Introduction
Cerebral ischemia is a condition in which there is
insufficient blood flow to the brain to meet metabolic demand. This
leads to poor oxygen supply or cerebral hypoxia and results in the
death of brain tissue or cerebral infarction/ischemic stroke.
Cerebral ischemia is a sub-type of stroke, as are subarachnoid and
intracerebral hemorrhages. Ischemic stroke remains a leading
contributor to mortality rates and long-term disability worldwide
(1). In China, cerebral ischemia
is a common cardiovascular disease and is the second most prevalent
non-cancer-associated disease (2).
The primary pathogenesis of cerebral ischemia includes an
inflammatory response, edema, hypoxia and neuronal apoptosis, which
result in several motor dysfunctions, including hemiplegia and
paraplegia (3–5). It has been identified that the
prognosis of cerebral ischemia is dependent on neuron survival and
improvement in terms of neurological outcome (5). Accordingly, the development of
innovative therapies to prevent neural damage caused by
hypoxic-ischemic injury is a high priority.
The Na+/K+-ATPase (NKA) pump
is an important plasma membrane protein, which comprises an
α-subunit (100 kDa) and a b-subunit (55 kDa), and is situated on
almost all animal cells. The primary function of NKA is the
exchange of three sodium ions out of the cell for two potassium
ions into the cell (6). Several
studies have demonstrated that, in addition to the characteristic
ion transportation, NKA can transfer extracellular binding
signaling into the cell through the regulation of protein tyrosine
phosphorylation (7–9).
A polyclonal antibody has been identified that
targets the DR region (Asp 897-Arg 911) of the NKA a-subunit and
stimulates NKA activity (10,11).
The stimulation not only enhances heart contractility through the
opening of L-type Ca2+ channels (12), but it also induces cardioprotective
effects against hypoxia/reperfusion (H/R) injury through activation
of the Src/phosphoinositide 3-kinase
(PI3K)/AKT/extracellular-signal regulated kinase (ERK) 1/2 pathways
(11).
ERK1/2 and PI3K/AKT are well-known pro-survival
protein kinases important in cerebral H/R injury and have been
reported to protect against transient focal cerebral H/R injury in
rats by dexmedetomidine (13). The
present study aimed to investigate the cerebral protective effect
of the DR region-specific antibody (DRSAb) and its underlying
mechanism in an H/R model of U251 cells.
Materials and methods
Animals
A total of 10 male Sprague-Dawley (SD) rats (250–300
g) were obtained from the Experimental Animal Center of Xi'an
Jiaotong University (Xi'an, China) and housed in a standardized
environment: Temperature 22°C, 50–60% humidity and 12:12 h
light-dark cycle with access to laboratory rodent chow and tap
water ad libitum under pathogen-free conditions in the
Experimental Animal Center of Xi'an Jiaotong University (Xi'an,
China). All protocols in the present study were approved by the
Institutional Animal Care and Use Committees of Xi'an Jiaotong
University.
Chemicals and reagents
The KLH-conjugated DR region peptide
(897DVEDSYGQQWTYEQR911) was synthesized by GL Biochem, Ltd.
(Shanghai, China). Rabbit anti-human NKAα1 subunit antibody
(ATP1A1; L2C00601; 1:500), was produced by Jimianshiye (Shanghai,
China), phosphorylated (P)-ERK1/2 (9106; 1:2,000), total (T)-ERK1/2
(9107; 1:1,000), P-AKT (4051; 1:1,000) and T-AKT antibodies (2920;
1:2,000) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Goat anti-rabbit secondary antibodies (SC-2004;
1:10,000), goat anti-rat secondary antibodies (SC-2032; 1:10,000)
and goat anti-mouse secondary antibodies (SC-2005; 1:10,000) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). LY-294002 (a PI3K inhibitor) and PD-98059 (an ERK1/2
inhibitor) were purchased from EMD Millipore (Billerica, MA, USA).
Complete Freund's adjuvant (CFA), incomplete Freund's adjuvant
(IFA), trypan blue dye and all other reagents used in the
experiments were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). All chemicals were dissolved in distilled
water with the exception of PD98059 and LY-294002, which were
dissolved in dimethyl sulfoxide (DMSO) at a final concentration of
0.1% (w/v).
Preparation of DRSAb
The 10 adult male SD rats (6–8 weeks) were immunized
with the KLH-conjugated DR region peptide (897DVEDSYGQQWTYEQR911)
four times each week. The initial dose was 100 µg protein
emulsified with CFA per rat followed by 50 µg protein emulsified
with IFA per rat every week. At 5 days following the final
immunization, the rats were anesthetized by intraperitoneal
injection of pentobarbital sodium (50 mg/kg; Abbott Laboratories,
North Chicago, IL, USA) and all blood was collected from the heart.
The blood was incubated at 37°C for 30 min and then centrifuged at
5,000 × g for 20 min. The sera were stored at −80°C until
use.
