Introduction
Primary osteoporosis is a systemic skeletal disease
that is characterized by low bone mass, microstructural damage of
bone tissue and weakened bone strength (1). It may lead to a significant increase
in fracture risk and may be a serious threat to the health of
patients. Postmenopausal osteoporosis (PMOP) is one of the most
common types of primary osteoporosis. Owing to the decrease of
estrogen levels, osteoclastogenesis is enhanced, which results in
dramatic bone loss and increased bone destruction (2–4).
Pharmacological interventions for osteoporosis include
antiresorptive agents that prevent bone resorption and anabolic
agents that aid in new bone formation. Despite the multiple
management options, there remain concerns for potential adverse
effects of these agents (5).
Discovery of the receptor activator of nuclear factor-κB
(RANK)/RANK ligand (RANKL) signaling pathway, which is a key
regulator of osteoclastogenesis and bone resorption, has led to a
novel therapy that targets this pathway in the treatment of
osteoporosis (6,7).
In Traditional Chinese Medicine (TCM) it is believed
that kidney deficiency is the main pathogenesis of PMOP, and spleen
deficiency is also involved (8,9). A
TCM formula, jiangugranule (JG; which includes calcined Os
Canitis, Epimediumbrevicornum Maxim and
Davalliamariesii Moore ex Bak), exhibited great efficacy in
the clinical treatment on PMOP by tonifying the kidney and
invigorating the spleen. The authors previous studies have
demonstrated that JG significantly improved both bone quantity and
bone quality of PMOP model rats by reducing bone calcium loss and
collagen degradation (10,11). Owing to the direct relationship
between bone decomposition and osteoclast activity, the present
study focused on osteoclast differentiation and the key regulators.
The effects of JG-containing serum on RANKL-induced
osteoclastogenesis and the expression of key molecules in the
RANK/RANKL signaling pathway were examined, including RANK, tumor
necrosis factor receptor-associated factor 6 (TRAF6), nuclear
factor-κB (NF-κB), c-Fos and nuclear factor of activated T cells,
cytoplasmic 1 (NFATc1), to investigate the mechanism of JG on
preventing and treating PMOP.
Materials and methods
Preparation of JG
JG is a TCM prescription that comprises calcined
Os Canitis (500 mg), E. brevicornum Maxim (12 g),
D. mariesii Moore ex Bak. (12 g), Cornusofficinalis
Sieb.et Zucc. (9 g), Lyciumchinense Mill. (9 g),
Dioscoreaopposita (9 g), Codonopsispilosula (10 g),
Dipsacusasperoides C.Y. Cheng et T.M. Ai (10 g), Crocus
sativus L. (2 g), Pericarpium Citri Reticulatae (6 g),
Curcuma longa L. (10 g) and Carapax Testudinis (400
mg). The medicinal materials were purchased from Fujian
Pharmaceutical Company (Fuzhou, China). All ingredients are made
into traditional Chinese medicine granules by Fujian Academy of
Traditional Chinese Medicine (Fujian, China). All the medicinal
materials were broken into coarse powder and mixed together. The
mixture was immersed in distilled water for 1 h and then boiled in
a distillation apparatus for 2 h. The extracting solution was
concentrated to extract under a vacuum in a 50°C water bath, then
cooled and stored at 4°C until use. A total of 1 gram of JG was
equivalent to 2.99 g of crude medicines.
Analysis of JG by high-performance
liquid chromatography (HPLC)
An Agilent 1260 Liquid Chromatography system
(Agilent Technologies, Inc., Santa Clara, CA, USA), equipped with a
G1311C quaternary solvent delivery system, a G7617B autosampler and
a G1315D diode array detector was used to detect icariin from E.
brevicornum Maxim, morroniside and loganin from
C.officinalis Sieb. et Zucc., andaurantiamarin from
Pericarpium Citri Reticulatae. An Agilent HC-C18 (25×4.6 mm;
5 µm) column connected with a Zorbax Extend guard column (20×4.6
mm; 5 µm) was used. The column temperature was set at 30°C. The
mobile phase consisted of (A) acetonitrile and (B) water (v/v)
using a linear gradient elution of 10–20% A at 0–15 min, 20–30% A
at 15–20 min and 30% A at 20–35 min. The flow rate was 1.0 ml/min,
and 10 µl of sample was injected. Ultraviolet detection wavelengths
were set at 240 nm at 0–15 min, 283 nm at 15–55 min and 270 nm at
25–35 min, and the absorption spectra of compounds were recorded
from 190 to 400 nm.
Preparation of JG-containing
serum
A total of 20 male specific-pathogen-free
Sprague-Dawley rats (age, 8–10 weeks; weight, 300±30 g) were
obtained from Shanghai Laboratory Animal Center (Shanghai, China)
and provided with food and water ad libitum, at 20°C and 50%
relative humidity, in a filtered clean atmosphere, under a12-h
dark/light cycle. Rats were randomly divided into 2 groups
(n=10/group): i) JG group, which received a dose of JG (2 g/kg/day)
intragastrically twice per day for 8 days; and ii) Blank control
group, which received a dose of standard saline (10 ml/kg/day)
intragastrically twice per day for 8 days. Rats were anesthetized 1
h following the last gavage, with an intraperitoneal injection of
2% pentobarbital sodium (0.2 ml/100 g body weight) and euthanized
by abdominal aorta exsanguination. The blood was collected and
centrifuged at 3,000 × g at room temperature for 15 min to obtain
the serum. Serum samples were collected from each group,
JG-containing serum or Blank serum, which were subsequently
filtered through a 0.22-µm filter membrane and stored in an
ultralow freezer at −80°C. All experimental procedures were
performed in accordance with the NIH Guidelines for the Care and
Use of Laboratory Animals and the National Animal Welfare Law of
China. The present study was approved by the Ethics Committee of
Fujian University of Traditional Chinese Medicine (Fuzhou,
China).
