Introduction
The mucus layer serves as the first line of defense
in the intestine against pathogenic infections and mechanical
injuries (1). The main component
of mucus is mucin, which is synthesized and secreted by intestinal
goblet cells (2). Intestinal mucin
is a high molecular weight glycoprotein composed of O-linked
glycosides connected with core proteins (3). The mucin core protein is called MUC,
and more than 20 MUC types have been already identified (4). Among these MUC isoforms, mucin 2,
oligomeric mucus/gel-forming (MUC2) is the main structural
component of the mucus gel and represents the main secretory mucin
in the small and large intestines (5,6).
Various evidences have indicated the importance of
intestinal mucin (4,6,7) and
its component MUC2 in maintaining intestinal homeostasis. The
deficiency of mucin or MUC2 was also reported to be harmful as a
result and/or a cause. In a mouse model, MUC2 deficiency led to the
development of spontaneous colitis (3), and in humans, decrease in the number
of mucin containing goblet cells was observed in patients with
aspirin-induced intestinal injury (8), inflammatory bowel disease (9–11),
and necrotizing enterocolitis (2).
Therefore, maintenance or increase in intestinal mucin secretion is
thought to be ideal for the prevention of or healing of many
intestinal diseases. However, no drugs have been reported to
increase mucin secretion in intestinal mucosa.
Rebamipide (Reb), a gastro muco-protective drug, has
been used widely for more than 25 years and its safety has been
confirmed (12,13). Reb has been reported to increase
mucin secretion in the conjunctiva (14) and stomach (15), but not in the intestine. However,
the results of recent human studies have shown that Reb exerts
protective effects against drug-induced intestinal injury (16–18).
Reb can prevent diclofenac sodium-induced decrease of mucin in the
small intestine (7); diclofenac
sodium is a non-steroidal anti-inflammatory drug. Therefore, Reb
can increase the secretion of intestinal mucin. In this study, we
focused on goblet cells, which are mucin-secreting cell, to
determine the possible use of Reb in increasing mucin secretion and
the mechanism behind these phenomena.
Materials and methods
Cell culture and culture medium
LS174T [CL-188™; American Type Culture Collection
(ATCC); Manassas, VA, USA], a human colon adenocarcinoma cell line,
exhibits characteristics of mucin-secreting intestinal epithelial
cells and is widely used as an intestinal goblet cell (6,19).
LS174T were grown for 1 week in Eagle's minimum essential medium
(EMEM) supplemented with 10% heat-inactivated fetal bovine serum
(FBS), 100 U/ml penicillin, and 100 U/ml streptomycin. The cells
were maintained at 37°C in a humidified incubator with 5%
CO2. LS174T were used in all experiments.
Prior to all experiments, the cells were
serum-starved for 6 h in glucose-free EMEM. We seeded LS174T cells
(2×105 cells/ml) in 6-well plates for protein assay and
24-well plates for polymerase chain reaction (PCR) and dot blot
assay.
Reagents
EMEM was purchased from ATCC and PBS and FBS were
purchased from Invitrogen (Carlsbad, CA, USA). Reb was obtained
from Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan). Epidermal
growth factor (EGF; cat. no. E9644) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal anti-MUC2
(cat. no. NBP1-31231; 1:3,000 dilution) was purchased from Novus
(St. Louis, MO, USA). Rabbit monoclonal anti-total EGF receptor
(EGFR) (D38B1; cat. no. 6627; 1:1,000 dilution), rabbit monoclonal
anti-phospho-EGFR Tyr1068 (cat. no. 3777; 1:1,000
dilution), rabbit monoclonal anti-total Akt (cat. no. 9272; 1:1,000
dilution), rabbit monoclonal anti-phospho-Akt
Ser473(cat. no. 4060; 1:2,000 dilution), and rabbit
monoclonal anti-phospho-Akt Thr308 (cat. no. 4060;
1:2,000 dilution) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Mouse monoclonal anti-β-actin antibody
(cat. no. A5316; 1:1,000 dilution) was purchased from
Sigma-Aldrich.
Cell viability
LS174T cells (2×104 cells/ml) were grown
in 96-well plates until confluence and incubated with Reb (0, 1,
10, and 100 µM) for 24 h. Cell viability was quantified using a
cell counting kit (Dojindo Laboratories, Tokyo, Japan) according to
the manufacturer's instructions. After washing two times with PBS,
the cells were incubated with methyl thiazolyl tetrazolium (MTT)
solution for 2 h at 37°C. The absorbance was measured at 450 nm
using a microplate reader (SpectraMax M2; Molecular Devices,
Sunnyvale, CA, USA). All experiments were performed in
triplicate.
