effect of DUSP1 on the expression of
OA-associated mediators, such as MMP-13 and COX-2, which occurs
through a mechanism involving the inhibition of the p38 MAPK/JNK
signaling pathway. Dexamethasone was used induce the expression of
DUSP1 in OA FLSs, which partially demonstrated the
anti-inflammatory mechanism of glucocorticoids in OA. The results
demonstrated the anti-inflammatory and anti-catabolic actions of
DUSP1 on OA FLSs, and DUSP1 was a potential target of treatment in
OA.
Materials and methods
Reagents
Reagents were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany) unless otherwise stated. Dexamethasone
was purchased from Shanghai GeneChem Co, Ltd. (cat. no.
A601187-0005; Shanghai, China). Antibodies against DUSP1 (cat. no.
AF1343a), COX-2 (cat. no. AJ1195b), MMP-13 (cat. no. AP13706c),
phosphorylated (p-) JNK (cat. no. AB208035), JNK (cat. no. AB4821)
and p38 MAPK (cat. no. AJ1201a) were purchased from Abgent, Inc.
(San Diego, CA, USA). Antibody against p-p38 MAPK was obtained from
Santa Cruz Biotechnology, Inc. (cat. no. sc101759; Dallas, TX,
USA). Antibodies against β-tubulin (cat. no. ab6406) and GAPDH
(cat. no. ab8245) were purchased from Abcam (Cambridge, MA,
USA).
Specimen selection and cell
culture
Human OA and normal synovial tissue specimens were
obtained from patients with OA requiring joint replacement surgery
and trauma patients undergoing post-traumatic amputation from June
2015 to June 2016, respectively. In the OA patients, two patients
were male and three patients were female, the age range was 57–76
years and the mean age ± standard error of the mean (SEM) was
65.2±3.2 years. All three trauma patients were male, the age range
was 22–44 years and the mean age (± SEM) was 31.0±5.9 years.
Informed consent was obtained from patients for the use of their
tissues for research purposes. The present study was approved by
the Ethics Committee of Tangdu Hospital, Fourth Military Medical
University, (Xi'an, China). Tissues were carefully minced and
digested with 0.2% collagenase I in Dulbecco's modified Eagle's
medium (DMEM; (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) for 4–6 h at 37°C, filtered through a 200-mesh sieve, and
finally cultured in DMEM supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences), 100 units penicillin and
100 µg/ml streptomycin. The cells were cultured up to 90%
confluence and then split in a 1/3 ratio up to passage 3–6. FLSs at
these passages were identified by flow cytometry as described
previously (27) and used for
subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc, Waltham, MA, USA)
according to the manufacturer's instructions. The cDNA was
synthesized using a Reverse Transcription kit (Thermo Fisher
Scientific, Inc.) and then was diluted 1:4 with RNase-free water.
cDNA (1 µl) was subjected to PCR analysis using SYBR Green Master
Mix (Invitrogen; Thermo Fisher Scientific, Inc.) with a Motor-Gene
Q RT-PCR instrument (Qiagen GmbH, Hilden, Germany). The primers
were purchased from Sangon Biotech Co, Ltd. (Shanghai, China) and
the sequences were as follows: Human DUSP1,
5′-AGTACCCCACTCTACGATCAGG-3′ (forward) and
5′-GAAGCGTGATACGCACTGC-3′ (reverse); human MMP-13,
5′-ACTGAGAGGCTCCGAGAAATC-3′ (forward) and 5′-GAACCCCGCATCTTGGCTT-3′
(reverse); human COX-2, 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ (forward)
and 5′-AGTTCATCTCTGCCTGAGTATCTT-3′ (reverse); and β-actin,
5′-TAGTTGCGTTACACCCTTTCTTG-3′ (forward) and
5′-TCACCTTCACCGTTCCAGTTT-3′ (reverse). The conditions of PCR
cycling were as follows: Denaturation step at 95°C for 10 min, then
40 cycles at 95°C for 15 sec, 65°C for 10 sec and 72°C for 15 sec.
Relative expression levels of target genes were calculated
according to the 2−∆∆Cq method (28).
Western blot analysis
At the indicated time points, cultured FLSs were
lysed in radioimmunoprecipitation containing proteinase inhibitor
and phosphatase inhibitor and boiled. GAPDH and β-tubulin were used
as internal reference protein. The protein was quantified by the
bicinchoninic method. The extracted proteins (20 µg/well) were
loaded onto a 10% SDS-PAGE gel and electrophoresed for 2.5 h at 80
V, then transferred to polyvinylidene fluoride (PVDF) membrane.
