Introduction
The most common type of chronic intermittent hypoxia
(CIH) is obstructive sleep apnea hypopnea syndrome (OSAHS), which
is characterized by repeated upper airway obstruction and
frequently-interrupted breathing during sleep; the condition is
potentially life-threatening (1).
By enhancing oxidative stress (OS), inflammation and sympathetic
nerve discharge, CIH, in addition to sleep structure disorders, may
stimulate a series of alterations in the molecular biology of the
patient (2).
The majority of patients with OSAHS additionally
exhibit hypertension, and more serious sleep apnea is associated
with increased hypertension, which is a risk factor for myocardial
and cerebral infarction (3).
Hypertension has been observed to be highly prevalent in patients
with OSAHS, with an incidence of 30–60% (4). According to previous studies, OSAHS
is present in 30–40% of patients with hypertension (5). Although OSAHS has been categorized as
one of the primary factors causing secondary hypertension, the
mechanisms of hypertension induced by OSAHS remain unclear. At
present, there are numerous processes which have been hypothesized
to contribute to hypertension, including abnormal nerve reflex
(6), humoral factors (7), vascular endothelial dysfunction
(8), inflammation (9) and vascular construction (10).
CIH is characterized by a low oxygen period,
distinct from a normal oxygen period (11). However, CIH, in addition to
continuous hypoxia, may induce inflammation. The underlying
mechanisms of OSAHS-induced hypertension remain to be elucidated.
It has previously been demonstrated that the circulating levels of
interleukin (IL)-6, tumor necrosis factor (TNF)-α and C-reactive
protein (CRP) are elevated in patients with OSAHS (12–15),
indicating that CIH may be associated with systemic inflammation.
In the initiation and progression of hypertension, endothelial
dysfunction occurs early, in response to the inflammatory cytokines
(16). This process may result in
an increase in intercellular adhesion molecules, leading to the
promotion of leukocyte adherence to the endothelium, in addition to
extravasation (16). Hypertension
may subsequently develop.
Additionally, the hypoxia-reoxygenation cycles
during CIH may induce the production of reactive oxygen species
(ROS) and OS (17). Following CIH
exposure, endothelin 1 and angiotensin II have been observed to
increase due to ROS-mediation, which may enhance chemosensitivity
in carotid bodies (18,19), while nitric oxide synthase (NOS)
may inhibit chemoreceptor function (20), including endothelial NOS (eNOS) and
neuronal NOS (nNOS).
There are numerous individuals belonging to the
Hakka population in Guangdong Province (China), and these
individuals are generally very careful about their health. Certain
common customs within this population, for example, stewing soup
for >2 h, making wine, plum liqueur or snake wine and cooking
with less salt or oil, are claimed to be beneficial to health and
in reducing diseases. Wine has been confirmed to soften the
vasculature and prevent cardiovascular diseases (21), and slow cooking soup has been
anecdotally claimed to maintain beauty and good health. However,
the incidence of CIH within the Hakka population has not previously
been investigated.
Therefore, the present study aimed to investigate
the association between inflammation, OS, CIH and hypertension by
comparing the levels of IL-6, TNF-α, CRP, nitric oxide (NO) and NOS
in Hakka patients from Huizhou City with non-Hakka patients.
Additionally, CIH rat models were established and the 5 factors
were detected in isolated lymphocytes and co-cultured
endotheliocytes.
Materials and methods
Patients
A total of 238 patients, including 106 Hakka
patients and 132 non-Hakka patients, from Huizhou (China), were
diagnosed with CIH combined with hypertension in Nanfang Hospital
(Guangzhou, China) between March 2013 and October 2016. A total of
156 volunteers (non-Hakka; from Huizhou) were recruited as healthy
controls during the same time periods as the patients. Clinical
data including age, sex, weight, systolic arterial pressure (SAP),
diastolic arterial pressure (DAP), mean arterial pressure (MAP),
heart rate (HR), smoking and type 2 diabetes (T2DM) history were
obtained for analysis. Blood samples were obtained at 9:00 a.m. at
the initial clinic visit and stored at −28°C.
Informed consent was obtained from each patient and
volunteer, and the present study was approved by the Medical Ethics
Committees of Nanfang Hospital (Guangzhou, China) and Renmin
Hospital of Wuhan University (Wuhan, China).
Animals
A total of 10 male Wistar rats (age, 60–70 days;
weight, 191.42±14.05 g) were obtained from Qingdao KangDa
Biotechnology Co., Ltd. (Qingdao, China) and housed individually in
polycarbonate cages with wire lids, under the conditions of 12-h
light/dark cycle, 22–25°C and 50–70% humidity. Standard laboratory
diet and water were supplied freely. The animal welfare and
experimental protocols were approved by the Animal Research Ethics
Committee of Renmin Hospital of Wuhan University. The experiments
were performed in accordance with the National Institutes of Health
Principles of Laboratory Animal Care (NIP Publication 85–23,
revised 1996), the European Guidelines for the Protection of
Animals used for Scientific Purposes (European directive
2010/63/EU) and Portuguese Law no. 113/2013.
CIH models
The 10 rats were randomly divided into 2 groups,
control and CIH. Rats in the CIH group were placed in a dynamic
O2/N2 controller (OxycyclerA84A-chamber;
BioSpherix, Ltd., New York, NY, USA) and exposed to 5 min cycles of
90 sec hypoxia (5% O2) and 210 sec normoxia (21%
O2), 8 h/day for 3 weeks during the light hours. Rats in
the control group were exposed to the same environment but with
normoxia (21% O2) maintained 24 h/day for 3 weeks. The
MouseOX pulse oximetry system (Starr Life Sciences Corp., Oakmont,
PA, USA) was used to measure the arterial oxygen saturation
(SaO2) as ~70%, according to the specifications of the
instrument.
Hemodynamic analysis
SAP, DAP, MAP and HR in human subjects were measured
according to the clinical protocol ‘Essential hypertension for
primary healthcare level’ (22).
Hemodynamic analysis in rats was performed using Mouse and Rat Tail
Cuff Blood Pressure system and analyzed using the equipped software
(3R22931; IITC Life Science, Inc., Woodland Hills, CA, USA),
according to the manufacturer's specifications, one day prior to
model establishment and at the 7th, 14th and 21st days of
experimentation.
Serum IL-6, TNF-α, CRP, NO and NOS
detection
For patients, blood samples were concentrated at
3,000 × g at 4°C for 10 min and serum was separated. For rats,
weights were measured and blood was collected at 9:00 a.m., one day
prior to model establishment and on the 7th, 14th and 21st day of
experiments. Blood samples were centrifuged at 3,000 × g for 10 min
at 4°C to separate the serum. ELISA kits for Serum IL-6 (CHC1263,
Thermo Fisher Scientific, Inc., Waltham, MA, USA), TNF-α (RAB0340)
and CRP (CYT298) (both from Sigma-Aldrich; Merck KGaA) were used.
NO and NOS levels were determined using Total Nitric Oxide Assay
and Nitric Oxide Synthase Assay kits (Beyotime Institute of
Biotechnology, Haimen, China), respectively.
Lymphocyte isolation
The lymphocytes of rats in the two groups were
isolated using Ficoll-Histopaque (Sigma-Aldrich; Merck KGaA) double
density gradient centrifugation. A total of 3 ml Histopaque 1119
was added to a 15 ml tube, and 3 ml Histopaque 1083 was added on
top. A total of 6 ml blood sample was added onto the Histopaque
1083, and centrifuged at 700 × g for 30 min. There were 6 levels
following centrifugation, including serum, monocytes, Histopaque
1083, neutrophils, Histopaque 1119 and erythrocytes. The serum,
monocytes and Histopaque 1083 were discarded to leave the
neutrophils at 0.5 cm thickness. The neutrophils were transferred
to a new tube and 10 ml 1X PBS was added to wash twice. The tube
was centrifuged at 200 × g for 10 min, then the supernatant
discarded, and the sample was resuspended with RPMI-1640 (Thermo
Fisher Scientific, Inc.) at a density of 1×106 cells/ml.
The lymphocytes were cultured with RPMI-1640 containing 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C, 5%
CO2 and saturated humidity.