Periodic acid-Schiff (PAS)
staining
LS174T cells (2×104 cells/ml) were grown
in 6-well plates until confluence and incubated with Reb (0, 1, 10,
and 100 µM) for 24 h. Next LS174T cells were fixed in 10.5%
formaldehyde at 4°C and stained using a PAS kit (Muto Pure
Chemicals Co., Tokyo, Japan), according to the manufacturer's
instructions.
Real-time PCR assay
Expression of MUC2, MUC5AC, and GAPDH mRNA in LS174T
cells were determined using real-time PCR. Total RNA was isolated
from LS174T cells using an RNA isolation reagent, Isogen (Nippon
Gene Co., Ltd., Tokyo, Japan). Extracted RNA (1 mg) was
reverse-transcribed into first-strand complementary DNA (cDNA)
using the High-capacity cDNA Reverse Transcription kit (Applied
Biosystems, Foster City, CA, USA). PCR reactions for MUC2, MUC5AC,
and GAPDH were performed with the 7300 Real-time PCR system
(Applied Biosystems) using the DNA-binding dye
SYBR−Green to detect PCR products. The primers were of
the following sequences: MUC2 sense, 5′-TGGGTGTCCTCGTCTCCTACA-3′
and antisense, 5′-TGTTGCCAAACCGGTGGTA-3′; MUC5AC sense,
5′-TGCACGAAGCCTATGATCACTT-3′ and antisense,
5′-GGCGCTGACATGGTAGTGGTA-3′; and GAPDH sense,
5′-ACCACAGTCCATGCCATCACT-3′ and antisense,
5′-CCATCACGCCACAGTTTCC-3′. All experiments were performed in
triplicate.
Dot blot analysis
Dot blot analysis was used to measure the mucin
concentration in a cell culture supernatant. Briefly, LS174T cells
were incubated with 10 µM Reb for 24 h in a 24-well plate. The
collected supernatant was centrifuged at 1,000 g for 20 min
at 20°C, and the cell pellet was removed. Each supernatant was
applied to each slot in a Bio-Dot SF® apparatus and
blotted onto a nitrocellulose membrane (0.45 µm; both from Bio-Rad
Laboratories, Inc., Berkeley, CA, USA) by aspiration. The blotted
membrane was incubated with 1% bovine serum albumin
(BSA)-Tris-buffered saline and incubated with anti-MUC2 antibody
(1:3,000; Novus) overnight. After washing three times with 0.05%
Tween-20-Tris-buffered saline (TBST), the membrane was incubated
with secondary antibody, goat anti-rabbit IgG (H + L)-AP (Bio-Rad
Laboratories, Inc.) for 2 h. The protein bands were visualized by
Immun-Blot Goat Anti-Rabbit IgG (H + L)-AP Assay kit (Bio-Rad
Laboratories, Inc.). The bands for MUC2 on the membrane were
quantified using Image J software (National Institutes of Health,
Bethesda, MD, USA).
Next, in order to confirm the involvement of
EGFR/Akt pathway in mucin secretion, we used two inhibitors; EGFR
kinase inhibitor (AG1478, 200 nM) and PI3 kinase/Akt inhibitor
(wortmannin, 10 µM). These inhibitors were added to LS174T cells 30
min prior to 10 µM Reb (after this experiment, we used 10 µM
concentration of Reb) addition and incubated for 24 h. The
densities of MUC2 in the supernatant was measured by dot blot
method above-mentioned and the bands for MUC2 on the membrane were
quantified using Image J software. All experiments were performed
in triplicate.
Western blot analysis
At first, we assessed the important signaling
pathway for mucin secretion, p-EGFR/p-Akt, after the addition of 10
µM Reb by western blotting. Treatment with 10 µM Reb for different
time periods (0, 15, 30, 60, 120, and 240 min), LS174T were
immediately rinsed with ice-cold PBS two times, and the cell pellet
was dissolved with lysis buffer (Cell Lytic M; Sigma-Aldrich).
