Introduction
Renal cell carcinoma (RCC) is a common cancer in the
world, which accounts to ~85% of renal malignancy (1). The incidence of RCC varies in
different region (2). Most of new
cases occur in developed countries, such as Europe and North
America (3). 20–30% of patients
with RCC are diagnosed at advanced stage with metastasis, and 30%
of those diagnosed at early stage will eventually developed
metastasis after resection (4).
Due to metastasis and resistance to chemotherapy and radiotherapy,
RCC has been reported to carry a poor prognosis (5). This emphasizes the importance to
develop new therapeutic approaches to improve prognosis and quality
of life of patients.
Aerobic glycolysis, also known as the Warburg
effect, is featured by the increased glucose utilization and
lactate production in tumor cells even at normal oxygen
concentrations (6). Recent studies
demonstrated that the Warburg effect serve an important role in
tumor development and progression (7,8).
Numerous studies reported that the expression of genes involved in
energetic metabolism displayed heterogeneity in RCC, suggesting
that the Warburg effect may participate in the development of RCC
(9,10). However, the function and mechanism
of the Warburg effect in RCC remains unknown. Lactate dehydrogenase
A (LDHA), a key enzyme in glycolysis, promotes the shift from
pyruvate to lactate. Previous studies showed that the level of LDHA
is upregulated in many human cancers (11,12).
Another group of investigators had observed that silencing or
inhibition of LDHA suppresses tumor growth and metastasis of human
cancer (13–15). All of this evidence implies the
underlying role of LDHA in cancer development and progression
(16). Recent studies manifested
that the expression of LDHA was up-regulated in RCC, and the high
level of LDHA was correlated with poor differentiation of tumor
cells and poor prognosis of RCC patients (17,18).
However, the mechanism remains unknown.
Epithelial-mesenchymal transition (EMT), a vital
event in invasion and metastasis of human cancer, allows tumor
cells to lose the epithelial phenotype and obtain mesenchymal
phenotype, which makes tumor cells acquire the ability of motility
(19,20). The role of EMT in tumor metastasis
has been widely accepted (21–23).
Downregulation of E-cadherin, which is the primary marker of EMT
process, is regarded as signaling an extremely poor prognosis.
Various research has demonstrated that dysregulated elements
leading to EMT can be potential targets in malignant tumor. Piva
et al (24) reported that
EMT contributed to RCC development and progression. Jiang et
al (25) reported that LDHA
was elevated in muscle-invasive bladder cancer, promoting malignant
progression via activation of EMT. The above findings prompted us
to investigate the effects of LDHA in EMT process in RCC.
In order to decipher the molecular mechanisms in the
present study, the authors examined the expression of LDHA in RCC
patients, and then analyzed the correlation between the LDHA and
the clinicopathological parameters of patients with RCC.
Furthermore, the relationship between LDHA expression and EMT
markers was evaluated. Different approaches were employed to
explore the role of LDHA expression in EMT process and metastasis
of tumor cells. Besides, LDHA specific inhibitor oxamate was used
to verify the function of LDHA in RCC. Finally, an orthotopic renal
xenograft model was established to determine the function of LDHA
on tumor metastasis in vivo. The present study elaborated
the molecular mechanisms of LDHA in EMT and progression of RCC, and
could represent a more promising strategy against RCC.
Materials and methods
Ethical review
The protocol of the research project had been
approved by the Ethics Committee of the Ruijin Hospital, Shanghai
Jiaotong University (Shanghai, China) and it conformed to the
provisions of the Declaration of Helsinki. The patients' informed
consents were all obtained.
Tissue specimens and
immunohistochemical staining analysis (IHC)
All formalin-fixed paraffin-embedded RCC tissue
specimens and normal renal tissue of RCC patients who underwent
radical nephrectomy or nephron-sparing surgery were obtained from
the Department of Urology, Ruijin Hospital, Shanghai Jiaotong
University School of Medicine (Shanghai, China) between 2009 and
2015. The diagnosis of RCC was made by at least two
contrast-enhanced imaging studies [ultrasound (US), computed
tomography (CT) and magnetic resonance imaging (MRI)], and
confirmed by US-assisted fine-needle biopsy. None of patients had
received chemotherapy or radiotherapy before the operation. Follow
up was performed from the date of tumor resection until May 2015 or
until patient mortality. All clinicopathological parameters were
collected from the medical records of the hospital.
Specimens were stained at 4°C overnight with LDHA
polyclonal antibody (cat. no. SC-27231; dilution, 1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), E-cadherin polyclonal
antibody (cat. no. 3195; dilution, 1:200), N-cadherin polyclonal
antibody (cat. no. 13116; dilution, 1:200), vimentin polyclonal
antibody (cat. no. 5741; dilution, 1:200) and Snail polyclonal
antibody (cat. no. 3879; dilution, 1:200) all obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). A biotinylated
secondary antibody (cat. no. 10K06A; dilution, 1:100; ZSGB-BIO;
OriGene Technologies, Inc.) was used to detect the primary antibody
at 37°C for 40 min. A score was calculated via multiplying the
extent of staining (1, 0–5%; 2, 5–25%; 3, 26–50%; 4, 50–75%; 5,
75–100%) by the staining intensity (0, negative; 1, weak; 2,
medium; 3, strong). For the purpose of statistical analyses, tumors
with a final staining score of >3 were considered as high
expression.
Western blot assay
Cells and tissues were lysed in lysis buffer
containing protease inhibitor cocktail (50 mM Tris pH 7.5, 150 mM
NaCl, 1% TritonX-100 and 5 mM ethylenediaminetetraacetic acid).
Protein concentration was determined using a Bio-Rad protein assay
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Western
blotting was performed following the routine protocol. Signals were
detected on X-ray film using the ECL detection system (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The relative
protein levels were calculated based on β-actin as the loading
control.