For antibody purification, a Protein L resin column
was first balanced with 10× volume of phosphate buffer (pH 7.2) and
the immune sera flowed automatically through the column. The
unspecific protein was washed away using washing buffer. The
antibody was eluted with 0.1 M glycine (pH 3.0) and the eluted
fractions were immediately adjusted to physiologic pH by adding 100
µl of 1 M phosphate (pH 8.0) to 1 ml of the eluate. The elution was
monitored by measuring the absorbance at 280 nm or by a protein
assay (BCA™ Protein Assay kit; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
To detect the titer of the immunized sera and
purified antibody, a 96-well-plate was coated with DR region
peptide at a concentration of 1 µg/well. The plate was blocked with
5% BSA for 1 h at room temperature following coating with the
peptide at 4°C overnight. The immunized sera and purified antibody
were diluted in a two-fold serial dilution and were then incubated
with the coated DR peptides at room temperature for 2 h. The bound
antibody was probed with goat anti-rat immunoglobulin G
(IgG)-labeled with horseradish peroxidase (SC-2032; 1:10,000; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature, followed by
incubation with 3,3′,5,5′-tetramethylbenzidine for 15–30 min and
reading of the optical density at 405 nm. The fractions of sera
were stored at −80°C for future use.
Flow cytometric analyses
To analyze whether DRSAb was able to bind with the
DR region of NKA in U251 cells, U251 cells (Cell Bank of the
Chinese Academy of Sciences, Shanghai, China) were cultivated in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% (v/v) FBS, penicillin (100
mg/ml) and streptomycin (10 mg/ml) in a humidified incubator at
37°C with 5% CO2 for 48 h, and then harvested and
divided into three samples, each containing 1×105 cells.
The cells were incubated with purified DRSAb or control purified
sera for 15 min at 37°C and then stained with the PE-conjugated
anti-IgG heavy and light chain (H+L) antibody (BD Pharmingen, San
Diego, CA, USA). The stained cells were run through a Becton
Dickinson FACSCalibur flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) and the resulting data were analyzed using FlowJo
software version 7.6 (Tree Star, Inc., Ashland, OR, USA).
H/R model of U251 cells
The U251 cells were randomly divided into three
groups as follows: Control group, treatment group and untreated
group. In the control group, the cells were seeded into 6-well
plates (1×105), the culture medium was deprived of
oxygen and glucose, and nitrogen-saturated D-hanks buffer solution
was added, following which the culture plates were incubated for 24
h in a Tri Gas incubator (Heal Force Bio-meditech Holdings Ltd.,
Shanghai, China) with humidified air containing 5% CO2
and 94% N2 at 37°C to simulate hypoxia (~1% oxygen).
Subsequently, complete medium was reintroduced and the culture
plates were incubated in a normal culture incubator at 37°C for 12
h to simulate re-oxygenation (14). In the treatment group, all
treatment procedures were the same as for H/R group; however, the
cells were incubated with either control sera or different
concentrations of DRSAb (0.1, 0.2, 0.3, 0.4 and 0.5 µM) during the
administration of H/P. The cells in the untreated group were
cultured in complete culture medium in a humidified air containing
5% CO2 at 37°C for 12 h without hypoxic exposure.
Measurement of cell viability
The U251 cells were plated at a density of
1×104 cells per well in 96-well plates and their
viability was determined using a 3-(4,5-dimethyl thiazol-2-yl)
−2,5-diphenyltetrazolium bromide (MTT) assay. The cells were
treated with control purified sera or DRSAb at different
concentrations and then subjected to H/R. Following H/R treatment,
the cells were incubated with MTT solution (0.5 mg/ml final
concentration) for 4 h at 37°C. The supernatant was then removed
and the formazan crystals were dissolved in DMSO. The quantity of
MTT formazan product was determined using a microplate reader
(Tecan; Thermo Fisher Scientific, Inc.) at a wavelength of 560
nm.
Annexin-V/propidium iodide (PI)
staining
To examine the effect of DRSAb on apoptosis, the
U251 cells treated with H/R were stained with 25 µg/ml Annexin
V-fluorescein isothiocyanate and 25 µg/ml PI. The staining was
performed according to the manufacturer's protocol. The cells were
analyzed using a flow cytometer (BD FACSCalibur; BD Biosciences).
Data acquisition and analysis were performed using FlowJo software
version 7.6 (Tree Star, Inc.).
SDS-PAGE and immunoblotting
SDS-PAGE and immunoblotting were performed as
described previously (8). The U251
cells were scraped in radio-immunoprecipitation assay lysis buffer
containing protease inhibitors. Subsequently, the extracted
proteins concentration was determined using the bicinchoninic acid
(BCA) protein assay kit (Thermo Fisher Scientific, Inc.) and
separated on a 10% SDS-PAGE gel (10–30 mg). The fractionated
proteins were then transferred onto a nitrocellulose membrane.