Detection of apoptosis rate and IL-6,
TNF-α, CRP, NO and iNOS levels in lymphocytes
Isolated lymphocytes in the logarithmic growth phase
were used for the following experiments (1×105
cells/ml). A propidium iodide staining kit (Beyotime Institute of
Biotechnology) was used to detect apoptosis in rat lymphocytes,
according to the manufacturer's protocol, and the results were
observed under a fluorescence microscope at 535 nm. Western
blotting was used to detect IL-6, TNF-α, CRP and iNOS levels in
lymphocytes. Radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.) was added to the lymphocytes according to the
manufacturer's protocol. The cells were incubated on ice for 10 min
and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant
was separated and 5X loading buffer (Beyotime Institute of
Biotechnology) was added. The total protein concentration was
determined using a bicinchoninic acid protein quantitation kit
(Beyotime Institute of Biotechnology). A total of 40 µg
protein/lane was separated by SDS-PAGE with a 5% stacking gel and a
10% separating gel. The proteins were transferred to polyvinylidene
fluoride membranes (Merck KGaA). Rabbit anti-IL-6 (21865–1-AP,
1:2,000), rabbit anti-TNF-α (17590-1-AP, 1:2,000), rabbit anti-CRP
(13432-1-AP, 1:2,000), rabbit anti-iNOS (18985-1-AP, 1:2,000),
mouse anti-β-actin (60008-1-AP, 1:10,000) (all from Wuhan Sanying
Biotechnology, Wuhan, China) were used to incubate at 4°C
overnight. The membranes were then incubated with goat anti-mouse
IgG HRP (223-005-024) or goat anti-rabbit secondary antibodies
(323-005-024, 1:3,000) (both from Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) at 25°C for 1 h. Enhanced
chemiluminescence (WBKLS0500; Merck KGaA) substrate was used to
visualize proteins. Semi-quantification was performed using ImageJ
1.48 (National Institutes of Health, Bethesda, MD, USA). NO
detection was performed using the Total Nitric Oxide Assay kit.
CIH induction and detection of IL-6,
TNF-α, CRP, NO, eNOS and iNOS levels in endotheliocytes
Rat aortic endotheliocytes were obtained from the
American Type Culture Collection (RAOEC; Manassas, VA, USA) and
cultured in RPMI-1640 with 10% FBS at 37°C, 5% CO2 and
saturated humidity. A three-gas incubator (C42; BioSpherix, Ltd.)
was used for to induce a CIH environment with hypoxia (1.5%
O2, 45 sec)-normoxia (21% O2, 135 sec), 20
times/h for a total of 60 cycles. Rat endotheliocytes were induced
for 1, 2 and 3 h.
The IL-6, TNF-α, CRP, eNOS and iNOS levels in
endotheliocytes were detected by western blotting and NO was
detected using the Total Nitric Oxide Assay kit.
Co-culturing of isolated lymphocytes
and endotheliocytes
A total of 4 groups were established: CIH-L+CIH-E
(CIH rat lymphocytes + CIH-induced rat endotheliocytes);
CIH-L+CON-E (CIH rat lymphocytes + endotheliocytes); CON-L+CIH-E
(lymphocytes + CIH-induced rat endotheliocytes); and CON-L+CON-E
(lymphocytes + endotheliocytes). Rat lymphocytes (1×105
cells/ml) were added to the rat endotheliocytes (1×105
cells/ml) with or without CIH induction, and co-cultured for 4
h.
Co-culturing supernatant IL-6, TNF-α,
CRP, NO and NOS levels detections
Subsequent to co-culturing, the supernatant of the
cells was collected. IL-6 (KRC0061C), TNF-α (KRC3011) and CRP
(88-7501-28) (all from Thermo Fisher Scientific, Inc.) were
measured using ELISA kits. NO and NOS were determined using the
Total Nitric Oxide Assay and Nitric Oxide Synthase Assay kits,
respectively.
Statistical analysis
The statistical analysis was performed using SPSS
19.0 software (IBM Corp., Armonk, NY, USA). The continuous
variables are expressed as the mean ± standard deviation. The
comparisons between groups were analyzed using one-way analysis of
variance. Multiple comparisons were analyzed using Dunnett's T3
post hoc test. The correlations between clinical data or serum
factors and CIH were evaluated using Pearson's correlation
coefficient. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical data and hemodynamic analysis
in Hakka patients
Comparisons between Hakka and non-Hakka patients
with CIH combined with hypertension and healthy volunteers are
presented in Table I and Fig. 1, including age, sex, body mass
index (BMI), smoking and T2DM history, hemodynamic changes (SAP,
DAP, MAP and HR), and serum markers (IL-6, TNF-α, CRP, NO and NOS).
The odds ratio and r (correlation coefficient) values are
representative of the entire cohort.
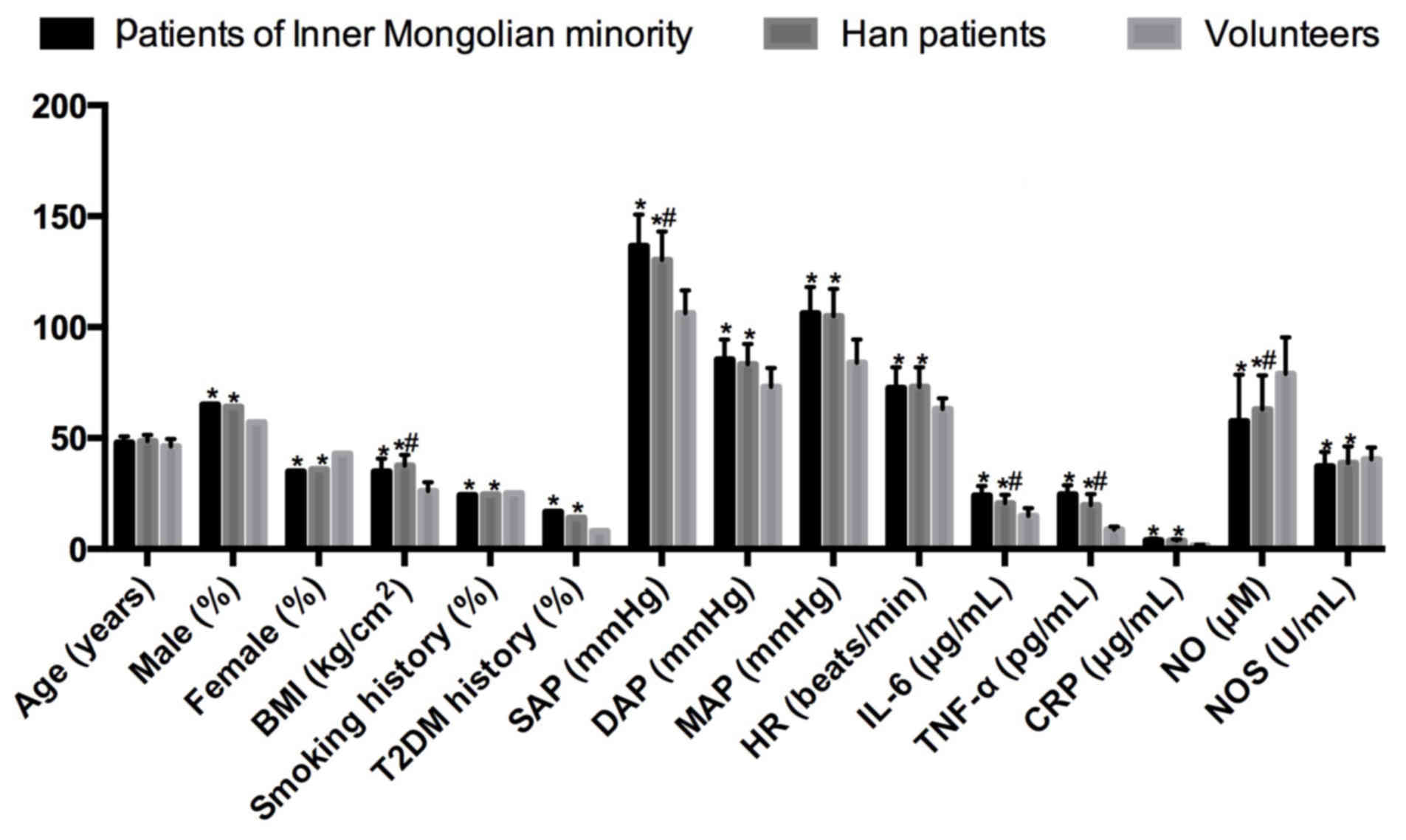 | Figure 1.Comparisons of clinical data,
hemodynamic changes, serum IL-6, TNF-α, CRP, NO and NOS in
non-Hakka and Hakka patients with CIH combined with hypertension,
and healthy volunteers. *P<0.05 vs. healthy volunteers;
#P<0.05 vs. Hakka patients. CIH, chronic intermittent
hypoxia; OR, odds ratio; r, correlation coefficient; BMI, body mass
index; T2DM, type 2 diabetes mellitus; SAP, systolic arterial
pressure; DAP, diastolic arterial pressure; MAP, mean arterial
pressure; HR, heart rate; IL-6, interleukin-6; TNF-α, tumor
necrosis factor-α; CRP, C-reactive protein; NO, nitric oxide; NOS,
nitric oxide synthase. |
 | Table I.Clinical data, hemodynamic
alterations, serum IL-6, TNF-α, CRP, NO and NOS in Hakka and
non-Hakka patients with CIH combined with hypertension, compared
with healthy volunteers (mean ± standard deviation). |
Table I.