Cell culture experiments
Osteoclast precursor RAW264.7 cells were obtained
from The Cell Bank of Type Culture Collection of Chinese Academy of
Science (Shanghai, China) and cultured in a differentiation medium
comprising: α-minimum essential medium (α-MEM) supplemented with
10% fetal bovine serum (FBS) (both from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 50 ng/ml RANKL (R&D
Systems, Inc., Minneapolis, MN, USA). The culture medium was
replaced every 48 h. Cells were incubated at 37°C in a humid
atmosphere containing 5% CO2 for 6 days to obtain
osteoclasts.
For differentiation assays, cells were seeded in
24-well plates at a density 1×104 cells/well in
differentiation medium (without FBS) with various concentrations
(2, 5, 10, 15 and 20%) of either JG-containing serum or Blank serum
during the entire culture period of 6 days. The differentiation
rate of each well was calculated and compared to determine the most
effective concentration of JG-containing serum.
For RANK/RANKL pathway tests, cells were divided
into 2 groups: i) The Blank group, which was treated with 50 ng/ml
RANKL and 10% Blank serum; and ii) the JG group, which was treated
with 50 ng/ml RANKL and 10% JG-containing serum. Following 24, 48
and 96 h incubation, the cells were harvested for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis. Cells cultured in common medium (α-MEM
supplemented with 10% Blank serum, without RANKL) as negative
control (0 h of Blank group). All experiments were repeated at
least 3 times.
Osteoclast differentiation assays
Osteoclast differentiation rate was measured by
counting the number of tartrate-resistant acid phosphatase
(TRAP)-positive stained cells, using the TRAP Staining kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China), according to
the manufacturer's protocol. RAW264.7 cells cultured in
differentiation medium at a density of 1×104 cells/well
for 6 days were collected and fixed with the fixative solution in
the kit at room temperature for 10 min, rinsed thoroughly with
deionized water and stained with naphthol AS-BI phosphate for 1 h
at 37°C, followed by hematoxylin counterstaining at room
temperature for 2 min. Osteoclasts were determined to be
TRAP-positive stained multinuclear (containing ≥3 nuclei) cells
under light microscopy. A total of 6 fields/well (magnification,
100x) were examined.
To detect the F-actin containing podosome belt of
osteoclasts, rhodaminephalloidin staining (1:200 dilution with PBS;
Cytoskeleton Inc., Denver, CO, USA) was performed, according to the
manufacturer's protocol. RAW264.7 cells cultured in a
differentiation medium at a density of 1×104 cells/well
for 6 days were collected, and fixed at room temperature with 4%
paraformaldehyde for 10 min, permeabilized with 1% Triton X-100 for
10 min, washed with PBS for 3 min and incubated with
rhodaminephalloidin at room temperature in the dark for 30 min,
followed by 3 washes with PBS for 5 min each. Nuclei were
counterstained with 100 nM DAPI in PBS at room temperature for 5
min. Osteoclasts were observed under a fluorescence microscope
(Leica DMI4000B; Leica Microsystems GmbH, Wetzlar, Germany).
RT-qPCR for RANK/RANKL pathway
components
Cells from the Blank control group, JG group and
negative control group were collected. RNA was extracted by TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) from cells at a
density of 1×107 cells/ml and reverse transcribed to
cDNA using Prime Script First-Strand cDNA Synthesis kit (Takara
Biotechnology Co., Ltd., Dalian, China) for use as qPCR template.
The primers were as follows: RANK forward,
5′-GGCTGGCTACCACTGGAACT-3′ and reverse,
5′-TCCTGTAGTAAACGCCGAAGA−3′; TRAF6 forward,
5′-TCATTATGATCTGGACTGCCCAAC-3′ and reverse,
5′-TTATGAACAGCCTGGGCCAAC-3′; NF-κB forward,
5′-ACCACTGCTCAGGTCCACTGTC-3′ and reverse,
5′-GCTGTCACTATCCCGGAGTTCA3-'; NFATc1,
5′-CAAGTCTCACCACAGGGCTCACTA-3′ and reverse,
5′-TCAGCCGTCCCAATGAACAG-3′;c-Fos forward,
5′-ACGTGGAGCTGAAGGCAGAAC-3′ and reverse,
5′-AGCCACTGGGCCTAGATGATG−3′; and β-actin forward,
5′-AGGCTGTGTTGTCCCTGTA-3′ and reverse, 5′-ATGTCACGCACGATTTCC−3′.
PCR was performed using SYBR Green qPCR Mix (Takara Biotechnology
Co., Ltd.) and a Real-Time PCR system (ABI7500; Thermo Fisher
Scientific, Inc.) with the following program: 1 cycle at 95°C for
30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 34 sec.
qPCR was carried out on three replicates per sample. β-actin was
used as a reference gene for RNA correction of all samples; the
relative standard curve method (2−ΔΔCq method) was used
for the calculation of fold changes in gene expression (12).
Western blot analysis for RANK/RANKL
pathway
Cells from the Blank control, JG and negative
control groups were collected. Total protein was extracted in
protein lysis buffer (Beyotime Institute of Biotechnology, Haimen,
China) from cells at a density of 5×107 cells/ml.