PVDF membranes were blocked in 5% skim milk in 1X Tris-buffered
saline Tween (TBST) for 1 h at room temperature, washed with TBST,
and the membranes were incubated for 24 h at 4°C with primary
antibodies against DUSP1 (1:500), p38 (1:500), p-p38 (1:500), JNK
(1:500), p-JNK (1:500), COX-2 (1:500), MMP-13 (1:500), GAPDH
(1:3,000) or β-tubulin (1:3,000). Then, the PVDF membranes were
washed in TBST and incubated with horseradish peroxidase
(HRP)-labeled secondary antibodies for 1 h at 37°C. The protein
bands were detected using Immobilon Western Chemiluminescent HRP
Substrate (EMD Millipore, Billerica, MA, USA) according to the
manufacturer's instructions. Densitometry was performed using
ImageJ software (Version 1.43; National Institutes of Health,
Bethesda, USA).
Immunofluorescence staining
FLSs were washed with PBS, fixed with 4%
paraformaldehyde, permeabilized with 0.2% Triton X-100 for 30 min
at room temperature and then blocked with 5% bovine serum albumin
for 1 h at room temperature. The cells were then incubated with
DUSP1 polyclonal antibody (1:200) in 1% bovine serum albumin
(Thermo Fisher Scientific, Inc.) at 4°C overnight. The bound
antibodies were detected by the cy3-conjugated secondary antibodies
(1:3,000; cat. no. CW0159; CWBio, Inc, Beijing, China) for 2 h at
room temperature. Cell nuclei were stained with DAPI (50 µg/ml) for
15 min at room temperature. The images were observed by inverted
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Overexpression of DUSP1 in OA
FLSs
The coding sequence of human DUSP1 was amplified by
RT-PCR and ligated into the GV358
(Ubi-MCS-3FLAG-SV40-EGFP-IRES-puromycin) lentiviral vector
(Shanghai GeneChem Co, Ltd, Shanghai, China) to produce LV-DUSP1.
The GV358 was used as a negative control. The DUSP1 coding sequence
was amplified from human genomic DNA by PCR using the primers:
forwards, 5′-GAGGATCCCCGGGTACCGGTCGCCACCATGGTCATGGAAGTGGGCAC-3′ and
reverse, 5′-TCCTTGTAGTCCATACCGCAGCTGGGAGAGGTCGTAATG-3′. cDNA (10
ng/µl, 1 µl) was subjected to PCR. The conditions of PCR cycling
were as follows: Denaturation step at 98°C for 5 min, then 30
cycles at 98°C for 10 sec, 65°C for 10 sec and 72°C for 60 sec. The
GV358 vector was cleaved at the AgeI/AgeI site by a
restriction endonuclease (10 units/µl, NEB) at 37°C for 3 h. The
amplified DUSP1 gene was cloned into GV358 vector (1 µg/µl, 2.5 µl)
using PrimeSTAR HS DNA polymerase (cat. no. R010B; Takara Bio, Inc,
Otsu, Japan) to generate LV-DUSP1. EndoFree midi Plasmid kit was
purchased from Tiangen Biotech, Co, Ltd, (cat. no. DP118-2;
Beijing, China). OA FLSs were infected with LV-DUSP1 and negative
control lentivirus of equal titers (1×108 TU/ml,
MOI=100) at 30% confluence and stable cells were selected with 2
µg/ml puromycin.
Statistical analysis
Data are presented as the mean ± standard error.