Clinical data, hemodynamic
alterations, serum IL-6, TNF-α, CRP, NO and NOS in Hakka and
non-Hakka patients with CIH combined with hypertension, compared
with healthy volunteers (mean ± standard deviation).
| Variable | Non-Hakka patients
(n=132) | Hakka patients
(n=106) | Healthy volunteers
(n=156) | OR | P-value | r |
|---|
| Age, years |
47.93±2.75a |
48.52±2.90a | 46.02±3.24 | 1.73 | <0.001 | 0.536 |
| Sex, n (%) |
|
Male | 86
(65.15a) | 68,
64.15a | 89 (57.05) | 2.07 | <0.001 | 0.681 |
|
Female | 46
(34.85a) | 38,
35.85a | 67 (42.95) | 0.85 | <0.001 | −0.326 |
| BMI,
kg/cm2 |
34.96±5.81a |
37.65±4.82a,b | 26.04±3.97 | 3.48 | <0.001 | 0.710 |
| Smoking history, n
(%) | 32
(24.24a) | 26,
24.53a | 25 (15.72) | 1.64 | 0.019 | 0.611 |
| T2DM history, n
(%) | 22
(16.67a) | 15,
14.15a | 8 (5.13) | 4.83 | <0.001 | 0.692 |
| SAP, mmHg |
136.58±14.18a |
130.17±12.93a,b | 106.24±10.37 | 2.14 | <0.001 | 0.542 |
| DAP, mmHg |
85.44±8.92a |
83.20±9.25a | 73.03±8.50 | 1.83 | <0.001 | 0.602 |
| MAP, mmHg |
106.25±11.77a |
104.92±12.35a | 83.96±10.45 | 1.95 | <0.001 | 0.575 |
| HR, bpm |
72.59±9.40a |
73.08±8.86a | 63.15±4.82 | 1.79 | 0.016 | 0.326 |
| IL-6, µg/ml |
24.13±4.22a |
20.65±3.82a,b | 14.85±3.60 | 2.37 | <0.001 | 0.787 |
| TNF-α, pg/ml |
24.51±4.26a |
19.79±5.04a,b | 8.67±1.33 | 2.54 | <0.001 | 0.619 |
| CRP, µg/ml |
3.94±0.85a |
3.70±0.73a | 1.56±0.38 | 1.60 | <0.001 | 0.328 |
| NO, µM |
57.64±20.86a |
62.90±15.37a,b | 78.94±16.55 | 0.72 | <0.001 | −0.206 |
| NOS, U/ml |
37.28±6.50a |
38.83±7.45a | 40.36±5.42 | 0.88 | 0.030 | −0.443 |
The patients with CIH combined with hypertension
were older (r=0.536), and more of them were males (r=0.681). The
patients exhibited increased BMI (r=0.710) compared with the
healthy volunteers, while the BMI of non-Hakka patients was
significantly decreased compared with Hakka patients (P<0.05).
More patients had a history of smoking (r=0.611) and T2DM (r=0.692)
compared with the healthy volunteers.
According to the results of the hemodynamic
analysis, the patients exhibited increased SAP (r=0.542), DAP
(r=0.602), MAP (r=0.575) and HR (r=0.326) compared with the healthy
volunteers. However, the SAP of Hakka patients was significantly
decreased compared with that of non-Hakka patients (P<0.05).
The inflammatory factors, IL-6 (r=0.787), TNF-α
(r=0.619) and CRP (r=0.328), were increased in the patients
compared with the healthy volunteers, while NO (r=-0.206) and NOS
(r=-0.443) were decreased. The IL-6 and TNF-α levels were increased
in non-Hakka patients compared with Hakka patients, while NO levels
were significantly decreased (P<0.05).
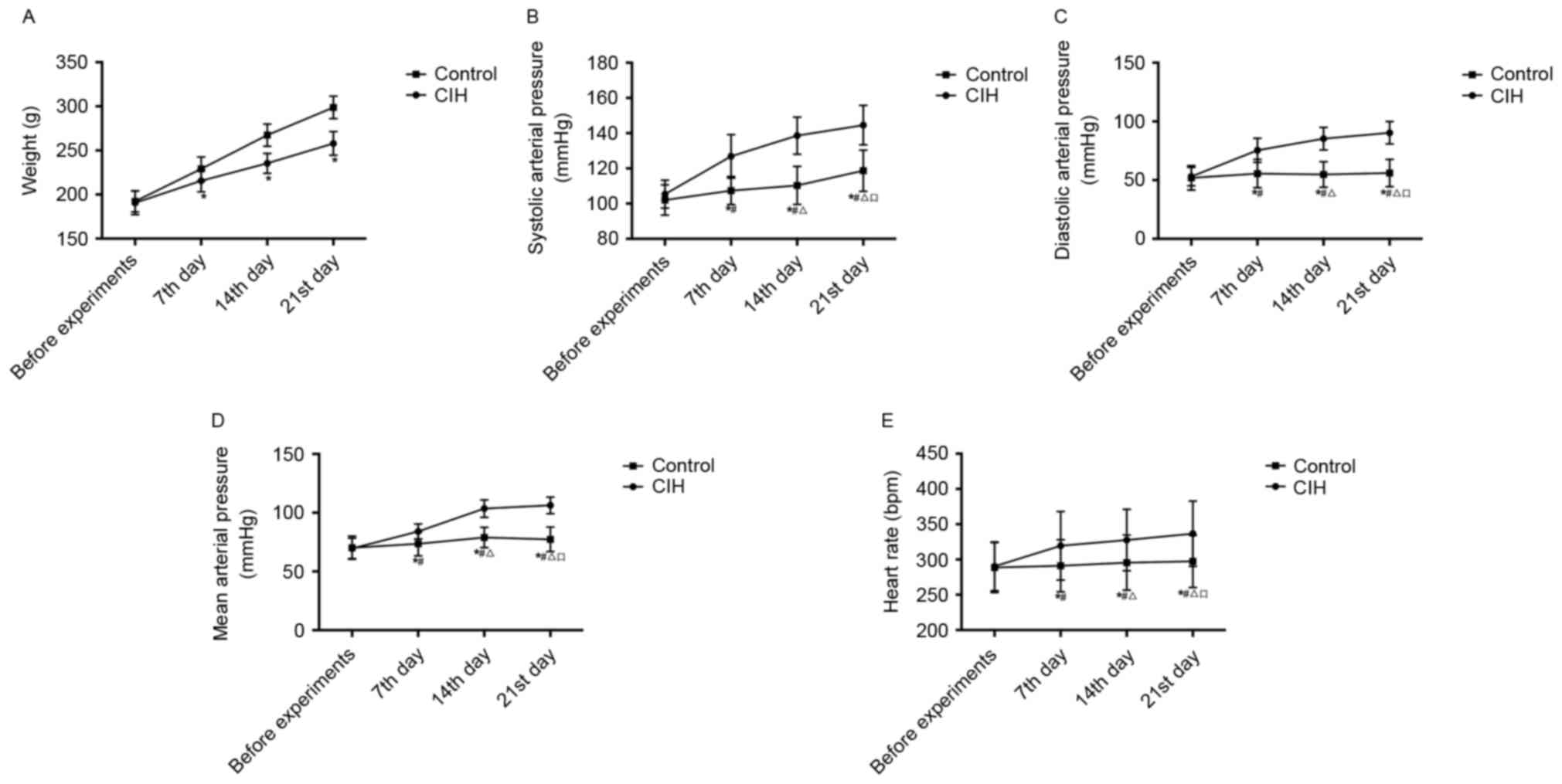
Rat weight and hemodynamic
alterations
The weight changes and comparisons between CIH and
healthy rats are presented in Table
II and Fig. 2A. Prior to the
experiments, the average weight of the CIH rats was not
significantly different from that of the control group. During the
experiments, the average weight of the CIH and healthy rats
increased gradually. However, the CIH rats exhibited decreased
activity and appetite, resulting in the average CIH rat weight
being significantly decreased compared with that of the healthy
rats on the 7th, 14th and 21st days (P<0.05).
 | Table II.Weight changes in rats with or
without CIH (n=10; mean ± standard deviation). |
Table II.
Weight changes in rats with or
without CIH (n=10; mean ± standard deviation).
|
|
|
| During experiments,
g |
|---|
|
|
|
|
|
|---|
| Group | No. of rats | Prior to
experiments, g | 7th day | 14th day | 21st day |
|---|
| CIH | 5 |
190.56±13.39 |
215.51±12.38a |
235.40±11.25a |
257.93±13.43a |
| Control | 5 |
192.27±12.04 |
229.07±13.58 |
267.36±12.60 |
298.83±12.81 |
The alterations in SAP, DAP, MAP and HR in CIH and
healthy rats are presented in Table
III and Fig. 2B-E. Prior to
experiments, there was no significant difference in SAP, DAP, MAP
or HR between CIH rats and healthy rats. During the experiments,
the average SAP, DAP, MAP and HR of the CIH rats increased,
gradually and significantly (P<0.05). Compared with the control,
SAP, DAP, MAP and HR in the CIH rats on the 7th, 14th and 21st days
were significantly increased (P<0.05).
 | Table III.Hemodynamic alterations in rats with
or without CIH (n=10; mean ± standard deviation). |
Table III.