Protein concentrations were determined by BCA Protein assay kit
(Beyotime Institute of Biotechnology). Equal amounts of proteins
(30 µg) were resolved by SDS-PAGE on a 12% gel and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Non-specific interactions were blocked with 5% skim milk at
4°C for 2 h and the membranes were incubated with 1:500 diluted
primary antibodies [rabbit anti-RANK polyclonal antibody (cat no.
ab200369), rabbit anti-TRAF6 monoclonal antibody (cat no. ab33915),
rabbit anti-NF-κB p105/p50 monoclonal antibody (cat no. ab32360),
rabbit anti-NFATc1 polyclonal antibody (cat no. ab25916), rabbit
anti-c-Fos polyclonal antibody (cat no. ab190289), Rabbit
anti-β-Actin monoclonal antibody (cat. no. ab8227) all Abcam,
Cambridge, MA, USA]. Rabbit anti-NF-κB p100/p52 monoclonal antibody
(cat no. 52583; Cell Signaling Technology, Inc., MA, USA) overnight
at 4°C, followed by incubation with horseradish peroxidase
(HRP)-conjugated secondary antibodies (goat anti-rabbit
immunoglobulin G H&L HRP; 1:5,000; cat no. ab6721; Abcam,
Cambridge, MA, USA). Protein bands were visualized with the
enhanced chemiluminescence reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Densitometric values were quantified for each
band with the Image Pro-Plus program (version 5.0; Media
Cybernetics, Inc., Rockville, MD, USA). Relative expression data
are expressed as a ratio of the optical intensity of the band of
the target protein over that of the internal control protein
(β-actin).
Statistical analysis
All calculations were performed using SPSS version
17.0 for Windows software (SPSS Inc., Chicago, IL, USA). Results
are presented as the mean ± standard deviation. All data were
analyzed using one-way analysis of variance and Fisher's least
significant difference test. P<0.05 were considered to indicate
a statistically significant difference.
Results
HPLC analysis of JG
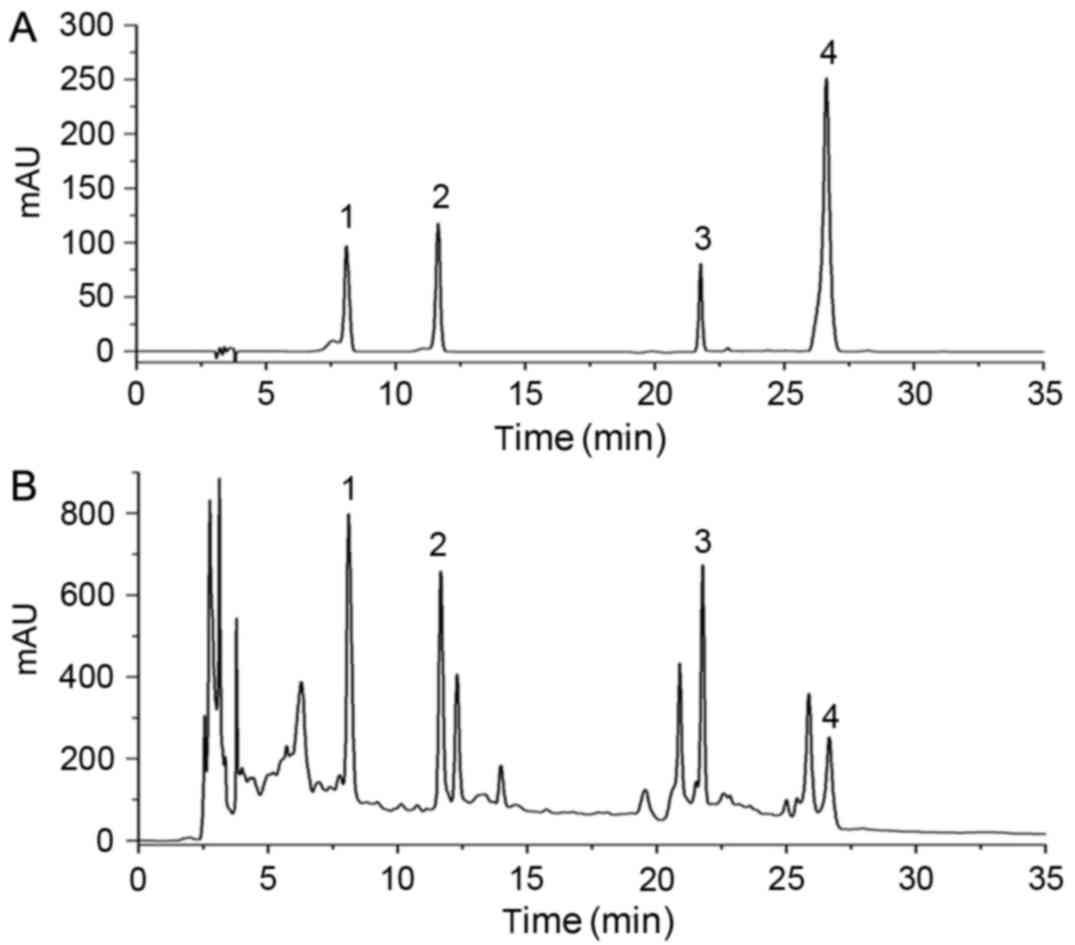
For HPLC analysis, four standard compounds (Fig. 1A) were used to determine the
composition of the JG extract (Fig.
1B), including morroniside and loganin from C.officinalis
Sieb. et Zucc., aurantiamarin from Pericarpium Citri
Reticulatae and icariin from E. brevicornum Maxim.