Cell culture and small interfering
(si)RNA transfection
Human RCC cell lines 786-O, OS-RC-2, Caki-1, A498,
HK-2 and ACHN were obtained from the Institute of Biochemistry and
Cell Biology, Chinese Academy of Sciences (Shanghai, China) and
were preserved in our institute. All the cells were cultured in
RPMI-1640 medium (HyClone Laboratories; GE Healthcare Life
Sciences, Chalfont, UK) with 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin and maintained at 37°C with 5% CO2.
Transfection of specific siRNAs targeting LDHA (Shanghai GenePharma
Co., Ltd., Shanghai, China) and negative control (NC)-siRNA into
RCC cells was performed using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Following this, the interference
efficiency was determined by western blotting. ACHN cells with
stable knockdown of LDHA and their control line were established
using the lentiviral transduction system (Santa Cruz Biotechnology,
Inc.) (26).
LDHA activity, glucose and lactate
measurement
RCC cells (1×106) were transfected with
siRNAs or cultured with 30 mM oxamate (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and prepared for LDHA activity, lactate
production and glucose utilization assay. LDHA activity and lactate
production assay were performed using the Lactate Dehydrogenase
Activity Assay kit (cat. no. MAK066) and Lactate Assay kit (cat.
no. MAK064; Sigma-Aldrich; Merck KGaA) according to the
manufacturer's protocol. Glucose concentration in the media was
measured using colorimetric glucose assay kit (BioVision, Milpitas,
CA, USA) and normalized according to cell number (27).
Invasion and migration assay
The scratch migration assay was performed in 24-well
plate, and cells were transfected with control or LDHA siRNA, or
cultured with 30 mM oxamate. Cells were scratched using a tip of
sterile 200 µl pipette in each well following 24 h. The plates were
washed twice with PBS, and then incubated at 37°C in 5%
CO2. Wound width was monitored at various time points
and quantified by the ratio of gap distance at 24 or 48 h to that
at 0 h. The experiment was conducted in triplicate. The migration
and invasion assay were both performed using Transwell (Corning
Incorporated, Corning, NY, USA) in 24-well plate. Filters coated
with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) were used
for invasion assay. At 48 h following RNA interference, cell
suspension (1×105 cells in 200 µl serum-free medium) was
plated into the upper chamber, while the lower chamber was filled
with 500 µl RPMI (HyClone Laboratories; GE Healthcare Life
Sciences) containing 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.). Following incubation at 37°C for 24 h, tumor cells adhere to
the lower surface of the membrane were fixed with methanol and
stained with crystal violet. The staining cells were photographed
and counted using Image J software.
In vivo metastasis analysis
All procedures for animal experiments were performed
in accordance with the Guide for the Care and Use of Laboratory
Animals (NIH publications Nos. 80-23, reversed 1996) and according
to the Ethical Guidelines for animal experiments of the Ruijin
Hospital, Shanghai Jiaotong University (Shanghai, China). The
orthotopic renal xenograft model was established in previous
research (26). RCC cells were
suspended in 20 µl RPMI-1640/Matrigel (1:1; R&D Systems, Inc.,
Minneapolis, MN, USA) and inoculated into the right subrenal
capsule of kidneys of nude mice. At 7 weeks, the two group of ten
mice were sacrificed by cervical dislocation and the lungs were
removed and embedded in paraffin for evaluating the frequency of
metastasis, serial sections from lung were stained with hematoxylin
and eosin (H&E) and then screened independently by two
researchers who were blinded to the treatment.
Statistical analysis
All data from at least three independent experiments
was presented as mean ± standard deviation and statistically
analyzed using SPSS software (version 18.0; SPSS, Inc., Chicago,
IL, USA). Statistical analysis was performed by Student's t-test.
The rate and constituent ratio were compared by the Chi-square
test. Kaplan-Meier survival curve was used to analyze patient's
overall survival (OS) rates and disease-free survival (DFS) rates,
and the differences were compared using the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association of expression of LDHA with
poor prognosis in RCC
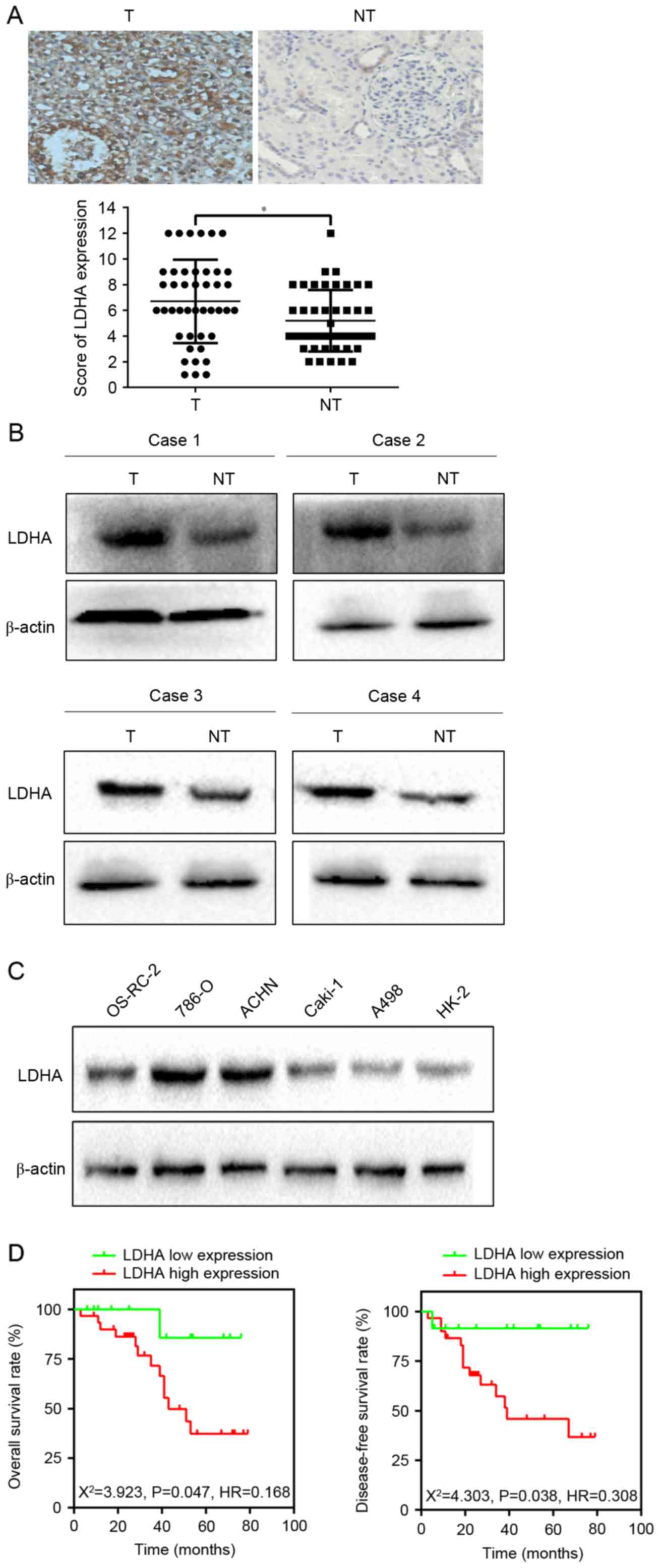
To verify the expression of LDHA in RCC, the authors
examined the level of LDHA in 43 RCC and corresponding
non-cancerous renal (CNR) tissues using immunohistochemical
staining and western blotting. Subsequent analyses revealed that
LDHA was upregulated in 55.8% (24/43) of RCC patients compared with
those of CNR tissues (P=0.016; Fig. 1A
and B). In addition, western blotting implied that LDHA
expression level was enhanced in 786-O and ACHN cells compared to
OS-RC-2, Caki-1, A498 and HK-2 cells (Fig. 1C). Therefore, 786-O and ACHN cells
were employed in the subsequent experiments. The above observations
suggested that LDHA was upregulated in RCC.
