Introduction
Atherosclerosis is the most common pathological
cause of cardiovascular diseases (1), and endothelial dysfunction has a
vital role in atherosclerosis (2).
Endothelial cells form the inner lining of the blood vessels and
regulate vascular function and homeostasis. Under pathological
conditions, endothelial cell apoptosis functions as an initiating
step for atherosclerosis, as apoptotic endothelial cells are
pro-thrombotic and pro-proliferative and contribute to the ensuing
atherogenic processes (3).
Oxidized low-density lipoprotein (ox-LDL), an essential
atherosclerotic risk factor, is reportedly to be crucial for
multiple functional alternations during the pathogenesis of
atherosclerosis, including inducing endothelial cell apoptosis
(4,5). The morphological changes of cultured
endothelial cells exposed to ox-LDL are similar to those observed
in the endothelium covering atherosclerotic lesions (6). Therefore, inhibiting endothelial cell
apoptosis induced by ox-LDL is a potential novel therapeutic
strategy against atherosclerosis.
Lectin-like ox-LDL receptor-1 (LOX-1), a small
transmembrane glycoprotein of 50 kDa encoded by the oxidized
low-density lipoprotein receptor 1 gene (7), is a scavenger receptor originally
identified as the primary receptor for ox-LDL uptake in endothelial
cells (8). Activation of LOX-1 by
ox-LDL mediates pro-atherogenic cellular responses implicated in
the pathogenesis of atherosclerosis, including endothelial
dysfunction characterized by reduced endothelium-dependent
relaxation, increased monocyte adhesion to endothelial cells, and
endothelial cell apoptosis and senescence (8). Basal LOX-1 expression in endothelial
cell is low, however it is induced by various stimuli associated
with atherosclerosis, including pro-inflammatory cytokines and
ox-LDL (9). In human
atherosclerotic lesions, LOX-1 is overexpressed in endothelial
cells (9). Therefore, LOX-1
represents an attractive therapeutic target for the treatment of
human atherosclerotic diseases.
A novel class of non-coding RNAs, longer than 200
nucleotides and termed long noncoding RNAs (lncRNAs), reportedly
are involved in the regulation of gene expression through
epigenetic mechanisms, including chromatin remodeling, regulation
of splicing and by acting as sponges for microRNAs (10,11).
Previous studies have revealed that lncRNAs are important in
vascular injury and remodeling, which are the leading causes of
cardiovascular diseases (12).
Long intergenic noncoding RNA p21 (lincRNA-p21) is a downstream
lncRNA transcript of p53. It has been previously reported that
lincRNA-p21 acts via several mechanisms ranging from repressing
genes in the p53 transcriptional network to regulating mRNA
translation and protein stability, and participates in diverse
biological processes, including apoptosis (13). Recent studies have demonstrated
that lincRNA-p21 inhibits proliferation and promotes apoptosis in
vascular endothelial cells and smooth muscle cells, suggesting that
this lncRNA may be useful as a therapeutic target for
atherosclerosis and associated cardiovascular disorders (14,15).
The present study aimed to investigate the role of
lincRNA-p21 in oxLDL-induced apoptosis and expression of LOX-1 in
human coronary artery endothelial cells (HCAECs).
Materials and methods
Cell culture
Primary HCAECs (cat. no. 300-05a) and MesoEndo cell
growth medium (cat. no. 212-500) were purchased from Cell
Applications, Inc. (San Diego, CA, USA). HCAECs were cultured in
MesoEndo cell growth medium supplemented with 5% fetal bovine serum
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 U/ml
penicillin-streptomycin (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) in an incubator with a humidified atmosphere of 95% air
and 5% CO2 at 37°C.
Preparation of ox-LDL and cell
treatment
Native LDL (density, 1.019–1.063 g/ml) was separated
from the fresh normolipidemic human serum purchased from Guangdong
Blood Bank (Guangzhou, China) by discontinuous density-gradient
ultracentrifugation as previously described (16). Isolated LDL was desalted using an
Econo-Pac 10 DG chromatography column (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and sterile-filtered (0.22 µm pore size; EMD
Millipore, Billerica, MA, USA). To oxidize LDL, the lipoprotein
(0.5 mg/ml in sterile PBS) was incubated with 5 µM CuSO4
at 37°C for 20 h. Oxidized LDL was concentrated by centrifuging in
Amicon Centriplus YM-100 tubes (EMD Millipore) for 2 h at 3,000 ×
g and 8°C, and subsequently sterile-filtered. The oxidation
was confirmed by measuring thiobarbituric acid-reactive substances
using tetraethoxypropane as a standard (17). HCAECs were treated with ox-LDL (30,
60 or 90 µg/ml) for 24 or 48 h with selective protein kinase C
(PKC) δ inhibitor, rottlerin (1 µM; cat. no. CAS 82-08-6; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) (18). Untreated HCAECs (0 µg/ml ox-LDL)
were used as controls.
Lentiviral transduction
Total RNA from cultured HCAECs was isolated using
miRNeasy kits (Qiagen China Co., Ltd., Shanghai, China) according
to the manufacturer's protocol, followed by purification with TURBO
DNA-free System (Ambion; Thermo Fisher Scientific Inc.). RT was
performed on 1,000 ng total RNA using MulV reverse transcriptase
(Thermo Fisher Scientific, Inc.) and random hexamer primers (Thermo
Fisher Scientific, Inc.) in a 20-µl reaction [2 µl RNA (500 ng/µl),
1 µl MulV reverse transcriptase (50 U/µl), 1 µl hexamer primers
(100 µM), 2 µl 10X RT reaction buffer, and 14 µl RNAse-free water]
and incubated at 42°C for 1 h. Total RNA from cultured HCAECs was
isolated using miRNeasy kits (Qiagen China Co., Ltd.) according to
the manufacturer's protocol, followed by purification with TURBO
DNA-free System (Ambion; Thermo Fisher Scientific Inc.). The
lincRNA-p21 cDNA was amplified by PCR using the extracted total RNA
and cloning primers with sequences as follows:
5′-TGGCAGTCTGACCCACACTCCCCACGCCC-3′ (forward) and
5′-ACAGTGCACAGACAATCATACACACGTGT-3′ (reverse). Lentivirus
expressing human lincRNA-p21 was generated by sub-cloning the above
amplified human lincRNA-p21 cDNA to the pLenti-GIII-CMV lentivirus
expression system (Applied Biological Materials, Inc., Richmond,
BC, Canada). The lincRNA-p21 lentiviral vector or blank control
lentivector was transfected with the packaging vectors psPAX2
(Addgene Inc., Cambridge, MA, USA) and pMD2.G (Addegene Inc.) into
293T cells (American Type Culture Collection, Manassas, VA, USA) by
calcium chloride to produce the lentivirus, which was subsequently
used to transduce HCAECs. Human lincRNA-p21-small interfering RNA
(siRNA) and scrambled control siRNA lentivirus particles were
designed and ordered from GenePharma Co., Ltd. (Shanghai, China).