Statistically significant differences among three groups were
identified by a one-way analysis of variance test followed by
Tukey's post-hoc test. Differences between two groups were analyzed
using Student's t test. All data were analyzed using SPSS version
19.0 (IBM Corp, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
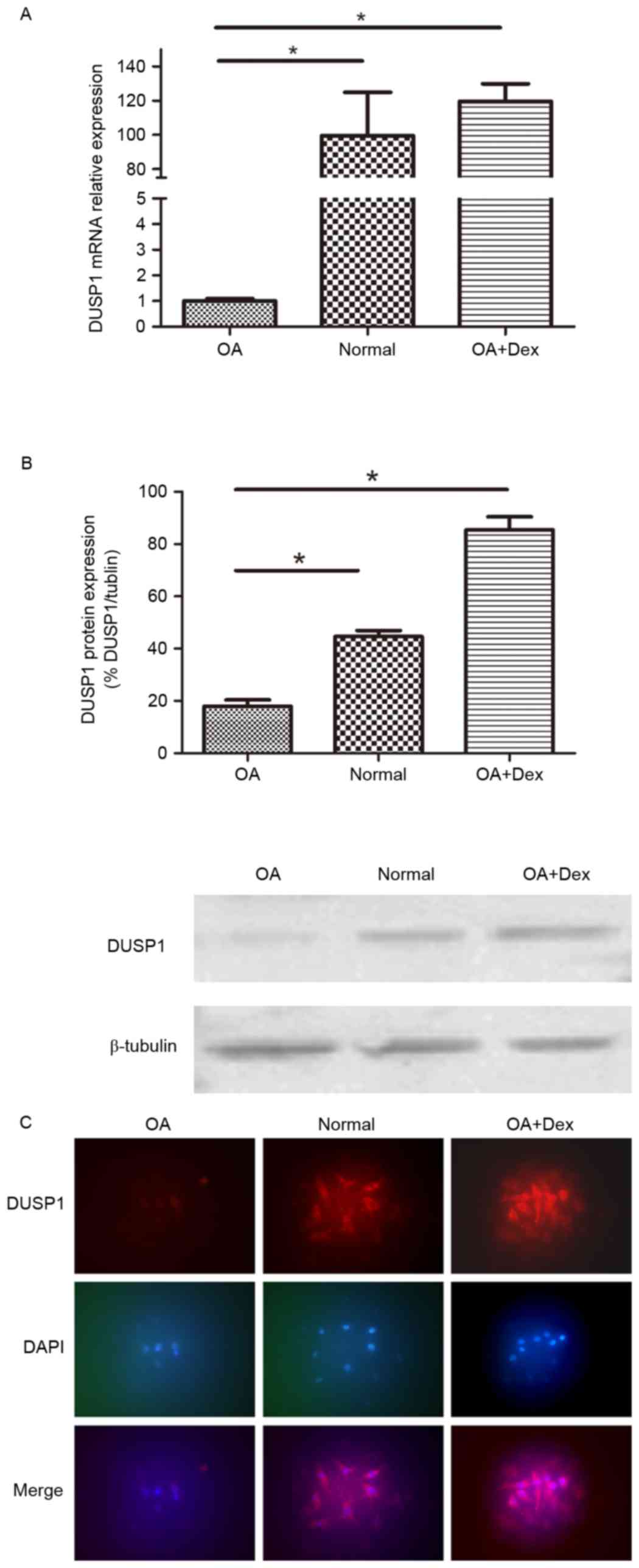
Expression of DUSP1 in OA FLSs, normal
FLSs and dexamethasone (Dex)-induced OA FLSs
The mRNA expression of DUSP1 in OA FLSs, normal FLSs
and OA FLSs pretreated with Dex (1 µM) for 24 h were measured by
RT-qPCR (Fig. 1A). The results
demonstrated that the mRNA expression levels of DUSP1 in normal
FLSs were significantly higher than in OA FLSs (P<0.01), and
that Dex significantly induced DUSP1 expression in OA FLSs
(P<0.01). The DUSP1 protein expression in the three groups was
also detected by western blot and immunofluorescence staining
(Fig. 1B and C). The findings
verified the RT-qPCR results.
p38 MAPK and JNK activation, and the
production of inflammatory and catabolic mediators in OA FLSs,
normal FLSs and Dex-induced OA FLSs
It is well-established that the p38 MAPK and JNK are
activated by phosphorylation. The level of p38, p-p38, JNK, p-JNK
were determined by western blot in the three groups of cells
(Fig. 2A and B). The results
demonstrated that the expression of p-p38 and p-JNK in OA FLSs were
higher than in normal FLSs and in OA FLSs pretreated with Dex
(P<0.01), while the expression of p38 and JNK were decreased
compared with normal FLSs and in OA FLSs pretreated with Dex, which
indicated that Dex could inhibit the activation of p38 and JNK.