RANKL induces osteoclastogenesis
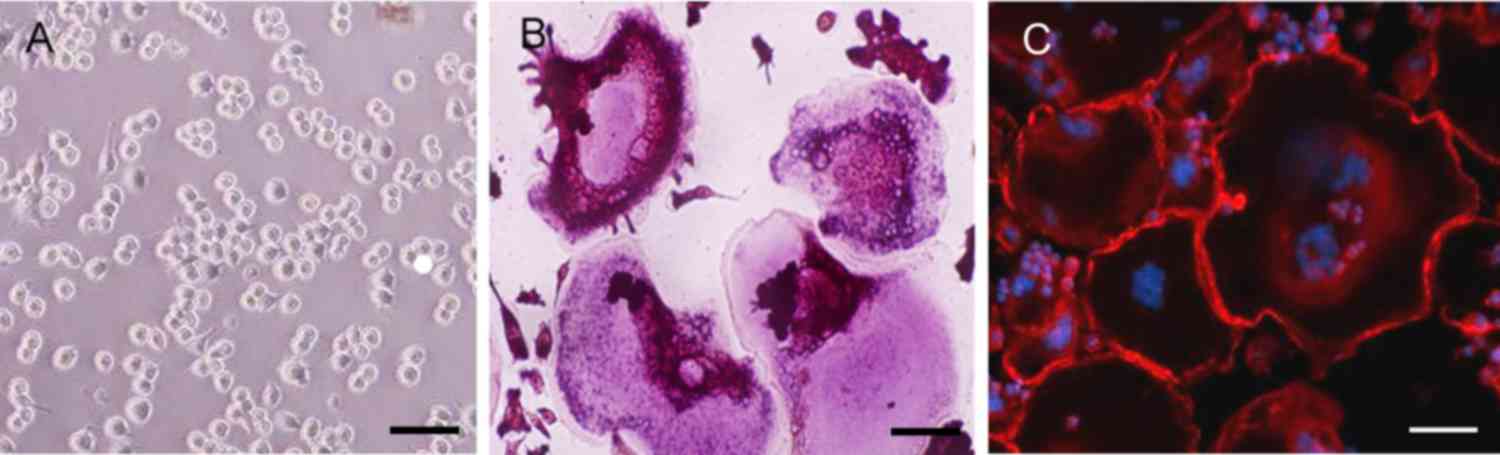
The results of TRAP staining demonstrated a notable
increase in TRAP-positive multinuclear cells in the RAW264.7 cells
incubated with RANKL for 6 days (Fig.
2A and B). Immunofluorescence staining with rhodaminephalloidin
revealed multinucleated giant cells with the characteristic
podosome belt of osteoclasts (Fig. 2B
and C). These results indicated that RANKL treatment induced
the formation of osteoclasts from RAW264.7 cells.
JG-containing serum inhibits
osteoclast differentiation induced by RANKL
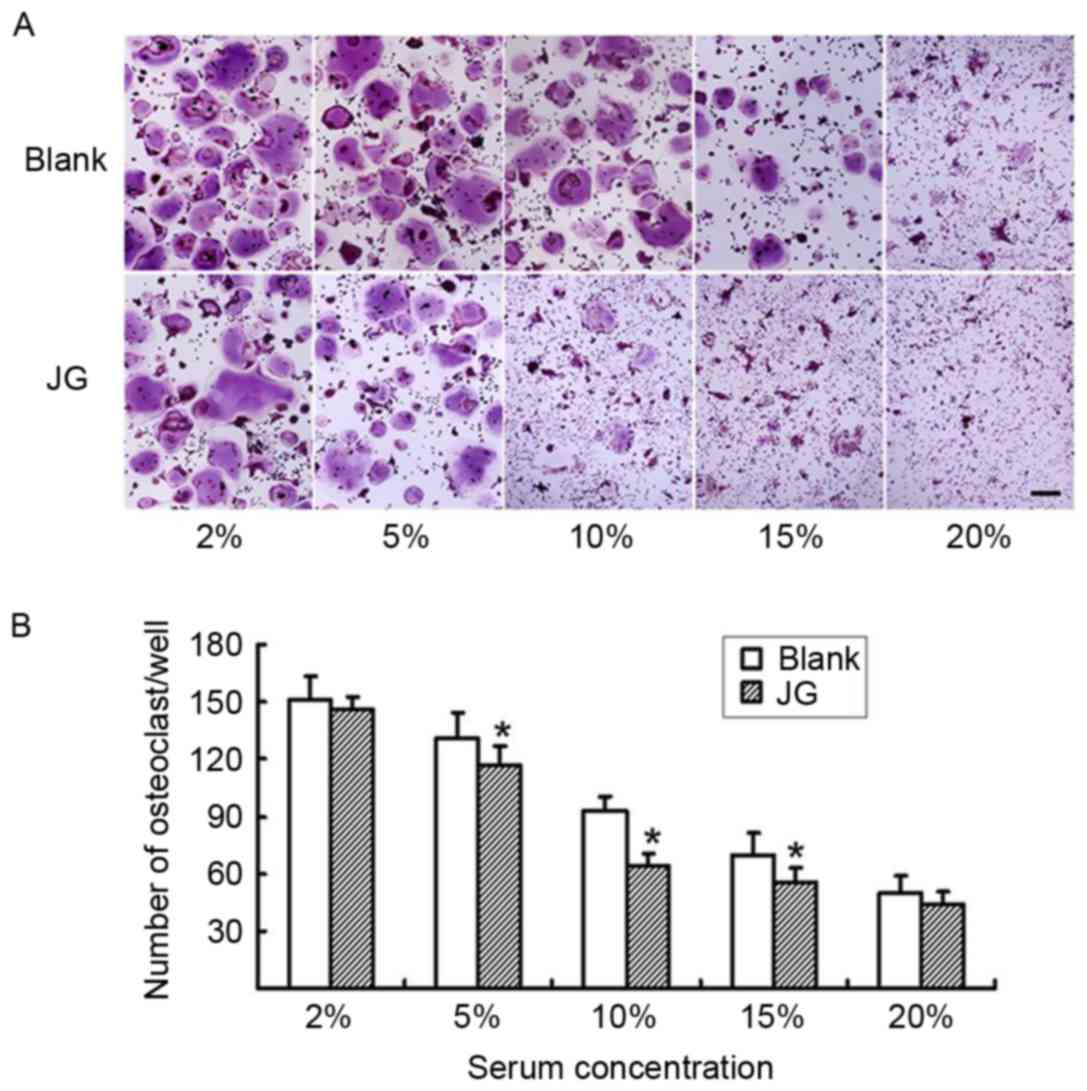
The differentiated osteoclasts were treated with
various concentrations of either JG-containing serum or Blank serum
for 6 days, and subsequently stained with TRAP (Fig. 3A). The number of TRAP-positive
stained cells in the 5, 10 and 15% JG-containing serum groups were
significantly decreased compared with the number of TRAP-positive
cells in the Blank group (P<0.05; Fig. 3B) particularly at the concentration
of 10%, which demonstrated that the serum dose-dependently
decreased the differentiation rate of osteoclasts.