These lysates were collected and incubated for 1 h on ice. After
centrifugation at 12,000 g for 15 min at 4°C, the
supernatants were extracted, and the protein concentration was
determined using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories,
Inc.). Protein (10 µg) from each sample was electrophoresed on 10%
SDS-PAGE gels for 30 min at 250 V and transferred to a
nitrocellulose membrane (Invitrogen Japan K.K., Tokyo, Japan) using
a semidry transfer system (Invitrogen Japan K.K.). The membrane was
incubated for 1 h with a blocking solution (5% BSA; Wako Pure
Chemical Industries, Ltd., Osaka, Japan) in TBST (10 mM Tris·Cl, pH
8.0, 150 mM NaCl, and 0.1% Tween-20) at room temperature. After
incubation with the appropriate primary antibody for 1 h at room
temperature, the membrane was washed three times with TBST. The
membrane was incubated in appropriate secondary antibody for 1 h at
room temperature. Immunoreactive proteins were detected using a
Western Blot Luminal Reagent kit (ECL plus; GE Health Bio-Sciences,
Tokyo, Japan), and densitometry was measured using Image Quant TL
software (GE Healthcare Life Sciences, Little Chalfont, UK).
During the next series of experiments, to confirm
the active involvement of EGFR/Akt pathway in mucin secretion, we
used three inhibitors; ERK1/2 kinase inhibitor (U0126, 1 µM), EGFR
kinase inhibitor (AG1478, 200 nM), and PI3 kinase/Akt inhibitor
(wortmannin, 10 µM). EGF (10 ng/ml, 15 min) was used as the
positive control to detect p-EGFR and p-Akt. Three inhibitors were
added to LS174T cells 30 min prior to 10 µM Reb addition and after
15 min LS174T whole cell lysates were collected. p-EGFR/p-Akt was
detected by western blotting according to the method
above-mentioned. All experiments were performed in triplicate.
Results
Reb upregulated mucin content in
LS174T cell line
The Reb concentration (1–100 µM) used in this study
did not affect cell viability for 24 h (data not shown). After
addition of Reb (1–100 µM) to LS174T cells for 24 h, the cells were
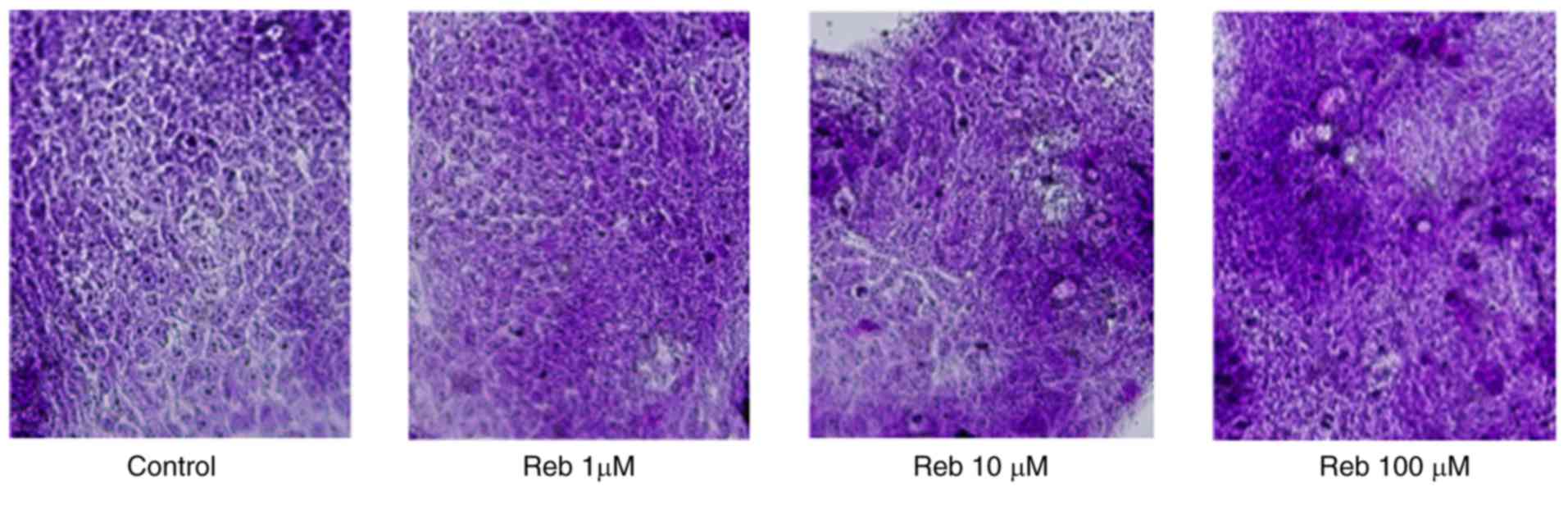
stained using the PAS staining method. We found that Reb strongly
upregulated the positivity of PAS staining in LS174T cells,
regardless of the concentration (Fig.