In order to investigate the relationship between
LDHA expression and clinicopathological characteristics of RCC, the
clinical data of 43 RCC patients were retrospectively analyzed.
Results showed that increased LDHA expression was significantly
correlated with lymph node metastasis (χ2=4.449,
P=0.025), distant metastasis (χ2=5.044, P=0.035), and
tumor size (χ2=6.894, P=0.009; Table I). The OS rate and DFS rate between
the two groups had significant differences, as evidenced by the
Kaplan-Meier survival curve and the log-rank test (OS,
χ2=3.923, P=0.0476; DFS, χ2=4.245, P=0.0394;
Fig. 1D). All of the data
indicated that LDHA might have an important role in the development
of RCC.
 | Table I.Association of LDHA expression with
clinicopathological factors of 43 RCC patients. |
Table I.
Association of LDHA expression with
clinicopathological factors of 43 RCC patients.
|
|
| LDHA
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | Cases | Low (n=12) | High (n=31) | P-value |
|---|
| Age (years) |
|
|
| 0.836 |
|
≤60 | 19 | 5 | 14 |
|
|
>60 | 24 | 7 | 17 |
|
| Gender |
|
|
| 0.099 |
|
Male | 20 | 8 | 12 |
|
|
Female | 23 | 4 | 19 |
|
| Histological
grade |
|
|
| 0.497 |
|
Well | 27 | 9 | 18 |
|
|
Moderate and poor | 16 | 3 | 13 |
|
| Tumor size |
|
|
| 0.009a |
| ≤5
cm | 22 | 10 | 12 |
|
| >5
cm | 21 | 2 | 19 |
|
| Lymph node
metastasis |
|
|
| 0.025b |
|
Positive | 10 | 0 | 10 |
|
|
Negative | 33 | 12 | 21 |
|
| Distant
metastasis |
|
|
| 0.035b |
|
Positive | 14 | 1 | 13 |
|
|
Negative | 29 | 11 | 18 |
|
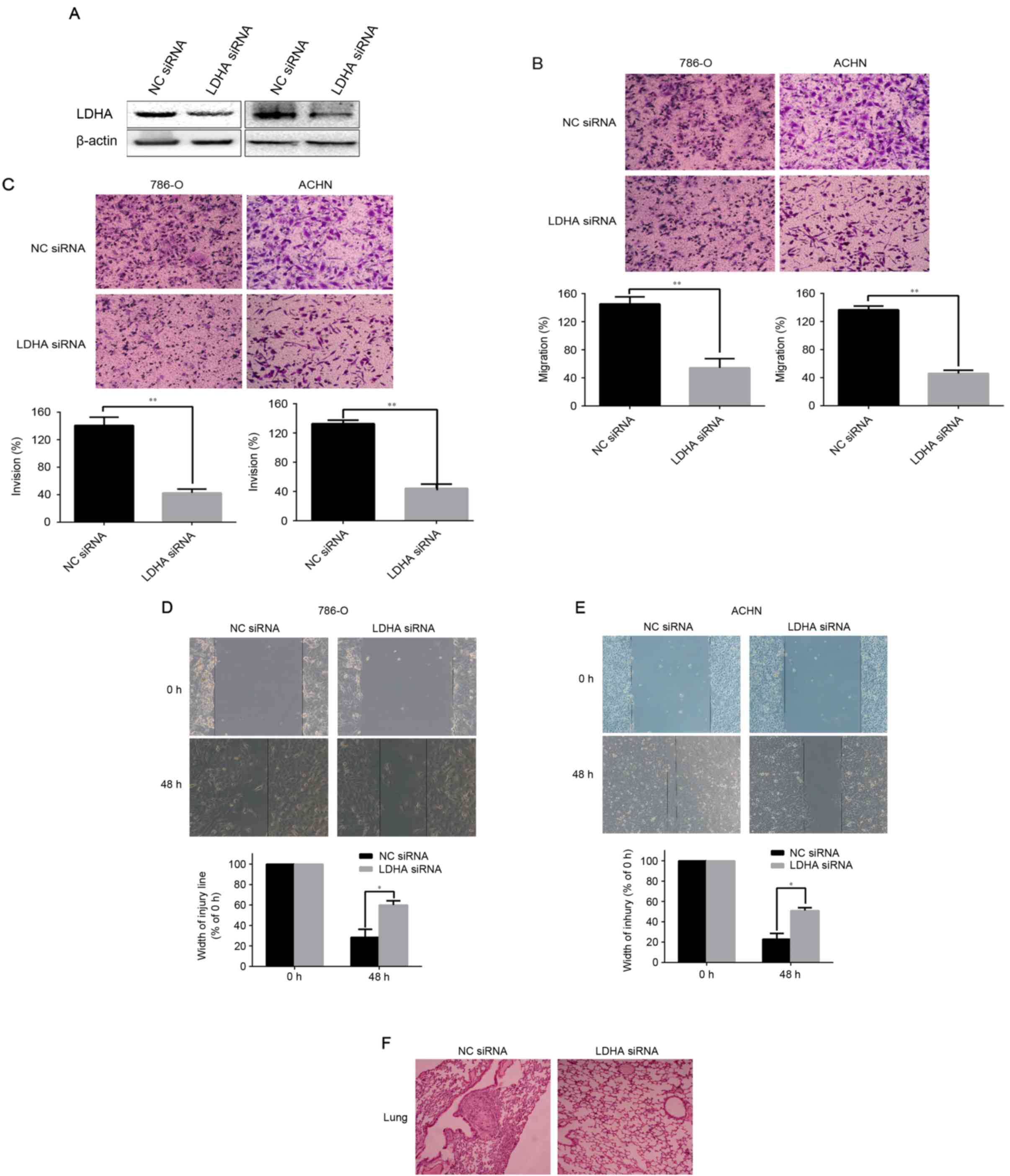
Downregulation of LDHA inhibited
migration and invasion of RCC cells
Following this, the authors hypothesized about the
functional role of LDHA in tumor cells. Western blotting revealed
that LDHA protein levels were obviously decreased in 786-O and ACHN
cells that transfected with LDHA-siRNA, compared with their control