Effects of JG-containing serum on
RANK/RANKL pathway
The results of RT-qPCR and western blot analysis
were consistent compared with the negative control (0 h of Blank
group), the mRNA and protein expression levels of NF-κB, NFATc1 and
c-Fos significantly increased in JG-containing serum group and the
Blank serum group following 24, 48 and 96 h of RANKL stimulation
(P<0.0l). Compared with Blank group, the mRNA and protein
expression of RANK, NFATc1 and c-Fos of JG group significantly
decreased following treatment for 24 and 48 h (P<0.0l). The
differences of TRAF6 and NF-κB expression between the Blank group
and JG group were not significant (Figs. 4 and 5).
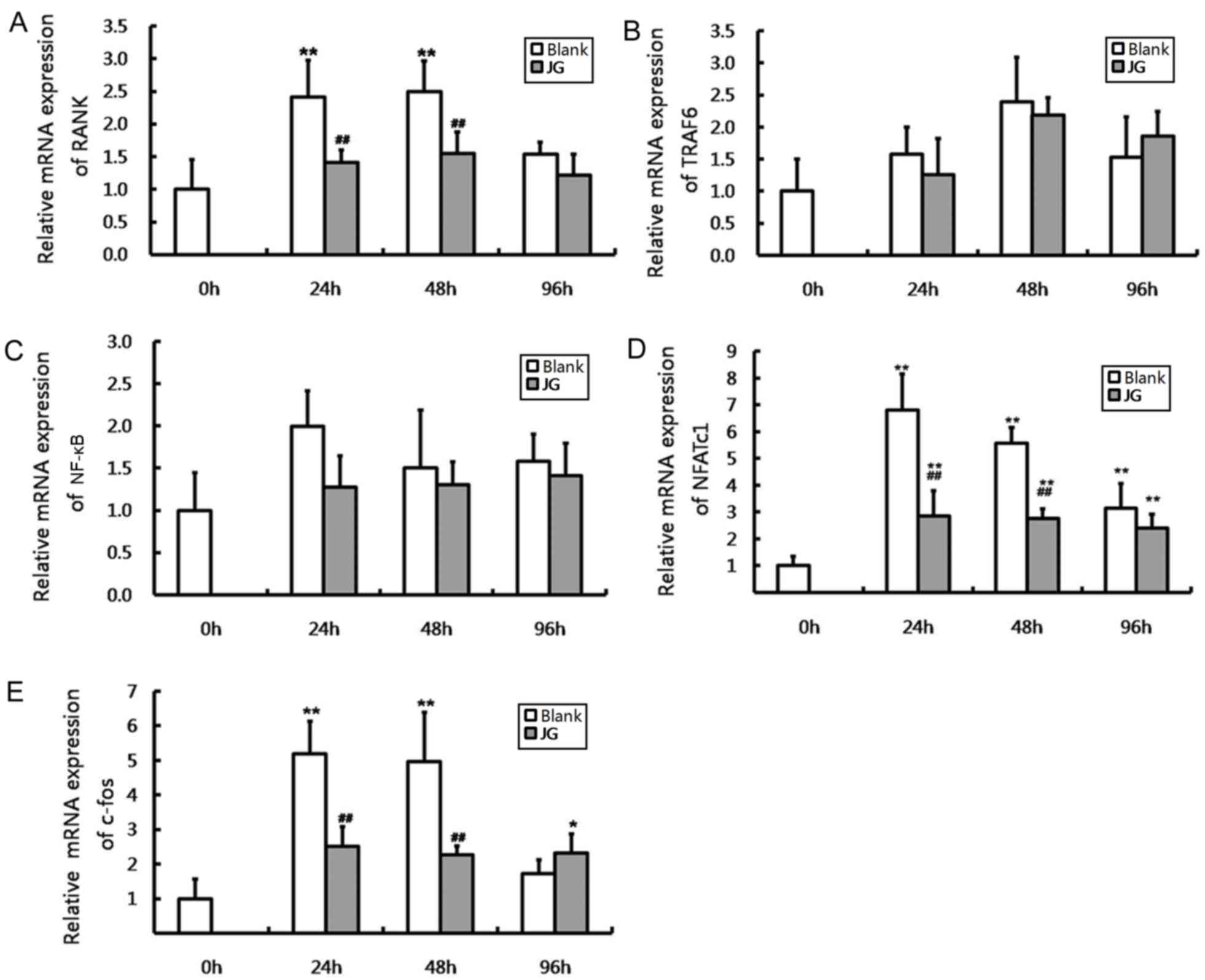 | Figure 4.Effects of JG-containing serum on mRNA
expression of RANK/RANKL pathway components in RAW264.7 cells.