1), thereby suggesting increased production of intracellular
mucin. The Reb concentration (10 µM) used in this study was thought
to be a clinical relevant concentration, since the concentration of
Reb in the human jejunum has been reported to be higher than 10 µM
at 3 h after oral intake of Reb (100 mg) (20), which is the dosage used in clinical
practice.
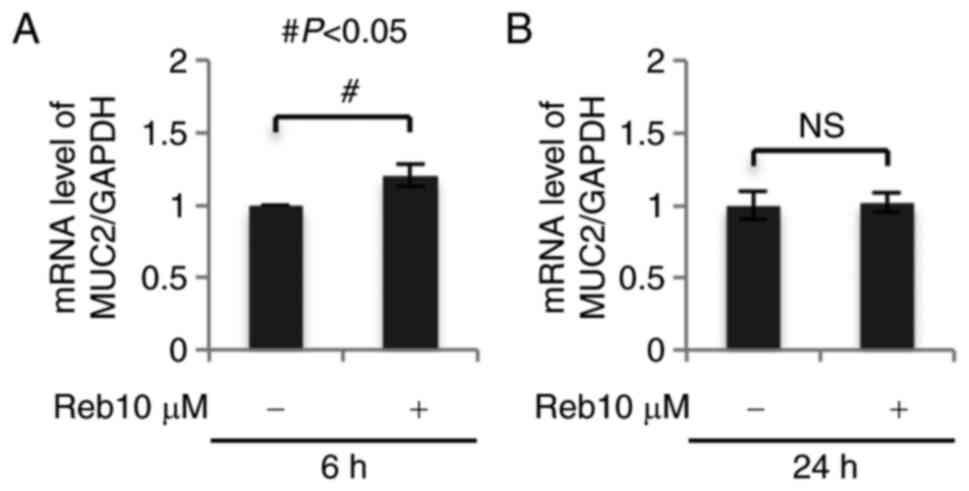
Reb significantly increased MUC2 mRNA
expression
To confirm the synthesis of MUC2 in Reb-treated
LS174T cells, we assessed MUC2 expression by real time-PCR and
found that Reb significantly increased MUC2 (intestinal mucin) mRNA
expression after 6 h (P=0.003; Fig.
2) without affecting MUC5AC (gastric mucin) mRNA expression
(data not shown). These results suggest that Reb increased the
synthesis of intestinal mucin, but not gastric mucin in LS174T
cells.
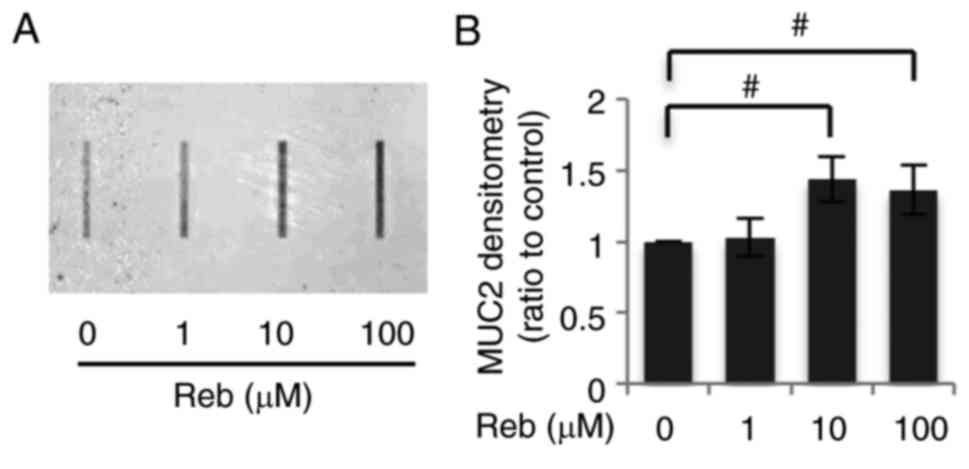
Reb significantly increased MUC2
secretion
In order to assess the secretion of mucin by
Reb-treated LS174T, we repeatedly assessed MUC2 protein secretion
in the supernatant using western blotting and enzyme-linked
immunosorbent assay (ELISA); however, we found it difficult to
assess MUC2 secretion using these methods. There might be two
possibilities for this difficulty; the western blotting for
proteins with very high molecular weight (MUC2, 520 kDa) might be
difficult to perform or the amount of MUC2 secreted in the
supernatant might be limited. To exclude the latter possibility, we
employed a dot blot method and found that Reb increased MUC2
secretion in a concentration-dependent manner. Reb, at the
concentration of more than 10 µM, significantly increased MUC2
secretion (Fig. 3).