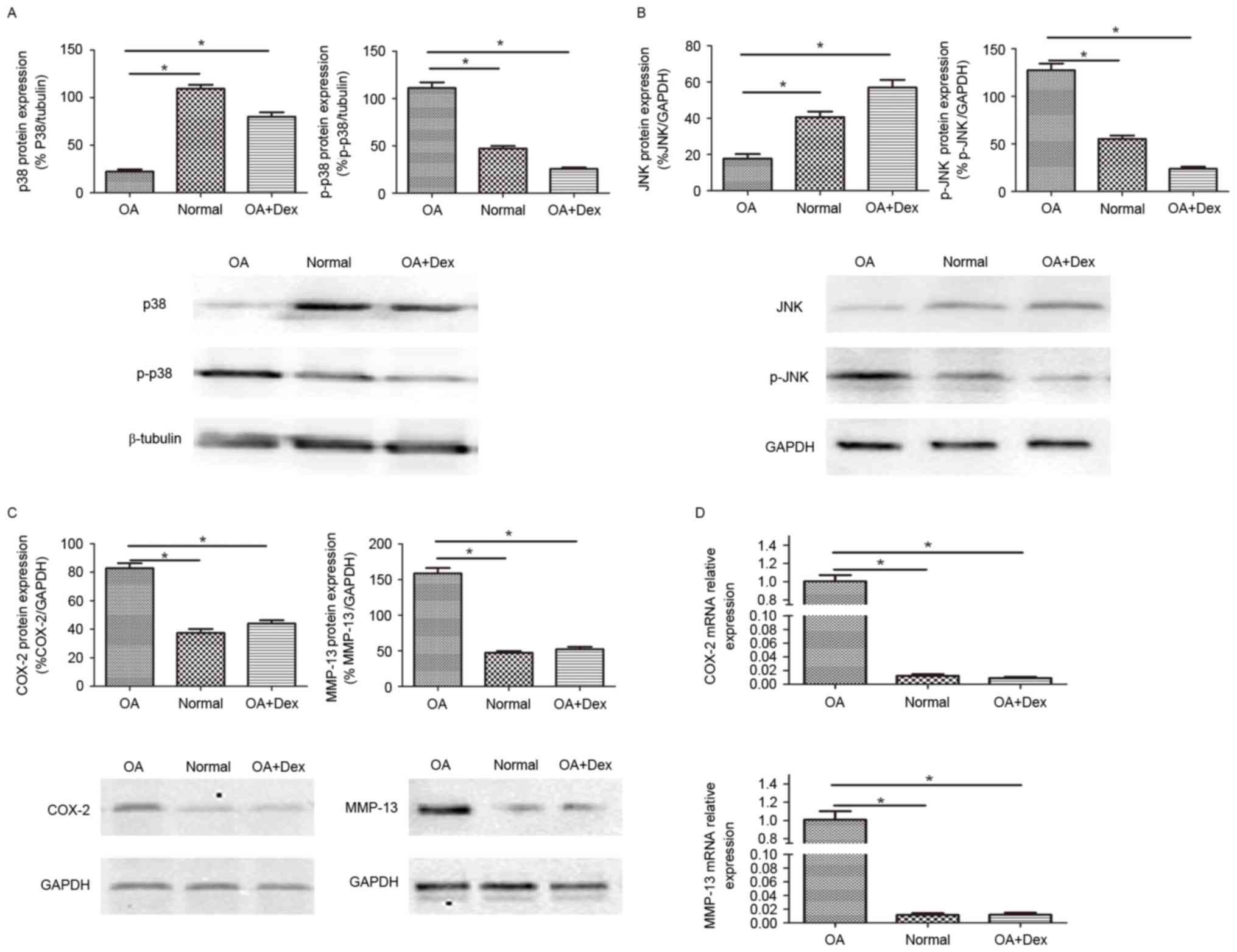 | Figure 2.p38 MAPK and JNK pathway activation
and the production of inflammatory and catabolic mediators in OA
FLSs, normal FLSs and Dex-induced OA FLSs. The protein expression
of (A) p38 MAPK, p-p38 MAPK, (B) JNK and p-JNK were measured by
western blot with β-tubulin and GAPDH as loading controls,
respectively. The expression of COX-2 and MMP-13 were detected by
(C) western blot (with β-tubulin as the loading control) and (D)
reverse transcription-quantitative polymerase chain reaction (with
β-actin as the reference gene). One-way analysis of variance test
was performed for the comparison of the results. Data are presented
as the mean ± standard error (n=3). *P<0.01. FLSs,
fibroblast-like synoviocytes; MAPK, mitogen-activated protein
kinase; OA, osteoarthritis; Dex, dexamethasone; p-, phosphorylated;
JNK, c-Jun NH2 terminal kinase; COX-2, cyclooxygenase-2; MMP,
matrix metalloproteinase. |
The expression of certain key mediators involved in
OA, such as MMP-13 and COX-2, was also determined by western blot
and RT-qPCR (Fig. 2C and D). The
results suggested that the expression levels of MMP-13 and COX-2 in
OA FLSs were higher than in normal FLSs and in OA FLSs pretreated
with Dex (P<0.01), and partially demonstrated that the
anti-inflammatory role of Dex in OA may act through a mechanism of
MAPK signaling pathway inhibition.