Results of reverse transcription-quantitative polymerase chain
reaction indicating the relative mRNA expression levels of (A)
RANK, (B) TRAF6, (C) NF-κB, (D) NFATc1 and (E) c-Fos. Data are
presented as the mean ± standard deviation of 3 cultures.
*P<0.05 vs. Blank group, **P<0.01 vs. Blank group;
#P<0.05 vs. negative control (0 h of Blank group),
##P<0.01 vs. negative control (0 h of Blank group).
JG, jiangu granule; NFATc1, nuclear factor of activated T cells,
cytoplasmic 1; NF-κB, nuclear factor-κB; RANK, receptor activator
of nuclear factor-κB ligand; RANKL, RANK ligand; TRAF6, tumor
necrosis factor receptor-associated factor 6. |
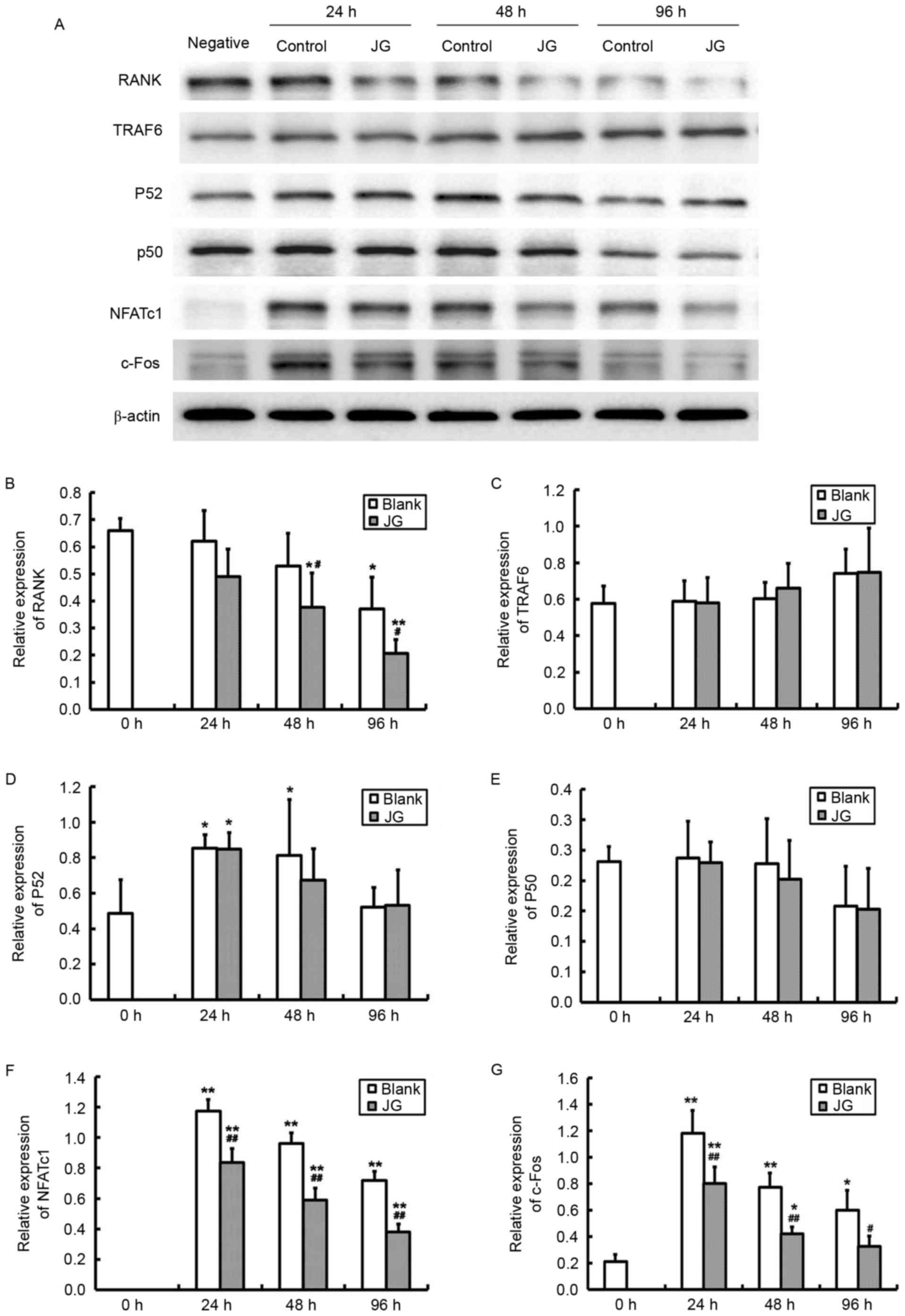 | Figure 5.Effects of JG-containing serum on
protein expression levels of RANK/RANKL pathway components. (A)
Images of the protein expression as analyzed by western blot assay.