Specific antigens were probed with corresponding monoclonal primary
antibodies, as detailed above, at 4°C overnight, followed by
incubation with the horseradish peroxidase-conjugated secondary
antibody (1:10,000) at room temperature for 1 h. Immunoreactivity
was detected using an ECL advanced western blot detection kit (GE
Healthcare Life Sciences, Chalfont, UK).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software for Windows (SPSS, Inc., Chicago, IL, USA). All results
are expressed as the mean ± standard deviation. One-way analysis of
variance was used to determine the differences between each DRSAb
concentration and control groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
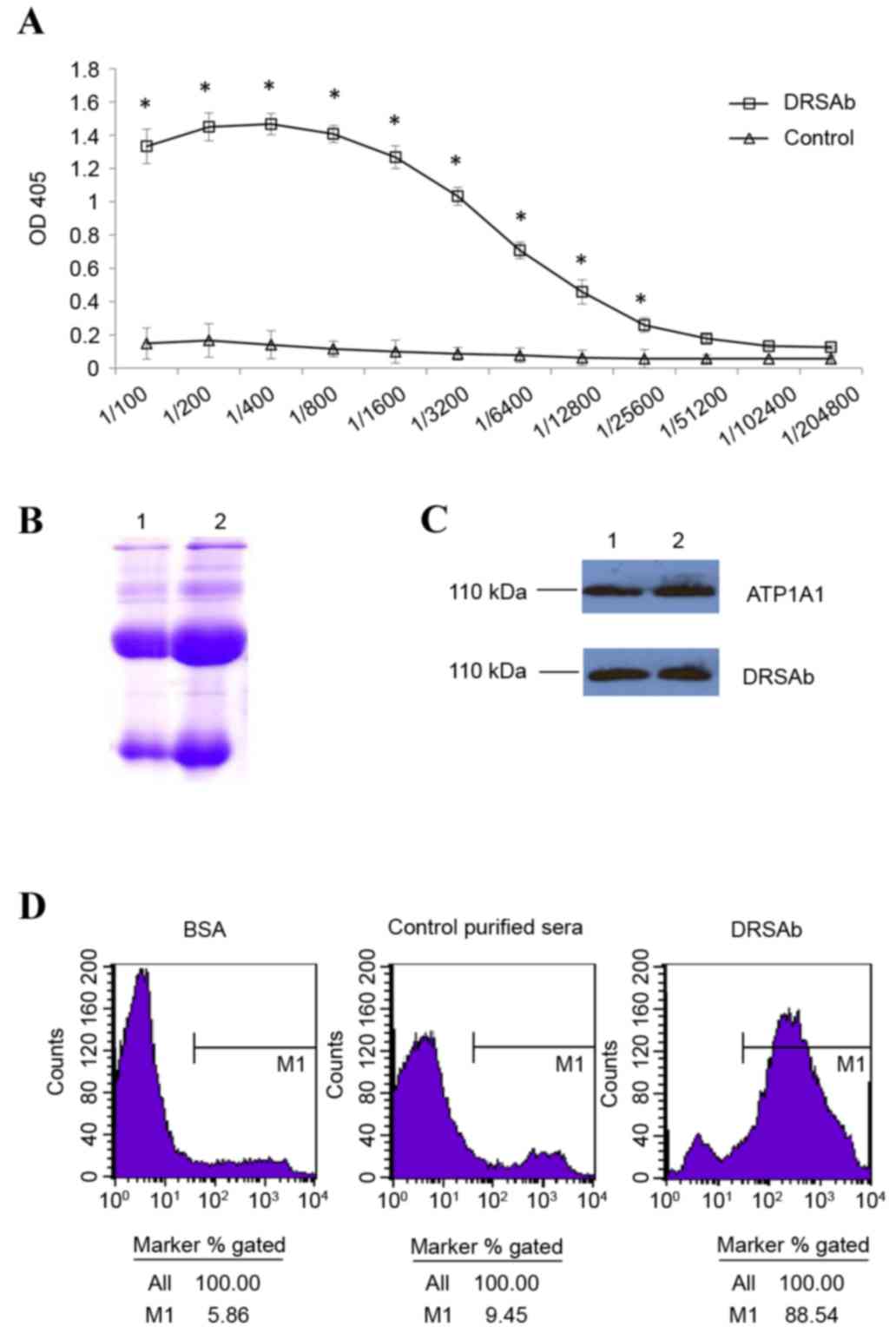
Immunologic properties of DRSAb
The titer of DRSAb was first examined. As presented
in Fig. 1A, the titer of immunized
sera was significantly higher compared with that of normal sera
between dilutions of 1:100 and 1:25,600 (Fig. 1A). This indicated that the sera
obtained were enriched with DRSAb. The SDS-PAGE data revealed two
distinct H+L chain bands of the purified antibody (Fig. 1B). DRSAb identified NKA in the U251
cells in addition to polyclonal rabbit anti human ATP1A1 (Fig. 1C). The binding of DRSAb to NKA was
revealed using flow cytometry. High signals were detected on the
U251 cells incubated with DRSAb, but not the control sera or BSA
(Fig. 1D).