Hemodynamic alterations in rats with
or without CIH (n=10; mean ± standard deviation).
|
|
|
|
| During
experiments |
|---|
|
|
|
|
|
|
|---|
| Hemodynamic
measurement | Group | No. of rats | Prior to
experiments | 7th day | 14th day | 21st day |
|---|
| SAP, mmHg | CIH | 5 |
105.36±7.93 |
126.84±12.37a,b |
138.60±10.54a–c |
144.58±11.23a–d |
|
| Control | 5 |
102.05±8.60 |
107.40±7.95 |
110.32±10.77 |
118.68±11.70 |
| DAP, mmHg | CIH | 5 |
53.05±7.90 |
75.44±10.28a,b |
85.33±9.61a–c |
90.37±9.55a–d |
|
| Control | 5 |
51.95±10.38 |
55.62±12.09 |
54.84±10.85 |
56.07±11.63 |
| MAP, mmHg | CIH | 5 |
69.59±8.70 |
84.06±6.33a,b |
103.52±7.47a–c |
106.27±7.05a–d |
|
| Control | 5 |
70.22±9.83 |
73.52±10.24 |
78.92±8.66 |
77.35±10.41 |
| HR, bpm | CIH | 5 |
290.33±34.51 |
319.45±48.52a,b |
327.66±43.57a–c |
336.58±46.30a–d |
|
| Control | 5 |
288.65±35.40 |
291.05±36.87 |
295.60±39.12 |
297.31±37.05 |
Serum IL-6, TNF-α, CRP, NO and NOS
alterations in rats
Serum IL-6, TNF-α, CRP, NO and NOS in CIH and
healthy rats are presented in Table
IV and Fig. 3. Prior to
experiments, there was no significant difference in serum IL-6,
TNF-α, CRP, NO or NOS between CIH rats and healthy rats. During the
experiments, serum IL-6, TNF-α and CRP in CIH rats increased, while
NO and NOS decreased, gradually and significantly (P<0.05).
Compared with the control, serum IL-6, TNF-α and CRP in CIH rats
were significantly increased, while NO and NOS were significantly
decreased, on the 7th, 14th and 21st days (P<0.05).
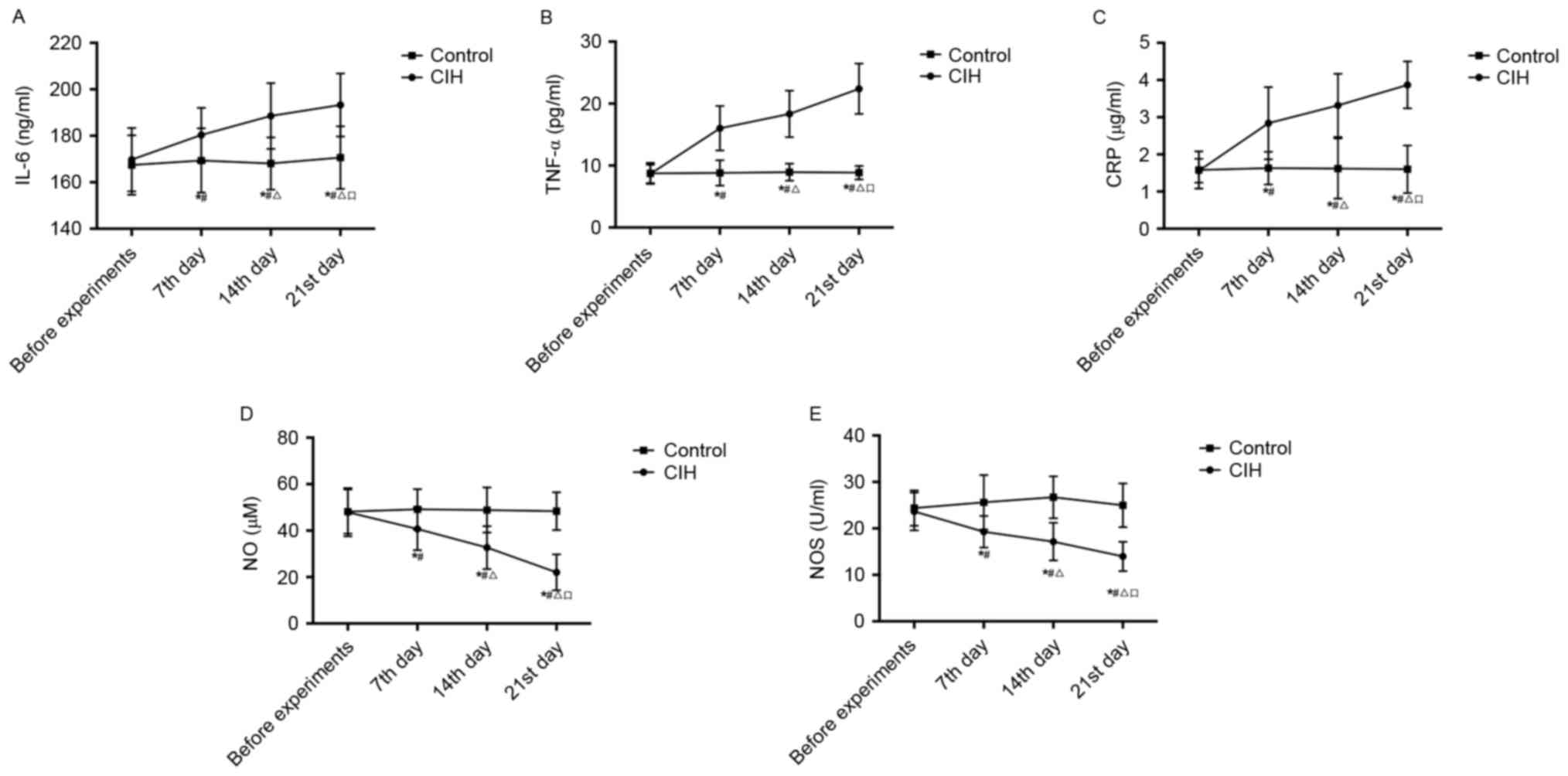 | Figure 3.Comparison of alterations in (A) IL-6,
(B) TNF-α, (C) CRP, (D) NO and (E) NOS in rats with or without CIH
prior to experiments and at the 7th, 14th and 21st day during
experiments. *P<0.05 vs. control; #P<0.05 vs. CIH
before experiments; ΔP<0.05 vs. CIH 7th day;
□P<0.05 vs. CIH 14th day. CIH, chronic intermittent
hypoxia-hypertension; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α; CRP, C-reactive protein; NO, nitric oxide; NOS, nitric
oxide synthase. |
 | Table IV.Serum IL-6, TNF-α, CRP, NO and NOS
alterations in rats with or without CIH (n=10; mean ± standard
deviation). |
Table IV.
Serum IL-6, TNF-α, CRP, NO and NOS
alterations in rats with or without CIH (n=10; mean ± standard
deviation).
|
|
|
|
| During
experiments |
|---|
|
|
|
|
|
|
|---|
| Variable | Groups | No. of rats | Prior to
experiments | 7th day | 14th day | 21st day |
|---|
| IL-6, ng/ml | CIH | 5 |
169.75±13.62 |
180.36±11.70a,b |
188.54±14.16a–c |
193.27±13.58a–d |
|
| Control | 5 |
167.44±12.79 |
169.38±13.83 |
168.07±11.25 |
170.62±13.46 |
| TNF-α, pg/ml | CIH | 5 |
8.66±1.49 |
16.02±3.58a,b |
18.34±3.75a–c |
22.40±4.07a–d |
|
| Control | 5 |
8.74±1.69 |
8.80±2.05 |
8.93±1.37 |
8.85±1.08 |
| CRP, µg/ml | CIH | 5 |
1.56±0.32 |
2.84±0.97a,b |
3.32±0.85a–c |
3.87±0.63a–d |
|
| Control | 5 |
1.58±0.50 |
1.63±0.44 |
1.62±0.81 |
1.60±0.64 |
| NO, µM | CIH | 5 |
47.92±10.36 |
40.73±9.18a,b |
32.67±9.22a–c |
22.03±7.71a–d |
|
| Control | 5 |
48.17±9.50 |
49.14±8.72 |
48.85±9.69 |
48.36±8.14 |
| NOS, U/ml | CIH | 5 |
23.65±4.06 |
19.28±3.41a,b |
17.16±4.04a–c |
13.97±3.15a–d |
|
| Control | 5 |
24.37±3.80 |
25.62±5.86 |
26.71±4.51 |
24.98±4.70 |
Correlation between IL-6, TNF-α, CRP,
NO and NOS, and CIH
The IL-6, TNF-α, CRP, NO and NOS levels were
correlated with CIH, including SAP, DAP, MAP and HR, which are
presented as the correlation coefficient (r) in Table V. IL-6 was positively correlated
with SAP (r=0.572), DAP (r=0.439), MAP (r=0.640) and HR (r=0.530),
in addition to TNF-α with SAP (r= 0.673), DAP (r=0.519), MAP
(r=0.436) and HR (r=0.448), and CRP with SAP (r=0.397), DAP
(r=0.418), MAP (r=0.607) and HR (r=0.528); NO and NOS were
negatively correlated with SAP (r=-0.568 and r=-0.473), DAP
(r=-0.372 and r=-0.460), MAP (r=-0.406 and r=-0.524) and HR
(r=-0.630 and r=-0.581).
 | Table V.Correlation coefficients of IL-6,
TNF-α, CRP, NO and NOS with SAP, DAP, MAP and HR in CIH rats. |
Table V.