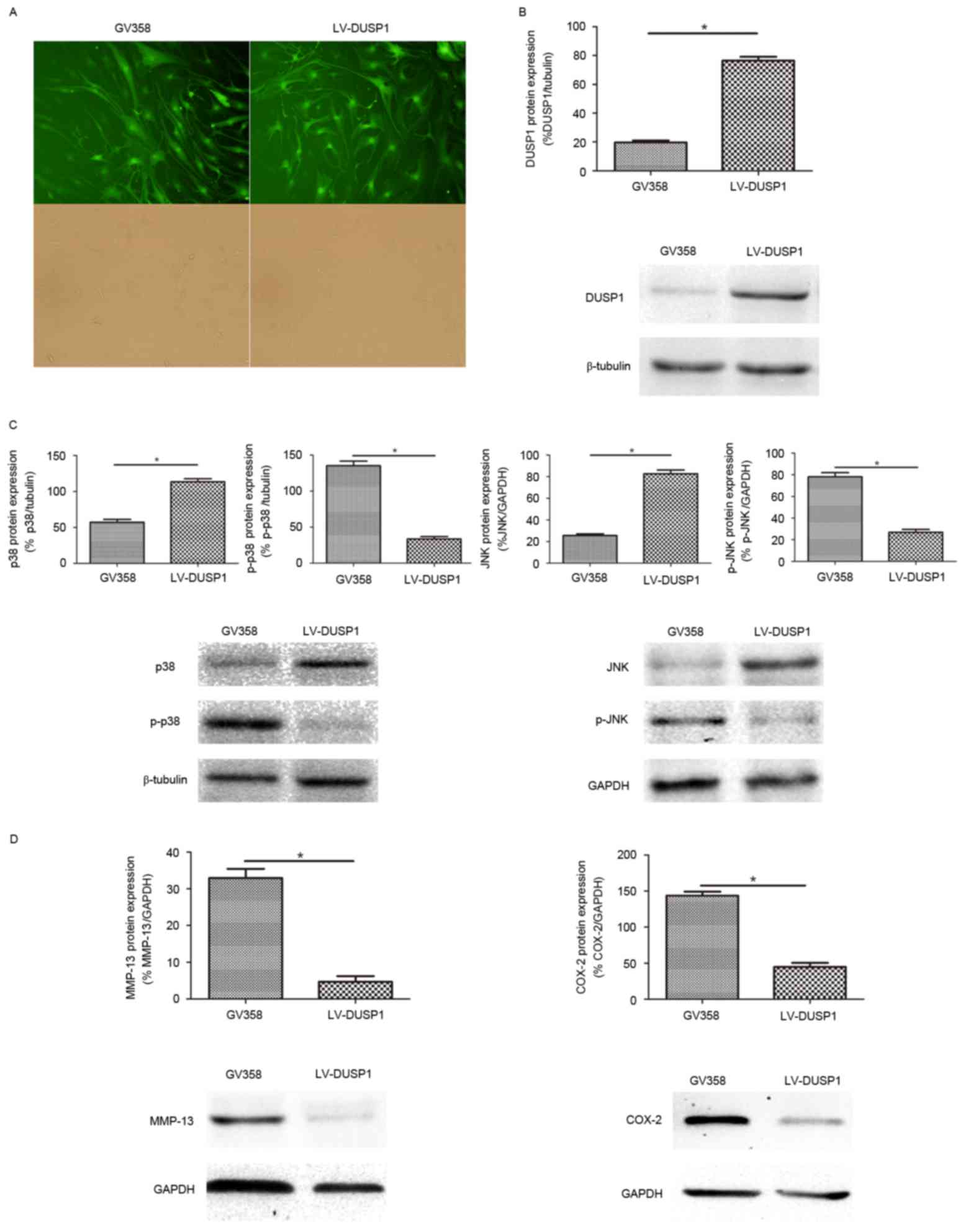
Effect of DUSP1 overexpression in OA
FLSs
To investigate the role of DUSP1 in OA FLSs, a
lentivirus vector (GV358) combined with DUSP1 gene (LV-DUSP1) was
used to infect OA FLSs, and GV358 was used as a control. The
infection efficiency was almost 90% (Fig. 3A). Western blot analysis confirmed
DUSP1 overexpression (Fig. 3B).