groups respectively (Fig. 2A).
Then assays were performed to investigate the role of LDHA in
migration of invasion of RCC cells. The Transwell assay indicated
that downregulation of LDHA significantly decreased the number of
786-O and ACHN cells crossing the membrane compared to the control
group, as presented in Fig. 2B
(P<0.01 for 786-O and ACHN). Matrigel invasion assay showed that
the number of LDHA-siRNA cells crossing Matrigel-coated membrane
significantly decreased compared to the control group (P<0.01
for 786-O and ACHN, Fig. 2C). The
same conclusions were reached following the scratch migration assay
(P=0.0374 for 786-O and P=0.0246 for ACHN, Fig. 2D and E). These data illustrated
that the low-expression of LDHA significantly suppresses the
ability of migration and invasion of RCC cells.
Knockdown of LDHA inhibits tumor
metastasis in vivo
To determine the function of LDHA on tumor
metastasis in vivo, an orthotopic renal xenograft model was
established in the present research. At 7 weeks, the mice were
sacrificed and the pulmonary metastasis foci were counted. Of note,
average metastatic nodules of lung were dramatically decreased in
the LDHA-siRNA group, compared to NC siRNA groups (P<0.033;
Table II). Hematoxylin and eosin
(H&E) staining of serial sections of lung were used to identify
metastasis nodules (Fig. 2F).
These results suggested that LDHA is a critical factor inhibiting
RCC metastasis in vivo.
 | Table II.Incidence of metastatic tumors of the
lung. |
Table II.
Incidence of metastatic tumors of the
lung.
| Group | Numbers | Incidence of renal
cell carcinoma | Metastatic
rates |
|---|
| LDHA-siRNA | 10 | 10/10 (100%) | 5/10 (50%) |
| NC siRNA | 10 | 10/10 (100%) | 10/10 (100%) |
| P-value |
|
| 0.033 |
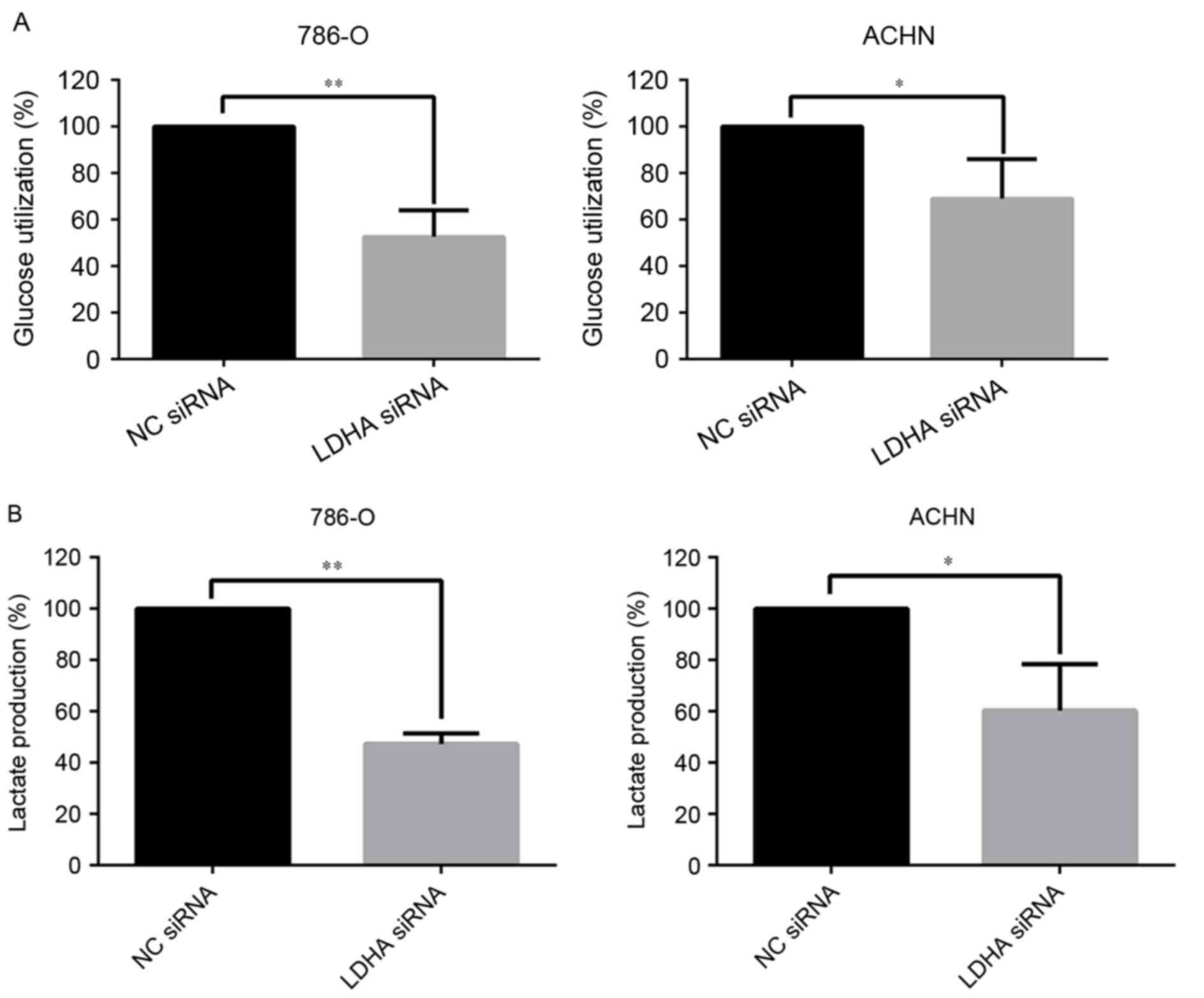
LDHA promoted the Warburg effect in