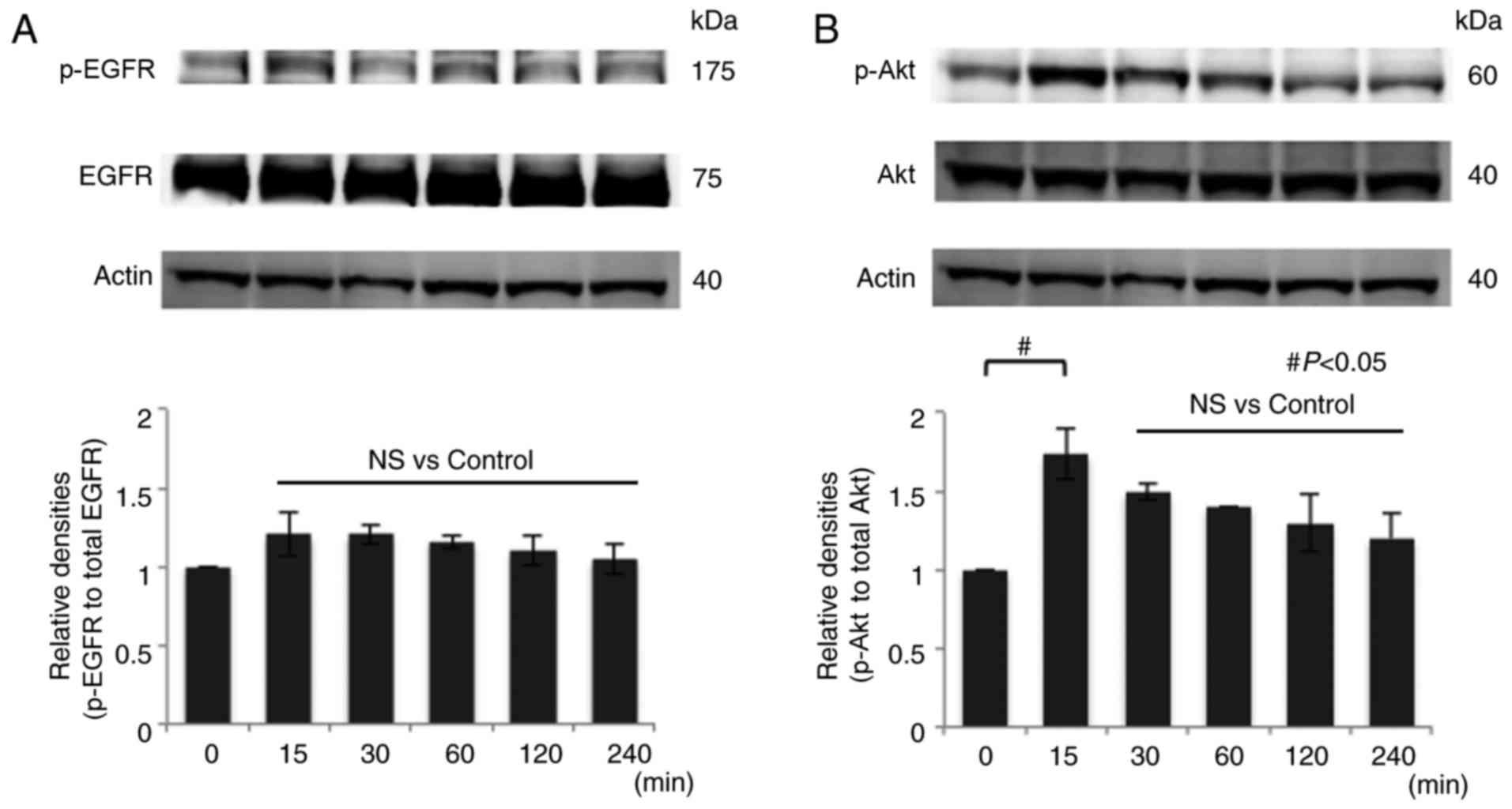
Reb significantly increased p-Akt
after 15 min
To elucidate the mechanism by which Reb increases
mucin secretion, we treated LS174T cells with 10 µM Reb for various
periods (0, 15, 30, 60, 120, and 240 min) and analyzed the
phosphorylation status of EGFR and Akt, a well-known target of EGF,
by western blotting. We found that Reb did not increase p-EGFR
(Fig. 4A). On the contrary, Reb
significantly increased p-Akt at serine 473 for 15 min (Fig. 4B). Since there are two
phosphorylation sites of Akt, serine and threonine, we also
examined phosphorylation at threonine 308 site, however Reb did not
induce phosphorylation at this site (data not shown). We also
examined phosphorylation of ERK1/2 after the treatment with 10 µM
Reb for different time periods (0, 15, 30, 60, 120, and 240 min) by
western blotting; however, Reb did not induce p-ERK1/2 (data not
shown). Taken together, we concluded that Reb induced
phosphorylation at only serine 473 of Akt.
In our additional experiment, the maximum expression