The activation of p38 MAPK and JNK pathways were detected by
western blot. The results demonstrated that the expression of p-p38
and p-JNK were increased in the GV358 group compared with the
LV-DUSP1 group. The expression of p38 and JNK was decreased in the
GV358 group compared with the LV-DUSP1 group, which indicated that
the activated p38 MAPK and JNK signaling pathway in OA FLSs was
significantly inhibited by LV-DUSP1 (Fig. 3C). The expression levels of MMP-13
and COX-2 were also decreased in the presence of LV-DUSP1 (Fig. 3D). Taken together, these findings
suggested that DUSP1 may inhibit inflammatory and catabolic
mediators via inhibition of the p38 MAPK and JNK signaling pathway
in OA FLSs.
Discussion
OA is one of the leading causes of physical
disability (29). While there have
been many surgical techniques, such as arthroplasty, used in the
treatment of OA, there is currently no effective treatment to
prevent or stop cartilage destruction. During the last several
years, more and more research has demonstrated the important role
of synovial inflammation in the pathogenesis of OA. Although there
have been many inconclusive clinical results of exogenous
anti-inflammatory therapy in OA (30). Anti-inflammatory treatment in OA,
especially in the early stages, is a promising therapeutic approach
to prevent cartilage degradation.
DUSP1 negatively regulates the MAPK signaling
pathway by dephosphorylating MAPKs, and is involved in various
cellular responses, including inflammation, cell proliferation,
differentiation, stress responses, apoptosis and immune defense
(19,31–33).
The current study focused on the role of DUSP1 in OA, and the
results demonstrated that DUSP1 has an anti-inflammation and
anti-catabolic role, which is potentially mediated via inhibition
of p38 MAPK and JNK signaling pathway in OA FLSs. These findings
suggest that DUSP1 can be a potential target for the treatment of
synovitis and preventing cartilage degradation in OA.
Initially, the DUSP1 mRNA and protein expression in
OA FLSs and normal FLSs were determined, and increased expression
levels of DUSP1in normal FLSs compared with in OA FLSs were
observed. Glucocorticoids are powerful anti-inflammatory agents
that reduce the expression of various inflammatory mediators and
have been successfully used in the treatment of inflammatory
diseases for many years (34). Dex
was used to induce OA FLSs in this current study, and the results
suggested that Dex induced the expression of DUSP1 at the mRNA and
protein level in OA FLSs. This result is consistent with the
literature data indicating that Dex is an important regulator of
DUSP1 (20,34,35).
It has been known that MAPK signaling pathway has a
critical role in the regulation of inflammatory and catabolic
mediators, such as COX-2 and MMP-13 (36,37).
In OA, MMP-13 and COX-2 have a critical role in maintaining
cartilage homeostasis (15,38).
MMP-13 is involved in type II collagen cleavage, which contributes
to the degradation of joint cartilage. COX-2 is likely responsible
for the elevated prostaglandin E2, which has a key role in OA
progression and pain (39).
Furthermore, the activation of p38 MAPK and JNK pathway, and the
expression of MMP-13 and COX-2 were investigated in the three
groups of FLSs in the present study. As illustrated in the results,
p38 MAPK and JNK were significantly activated and the expression of
MMP-13 and COX-2 in OA FLSs were higher than the other two groups
cells. These findings suggest that Dex can inhibit the activation
of MAPKs, and exhibit anti-inflammatory and anti-catabolic effects
in OA FLSs, and the effects of Dex may be dependent on the
induction of DUSP1; the specific evidence requires further
investigation.