RCC cells
Accumulating evidence has demonstrated that the
Warburg effect played an important role in the development of
tumor, and may have potential clinical applications (24). The influence of LDHA on the Warburg
effect in RCC cells was also investigated. In the present study,
the effect of LDHA on glucose utilization and lactate production
was assessed. Significant decreasing in glucose utilization
(P=0.0019 for 786-O cells and P=0.035 for ACHN cells) and lactate
production (P<0.01 for 786-O cells and P=0.019 for ACHN cells)
in LDHA-siRNA RCC cells were observed (Fig. 3A and B). These data indicated that
LDHA could affect the Warburg effect, which influences the
development of RCC.
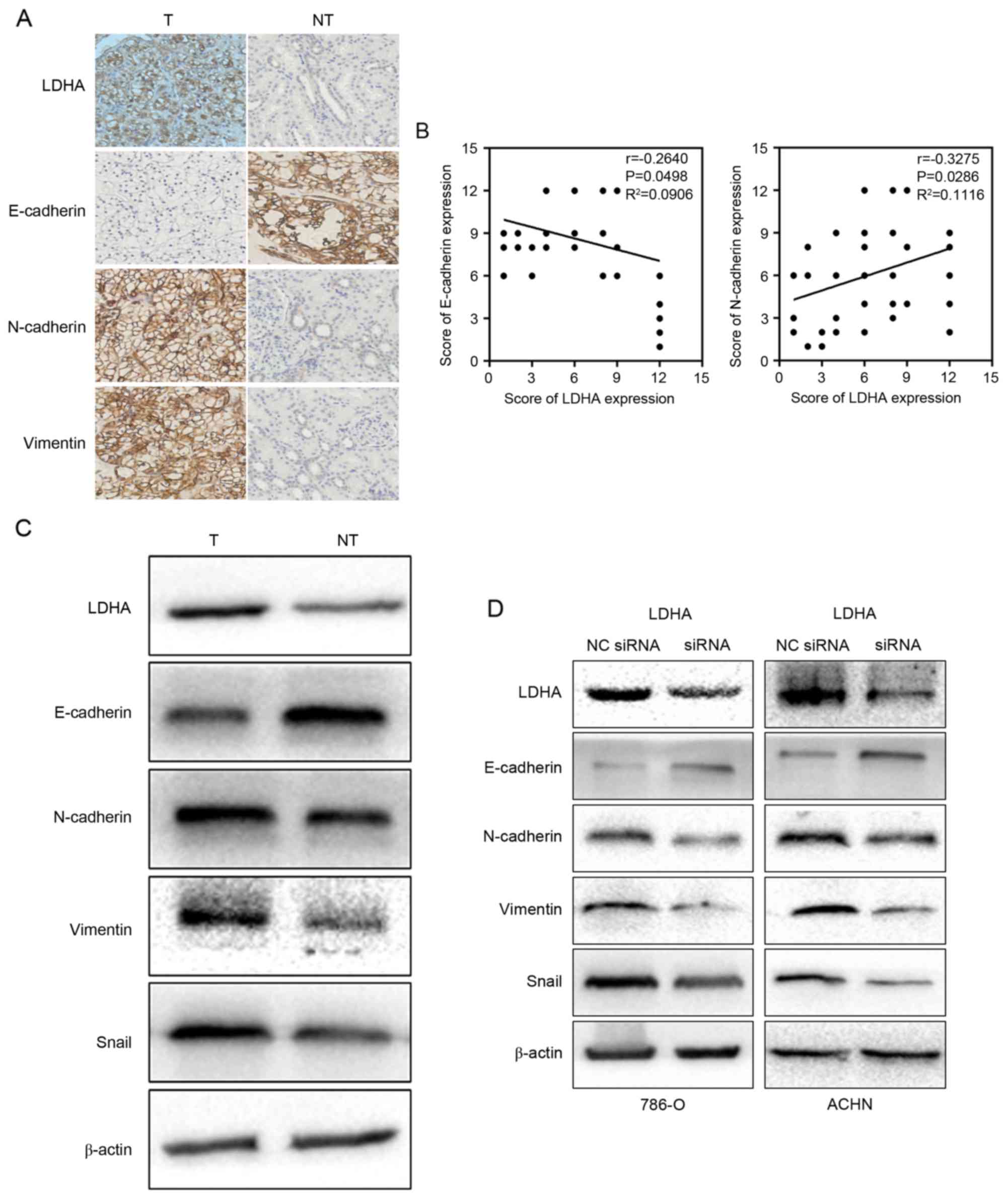
LDHA suppressed epithelial phenotype
and promoted mesenchymal transition
Limited investigation attempted to elucidate that
LDHA could promote EMT in muscle-invasive bladder cancer (25). In the current study, retrospective
analyses showed that LDHA expression was related to tumor
metastasis. Therefore, the authors ask whether LDHA expression was
related to EMT process. To this end, the correlation between the
expression of LDHA and EMT markers was tested. The results of IHC
indicated that the level of reduction of E-cadherin (P=0.0498) and
elevation of N-cadherin (P=0.0286) and vimentin (P=0.033) were
dramatically correlated with the upregulation of LDHA (Fig. 4A). In addition, a negative
correlation between LDHA expression and E-cadherin expression and a
positive correlation between LDHA expression and N-cadherin were
explored using Pearson's correlation coefficient (Fig. 4B). Western blotting of RCC tissues
revealed that the downregulation of LDHA was negatively associated
with epithelial phenotype and positively associated with
mesenchymal phenotype, which was consistent with the IHC
observations (Fig. 4C).