Correlation coefficients of IL-6,
TNF-α, CRP, NO and NOS with SAP, DAP, MAP and HR in CIH rats.
|
| Correlation
coefficient (r) |
|---|
|
|
|
|---|
| Variable | SAP | DAP | MAP | HR |
|---|
| IL-6 | 0.572b | 0.439a | 0.640b | 0.530b |
| TNF-α | 0.673b | 0.519b | 0.436a | 0.448a |
| CRP | 0.397a | 0.418a | 0.607b | 0.528b |
| NO | −0.568b | −0.372a | −0.406a | −0.630b |
| NOS | −0.473a | −0.460a | −0.524b | −0.581b |
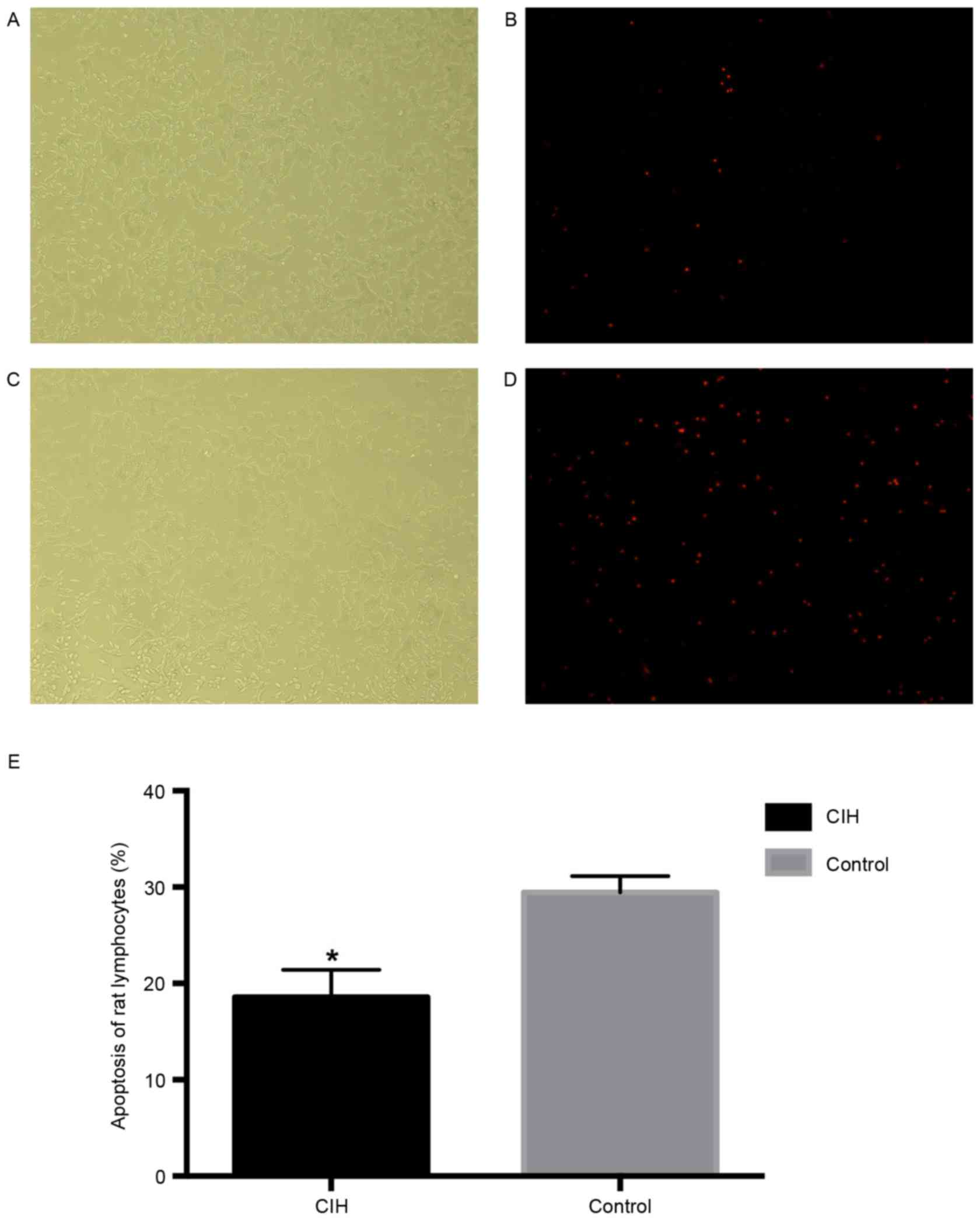
Apoptosis rate, IL-6, TNF-α, CRP, NO
and iNOS in rat lymphocytes
Apoptosis rate, IL-6, TNF-α, CRP, NO and iNOS in CIH
and control rats are presented in Table VI and Figs. 4 and 5. The apoptosis rat in CIH rats was
significantly decreased compared with healthy rats (P<0.05;
Fig. 4). The levels of IL-6,
TNF-α, CRP, NO and iNOS were significantly increased in CIH rats
compared with healthy rats (P<0.05; Fig. 5).
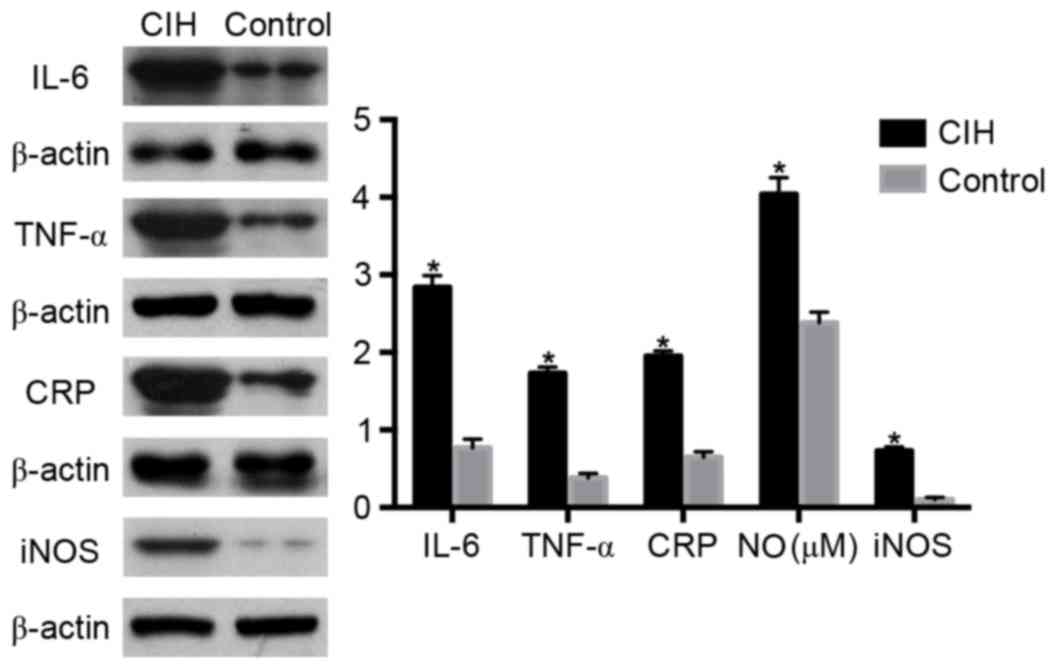 | Figure 5.Alterations in IL-6, TNF-α, CRP, NO
and iNOS in rat lymphocytes in the CIH or control groups, assessed
by western blotting analysis. *P<0.05 vs. control. CIH, chronic
intermittent hypoxia; IL-6, interleukin-6; TNF-α, tumor necrosis
factor-α; CRP, C-reactive protein; NO, nitric oxide; iNOS,
inducible nitric oxide synthase. |
 | Table VI.Apoptosis, IL-6, TNF-α, CRP, NO and
iNOS in rat lymphocytes in rats with or without CIH. |
Table VI.
Apoptosis, IL-6, TNF-α, CRP, NO and
iNOS in rat lymphocytes in rats with or without CIH.
| Variable | CIH | Control |
|---|
| Apoptosis rate,
% |
18.59±2.83a |
29.46±1.70 |
| IL-6, ng/ml |
2.84±0.15a |
0.77±0.11 |
| TNF-α, pg/ml |
1.73±0.08a |
0.38±0.06 |
| CRP, µg/ml |
1.95±0.07a |
0.65±0.07 |
| NO, µM |
4.04±0.21a |
2.38±0.14 |
| iNOS, U/ml |
0.73±0.05a |
0.10±0.03 |
IL-6, TNF-α, CRP, NO, eNOS and iNOS in
rat endotheliocytes
IL-6, TNF-α, CRP, NO, eNOS and iNOS in rat
endotheliocytes prior to and following CIH induction are presented
in Table VII and Fig. 6. During the 3 h of CIH induction,
IL-6, TNF-α, CRP and iNOS in rat endotheliocytes increased, while
NO and eNOS decreased, gradually and significantly (P<0.05).