The siRNA sequence targeting lincRNA-p21 was as follows:
5′-GGAGGACACAGGAGAGGCA-3′. The scrambled control sequence was as
follows: 5′-GGCGCAGAGACGGAGAGAA-3′. At 6 h after lentiviral
transduction, HCAECs were subject to ox-LDL treatment.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cultured HCAECs was isolated using
miRNeasy kits (Qiagen China Co., Ltd.) according to the
manufacturer's protocol, followed by purification with TURBO
DNA-free System (Ambion; Thermo Fisher Scientific, Inc.). For
measuring lncRNAs, RT was performed on 1,000 ng total RNA using
MulV reverse transcriptase (Thermo Fisher Scientific, Inc.) and
random hexamer primers (Thermo Fisher Scientific, Inc.) in a 20-µl
reaction [2 µl RNA (500 ng/µl), 1 µl MulV reverse transcriptase (50
U/µl), 1 µl hexamer primers (100 µM), 2 µl 10X RT reaction buffer,
and 14 µl RNAse-free water] and incubated at 42°C for 1 h. cDNA was
used as template for qPCR using an ABI-Prism 7700 Sequence
Detection system and the Fast SYBR Green (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Human ribosomal P0 (RPLP0) mRNA
was as the reference gene. Primer sequences used are as follows:
LincRNA-p21, 5′-CCTGTCCCACTCGCTTTC-3′ (forward) and
5′-GGAACTGGAGACGGAATGTC-3′ (reverse); LOX-1,
5′-TTACTCTCCATGGTGGTGGTGCC-3′ (forward) and
5′-AGCTTCTTCTGCTTGTTGCC-3-3′ (reverse); RPLP0,
5′-TCGACAATGGCAGCATCTAC-3′ (forward) and 5′-ATCCGTCTCCACAGACAAGG-3′
(reverse). Analysis of relative gene expression levels was
performed using the formula 2−ΔCq, with
ΔCq=Cq(target gene)-Cq(control) (19).
Cell apoptosis assay
HCAECs were cultured at 5×104 cells per
well in 96-well tissue culture plates and subject to ox-LDL
treatment (30, 60 or 90 µg/ml) for 24 or 48 h. Cell apoptosis was
measured at 24 or 48 h of ox-LDL treatment with a microplate
reader-based TiterTACS in situ apoptosis detection kit (cat.
no. 4822-96-K; R&D Systems, Inc., Minneapolis, MN, USA) as
described by the manufacturer. Each experiment was repeated three
times in triplicates.
Western blot analysis
Cells was lysed with a hypotonic buffer containing
2% Nonidet-P and a protease inhibitor cocktail (Sigma-Aldrich;
Merck Millipore) by sonication three times for 3 sec on ice. The
supernatant obtained after centrifugation at 2,000 × g for
15 min at 4°C was used for protein concentration determination by
the Coomassie blue method (Thermo Fisher Scientific, Inc.) and for
subsequent steps. Equal amount of proteins (5 µg) for each sample
were separated by 10% SDS-PAGE and blotted onto a polyvinylidene
difluoride microporous membrane (EMD Millipore). The membranes were
blocked with 5% skim milk powder in TBS-Tween (0.1%) for 2 h and
incubated for 1 h with a 1:1,000 dilution of goat anti-human LOX-1
polyclonal antibody (cat. no. sc-11653; Santa Cruz Biotechnology,
Inc.), mouse anti-human β-actin monoclonal antibody (cat. no.
sc-81178; Santa Cruz Biotechnology, Inc.), goat anti-human
phosphorylated PKCδ (p-PKCδ) polyclonal antibody (Tyr 155; cat. no.
sc-18367; Santa Cruz Biotechnology, Inc.), or rabbit anti-human
PKCδ polyclonal antibody (cat. no. sc-937; Santa Cruz
Biotechnology, Inc.). The membranes were then washed with TBS-Tween
(0.1%) and probed using bovine anti-goat (cat. no. sc-2350; Santa
Cruz Biotechnology, Inc.), anti-rabbit (cat. no. sc-2370; Santa
Cruz Biotechnology, Inc.) or anti-mouse (cat. no. sc-2371; Santa
Cruz Biotechnology, Inc.) secondary antibody (1:5,000 dilution for
1 h) conjugated to horseradish peroxidase. Peroxidase was detected
with a GE Healthcare enhanced chemiluminescence kit (GE Healthcare
Life Sciences, Shanghai, China) using the ChemiDoc Touch Imaging
System (Bio-Rad Laboratories Inc.) and Image Lab™ Touch Software
(Bio-Rad Laboratories Inc.) for image acquisition and onboard image
analysis. Three independent experiments were performed.
PKCδ activity assay
PKCδ activity assays were performed as previously
described (20). Briefly, HCAEC
lysate was pre-cleaned with protein A/G plus-agarose (Santa Cruz
Biotechnology, Inc.) and then incubated with rabbit anti-human PKCδ
polyclonal antibody (cat. no. sc-937; Santa Cruz Biotechnology,
Inc.) overnight at 4°C. PKCδ immunocomplexes were then pulled down
by 3 h of incubation with 30 µl protein A/G plus-agarose. The
immunocomplexes were resuspended in 2X kinase reaction buffer [300
mM NaCl, 8 mM MnCl2, 12 mM MgCl2, 20%
(vol/vol) glycerol, 20 µM ATP, 2 mM dithiothreitol, 200 µM
Na3VO4, and 100 mM Hepes (pH 7.5)]. The
protein kinase reaction was initiated by adding 5 µCi
[γ-32P] ATP (GE Healthcare Life Sciences) and 100 ng
histone mixture extracted from calf thymus (Sigma-Aldrich; Merck
Millipore) into 20 µl reaction buffer containing immunoprecipitated
PKCδ. After 20 min of incubation at 30°C, the reaction was
terminated by adding 2% SDS gel loading buffer. The samples were
then subjected to gel electrophoresis, and phosphorylated histone
was revealed by autoradiography to indicate PKCδ kinase
activity.