Relative protein expression levels of (B) RANK, (C) TRAF6, (D)
NF-κBp52, (E) NF-κBp50, (F) NFATc1 and (G) c-Fos. Data are
presented as the mean ± standard deviation of 3 cultures.
*P<0.05 vs. Blank group; **P<0.01 vs. Blank group;
#P<0.05 vs. negative control (0 h of Blank group);
##P<0.01 vs. negative control (0 h of Blank group).
JG, jiangu granule; NFATc1, nuclear factor of activated T cells,
cytoplasmic 1; NF-κB, nuclear factor-κB; RANK, receptor activator
of nuclear factor-κB ligand; RANKL, RANK ligand; TRAF6, tumor
necrosis factor receptor-associated factor 6. |
Discussion
In TCM it is believed that the kidney is the origin
of congenital constitution. Bones are governed and nourished by the
kidney, which means that the development and the quality of bones
depend on the functions of the kidneys (13). It is been reported that several
traditional Chinese kidney-tonifying herbals may have
bone-strengthening effects (14).
It is also believed in TCM that the spleen is the origin of the
acquired constitution, which nourishes the kidney. The kidney and
spleen complement each other, so as to maintain bone health. A
summary of ancient literatures on constitution during menopause has
indicated that a shared pathogenesis in PMOP may be kidney
deficiency, and is frequently accompanied with spleen deficiency;
hence, the basic law of bone strengthening is to reinforce them
(8,9). Based on its pharmacological effects
of tonifying kidney and spleen, the Chinese formula JG was applied
to prevent and treat PMOP.
Bone is a dynamic organ that undergoes continuous
remodeling; that is, the osteoclasts resorb old and damaged bone,
which is replaced with new bone by osteoblasts (15,16).
The activity and balance of osteoclasts and osteoblasts maintain
the structural integrity and bone mass. When bone resorption
surpasses formation, bone mass decreases and osteoporosis may
occur. PMOP is a disease that results from dramatic bone loss and
increased bone destruction, owing to enhanced osteoclastogenesis
without a corresponding increase in osteoblastic activity (2–4). The
mouse monocyte/macrophage cell line RAW264.7, widely used as
osteoclast precursor, exhibits a strong potential to differentiate
into osteoclasts in the presence of RANKL (17). TRAP staining and F-actin
immunofluorescence staining are usually used for osteoclast
identification due to the abundant TRAP expression and podosome
belt formation of mature osteoclasts (18). Results from the present study
indicated that JG-containing serum treatment reduced the
RANKL-induced osteoclast differentiation from RAW264.7, which
indicated that JG may prevent osteoporosis by inhibiting
osteoclastogenesis and osteoclastic bone resorption.
Osteoclasts are large, multinucleated cells that are
derived from the monocyte/macrophage lineage. The RANK/RANKL
pathway serves a crucial role in osteoclast differentiation and
bone resorption (6,7). RANKL induces intracellular signals
through its receptor, RANK, and regulates the expression of various
down stream signaling molecules to exert their osteoclast-inducing
effect (19,20). The cytoplasmic domain of RANK binds
TRAF1, TRAF2, TRAF3, TRAF5, and TRAF6 to mediate the signals
(21). TRAF6 is the most important
of the TRAFs (22), which
transmits signals to downstream targets such as NF-κB and the
mitogen-activated protein kinase (MAPK) signaling pathways
(23). NF-κB regulates target
genes with its binding sites through classical or non-classical
pathway activation. There are five members of the NF-κB family in
mammals: p50/p105, p65/RelA, c-Rel, RelB and p52/p100. p50 and p52
expression is required for osteoclast precursors to differentiate
into osteoclasts in response to RANKL (24,25).
MAPK signaling pathways activate transcription factors of the
activator protein 1 family, which includes Jun, Fos and Fos-related
antigen, which subsequently regulate the expression of necessary
and important genes for osteoclast differentiation. c-Fos is
activated by a number of growth factors and cytokines, and serves a
key role in RANKL-induced osteoclast differentiation. c-Fos gene
deficient mice exhibited serious bone sclerosis owing to a complete
block of osteoclast differentiation (26). In addition, c-Fos induces and
activates NFATc1, which is another key transcription factor that
affects osteoclast differentiation (27). NFATc1 is activated by calcium
signaling and binds to its own promoter, thus turning on an
autoregulatory loop. NFATc1 also promotes expression and activation
of osteoclast-specific genes and proteins like TRAP, calcitonin
receptor and cathepsin, which results in the termination of
osteoclastic differentiation.
In the present study, key molecules of the RANK
signaling pathway, including RANK, TRAF6, NF-κB (p50 and p52),
NFATc1 and c-Fos were examined to investigate the effects of JG
treatment on RANKL-induced osteoclastogenesis. The results
demonstrated that RANKL stimulation increased mRNA and protein
expression of NF-κB, c-Fos and NFATc1, and consequently led to
osteoclast differentiation, which was consistent with the results
of previous studies (19,20). It was also revealed that
JG-containing serum significantly reduced the expression of RANK,
c-Fos and NFATc1, and consequently inhibited RANKL-induced
osteoclastogenesis. Decreased osteoclastogenesis reduces bone
resorption and ultimately increases bone mass. Therefore, the
results indicated a possible mechanism of JG on preventing and
treating PMOP.
In conclusion, the present study demonstrated the
effects of JG treatment on inhibiting osteoclast differentiation,
which may be achieved through the RANK/RANKL signaling pathway.
This study provides an experimental rationale for the application
of JG in clinical therapy of PMOP.