Effect of DRSAb on cell viability and
apoptosis in H/P-treated U251 cells
The U251 cells were incubated with control sera,
ATP1A1 or DRSAb at different concentrations (0.1, 0.2, 0.3, 0.4 and
0.5 µM, respectively), underwent hypoxia for 24 h and then
re-oxygenation with complete medium in normal conditions for 12 h.
The cells in the untreated group were cultured in complete culture
medium in humidified air containing 5% CO2 at 37°C for
12 h without hypoxic treatment (Fig.
2A).
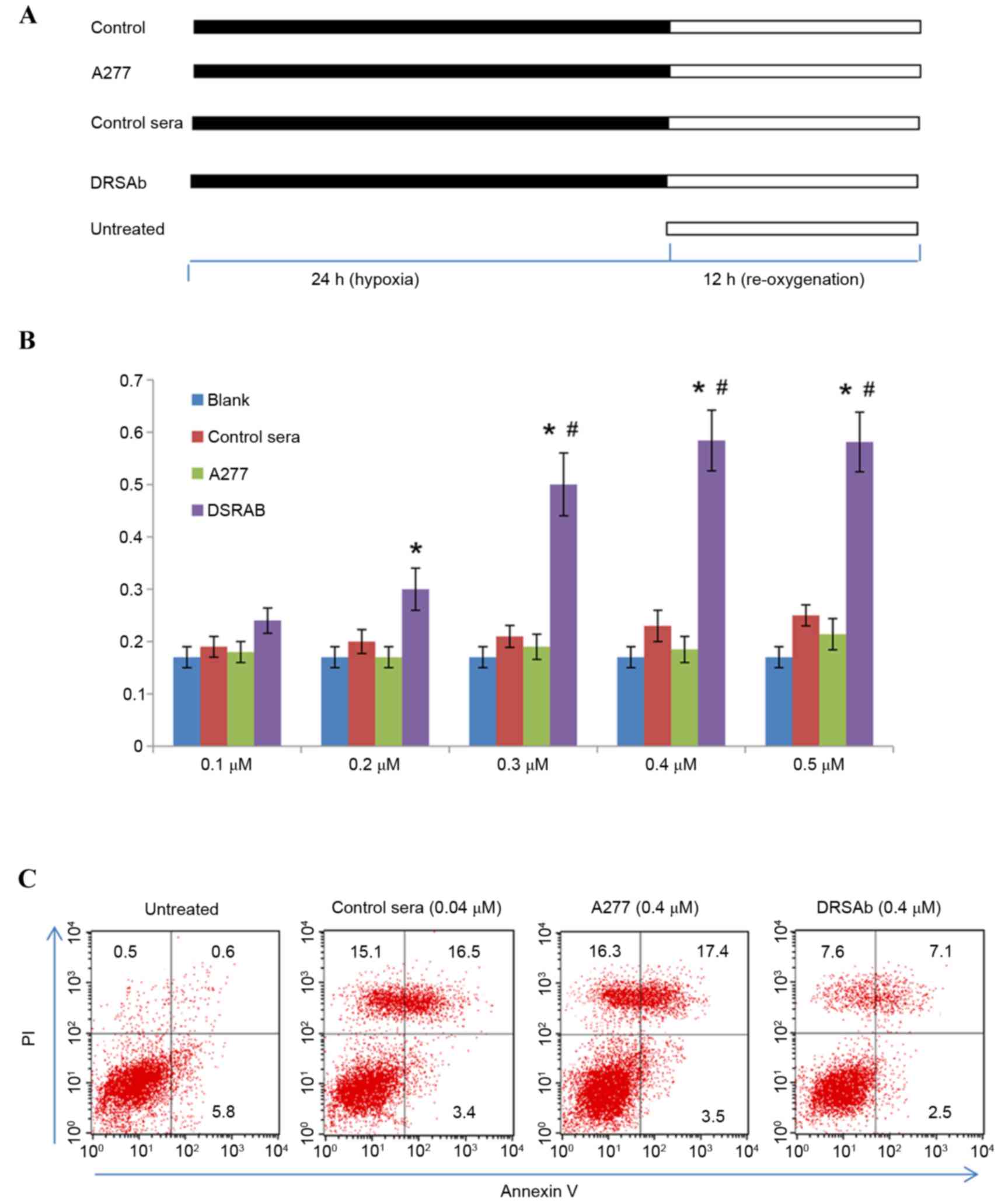 | Figure 2.Protective effect of DRSAb in U251
cells subjected to H/R. (A) Experimental protocols. U251 cells were
incubated with control sera, ATP1A1 or DRSAb at different
concentrations (0.1, 0.2, 0.3, 0.4 and 0.5 µM) in culture medium
deprived of oxygen and glucose, treated with hypoxia (5%
CO2, 1% oxygen and 94% N2) for 24 h and then
re-oxygenated with complete medium in normal conditions for 12 h.