Statistical analysis
Statistical analyses were performed using SPSS for
Windows 19.0 (IBM SPSS, Armonk, NY, USA). All values are expressed
as the mean ± standard deviation. Comparisons of means among
multiple groups were performed with one-way analysis of variance
followed by post hoc pairwise comparisons using Tukey's
tests. Two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Ox-LDL induces expression of
lincRNA-p21 and LOX-1, and induces apoptosis in HCAECs
HCAECs were treated with ox-LDL (30, 60 or 90 µg/ml)
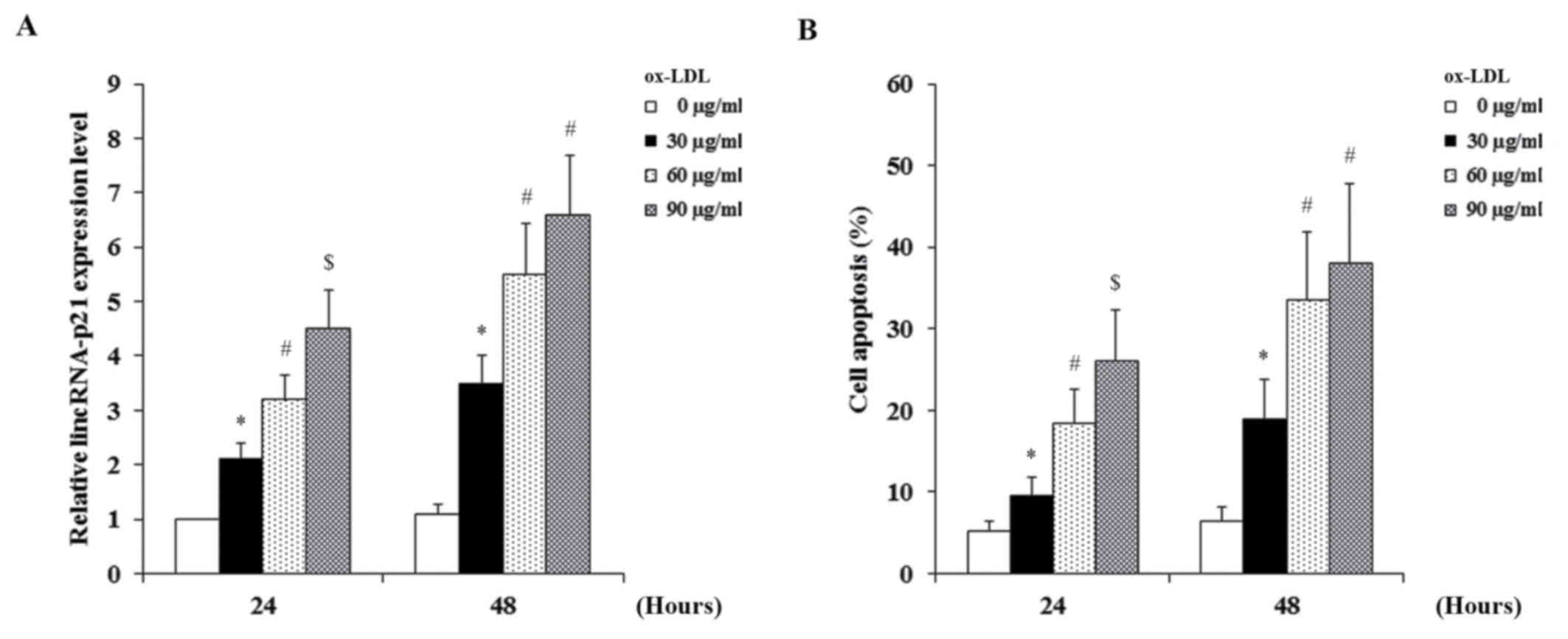
for 24 or 48 h. As demonstrated in Fig. 1, ox-LDL dose- and time-dependently
induced the expression of lincRNA-p21 (Fig. 1A) and apoptosis (Fig. 1B) in HCAECs. As demonstrated in
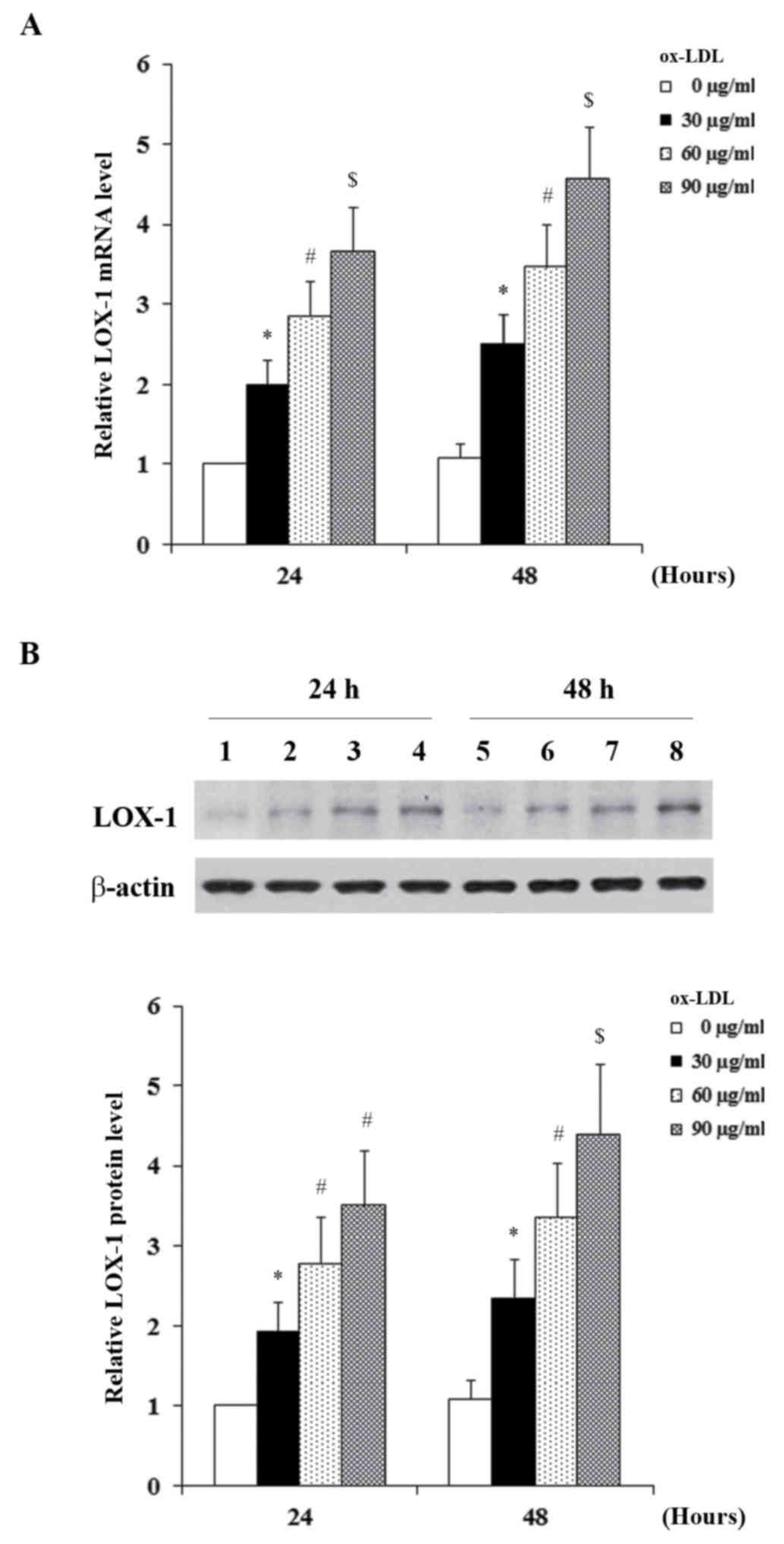
Fig. 2, the constitutive
expression level of LOX-1 in HCAECs was low; ox-LDL dose- and
time-dependently induced the mRNA (Fig. 2A) and protein (Fig. 2B) expression of LOX-1 in HCAECs.
The similar trends of oxLDL-induced expression of lincRNA-p21 and
LOX-1, and apoptosis in HCAECs suggested that lincRNA-p21 may be
associated with oxLDL-induced expression of LOX-1 and apoptosis in
HCAECs.
Effect of lincRNA-p21 on oxLDL-induced
apoptosis and expression of LOX-1 in HCAECs
Subsequently, HCAECs were transduced with human
lincRNA-p21 lentivirus or human lincRNA-p21 siRNA lentivirus to
overexpress or knockdown lincRNA-p21, respectively. HCAECs without
lentiviral transduction and those transduced with the blank control
lentivirus or a scrambled siRNA lentivirus were used as controls.
The cells in all groups were treated with 90 µg/ml ox-LDL for 48 h
with or without 1 µM selective PKCδ inhibitor, rottlerin (18). As presented in Fig. 3A, lentiviral transduction of
lincRNA-p21 and lincRNA-p21-siRNA resulted in significant increase
and decrease of the expression of lincRNA-p21, respectively
compared with the control groups (P=0.0032). Inhibition of PKCδ by
rottlerin exhibited no significant effect on the expression of
lincRNA-p21 compared with the control groups. As demonstrated in
Fig. 3B, overexpression of
lincRNA-p21 significantly enhanced oxLDL-induced apoptosis from
~40% in the controls to ~93% (P=0.0007). Inhibition of PKCδ
signaling by rottlerin combined with lincRNA-21 overexpression
decreased the rate back to ~60%. However, knockdown of lincRNA-p21
significantly decreased oxLDL-induced apoptosis from ~40% in the
control group to ~14% (P=0.0014; Fig.
3B).