In order to substantiate the hypothesis that LDHA
may inhibit EMT, we further tested the EMT makers in LDHA-siRNA
cells and the control group, which yielded similar results. An
increased level of E-cadherin was observed as a response to the
treatment of LDHA-siRNA in RCC cells. On the contrary, N-cadherin
and vimentin were attenuated in LDHA-siRNA cells compared to the
control group (Fig. 4D).
Furthermore, downregulation of LDHA suppressed the expression of
EMT inducer Snail (Fig. 4C and D).
These findings make clear that EMT process plays an important role
in LDHA induced metastasis in RCC cells.
Oxamate suppressed tumor metastasis by
inhibiting LDHA activity and EMT process
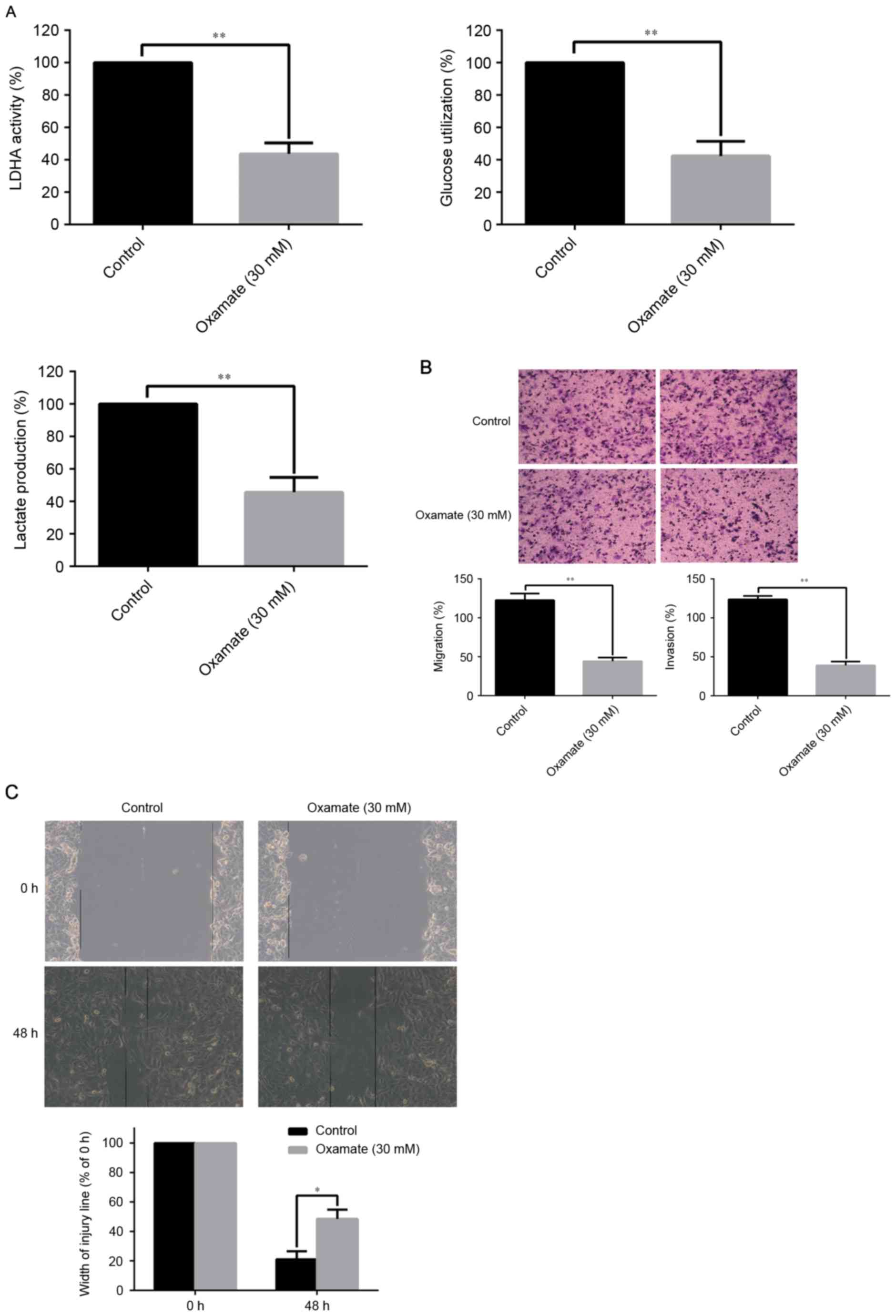
Considering the clinical application of target
therapy, the authors treated the RCC cells with oxamate to
investigate the contribution of LDHA inhibitor to LDHA activity and
LDHA-driven EMT. The concentration of oxamate was 30 mM according
to the authors' previous study. The results demonstrated that
oxamate significantly decreased LDHA activity, glucose utilization,
and lactate production of RCC cells compared with the control group
(P<0.01; Fig. 5A). As
illustrated in Fig. 5B, oxamate
apparently inhibited the ability of migration and invasion of 786-O
RCC cells by Transwell assays compared to the control group
(P<0.01). The scratch migration assay, in line with Transwell
assays confirmed that oxamate significantly suppressed the
migration of 786-O RCC cells (P<0.05; Fig. 5C). The above observations
increasingly support the notion that LDHA promote RCC metastasis by
mechanisms orchestrated by EMT.