Cells in the untreated group were cultured in complete culture
medium in humidified air containing 5% CO2 at 37°C for
12 h without hypoxic exposure. (B) Optimal concentration of DRSAb
for protection was 0.4 µM (n=3). Data are presented as the mean ±
standard error of the mean. *P<0.01 vs. control;
#P<0.05 vs. 0.2 µM. (C) Flow cytometry of
Annexin-V/PI staining in U251 cells with or without DRSAb (0.4 µM)
treatment. The administration of DRSAb markedly reduced the number
of apoptotic cells, compared with the A277 and control sera. DRSAb,
DR region-specific antibody; H/R, hypoxia/reperfusion; ATP1A1;
Na+/K+ATPase α1 subunit antibody; PI,
propidium iodide. |
Treatment with H/R significantly
decreased cell viability
DRSAb attenuated this effect at 0.3, 0.4 and 0.5 µM
(Fig. 2B), however, it was not
attenuated in the control sera or with ATP1A1, suggesting that
DRSAb may have protected the cells against H/R-induced cell
injury.
The present study also investigated the effect of
DRSAb on the apoptosis of U251 cell following H/R treatment by
performing Annexin V/PI staining. As presented in Fig. 2C, the data revealed that the
administration of 0.4 µM DRSAb markedly reduced the number of
apoptotic cells, whereas the numbers of apoptotic cells were
markedly increased in the cell cultures treated with control sera
or ATP1A1.
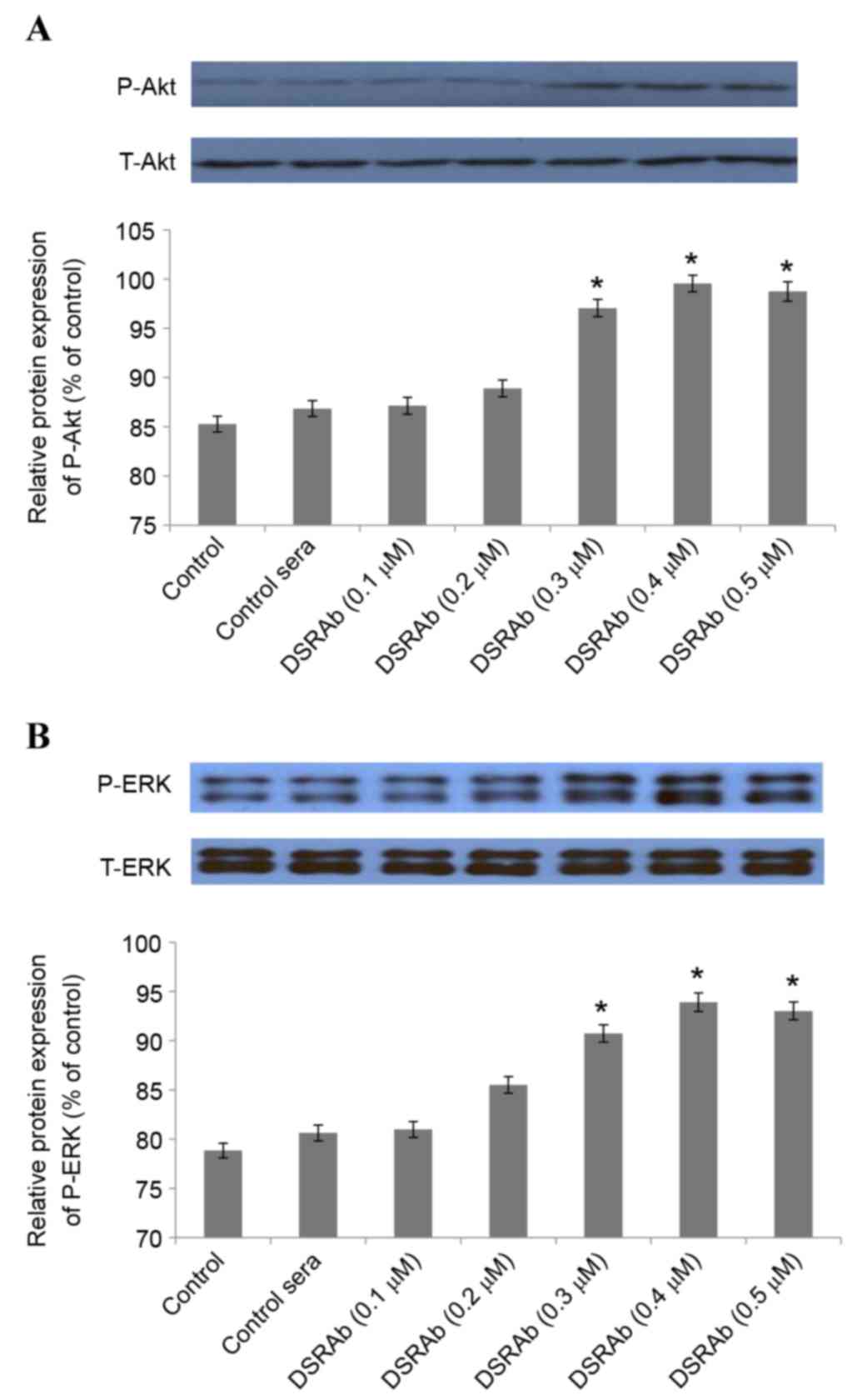
PI3K/AKT and ERK1/2 are involved in
the protective effect of DRSAb
To investigate the involvement of PI3K/AKT and
ERK1/2 in the protective effect of DRSAb, several experiments were
performed. Western blot analysis demonstrated that the
DRSAb-induced activation of PI3K/AKT and ERK1/2 increased depending
on the concentration of DRSAb. Significant differences were
identified at concentrations of 0.3, 0.4 and 0.5 µM (Fig. 3A and B).