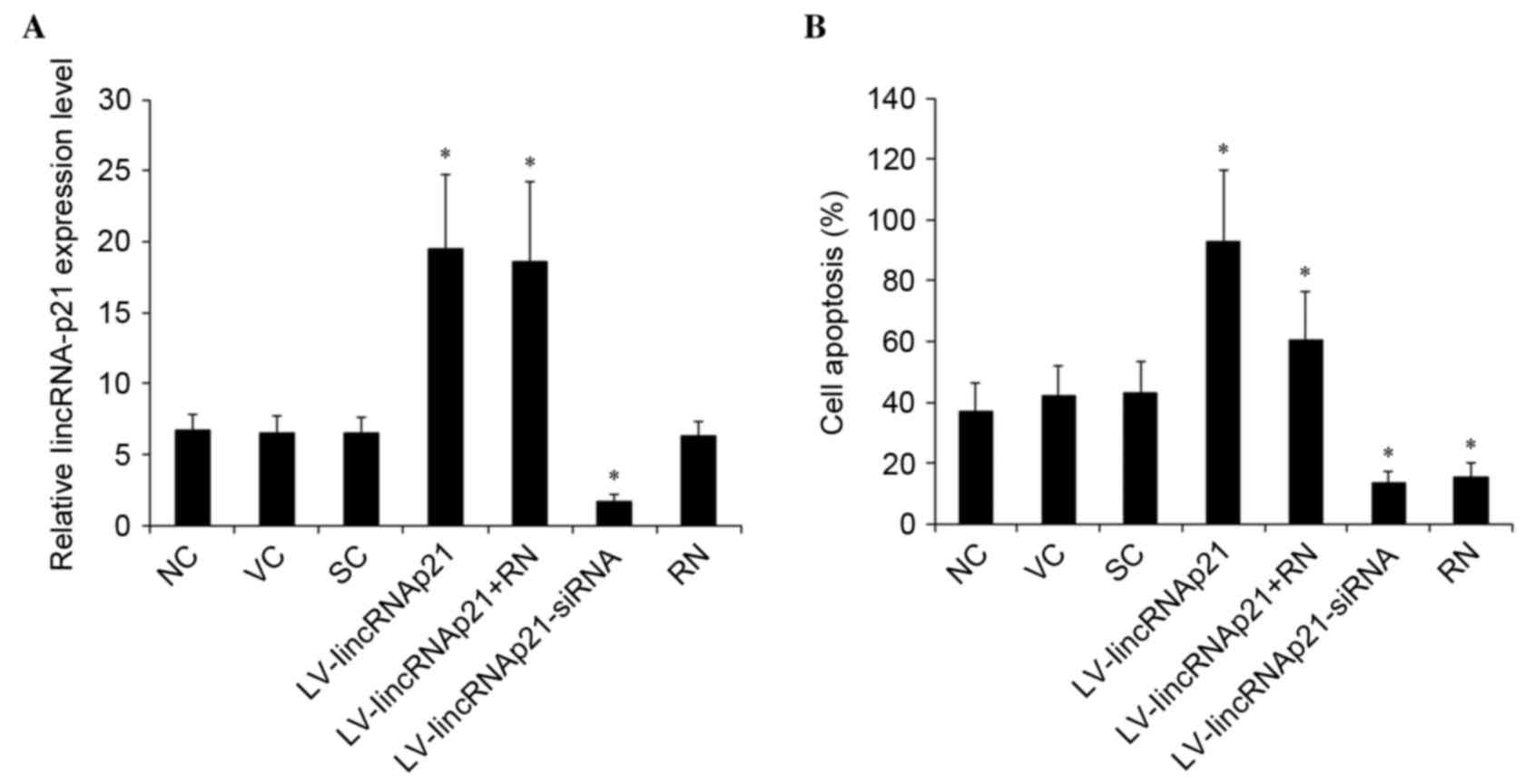 | Figure 3.Effect of linkRNA-p21 on apoptosis in
HCAECs under ox-LDL treatment. HCAECs were transduced with
LV-lincRNA-p21 or LV-lincRNA-p21-siRNA to overexpress or knockdown
lincRNA-p21, respectively. NC, VC and SC were used as controls. The
cells were treated with 90 µg/ml ox-LDL for 48 h with or without 1
µM selective protein kinase C-δ inhibitor, RN. (A) The expression
of lincRNA-p21 was measured with reverse transcription-quantitative
polymerase chain reaction and expressed as fold-changes to that of
HCAECs under normal culture conditions (0 µg/m ox-LDL treatment)
for 24 h (designated as 1) (B) The apoptosis rate of HCAECs was
measured with a microplate reader-based apoptosis detection kit.
*P<0.05 vs. NC, VC and SC. HCAECs, human coronary artery
endothelial cells; ox-LDL, oxidized low-density lipoprotein; LV,
lentivirus; NC, no LV transduction; VC, blank control lentivirus;
SC, scramble control lentivirus; lincRNA-p21, long intergenic
noncoding RNA p21; RN, Rottlerin; siRNA, small interfering RNA. |
In HCAECs treated with 90 µg/ml ox-LDL for 48 h, the
mRNA and protein expression of LOX-1 was measured and expressed as
fold-changes to that of HCAECs under normal culture conditions (0
µg/ml ox-LDL treatment) for 24 h (designated as 1). As demonstrated
in Fig. 4, ox-LDL significantly
induced the expression of LOX-1 in HCAECs, as the control groups
exhibited a >4-fold increase in expression of LOX-1 at both the
mRNA (Fig. 4A) and the protein
(Fig. 4B) levels. Compared with
the controls, overexpression of lincRNA-p21 significantly enhanced
the oxLDL-induced expression of LOX-1 (P=0.0025). The enhancing
effect of lincRNA-p21 on LOX-1 expression at the mRNA and the
protein levels was blocked by ~57% by rottlerin (Fig. 4). However, knockdown of lincRNA-p21
significantly decreased oxLDL-induced expression of LOX-1 at the
mRNA and the protein levels by ~72% compared with the control
groups (P=0.0006; Fig. 4).
Rottlerin alone blocked ~40% of oxLDL-induced apoptosis, suggesting
that PKCδ is important in oxLDL-induced endothelial apoptosis.
Taken together, the findings suggested that ox-LDL induced
apoptosis and the expression of LOX-1 in HCAECs partially through
lincRNA-p21 by a PKCδ-dependent mechanism.
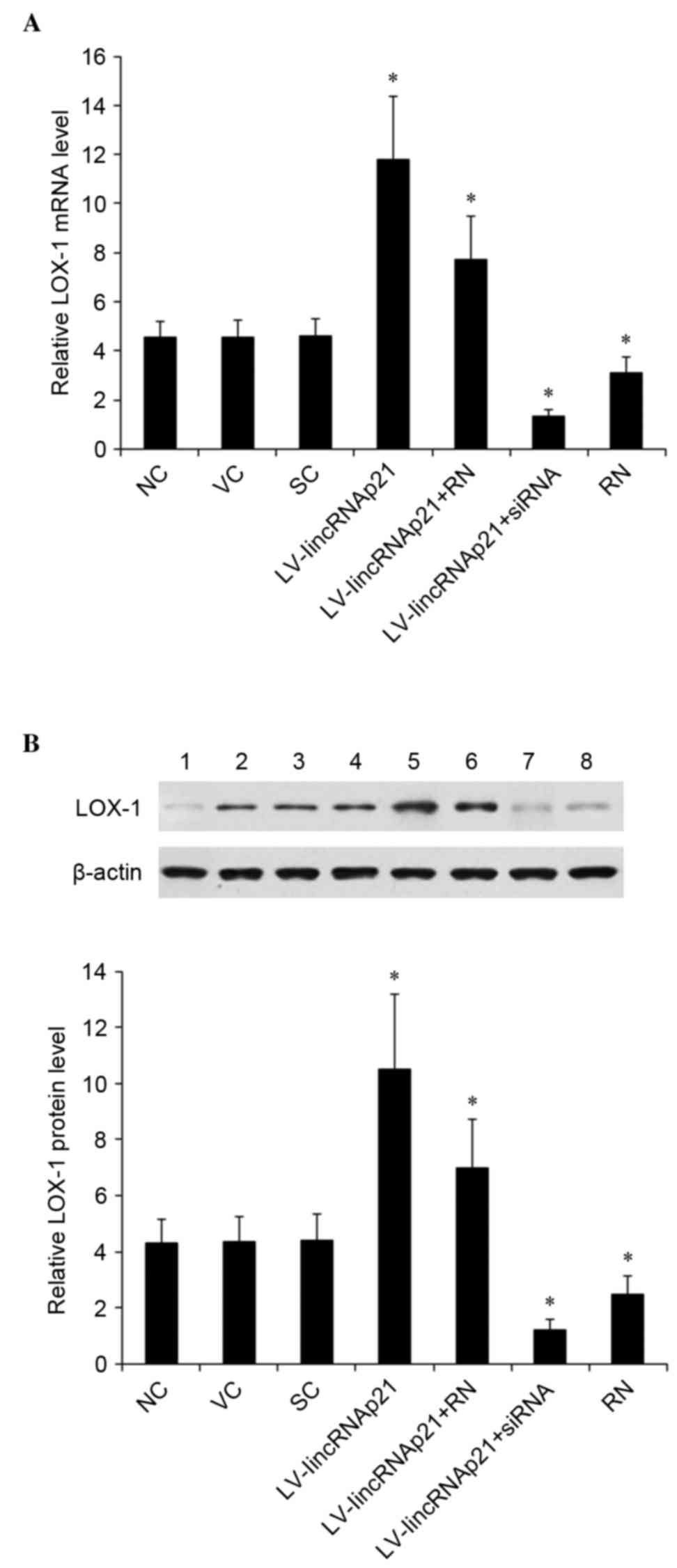 | Figure 4.Effect of lincRNA-p21 on expression of
LOX-1 in HCAECs under ox-LDL treatment. HCAECs were transduced with
LV-lincRNA-p21 or LV-lincRNA-p21-siRNA to overexpress or knockdown