Discussion
RCC is the leading cause of cancer-death worldwide.
Surgical treatment at early stage is the only curative therapy for
RCC patients. Unfortunately, due to high frequency of intrarenal
and extrarenal metastasis, most of RCC patients are diagnosed at
the advanced stage and no suitable to receive the curative therapy,
which leads to poor prognosis of RCCs. Hence, it is urgent to
explore the molecular mechanism of RCC progression and discover the
novel RCC markers and target agents.
Gradually acquiring a number of genetic mutations is
necessary for the malignant transformation of cells. LDHA, a key
enzyme in aerobic glycolysis, has a primary function to maintain
the rapid regeneration of NAD+ (28). A growing body of evidence has
suggested that LDHA was overexpressed and associated with poor
prognosis of various cancers including pancreatic cancer, breast
cancer and ovarian cancer (29,30).
In the current study, on the basis of gathered specimens from RCCs
and the relevant clinical information, the authors detected that
LDHA was overexpressed in RCC tissues significantly and predicted
worse survival following renal resection, which was in agreement
with published observations (31).
These data supported that LDHA could function as an oncogenic
protein in RCC.
Tumor cells are characterized by increased glucose
uptake, and increased lactate production, which are known as the
Warburg effect. In tumor cells, lactate is exported by
monocarboxylate transporters leading to the acidification of
microenvironment, however this alternation results in cell death in
normal cells (32). Numerous
attempts have been made to explore the changes of gene related with
the Warburg effect (33). In the
present research, a decreased level of consumption of glucose and
production of lactate were observed as a response to downregulation
of LDHA, which implied that LDHA may trigger aggressive forms of
malignancy via regulating the Warburg effect. But the role of the
change of the Warburg effect has not been illuminated.
Tumor metastasis is a primary factor affecting the
prognosis of RCC patients (1).
Therefore, it is critical to elucidate the mechanism of metastasis
of RCC. The transition of epithelial cells to a mesenchymal
phenotype, is the EMT (22). The
role of EMT contributing to the metastatic potential of tumors has
been widely accepted (26,34). A recent study showed that EMT
played a crucial role in RCC development and progression, it could
be a therapeutic target in RCC patients (35). Multiple intracellular signaling
pathways have been implicated in mediating EMT. Wang et al
(36) showed that downregulation
of LDHA would suppress tumor metastasis by downregulating the
expression of matrix metalloproteinase (MMP)-9, MMP-2 and vascular
endothelial growth factor. Therefore, the authors hypothesized that
EMT might be implicated in the metastasis induced by the factor
LDHA. In the present investigation, the authors firstly confirmed
that LDHA level was positively related to tumor metastasis by
clinical data analysis. Further studies showed that LDHA expression
has a negative correlation with epithelial phenotype and a positive
correlation with mesenchymal phenotype. It is worthwhile to note
that LDHA was positively associated with Snail, a transcription
factor regulating EMT. Furthermore, LDHA expression was silenced by
LDHA-siRNA in RCC cells and obtained a reversed EMT phenotype
including lower cell mobility, weaker invasion capacity, more
epithelial maker expression and less expression of mesenchymal
markers, which was in concert with the IHC result. Besides,
experiments in vivo further confirmed the role of LDHA in
facilitating RCC metastasis. These data strongly suggest that the
dysregulation of LDHA is responsible for the initiation of EMT in
RCC.
To establish the potential role of LDHA in clinical
application and corroborate these data further, the authors
extended their studies with treating LDHA inhibitor oxamate to the
RCC cells, of which results indicated that oxamate could inhibit
the activity of LDHA, the utilization of glucose and the production
of lactate. Oxamate can also suppress the migration and invasion of
RCC cells by affecting the EMT process. In summary, the study
indicated that the high level of LDHA can promote tumor metastasis
by stimulating initiation of EMT in renal cell carcinoma. Based on
the combination of these data, the authors believe that LDHA would
have therapeutic implications. However, the molecular mechanisms
need further to fully elucidated.
Glossary
Abbreviations
Abbreviations:
|
RCC
|
renal cell carcinoma
|
|
siRNA
|
small interfering RNA
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
US
|
ultrasound
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
SD
|
standard deviation
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
T
|
tumor tissue
|
|
NT
|
corresponding non-tumor tissue
|
References
|
1
|
Ljungberg B, Campbell SC, Choi HY, Jacqmin
D, Lee JE, Weikert S and Kiemeney LA: The epidemiology of renal
cell carcinoma. Eur Urol. 60:615–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kabaria R, Klaassen Z and Terris MK: Renal
cell carcinoma: Links and risks. Int J Nephrol Renovasc Dis.
9:45–52. 2016.PubMed/NCBI
|
|
4
|
Moch H, Artibani W, Delahunt B, Ficarra V,
Knuechel R, Montorsi F, Patard JJ, Stief CG, Sulser T and Wild PJ:
Reassessing the current UICC/AJCC TNM staging for renal cell
carcinoma. Eur Urol. 56:636–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bilim V, Ougolkov A, Yuuki K, Naito S,
Kawazoe H, Muto A, Oya M, Billadeau D, Motoyama T and Tomita Y:
Glycogen synthase kinase-3: A new therapeutic target in renal cell
carcinoma. Br J Cancer. 101:2005–2014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuen CA, Asuthkar S, Guda MR, Tsung AJ and
Velpula KK: Cancer stem cell molecular reprogramming of the Warburg
effect in glioblastomas: A new target gleaned from an old concept.