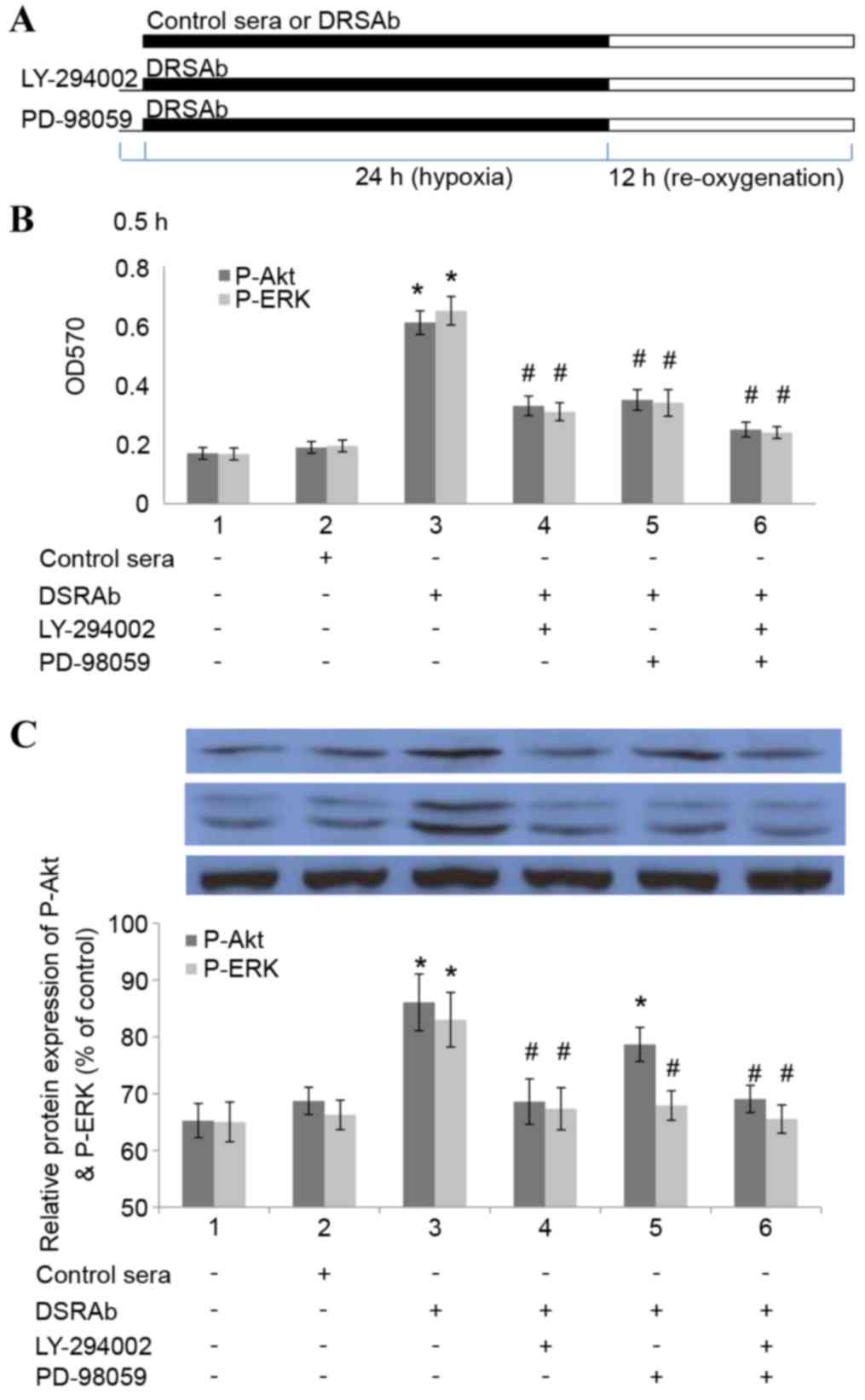
In order to determine whether inhibiting the
activation of PI3K/AKT and ERK1/2 eradicated the protective effect
of DRSAb, the U251 cells were pre-incubated with LY-294002 (15 mM
for 30 min), a selective AKT inhibitor, or PD98059 (30 µM; for 30
min), a selective pERK1/2 inhibitor, prior to treatment with DRSAb
(Fig. 4A). It was identified that
the two inhibitors eliminated the protective effects of DRSAb,
which indicated that the protective effects were mediated via the
PI3K/AKT and ERK1/2 pathways (Fig.