lincRNA-p21, respectively. NC, VC and SC were used as controls. The
cells were treated with 90 µg/ml ox-LDL for 48 h with or without 1
µM of selective protein kinase C-δ inhibitor, RN. (A) The LOX-1
mRNA levels was measured with reverse transcription-quantitative
polymerase chain reaction and expressed as fold-changes to that of
HCAECs under normal culture conditions (0 µg/ml ox-LDL treatment)
for 24 h (designated as 1). (B) The LOX-1 protein levels were
measured with western blot analyses: Lane 1, HCAECs under normal
culture conditions (0 µg/ml ox-LDL treatment) for 24 h; lanes 2–8,
HCAECs treated with 90 µg/ml ox-LDL for 48 h; and plus, lane 2, NC;
lane 3, VC; lane 4, SC; lane 5, LV-lincRNA-p21; lane 6,
LV-lincRNA-p21 + RN; lane 7, lincRNA-p21-siRNA; lane 8, RN. b-actin
blotting was used as a loading control. Density of the LOX-1 blot
was normalized against that of the β-actin blot to obtain a
relative blot density, which was expressed as fold-changes to that
of HCAECs under normal culture conditions (0 µg/ml ox-LDL
treatment) for 24 h (designated as 1). *P<0.05 vs. NC, VC and
SC. HCAECs, human coronary artery endothelial cells; ox-LDL,
oxidized low-density lipoprotein; LV, lentivirus; NC, no LV
transduction; VC, blank control lentivirus; SC, scramble control
lentivirus; lincRNA-p21, long intergenic noncoding RNA p21; RN,
Rottlerin; siRNA, small interfering RNA. |
Effect of lincRNA-p21 on PKCδ activity
and phosphorylation in HCAECs under ox-LDL treatment
In HCAECs treated with 90 µg/ml ox-LDL for 48 h, the
PKCδ activity was measured and expressed as fold-changes to that of
HCAECs under normal culture conditions (0 µg/ml ox-LDL treatment)
for 24 h (designated as 1). As demonstrate in Fig. 5A, ox-LDL significantly induced the
PKCδ activity in HCAECs, as the control groups exhibited a
~3.65-fold increase in PKCδ activity compared with 0 µg/ml ox-LDL.
Compared with the controls, overexpression of lincRNA-p21
significantly enhanced the oxLDL-induced PKCδ activity (P<0.05).
The enhancing effect of lincRNA-p21 and ox-LDL was completely
blocked by rottlerin (Fig. 5A).
However, knockdown of lincRNA-p21 significantly decreased
oxLDL-induced PKCδ activity by ~69% compared with the control
groups (Fig. 5A). A similar data
trend was observed with the PKCδ phosphorylation at Tyr 155
(Fig. 5B) (21,22).
The findings indicated that PKCδ is a downstream effector of
lincRNA-p21 in HCAECs.