Compared with the control, IL-6, TNF-α, CRP and iNOS in rat
endotheliocytes with CIH induction were significantly increased,
while NO and eNOS were decreased, at 1, 2 and 3 h (P<0.05).
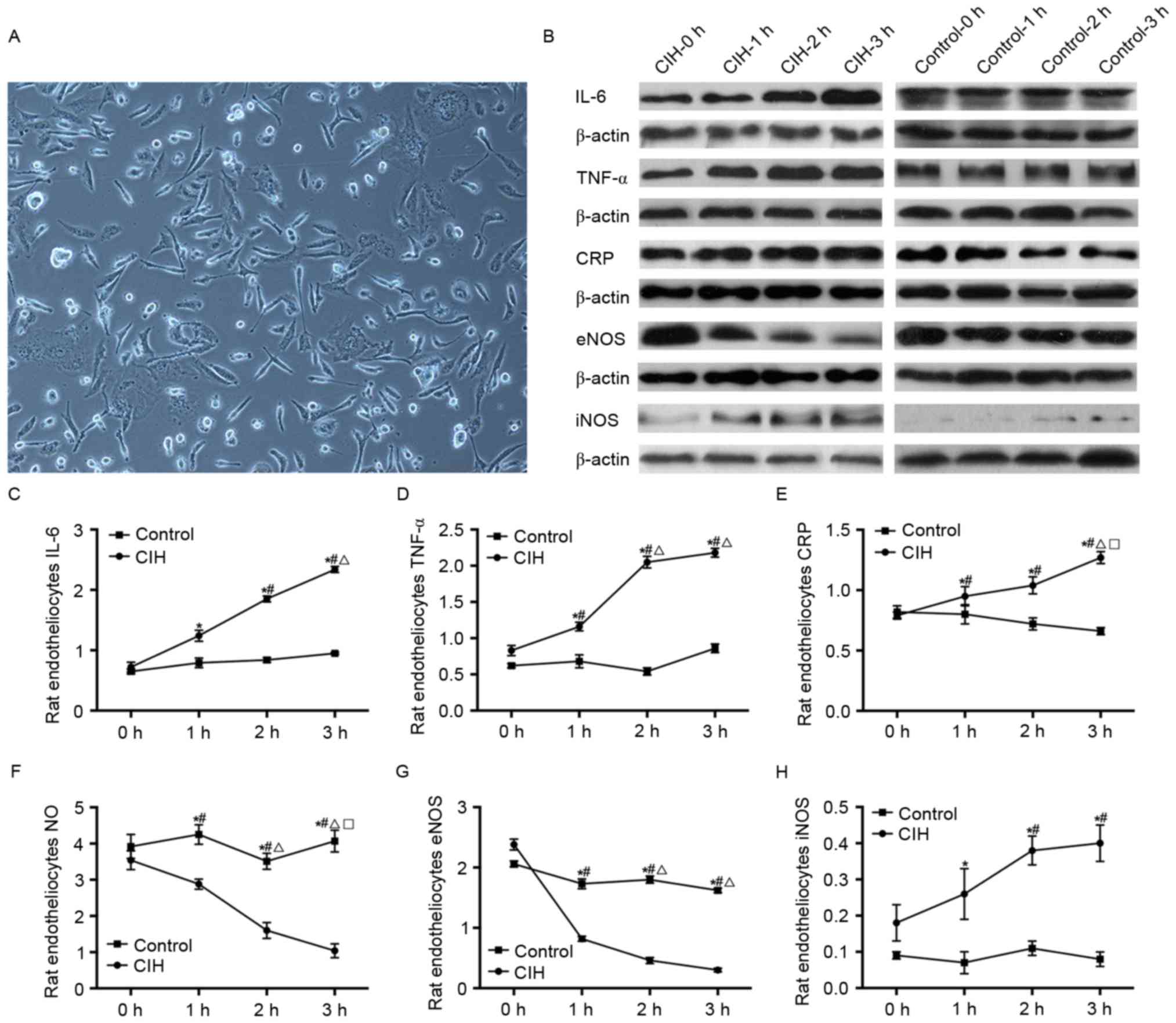 | Figure 6.Alterations in IL-6, TNF-α, CRP, NO,
eNOS and iNOS in rat endotheliocytes prior to and following CIH
induction. (A) Rat endotheliocytes (original magnification, ×100).
(B) Protein levels of IL-6, TNF-α, CRP, eNOS and iNOS in rat
endotheliocytes prior to and following CIH induction, ssessed by
western blotting. (C) Rat endotheliocytes IL-6. (D) Rat
endotheliocytes TNF-α. (E) Rat endotheliocytes CRP. (F) Rat
endotheliocytes NO. (G) Rat endotheliocytes eNOS. (H) Rat
endotheliocytes iNOS. *P<0.05 vs. control; #P<0.05
vs. CIH-0 h; ΔP<0.05 vs. CIH-1; □P<0.05
vs. CIH-2 h. CIH, chronic intermittent hypoxia; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α; CRP, C-reactive
protein; NO, nitric oxide; eNOS, endothelial nitric oxide synthase;
iNOS, inducible nitric oxide synthase. |
 | Table VII.IL-6, TNF-α, CRP, NO, eNOS and iNOS
in rat endotheliocytes prior to and following CIH induction. |
Table VII.
IL-6, TNF-α, CRP, NO, eNOS and iNOS
in rat endotheliocytes prior to and following CIH induction.
|
|
| CIH induction
time |
|---|
|
|
|
|
|---|
| Factor | Groups | 0 h | 1 h | 2 h | 3 h |
|---|
| IL-6, ng/ml | CIH |
0.72±0.08 |
1.24±0.09a |
1.85±0.05a,b |
2.34±0.05a–c |
|
| Control |
0.65±0.04 |
0.79±0.08 |
0.84±0.04 |
0.95±0.02 |
| TNF-α, pg/ml | CIH |
0.83±0.07 |
1.16±0.06a,b |
2.05±0.08a–c |
2.18±0.06a–c |
|
| Control |
0.62±0.03 |
0.68±0.09 |
0.54±0.05 |
0.86±0.06 |
| CRP, µg/ml | CIH |
0.79±0.03 |
0.95±0.08a,b |
1.04±0.07a,b |
1.27±0.05a–d |
|
| Control |
0.82±0.05 |
0.80±0.08 |
0.72±0.05 |
0.66±0.03 |
| NO, µM | CIH |
3.54±0.26 |
2.88±0.14a,b |
1.60±0.22a–c |
1.04±0.19a–d |
|
| Control |
3.92±0.33 |
4.25±0.27 |
3.51±0.22 |
4.07±0.30 |
| eNOS, U/ml | CIH |
2.38±0.09 |
0.82±0.04a,b |
0.46±0.05a–c |
0.30±0.03a–d |
|
| Control |
2.06±0.05 |
1.73±0.08 |
1.80±0.06 |
1.62±0.04 |
| iNOS, U/ml | CIH |
0.18±0.05 |
0.26±0.07a |
0.38±0.04a,b |
0.40±0.05a,b |
|
| Control |
0.09±0.01 |
0.07±0.03 |
0.11±0.02 |
0.08±0.02 |
IL-6, TNF-α, CRP, NO and NOS in
co-culturing supernatant of lymphocytes and endotheliocytes
IL-6, TNF-α, CRP, NO and NOS levels in the
supernatant of co-cultured rat lymphocytes and endotheliocytes are
presented in Table VIII and
Fig. 7. Compared with the
CON-L+CON-E group, the IL-6, TNF-α and CRP levels in the
CIH-L+CIH-E, CIH-L+CON-E and CON-L+CIH-E groups increased, and were
significantly increased in the CIH-L+CIH-E compared with the
CIH-L+CON-E and CON-L+CIH-E groups (P<0.05). The total NO and
NOS in the CON-L+CON-E group was decreased compared with the
CIH-L+CIH-E, CIH-L+CON-E and CON-L+CIH-E groups (P<0.05).