4B). It was also identified that LY294002 eliminated the
phosphorylation of ERK1/2, whereas PD98059 did not alter the effect
of DRSAb on the activation of AKT. These data suggested that
PI3K/AKT was activated prior to the activation of ERK1/2 in the
conditions in the present study (Fig.
4C).
Discussion
NKA is a plasma enzyme protein responsible for the
active transport or pumping of Na+ and K+
ions across the plasma membrane. Previous studies have revealed
that NKA has dual functions. In addition to pumping Na+
and K+ ions across cell membranes, it relays an
extracellular ouabain signal to intracellular compartments via the
activation of different protein kinases (15). The binding of ouabain to the
signaling NKA activates the cytoplasmic tyrosine kinase, resulting
in the phosphorylation of other proteins into different signaling
modules. This, in turn, activates multiple protein kinase cascades,
including mitogen-activated protein kinases (MAPK) and protein
kinase C isozymes (16). It also
increases the mitochondrial production of reactive oxygen species
(17) and regulates intracellular
calcium concentrations.
The extracellular DR region (897DVEDSYGQQ WTYEQR911)
of the H7-H8 domain in the α-subunit is an important activation
site of NKA, which is capable of promoting the catalytic function
of the enzyme and regulating cardiac contractility (10). It has been previously reported that
the activation of NKA with DRSAb produces protective effects via
the PI3K/AKT and ERK pathways in the heart (11). It has also been reported that
DRSAbs can statistically extend the survival of isolated cardiac
myocytes and can protect cardiac myocytes against H/R injury by
activating NKA and its subsequent signaling pathway (11). Therefore, the present study
hypothesized that DRSAb may have the ability to induce a protective
effect on U251 cells, similar to that elicited by the activator
against the DR region. In the present study, a polyclonal antibody
against the NKA DR region was constructed and it was identified
that DRSAb was able to bind with U251 cells. Furthermore, it was
identified that incubation with DRSAb produced protective effects
in U251 cells and attenuated the appostosis induced by H/R.
The ERK signaling pathway is involved in the
regulation of normal cell proliferation, survival, growth and
differentiation (18). Studies
have confirmed that the phosphorylation of ERK1/2 in cardiac
myocytes and brain cells during early reperfusion serves as a
protective mechanism against ischemic stress stimuli in the heart
and brain (11,19).
In addition to ERK1/2, PI3K/AKT also serves as an
important signaling pathway in effecting alterations in several
cellular functions in response to extracellular signals (20). AKT, as a key downstream effector of
PI3K, is activated in response to PI3K activation and regulates the
activity of a number of targets, including kinases, transcription
factors and other regulatory molecules, involved in the regulation
of cell growth, proliferation and anti-apoptotic mechanisms
(19,21,22).
In the present study, the involvement of the
PI3K/AKT and ERK pathways in the DRSAb-induced protective effect on
H/R injury was examined in U251 cells, as PI3K/AKT and ERK have
been reported to be essential proliferative and survival molecules
in different tissues (11,19,23).
The present study examined the protective effect of DRSAb in the
presence of LY-294002, a selective AKT inhibitor, or PD98059, a
selective p42/44 MAPK inhibitor. LY-294002 and PD98059 successfully
attenuated the protective effect induced by DRSAb, indicating that
the protective effect of DRSAb was mediated by PI3K/AKT and
ERK.
The signaling sequence of DRSAb was also examined.
It was identified that the inhibition of PI3K/AKT by LY294002
attenuated the DRSAb-induced phosphorylation of ERK1/2. By
contrast, the inhibition of ERK1/2 by PD98059 failed to attenuate
the phosphorylation of PI3K/AKT. These data revealed that PI3K/AKT
may be activated earlier than ERK1/2 in the conditions in the
present study.
Taken together, the data obtained in the present
study suggested that DRSAb provided cytoprotection in the H/R model
through the PI3K/AKT and ERK signaling pathway.
The inhibition of PI3K/AKT and ERK contributed to
the beneficial effects of DRSAb on U251 cell survival. These
findings indicated that DRSAb produced important neuroprotective
effects against neural injury, including ischemia in the brain.
These findings suggested that there is potential for the
development of DRSAb-based neuroprotectant therapies against neural
damage from ischemia.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of Shaanxi Province (grant no. 2015JM8392), the
International Cooperation Project of Shaanxi Province (grant no.
2014KW21-01) and the National Natural Science Foundation of China
(grant no. 3111100399).
References
|
1
|
Pendlebury ST and Rothwell PM: Prevalence,
incidence, and factors associated with pre-stroke and post-stroke
dementia: A systematic review and meta-analysis. Lancet Neurology.
8:1006–1018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chinese Society of Neurology, . Guidelines
for the diagnosis and treatment of cerebral hemorrhage in China
(2014). Chinese J Neurology. 48:435–444. 2015.
|
|
3
|
Gorelick PB: Stroke prevention therapy
beyond antithrombotics: Unifying mechanisms in ischemic stroke
pathogenesis and implications for therapy: An invited review.