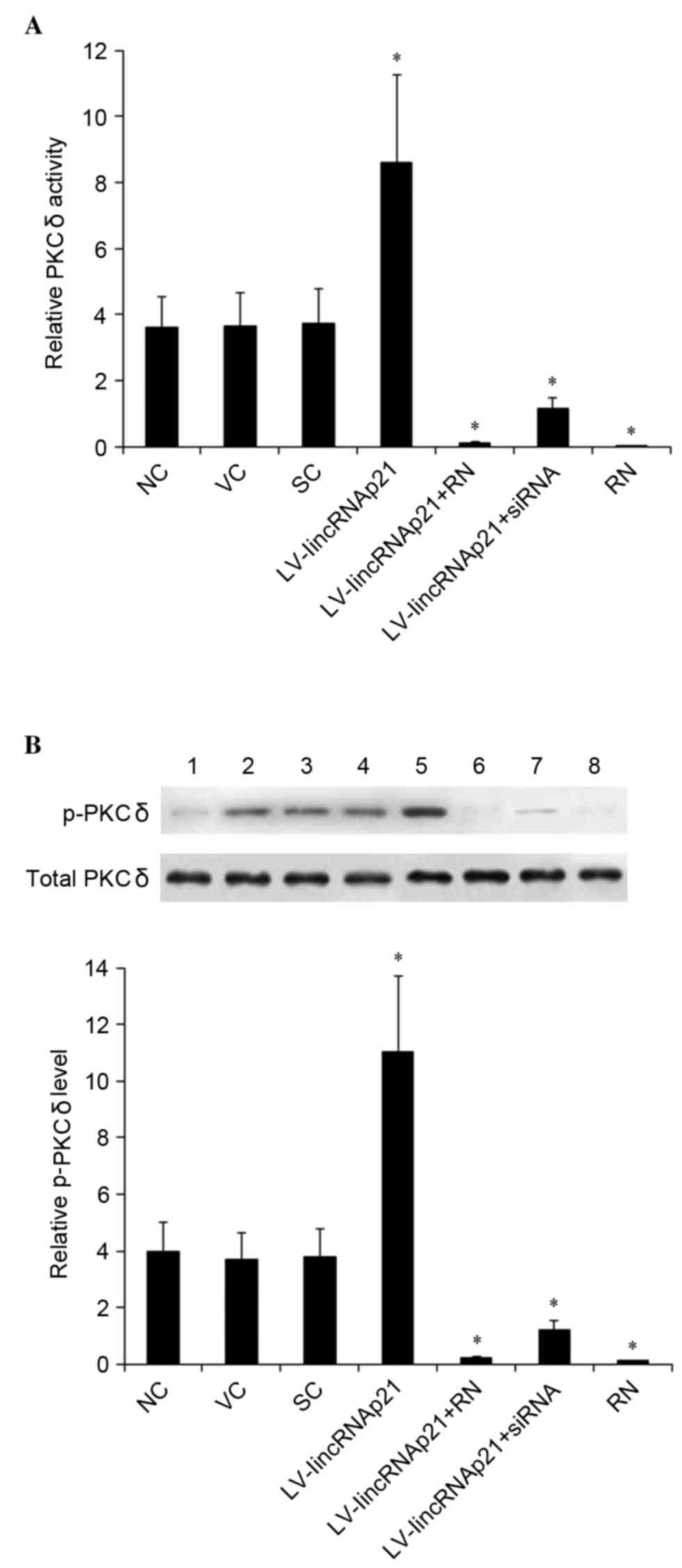 | Figure 5.Effect of linkRNA-p21 on PKCδ activity
and p-PKCδ levels in in HCAECs under ox-LDL treatment. HCAECs were
transduced with LV-lincRNA-p21 or LV-lincRNA-p21-siRNA to
overexpress or knockdown lincRNA-p21, respectively. NC, VC and SC
were used as controls. The cells were treated with 90 µg/ml ox-LDL
for 48 h with or without 1 µM selective PKCδ inhibitor, RN. (A) The
PKCδ activity was measured and expressed as fold-changes to that of
HCAECs under normal culture conditions (0 µg/ml ox-LDL treatment)
for 24 h (designated as 1) (B) Levels of total PKCδ and p-PKCδ at
Tyr 155 were determined by western blot analyses: Lane 1, HCAECs
under normal culture conditions (0 µg/ml ox-LDL treatment) for 24
h; lanes 2–8, HCAECs treated with 90 µg/ml ox-LDL for 48 h; and
plus, lane 2, NC; lane 3, VC; lane 4, SC; lane 5, LV-lincRNA-p21;
lane 6, LV-lincRNA-p21 + RN; lane 7, lincRNA-p21-siRNA; lane 8, RN.
The total PKCδ level was not significantly altered by ox-LDL
treatment. Density of the p-PKCδ (Tyr 155) blot was normalized
against that of total PKCδ to obtain a relative blot density, which
was expressed as fold-changes to that of HCAECs under normal
culture conditions (0 µg/ml ox-LDL treatment) for 24 h (designated
as 1). *P<0.05 vs. NC, SC and VC. HCAECs, human coronary artery
endothelial cells; ox-LDL, oxidized low-density lipoprotein; PKCδ,
protein kinase C δ; p-, phosphorylated; LV, lentivirus; NC, no LV
transduction; VC, blank control lentivirus; SC, scramble control
lentivirus; lincRNA-p21, long intergenic noncoding RNA p21; RN,
Rottlerin; siRNA, small interfering RNA. |
Discussion
It has been previous suggested that inhibiting
oxLDL-induced endothelial cell apoptosis is a potential novel
therapeutic strategy against atherosclerosis (23). Recent studies have revealed that
endothelial LOX-1 and lincRNA-p21 may serve as therapeutic targets
for atherosclerosis and associated cardiovascular disorders
(8,14,15).
To the best of our knowledge, the present study provides the first
evidence suggesting that lincRNA-p21 mediates oxLDL-induced
apoptosis and expression of LOX-1 in human vascular endothelial
cells, using HCAECs as an in vitro cell model.
In support of previous studies (9), the present study demonstrated that
ox-LDL induced increased expression of LOX-1 and apoptosis in
HCAECs. It was also demonstrated that ox-LDL induced increased
expression of lincRNA-p21 in parallel, suggesting that lincRNA-p21