Acknowledgements
This work was supported by the Natural Science
Foundation of China (grant nos. 81574003 and 81473706), the Guiding
Project Foundation of Fujian Science and Technology Department
(grant no. 2015Y0069) and the Science Foundation of Fujian Province
(grant nos. 2015J01690 and 2014J01355).
References
|
1
|
Consensus development conference:
Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med.
94:646–650. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pacifici R: Estrogen, cytokines, and
pathogenesis of postmenopausal osteoporosis. J Bone Miner Res.
11:1043–1051. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seifert-Klauss V, Fillenberg S, Schneider
H, Luppa P, Mueller D and Kiechle M: Bone loss in premenopausal,
perimenopausal and postmenopausal women: Results of a prospective
observational study over 9 years. Climacteric. 15:433–440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Villiers TJ: Bone health and
osteoporosis in postmenopausal women. Best Pract Res Clin Obstet
Gynaecol. 23:73–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tella SH and Gallagher JC: Prevention and
treatment of postmenopausal osteoporosis. J Steroid Biochem Mol
Biol. 142:155–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lacey DL, Boyle WJ, Simonet WS, Kostenuik
PJ, Dougall WC, Sullivan JK, San Martin J and Dansey R: Bench to
bedside: Elucidation of the OPG-RANK-RANKL pathway and the
development of denosumab. Nat Rev Drug Discov. 11:401–419. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bridgeman MB and Pathak R: Denosumab for
the reduction of bone loss in postmenopausal osteoporosis: A
review. Clin Ther. 33:1547–1559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang XX, Zhang YL and Huang QF: Discussion
on the main pathogenesis in traditional Chinese medicine and
etiology about primary osteoporosis. Zhong Xi Yi Jie He Xue Bao.
8:1119–1123. 2010.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li DT, Li FY, Wang J, Liu JH, Yan N, Cheng
YM, Hu AH, Jiang HY, Shi FL, Zhang MZ, et al: A study of diagnostic
criteria for traditional Chinese medicine syndromes in
osteoporosis. Zhong Xi Yi Jie He Xue Bao. 9:1326–1332. 2011.(In
Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin YP, Zhou RX and Guo SM: Effect of
jiangu granule on quality of bone in model rats with osteoporosis
induced by ovariectomy. Zhongguo Zhong Xi Yi Jie He Za Zhi.
24:431–434. 2004.(In Chinese). PubMed/NCBI
|
|
11
|
Lin YP, Ma JH and Feng EY: Study on
preventive effect of jiangu granule on osteoporosis in
ovariectomized rats. Zhongguo Zhong Xi Yi Jie He Za Zhi.
22:369–371. 2002.(In Chinese). PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ju D, Liu M, Zhao H and Wang J: Mechanisms
of ‘kidney governing bones’ theory in traditional Chinese medicine.
Front Med. 8:389–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Tong J, Zhou YJ and Xu XY: Research
progress on anti-osteoporotic active ingredients and
pharmacological action mechanism of traditional Chinese
kidney-tonifying and bone-strengthening drugs. Zhongguo Zhong Yao
Za Zhi. 40:1038–1043. 2015.(In Chinese). PubMed/NCBI
|
|
15
|
Crockett JC, Rogers MJ, Coxon FP, Hocking
LJ and Helfrich MH: Bone remodelling at a glance. J Cell Sci.
124:991–998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann NY Acad Sci. 1092:385–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kartsogiannis V and Ng KW: Cell lines and
primary cell cultures in the study of bone cell biology. Mol Cell
Endocrinol. 228:79–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Väänänen HK, Zhao H, Mulari M and Halleen
JM: The cell biology of osteoclast function. J Cell Sci.
113:377–381. 2000.PubMed/NCBI
|
|
19
|
Liu C, Walter TS, Huang P, Zhang S, Zhu X,
Wu Y, Wedderburn LR, Tang P, Owens RJ, Stuart DI, et al: Structural
and functional insights of RANKL-RANK interaction and signaling. J
Immunol. 184:6910–6919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuroda Y and Matsuo K: Molecular
mechanisms of triggering, amplifying and targeting RANK signaling
in osteoclasts. World J Orthop. 3:167–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue Ji, Ishida T, Tsukamoto N, Kobayashi
N, Naito A, Azuma S and Yamamoto T: Tumor necrosis factor
receptor-associated factor (TRAF) family: Adapter proteins that
mediate cytokine signaling. Exp Cell Res. 254:14–24. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Armstrong AP, Tometsko ME, Glaccum M,
Sutherland CL, Cosman D and Dougall WC: A RANK/TRAF6-dependent
signal transduction pathway is essential for osteoclast
cytoskeletal organization and resorptive function. J Biol Chem.
277:44347–44356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xing L, Bushnell TP, Carlson L, Tai Z,
Tondravi M, Siebenlist U, Young F and Boyce BF: NF-kappaB p50 and
p52 expression is not required for RANK-expressing osteoclast
progenitor formation but is essential for RANK- and
cytokine-mediated osteoclastogenesis. J Bone Miner Res.
17:1200–1210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soysa NS and Alles N: NF-kappaB functions
in osteoclasts. Biochem Biophys Res Commun. 378:1–5. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsuo K, Owens JM, Tonko M, Elliott C,
Chambers TJ and Wagner EF: Fosl1 is a transcriptional target of
c-Fos during osteoclast differentiation. Nat Genet. 24:184–187.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao Q, Wang X, Liu Y, He A and Jia R:
NFATc1: Functions in osteoclasts. Int J Biochem Cell Biol.
42:576–579. 2010. View Article : Google Scholar : PubMed/NCBI
|