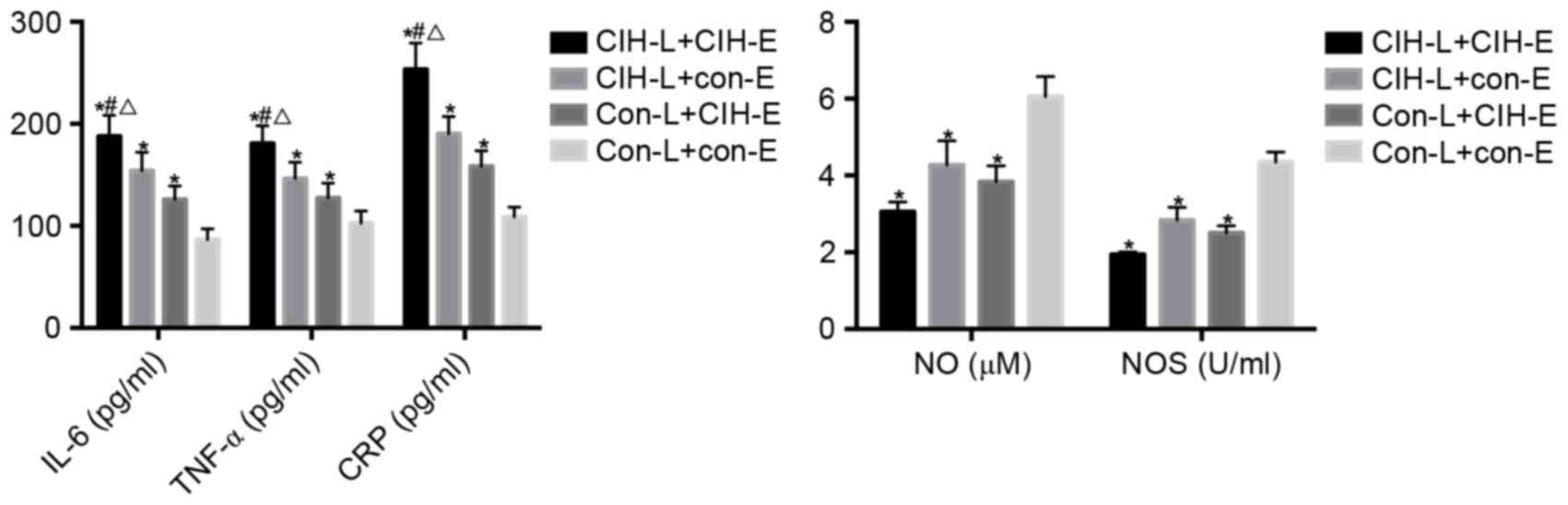 | Figure 7.IL-6, TNF-α, CRP, NO and NOS in the
co-culture supernatant of rat lymphocytes and rat endotheliocytes.
*P<0.05 vs. CON-L+CON-E; #P<0.05 vs. CIH-L+CON-E;
ΔP<0.05 vs. CON-L+CIH-E. CIH, chronic intermittent
hypoxia; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; CRP,
C-reactive protein; NO, nitric oxide; NOS, nitric oxide synthase;
CON, control; L, lymphocytes; E, endotheliocytes. |
 | Table VIII.IL-6, TNF-α, CRP, NO and NOS in the
co-culture supernatant of rat lymphocytes and endotheliocytes. |
Table VIII.
IL-6, TNF-α, CRP, NO and NOS in the
co-culture supernatant of rat lymphocytes and endotheliocytes.
| Variable | CIH-L+CIH-E | CIH-L+CON-E | CON-L+CIH-E | CON-L+CON-E |
|---|
| IL-6, pg/ml |
187.94±20.36a–c |
154.08±17.93a |
125.93±13.20a |
86.51±10.68 |
| TNF-α, pg/ml |
180.77±17.35a–c |
146.38±15.90a |
127.37±14.62a |
102.64±11.86 |
| CRP, pg/ml |
253.35±25.89a–c |
190.28±16.72a |
158.44±15.20a |
108.95±9.54 |
| NO, µM |
3.06±0.25a |
4.27±0.63a |
3.84±0.41a |
6.06±0.52 |
| NOS, U/ml |
1.94±0.07a |
2.84±0.33a |
2.50±0.19a |
4.35±0.26 |
Discussion
Comparison between Hakka CIH patients
and non-Hakka CIH patients
According to the analysis of hemodynamic
alterations, the patients with CIH exhibited hypertension. From
analysis of the clinical data in the patients with CIH combined
with hypertension, it was observed that older people exhibited an
increased incidence, as did males. The results of the analysis of
BMI and T2DM history indicated that obesity was associated with an
increased incidence of CIH combined with hypertension. However, the
BMI of the non-Hakka patients was decreased, which indicated that
the Hakka patients with increased BMI may be at increased risk of
CIH combined with hypertension. Obesity results in excessive fat
around the neck, a softened airway and relative macroglossia,
resulting in snoring (23). It was
hypothesized that certain factors may protect the Hakka people,
even with their higher average BMI, from obesity. In addition,
individuals who smoke may be at an increased risk of CIH combined
with hypertension, demonstrating that smoking is a risk factor for
CIH.
Through hemodynamic analysis, it was observed that
patients with CIH exhibited increased SAP, DAP, MAP and HR compared
with healthy people. However, the non-Hakka patients exhibited
increased SAP compared with Hakka patients, suggesting that the
Hakka people may have a decreased SAP in CIH combined with
hypertension, or that there are other factors influencing their
SAP.
Through the comparisons of serum markers, it was
demonstrated that patients with CIH combined with hypertension
exhibited increased serum IL-6, TNF-α and CRP levels, and decreased
NO and NOS levels, compared with healthy volunteers. When comparing
between different patients, the IL-6 and TNF-α levels were
increased in non-Hakka patients compared with Hakka patients, while
the NO level was decreased. The results of the present study
indicated decreased inflammation in Hakka patients with CIH
combined with hypertension. Therefore, there may be factors which
protect the Hakka people from inflammation.
It was previously demonstrated that CIH may
stimulate neuromodulatory adaptive changes, influencing the control
of breathing, the autonomic nervous system and the cardiovascular
system (24). CIH may present with
interstitial lung disease (25)
and sleep-disordered breathing (26). In addition, CIH has been observed
to occur in Hakka patients with hepatopulmonary syndromes (27). Increasing attention has been paid
to the association between CIH and breathing disorders associated
with sleep, including obstructive sleep apnea (OSA) (28). OSA is associated with a number of
cardiovascular conditions, and one of the closest associations was
observed with systemic hypertension (29). The majority of patients with OSA
exhibit sleep disruption, which may induce sympathetic disorders of
nerve activity and blood pressure (30). The results of the present study
indicated the differences between patients with CIH in different
populations. These data may provide novel insights into the
diagnosis, treatment and prevention of CIH, particularly in
different populations.
CIH models
In previous studies, the important role of hypoxia
in promoting an increase in blood pressure was investigated in
humans (31) or by establishing
animal models (32). There are a
number of advantages to using animal models, including the fact
that a single component may be evaluated accurately in a
homogeneous population (33). A
CIH model in rats was established in the present study. By
detecting the SAP, DAP, MAP and HR at different timepoints, it was
successfully confirmed that CIH was able to induce hypertension.
The SAP, DAP, MAP and HR increased gradually, which indicated that
CIH may promote an increase in blood pressure in rats in a
time-dependent manner.
The CIH rats were less active and exhibited a
decreased appetite, resulting in decreased weight gain compared
with healthy rats. OSA was not detected in CIH rats in the present
study; it was hypothesized that there were sleep-related disorders
in the CIH rats, although this requires further investigation.
In a previous study (34), it was demonstrated that OSA may
cause repetitive episodes of airway occlusion with hypoxia,
hypercapnia, and alterations in intrathoracic pressure, which may
lead to diverse autonomic, humoral, neurohumoral and hemodynamic
responses. Indeed, serum IL-6, TNF-α and CRP have been observed to
be elevated in patients with OSA (35,36),
although there is little evidence to identify a direct association
between serum IL-6, TNF-α and CRP, and the effect of CIH on SAP,
DAP, MAP and HR. In the present study, it was observed that serum
IL-6, TNF-α and CRP levels increased in CIH rats and lymphocytes,
in addition to in rat endotheliocytes following CIH induction,
compared with the control. Additionally, serum IL-6, TNF-α and CRP
were demonstrated to be positively correlated with the incidence of
CIH combined with hypertension in humans, in addition to the SAP,
DAP, MAP and HR in CIH rats, suggesting that serum IL-6, TNF-α and
CRP may be biomarkers of CIH combined with hypertension.
NO and NOS have been demonstrated to be involved in
important regulatory on autonomic functions, particularly in
regulating the expression of genes associated with hypoxia
(37,38). The present study detected the serum
NO and NOS in rats at different timepoints, and it was observed
that NO and NOS decreased gradually. The present study additionally
analyzed the association between NO and NOS, and CIH combined with
hypertension, and confirmed that NO and NOS were negatively
correlated with SAP, DAP, MAP and HR in CIH rats. The results of
the present study indicated that NO and NOS may be taken to be
biomarkers for the diagnosis of CIH combined with hypertension.
However, the levels of NO and iNOS were increased in
CIH rat lymphocytes compared with control cells, as did iNOS in rat
endotheliocytes following CIH induction, while NO and eNOS
decreased significantly in rat endotheliocytes following CIH
induction. These results illustrated that the serum NO and NOS were
predominantly derived from endotheliocytes. Although CIH induction
induced the expression of iNOS, the influence on the total NO was
less compared with decreasing eNOS expression.
However, there were limitations to the present
study. The sample size in the investigation was small, and was
focused on the Hakka people of Huizhou City without investigation
other regions, which is likely to result in regional differences
and inaccuracy of the correlations between CIH combined with
hypertension and clinical data, hemodynamic alterations and serum
factors. In addition, due to the limited time of the experiments,
the rats were only fed for 21 days for the present study. Further
investigations with longer experimental time and more measures are
required.