may be associated with oxLDL-induced expression of LOX-1 and
apoptosis in HCAECs. Lentiviral overexpression and knockdown of
lincRNA-p21 markedly increased and decreased oxLDL-induced
apoptosis and the expression of LOX-1, respectively, suggesting
that lincRNA-p21 is a major mediator of the effects of ox-LDL on
the expression of LOX-1 and apoptosis in human vascular endothelial
cells.
LOX-1 binds and internalizes ox-LDL by
receptor-mediated endocytosis, which is the initial step that leads
to oxLDL-induced apoptosis (7). In
the current study, ox-LDL induced the expression of lincRNA-p21,
which enhanced oxLDL-induced expression of LOX-1. The findings
suggest that the oxLDL/LOX-1/lincRNA-p21 signaling may form a
positive feedback loop, which facilitates the effect of ox-LDL on
human vascular endothelial cells as manifested by enhanced
apoptosis of HCAECs under ox-LDL treatment. Notably, although
rottlerin, a selective PKCδ inhibitor, did not induce a significant
effect on the expression of lincRNA-p21, it largely blocked the
enhancing effect of lincRNA-p21 on oxLDL-induced expression of
LOX-1 and apoptosis, suggesting that lincRNA-p21 mediates the
effects of ox-LDL on human vascular endothelial cells partially via
a PKCδ-dependent mechanism. This was subsequently corroborated by
PKCδ activity assay results and the activation phosphorylation
levels of PKCδ, confirming that PKCδ is a downstream effector of
lincRNA-p21 in HCAECs.
It has been previously reported that lincRNA-p21
acts via several mechanisms, ranging from repressing genes in the
p53 transcriptional network, to regulating mRNA translation and
protein stability (13). Wu et
al (15) demonstrated that
lincRNA-p21, a transcriptional target of p53, regulates vascular
smooth muscle cell apoptosis and atherosclerosis by feeding back to
enhance p53 transcriptional activity. Previous studies also have
reported that PKCδ mediates activation of p53 and promotes cell
apoptosis (24–26). The findings of the current study
suggest that lincRNA-p21 activates PKCδ. This may enhance
oxLDL-induced HCAEC apoptosis by: i) Increasing oxLDL-induced
expression of LOX-1 and facilitating the positive feedback loop of
oxLDL/LOX-1/lincRNA-p21 signaling; and/or ii) directly activating
p53. As inhibition of PKCδ activity blocked the majority, but not
all, of the enhancing effect of lincRNA-p21 on oxLDL-induced
expression of LOX-1 and apoptosis, other mechanisms may be
involved. Future studies are required to determine this.