CNS Oncol. 5:101–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taniguchi K, Sakai M, Sugito N, Kumazaki
M, Shinohara H, Yamada N, Nakayama T, Ueda H, Nakagawa Y, Ito Y, et
al: PTBP1-associated microRNA-1 and −133b suppress the Warburg
effect in colorectal tumors. Oncotarget. 7:18940–18952. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soltysova A, Breza J, Takacova M,
Feruszova J, Hudecova S, Novotna B, Rozborilova E, Pastorekova S,
Kadasi L and Krizanova O: Deregulation of energetic metabolism in
the clear cell renal cell carcinoma: A multiple pathway analysis
based on microarray profiling. Int J Oncol. 47:287–295. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim HY, Yip YM, Chiong E, Tiong HY,
Halliwell B, Esuvaranathan K and Wong KP: Metabolic signatures of
renal cell carcinoma. Biochem Biophys Res Commun. 460:938–943.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldman RD, Kaplan NO and Hall TC: Lactic
dehydrogenase in human neoplastic tissues. Cancer Res. 24:389–399.
1964.PubMed/NCBI
|
|
12
|
Sun X, Sun Z, Zhu Z, Guan H, Zhang J,
Zhang Y, Xu H and Sun M: Clinicopathological significance and
prognostic value of lactate dehydrogenase A expression in gastric
cancer patients. PloS One. 9:e910682014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ,
Xue J, Yang HQ, Li JL, Liu XF and Kuang SJ: Inhibition of LDHA
suppresses tumor progression in prostate cancer. Tumour Biol.
36:8093–8100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu H, Jackson AL, Kilgore JE, Zhong Y,
Chan LL, Gehrig PA, Zhou C and Bae-Jump VL: JQ1 suppresses tumor
growth through downregulating LDHA in ovarian cancer. Oncotarget.
6:6915–6930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao F, Zhao T, Zhong C, Zhu J and Zhao H:
LDHA is necessary for the tumorigenicity of esophageal squamous
cell carcinoma. Tumour Biol. 34:25–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fantin VR, St-Pierre J and Leder P:
Attenuation of LDH-A expression uncovers a link between glycolysis,
mitochondrial physiology, and tumor maintenance. Cancer Cell.
9:425–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Girgis H, Masui O, White NM, Scorilas A,
Rotondo F, Seivwright A, Gabril M, Filter ER, Girgis AH, Bjarnason
GA, et al: Lactate Dehydrogenase A is a potential prognostic marker
in clear cell renal cell carcinoma. Mol Cancer. 13:1012014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Xu L, Wu Q, Liu M, Tang F, Cai Y,
Fan W, Huang H and Gu X: Inhibition of LDHA deliver potential
anticancer performance in renal cell carcinoma. Urol Int. 2016.
|
|
19
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao J, Wang H, Chen F, Fang J, Xu A, Xi W,
Zhang S, Wu G and Wang Z: Galangin inhibits cell invasion by
suppressing the epithelial-mesenchymal transition and inducing
apoptosis in renal cell carcinoma. Mol Med Rep. 13:4238–4244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giannelli G, Koudelkova P, Dituri F and
Mikulits W: Role of epithelial to mesenchymal transition in
hepatocellular carcinoma. J Hepatol. 65:798–808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakazawa M and Kyprianou N:
Epithelial-mesenchymal-transition regulators in prostate cancer:
Androgens and beyond. J Steroid Biochem Mol Biol. 166:84–90. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:E172016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piva F, Giulietti M, Santoni M, Occhipinti
G, Scarpelli M, Lopez-Beltran A, Cheng L, Principato G and
Montironi R: Epithelial to mesenchymal transition in renal cell
carcinoma: Implications for cancer therapy. Mol Diagn Ther.
20:111–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang F, Ma S, Xue Y, Hou J and Zhang Y:
LDH-A promotes malignant progression via activation of
epithelial-to-mesenchymal transition and conferring stemness in
muscle-invasive bladder cancer. Biochem Biophys Res Commun.
469:985–992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Sun Y, Rao Q, Xu H, Li L and Chang
C: Androgen receptor (AR) suppresses miRNA-145 to promote renal
cell carcinoma (RCC) progression independent of VHL status.
Oncotarget. 6:31203–31215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hatzivassiliou G, Zhao F, Bauer DE,
Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA and
Thompson CB: ATP citrate lyase inhibition can suppress tumor cell
growth. Cancer Cell. 8:311–321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaplan NO: Lactate dehydrogenase-structure
and function. Brookhaven Symp Biol. 17:131–153. 1964.PubMed/NCBI
|
|
29
|
Weide B, Elsasser M, Buttner P,
Pflugfelder A, Leiter U, Eigentler TK, Bauer J, Witte M, Meier F
and Garbe C: Serum markers lactate dehydrogenase and S100B predict
independently disease outcome in melanoma patients with distant
metastasis. Br J Cancer. 107:422–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brown JE, Cook RJ, Lipton A and Coleman
RE: Serum lactate dehydrogenase is prognostic for survival in
patients with bone metastases from breast cancer: A retrospective
analysis in bisphosphonate-treated patients. Clin Cancer Res.
18:6348–6355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Girgis H, Masui O, White NM, Scorilas A,
Rotondo F, Seivwright A, Gabril M, Filter ER, Girgis AH, Bjarnason
GA, et al: Lactate dehydrogenase A is a potential prognostic marker
in clear cell renal cell carcinoma. Mol Cancer. 13:1012014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han T, Kang D, Ji D, Wang X, Zhan W, Fu M,
Xin HB and Wang JB: How does cancer cell metabolism affect tumor
migration and invasion? Cell Adh Migr. 7:395–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sanders E and Diehl S: Analysis and
interpretation of transcriptomic data obtained from extended
Warburg effect genes in patients with clear cell renal cell
carcinoma. Oncoscience. 2:151–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garber K: Energy deregulation: Licensing
tumors to grow. Science. 312:1158–1159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sanders E and Diehl S: Analysis and
interpretation of transcriptomic data obtained from extended
Warburg effect genes in patients with clear cell renal cell
carcinoma. Oncoscience. 2:151–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Xu L, Wu Q, Liu M, Tang F, Cai Y,
Fan W, Huang H and Gu X: Inhibition of LDHA deliver potential
anticancer performance in renal cell carcinoma. Urol Int. 2016.
|