In conclusion, age, male gender, BMI, smoking and
T2DM history, and serum IL-6, TNF-α and CRP were positively
correlated with CIH combined with hypertension, while NO and NOS
were negatively correlated with CIH. Hakka patients exhibited less
inflammation and OS in CIH combined with hypertension. Serum IL-6,
TNF-α, CRP, NO and NOS may be biomarkers for the diagnosis of CIH
combined with hypertension, in addition to targets for the
treatment or prevention of CIH or hypertension.
References
|
1
|
Caples SM, Gami AS and Somers VK:
Obstructive sleep apnea. Ann Intern Med. 142:187–197. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khayat R, Patt B and Hayes D Jr:
Obstructive sleep apnea: The new cardiovascular disease. Part I:
Obstructive sleep apnea and the pathogenesis of vascular disease.
Heart Fail Rev. 14:143–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dempsey JA, Veasey SC, Morgan BJ and
O'Donnell CP: Pathophysiology of sleep apnea. Physiol Rev.
90:47–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fletcher EC: The relationship between
systemic hypertension and obstructive sleep apnea: Facts and
theory. Am J Med. 98:118–128. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Das AM and Khayat R: Hypertension in
obstructive sleep apnea: Risk and therapy. Expert Rev Cardiovasc
Ther. 7:619–626. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sala-Mercado JA, Spranger MD, Abu-Hamdah
R, Kaur J, Coutsos M, Stayer D, Augustyniak RA and O'Leary DS:
Attenuated muscle metaboreflex-induced increases in cardiac
function in hypertension. Am J Physiol Heart Circ Physiol.
305:H1548–H1554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ito T, Ishikawa E, Fujimoto N, Okubo S,
Ito G, Ichikawa T, Nomura S and Ito M: Effects of aliskiren on
blood pressure and humoral factors in hypertensive hemodialysis
patients previously on angiotensin II receptor antagonists. Clin
Exp Hypertens. 36:497–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiasson VL, Munshi N, Chatterjee P, Young
KJ and Mitchell BM: Pin1 deficiency causes endothelial dysfunction
and hypertension. Hypertension. 58:431–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morishita R: Is vascular endothelial
growth factor a missing link between hypertension and inflammation?
Hypertension. 44:253–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mikami S, Nihira S and Takemori K: The
construction of vascular information data base for efficient blood
pressure measurement. Hirosaki Med J. 41:282–289. 1989.
|
|
11
|
Peng Y, Kline DD, Dick TE and Prabhakar
NR: Chronic intermittent hypoxia enhances carotid body
chemoreceptor response to low oxygen. Adv Exp Med Biol. 499:33–38.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karamanlı H, Özol D, Ugur KS, Yıldırım Z,
Armutçu F, Bozkurt B and Yigitoglu R: Influence of CPAP treatment
on airway and systemic inflammation in OSAS patients. Sleep Breath.
18:251–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Svensson M, Venge P, Janson C and Lindberg
E: Relationship between sleep-disordered breathing and markers of
systemic inflammation in women from the general population. J Sleep
Res. 21:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaviraj B, Bai SC, Liang SU, Zheng XO,
Huang R, Li TP and Xu DL: Effect of obstructive sleep apnea
syndrome on serum C-reactive protein level, left atrial size and
premature atrial contraction. Nan Fang Yi Ke Da Xue Xue Bao.
31:197–200. 2011.PubMed/NCBI
|
|
15
|
Chen J, Zhang J and Chen Y: Relationship
between hs-CRP and obstructive sleep apnea in hypertension
patients. Chin J Integr Med Cardio-/Cerebrovasc Dis. 2012.
|
|
16
|
McNicholas WT: Chronic obstructive
pulmonary disease and obstructive sleep apnea: Overlaps in
pathophysiology, systemic inflammation, and cardiovascular disease.
Am J Respir Crit Care Med. 180:692–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jordan W, Cohrs S, Degner D, Meier A,
Rodenbeck A, Mayer G, Pilz J, Rüther E, Kornhuber J and Bleich S:
Evaluation of oxidative stress measurements in obstructive sleep
apnea syndrome. J Neural Transm (Vienna). 113:239–254. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pawar A, Nanduri J, Yuan G, Khan SA, Wang
N, Kumar GK and Prabhakar NR: Reactive oxygen species-dependent
endothelin signaling is required for augmented hypoxic sensory
response of the neonatal carotid body by intermittent hypoxia. Am J
Physiol Regul Integr Comp Physiol. 296:R735–R742. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lam SY and Leung PS: A locally generated
angiotensin system in rat carotid body. Regul Pept. 107:97–103.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iturriaga R, Villanueva S and Mosqueira M:
Dual effects of nitric oxide on cat carotid body chemoreception. J
Appl Physiol (1985). 89:1005–1012. 2000.PubMed/NCBI
|
|
21
|
Alissa EM and Ferns GA: Functional foods
and nutraceuticals in the primary prevention of cardiovascular
diseases. J Nutr Metab. 2012:5694862012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knox S, Theorell T, Malmberg BG and
Lindqvist R: Stress management in the treatment of essential
hypertension in primary health care. Scand J Prim Health Care.
4:175–181. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Resta O, Foschino-Barbaro MP, Legari G,
Talamo S, Bonfitto P, Palumbo A, Minenna A, Giorgino R and De
Pergola G: Sleep-related breathing disorders, loud snoring and
excessive daytime sleepiness in obese subjects. Int J Obes Relat
Metab Disord. 25:669–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Foster GE, Hanly PJ, Ahmed SB, Beaudin AE,
Pialoux V and Poulin MJ: Intermittent hypoxia increases arterial
blood pressure in humans through a Renin-Angiotensin
system-dependent mechanism. Hypertension. 56:369–377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fletcher EC, Lesske J, Qian W, Miller CC
III and Unger T: Repetitive, episodic hypoxia causes diurnal
elevation of blood pressure in rats. Hypertension. 19:555–561.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nanduri J, Peng YJ, Yuan G, Kumar GK and
Prabhakar NR: Hypoxia-inducible factors and hypertension: Lessons
from sleep apnea syndrome. J Mol Med (Berl). 93:473–480. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palma DT, Philips GM, Arguedas MR, Harding
SM and Fallon MB: Oxygen desaturation during sleep in
hepatopulmonary syndrome. Hepatology. 47:1257–1263. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Drager LF, Jun JC and Polotsky VY:
Metabolic consequences of intermittent hypoxia: Relevance to
obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab.
24:843–851. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kapa S, Kuniyoshi FH Sert and Somers VK:
Sleep apnea and hypertension: Interactions and implications for
management. Hypertension. 51:605–608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morgan BJ, Crabtree DC, Puleo DS, Badr MS,
Toiber F and Skatrud JB: Neurocirculatory consequences of abrupt
change in sleep state in humans. J Appl Physiol (1985).
80:1627–1636. 1996.PubMed/NCBI
|
|
31
|
Tamisier R, Pépin JL, Rémy J, Baguet JP,
Taylor JA, Weiss JW and Lévy P: 14 nights of intermittent hypoxia
elevate daytime blood pressure and sympathetic activity in healthy
humans. Eur Respir J. 37:119–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wan NS, Chen BY, Feng J, Li S, Zhou W,
Zhang Z and Guo R: The effects of chronic intermittent hypoxia on
blood pressure and sympathetic nerve activity in rats. Zhonghua Jie
He He Hu Xi Za Zhi. 35:29–32. 2012.(In Chinese). PubMed/NCBI
|
|
33
|
Levy P, Tamisier R, Arnaud C, Monneret D,
Baguet JP, Stanke-Labesque F, Dematteis M, Godin-Ribuot D, Ribuot C
and Pepin JL: Sleep deprivation, sleep apnea and cardiovascular
diseases. Front Biosci (Elite Ed). 4:2007–2021. 2012.PubMed/NCBI
|
|
34
|
Somers VK, White DP, Amin R, Abraham WT,
Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, et
al: Sleep apnea and cardiovascular disease: An American heart
association/American college of cardiology foundation scientific
statement from the American hear association council for high blood
pressure research professional education committee, council on
clinical cardiology, stroke council, and council on cardiovascular
nursing. J Am Coll Cardiol. 52:686–717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karamanlı H, Özol D, Ugur KS, Yıldırım Z,
Armutçu F, Bozkurt B and Yigitoglu R: Influence of CPAP treatment
on airway and systemic inflammation in OSAS patients. Sleep Breath.
18:251–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Svensson M, Venge P, Janson C and Lindberg
E: Relationship between sleep-disordered breathing and markers of
systemic inflammation in women from the general population. J Sleep
Res. 21:147–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rus A, Del Moral ML, Molina F and Peinado
MA: Upregulation of cardiac NO/NOS system during short-term hypoxia
and the subsequent reoxygenation period. Eur J Histochem.
55:e172011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren W, Yang X, Jiang X, Li Z and Zhang Z:
Chronic hypoxia and exercise training affect the NO content and NOS
activity of rat skeletal muscle: Original research article. Int
SportMed J. 11:244–257. 2010.
|