Other than atherosclerosis, LOX-1 is reportedly also
critical in the pathogenesis of hypertension, myocardial
infarction, congestive heart failure, vascular diseases and
thrombosis (7). It will be
intriguing to determine the role of oxLDL/LOX-1/lincRNA-p21
signaling in other cardiovascular diseases in addition to
atherosclerosis in future studies. In conclusion, the findings of
the present study suggest that lincRNA-p21 is a major mediator of
oxLDL-induced expression of LOX-1 and apoptosis in human vascular
endothelial cells, predominantly by activating PKCδ. This provides
novel insights into the role of lincRNA-p21 in the pathogenesis of
atherosclerosis.
References
|
1
|
Chen Y, Li D, Xu Y, Zhang Y, Tao L, Li S,
Jiang Y and Shen X: Essential oils from Fructus A. zerumbet protect
human aortic endothelial cells from apoptosis induced by Ox-LDL in
vitro. Evid Based Complement Alternat Med. 2014:9568242014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Zhang Y, Zhu Y and Zhang P:
Lipolytic inhibitor G0/G1 switch gene 2 inhibits reactive oxygen
species production and apoptosis in endothelial cells. Am J Physiol
Cell Physiol. 308:C496–C504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang HC, Chen TG, Tai YT, Chen TL, Chiu
WT and Chen RM: Resveratrol attenuates oxidized LDL-evoked Lox-1
signaling and consequently protects against apoptotic insults to
cerebrovascular endothelial cells. J Cereb Blood Flow Metab.
31:842–854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitra S, Deshmukh A, Sachdeva R, Lu J and
Mehta JL: Oxidized low-density lipoprotein and atherosclerosis
implications in antioxidant therapy. Am J Med Sci. 342:135–142.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taye A and El-Sheikh AA: Lectin-like
oxidized low-density lipoprotein receptor 1 pathways. Eur J Clin
Invest. 43:740–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu S, Ogura S, Chen J, Little PJ, Moss J
and Liu P: LOX-1 in atherosclerosis: Biological functions and
pharmacological modifiers. Cell Mol Life Sci. 70:2859–2872. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pirillo A, Norata GD and Catapano AL:
LOX-1, OxLDL, and atherosclerosis angela. Mediators Inflamm.
2013:1527862013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercer TR and Mattick JS: Structure and
function of long noncoding RNAs in epigenetic regulation. Nat
Struct Mol Biol. 20:300–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu W, Alvarez-Dominguez JR and Lodish HF:
Regulation of mammalian cell differentiation by long non-coding
RNAs. EMBO Rep. 13:971–983. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song X, Shan D, Chen J and Jing Q: miRNAs
and lncRNAs in vascular injury and remodeling. Sci China Life Sci.
57:826–835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang N, Fu Y, Zhang H, Sima H, Zhu N and
Yang G: LincRNA-p21 activates endoplasmic reticulum stress and
inhibits hepatocellular carcinoma. Oncotarget. 6:28151–28163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He C, Ding JW, Li S, Wu H, Jiang YR, Yang
W, Teng L and Yang J and Yang J: The role of long intergenic
noncoding RNA p21 in vascular endothelial cells. DNA Cell Biol.
34:677–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen
C, Cai Y, Huang H, Yang Y, Liu Y, et al: LincRNA-p21 regulates
neointima formation, vascular smooth muscle cell proliferation,
apoptosis and atherosclerosis by enhancing p53 activity.
Circulation. 130:1452–1465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mackness MI and Durrington PN: Lipoprotein
separation and analysis for clinical studiesLipoprotein Analysis: A
Practical Approach. Converse CA and Skinner ER: Oxford University
Press; Oxford: pp. 1–42. 1992
|
|
17
|
Sparrow CP, Parthasarathy S and Steinberg
D: Enzymatic modification of low density lipoprotein by purified
lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative
modification. J Lipid Res. 29:745–753. 1988.PubMed/NCBI
|
|
18
|
Perissi V, Scafoglio C, Zhang J, Ohgi KA,
Rose DW, Glass CK and Rosenfeld MG: TBL1 and TBLR1 phosphorylation
on regulated gene promoters overcomes dual CtBP and NCoR/SMRT
transcriptional repression checkpoints. Mol Cell. 29:755–766. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmitten TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Pabla N, Wei Q, Dong G, Messing RO,
Wang CY and Dong Z: PKC-delta promotes renal tubular cell apoptosis
associated with proteinuria. J Am Soc Nephrol. 21:1115–1124. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Konishi H, Tanaka M, Takemura Y, Matsuzaki
H, Ono Y, Kikkawa U and Nishizuka Y: Activation of protein kinase C
by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci
USA. 94:pp. 11233–11237. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramnath RD, Sun J, Adhikari S, Zhi L and
Bhatia M: Role of PKC-delta on substance P-induced chemokine
synthesis in pancreatic acinar cells. Am J Physiol Cell Physiol.
294:C683–C692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin B, Cao Y, Yang H, Xiao B and Lu Z:
MicroRNA-221/222 regulate ox-LDL-induced endothelial apoptosis via
Ets-1/p21 inhibition. Mol Cell Biochem. 405:115–124. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coutinho I, Pereira C, Pereira G,
Gonçalves J, Côrte-Real M and Saraiva L: Distinct regulation of
p53-mediated apoptosis by protein kinase Cα, δ, ε and ζ: Evidence
in yeast for transcription-dependent and -independent p53 apoptotic
mechanisms. Exp Cell Res. 317:1147–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu HM, Schally AV, Cheng JC, Zarandi M,
Varga J and Leung PC: Growth hormone-releasing hormone antagonist
induces apoptosis of human endometrial cancer cells through
pkcδ-mediated activation of p53/p21. Cancer Lett. 298:16–25. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schisano B, Tripathi G, McGee K, McTernan
PG and Ceriello A: Glucose oscillations, more than constant high
glucose, induce p53 activation and a metabolic memory in human
endothelial cells. Diabetologia. 54:1219–1226. 2011. View Article : Google Scholar : PubMed/NCBI
|