Stroke. 33:862–875. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma LL, Xing GP, Yu Y, Liang H, Yu TX,
Zheng WH and Lai TB: Sulforaphane exerts neuroprotective effects
via suppression of the inflammatory response in a rat model of
focal cerebral ischemia. Int J Clin Exp Med. 8:17811–17817.
2015.PubMed/NCBI
|
|
5
|
Sherzai AZ and Elkind MS: Advances in
stroke prevention. Ann N Y Acad Sci. 1338:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaplan JH: Biochemistry of Na,K-ATPase.
Annu Rev Biochem. 71:511–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Tian J, Haas M, Shapiro JI, Askari
A and Xie Z: Ouabain interaction with cardiac
Na+/K+-ATPase initiates signal cascades
independent of changes in intracellular Na+ and
Ca2+ concentrations. J Biol Chem. 275:27838–27844.
2000.PubMed/NCBI
|
|
8
|
Kominato R, Fujimoto S, Mukai E, Nakamura
Y, Nabe K, Shimodahira M, Nishi Y, Funakoshi S, Seino Y and Inagaki
N: Src activation generates reactive oxygen species and impairs
metabolism-secretion coupling in diabetic Goto-Kakizaki and
ouabain-treated rat pancreatic islets. Diabetologia. 51:1226–1235.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Zhan Y, Xu R, Shao R, Jiang J and
Wang Z: Src mediates extracellular signal-regulated kinase 1/2
activation and autophagic cell death induced by cardiac glycosides
in human non-small cell lung cancer cell lines. Mol Carcinog. 54
Suppl 1:E26–E34. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu KY: Activation of (Na+ +
K+)-ATPase. Biochem Biophys Res Commun. 338:1669–1677.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng J, Koh X, Hua F, Li G, Larrick JW
and Bian JS: Cardioprotection induced by Na(+)/K(+)-ATPase
activation involves extracellular signal-regulated kinase 1/2 and
phosphoinositide 3-kinase/Akt pathway. Cardiovasc Res. 89:51–59.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee DI, Klein MG, Zhu W, Xiao RP,
Gerzanich V and Xu KY: Activation of
(Na++K+)-ATPase modulates cardiac L-type
Ca2+ channel function. Mol Pharmacol. 75:774–781. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL,
Yu J, Zhu MH, Wen CY, Yu LN and Yan M: Both PI3K/Akt and ERK1/2
pathways participate in the protection by dexmedetomidine against
transient focal cerebral ischemia/reperfusion injury in rats. Brain
Res. 1494:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan C, Prentice H, Price AL and Wu JY:
Beneficial effect of taurine on hypoxia- and glutamate-induced
endoplasmic reticulum stress pathways in primary neuronal culture.
Amino Acids. 43:845–855. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Z and Askari A: Na(+)/K(+)-ATPase as a
signal transducer. Eur J Biochem. 269:2434–2439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohammadi K, Kometiani P, Xie Z and Askari
A: Role of protein kinase C in the signal pathways that link
Na+/K+-ATPase to ERK1/2. J Biol Chem.
276:42050–42056. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pasdois P, Quinlan CL, Rissa A, Tariosse
L, Vinassa B, Costa AD, Pierre SV, Dos Santos P and Garlid KD:
Ouabain protects rat hearts against ischemia-reperfusion injury via
pathway involving src kinase, mitoKATP and ROS. Am J Physiol Heart
Circ Physiol. 292:H1470–H1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee
JC, Feuerstein GZ, Thomas H, Maleeff B and Ohlstein EH: Inhibition
of extracellular signal-regulated kinase enhances
Ischemia/Reoxygenation-induced apoptosis in cultured cardiac
myocytes and exaggerates reperfusion injury in isolated perfused
heart. Circ Res. 86:692–699. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Zhang X, Cui H, Zhang C, Zhu C and
Li L: Apelin-13 protects the brain against ischemia/reperfusion
injury through activating PI3K/Akt and ERK1/2 signaling pathways.
Neurosci Lett. 568:44–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han XH, Cheng MN, Chen L, Fang H, Wang LJ,
Li XT and Qu ZQ: 7,8-dihydroxyflavone protects PC12 cells against
6-hydroxydopamine-induced cell death through modulating PI3K/Akt
and JNK pathways. Neurosci Lett. 581:85–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hausenloy DJ and Yellon DM: Survival
kinases in ischemic preconditioning and postconditioning.
Cardiovasc Res. 70:240–253. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke
TF and Fisher PB: Astrocyte elevated gene-1 activates cell survival
pathways through PI3K-Akt signaling. Oncogene. 27:1114–1121. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Turjanski AG, Vaque JP and Gutkind JS: MAP
kinases and the control of nuclear events. Oncogene. 26:3240–3253.
2007. View Article : Google Scholar : PubMed/NCBI
|