Additionally, to elucidate the role of DUSP1 in OA
FLSs, LV-DUSP1 and GV358 were constructed to infect OA FLSs, and
the activation of the MAPK pathway, and the expression of MMP-13
and COX-2 were detected. The results demonstrated that the
activated MAPKs were inhibited in the presence of LV-DUSP1 in OA
FLSs, and the expression levels of OA-associated mediators, MMP-13
and COX-2, were decreased. The findings are consistent with the
role of DUSP1 as a negative regulator of the MAPK pathway, and
suggest that DUSP1 has an anti-inflammatory role in certain
inflammation-associated diseases.
In conclusion, the results demonstrated that the
expression of DUSP1 was lower in OA FLSs than in normal FLSs, and
DUSP1 may inhibit the expression of OA-associated mediators, MMP-13
and COX-2, by suppressing the activation of p38 MAPK and JNK
pathways in OA FLSs. In addition, the anti-inflammatory role of Dex
may partially depend on induction of DUSP1. The findings of the
present study suggest that DUSP1 may be a promising therapeutic
target in OA.
Acknowledgements
This work was supported by Institute of
Osteosarcoma, Tangdu Hospital, The Fourth Military Medical
University (Xi'an, China).
References
|
1
|
Cross M, Smith E, Hoy D, Nolte S, Ackerman
I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, et al:
The global burden of hip and knee osteoarthritis: Estimates from
the global burden of disease 2010 study. Ann Rheum Dis.
73:1323–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dieppe PA and Lohmander LS: Pathogenesis
and management of pain in osteoarthritis. Lancet. 365:965–973.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berenbaum F: Osteoarthritis as an
inflammatory disease (osteoarthritis is not osteoarthrosis!).
Osteoarthritis Cartilage. 21:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ayral X, Pickering EH, Woodworth TG,
Mackillop N and Dougados M: Synovitis: A potential predictive
factor of structural progression of medial tibiofemoral knee
osteoarthritis - results of a 1 year longitudinal arthroscopic
study in 422 patients. Osteoarthritis Cartilage. 13:361–367. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scanzello CR, McKeon B, Swaim BH, DiCarlo
E, Asomugha EU, Kanda V, Nair A, Lee DM, Richmond JC, Katz JN, et
al: Synovial inflammation in patients undergoing arthroscopic
meniscectomy: Molecular characterization and relationship to
symptoms. Arthritis Rheum. 63:391–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang L and Karin C: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rincón M and Davis RJ: Regulation of the
immune response by stress-activated protein kinases. Immunol Rev.
228:212–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–3675. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fechir M, Linker K, Pautz A, Hubrich T,
Förstermann U, Rodriguez-Pascual F and Kleinert H: Tristetraprolin
regulates the expression of the human inducible nitric-oxide
synthase gene. Mol Pharmacol. 67:2148–2161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashwell JD: The many paths to p38
mitogen-activated protein kinase activation in the immune system.
Nat Rev Immunol. 6:532–540. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ono K and Han J: The p38 signal
transduction pathway: Activation and function. Cell Signal.
12:1–13. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng L, Wang W, Rong XF, Zhong Y, Jia P,
Zhou GQ and Li RH: Chondroprotective effects and multi-target
mechanisms of Icariin in IL-1 beta-induced human SW 1353
chondrosarcoma cells and a rat osteoarthritis model. Int
Immunopharmacol. 18:175–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Q, Shepherd EG, Manson ME, Nelin LD,
Sorokin A and Liu Y: The role of mitogen-activated protein kinase
phosphatase-1 in the response of alveolar macrophages to
lipopolysaccharide: Attenuation of proinflammatory cytokine
biosynthesis via feedback control of p38. J Biol Chem.
280:8101–8108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA,
Bennett AM and Flavell RA: Dynamic regulation of pro- and
anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate
immune responses. Proc Natl Acad Sci USA. 103:pp. 2274–2279. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Shepherd EG and Nelin LD: MAPK
phosphatases-regulating the immune response. Nat Rev Immunol.
7:202–212. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boutros T, Chevet E and Metrakos P:
Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase
regulation: Roles in cell growth, death, and cancer. Pharmacol Rev.
60:261–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kassel O, Sancono A, Krätzschmar J, Kreft
B, Stassen M and Cato AC: Glucocorticoids inhibit MAP kinase via
increased expression and decreased degradation of MKP-1. EMBO J.
20:7108–7116. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nieminen R, Korhonen R, Moilanen T, Clark
AR and Moilanen E: Aurothiomalate inhibits cyclooxygenase 2, matrix
metalloproteinase 3, and interleukin-6 expression in chondrocytes
by increasing MAPK phosphatase 1 expression and decreasing p38
phosphorylation: MAPK phosphatase 1 as a novel target for
antirheumatic drugs. Arthritis Rheum. 62:1650–1659. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McAbee J, Li Q, Yu H and Kirkwood KL:
Sexual dimorphism in periapical inflammation and bone loss from
mitogen-activated protein kinase phosphatase-1 deficient mice. J
Endod. 38:1097–1100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashizume M and Mihara M: High molecular
weight hyaluronic acid inhibits IL-6-induced MMP production from
human chondrocytes by up-regulating the ERK inhibitor, MKP-1.
Biochem Biophys Res Commun. 403:184–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smallie T, Ross EA, Ammit AJ, Cunliffe HE,
Tang T, Rosner DR, Ridley ML, Buckley CD, Saklatvala J, Dean JL and
Clark AR: Dual-specificity phosphatase 1 and tristetraprolin
cooperate to regulate macrophage responses to lipopolysaccharide. J
Immunol. 195:277–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matta R, Barnard JA, Wancket LM, Yan J,
Xue J, Grieves J, Frazier WJ, Nelin L, Cato AC and Liu Y: Knockout
of Mkp-1 exacerbates colitis in Il-10-deficient mice. Am J Physiol
Gastrointest Liver Physiol. 302:G1322–G1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vattakuzhi Y, Abraham SM, Freidin A, Clark
AR and Horwood NJ: Dual-specificity phosphatase 1-null mice exhibit
spontaneous osteolytic disease and enhanced inflammatory osteolysis
in experimental arthritis. Arthritis Rheum. 64:2201–2210. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lattuada D, Gualtierotti R, Crotta K,
Seneci P, Ingegnoli F, Corradini C, Viganò R, Marelli O and Casnici
C: Smac127 has proapoptotic and anti-inflammatory effects on
rheumatoid arthritis fibroblast-like synoviocytes. Mediators
Inflamm. 2016:69056782016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buckwalter JA, Mankin HJ and Grodzinsky
AJ: Articular cartilage and osteoarthritis. Instr Course Lect.
54:465–480. 2005.PubMed/NCBI
|
|
30
|
Laev SS and Salakhutdinov NF:
Anti-arthritic agents: Progress and potential. Bioorg Med Chem.
23:3059–3080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawan A, Shi H, Gatzke F and Bennett AM:
Diversity and specificity of the mitogen-activated protein kinase
phosphatase-1 functions. Cell Mol Life Sci. 70:223–237. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wancket LM, Frazier WJ and Liu Y:
Mitogen-activated protein kinase phosphatase (MKP)-1 in immunology,
physiology, and disease. Life Sci. 90:237–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mahalingam CD, Datta T, Patil RV, Kreider
J, Bonfil RD, Kirkwood KL, Goldstein SA, Abou-Samra AB and Datta
NS: Mitogen-activated protein kinase phosphatase 1 regulates bone
mass, osteoblast gene expression, and responsiveness to parathyroid
hormone. J Endocrinol. 211:145–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Newton R: Molecular mechanisms of
glucocorticoid action: What is important? Thorax. 55:603–613. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abraham SM, Lawrence T, Kleiman A, Warden
P, Medghalchi M, Tuckermann J, Saklatvala J and Clark AR:
Antiinflammatory effects of dexamethasone are partly dependent on
induction of dual specificity phosphatase 1. J Exp Med.
203:1883–1889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mariani E, Pulsatelli L and Facchini A:
Signaling pathways in cartilage repair. Int J Mol Sci.
15:8667–8698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amin AR, Attur M, Patel RN, Thakker GD,
Marshall PJ, Rediske J, Stuchin SA, Patel IR and Abramson SB:
Superinduction of cyclooxygenase-2 activity in human
osteoarthritis-affected cartilage. Influence of nitric oxide. J
Clin Invest. 99:1231–1237. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ying X, Peng L, Chen H, Shen Y, Yu K and
Cheng S: Cordycepin prevented IL-β-induced expression of
inflammatory mediators in human osteoarthritis chondrocytes. Int
Orthop. 38:1519–1526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee AS, Ellman MB, Yan D, Kroin JS, Cole
BJ, van Wijnen AJ and Im HJ: A current review of molecular
mechanisms regarding osteoarthritis and pain. Gene. 527:440–447.
2013. View Article : Google Scholar : PubMed/NCBI
|