of p-EGFR or p-Akt was obtained 3–5 min or 5–15 min after EGF
stimulation respectively (Fig. 5),
suggesting that the signaling pathway of p-EGFR is upstream of
p-Akt. Therefore, it is possible that Reb might induce maximum
expression of p-EGFR at shorter than 15 min, and we could not
detect p-EGFR both in Reb and EGF stimulated LS174T cells at 15 min
(Figs. 4 and 6).
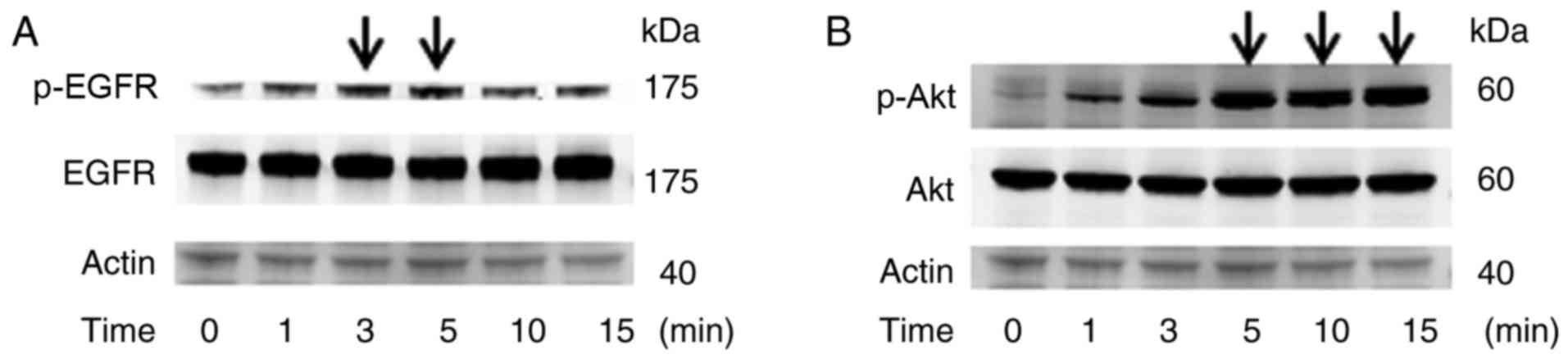 | Figure 5.The peak of p-EGFR is shorter than
p-Akt. EGF (10 ng/ml) was added to LS174T and the expression of
EGFR/Akt and the phosphorylation of EGFR/Akt at various periods (0,
1, 3, 5, 10, and 15 min) were assessed by western blotting. (A) The
peak of phosphorylation of EGFR is 3–5 min after EGF addition,
whereas (B) the peak of phosphorylation of Akt is 5–15 min after
EGF addition. Actin was used as an internal standard (arrows
indicate peaks). A representative image was presented. EGFR,
epidermal growth factor receptor; p-EGFR, phosphorylated EGFR; EGF,
epidermal growth factor; p-Akt, phosphorylated Akt. |
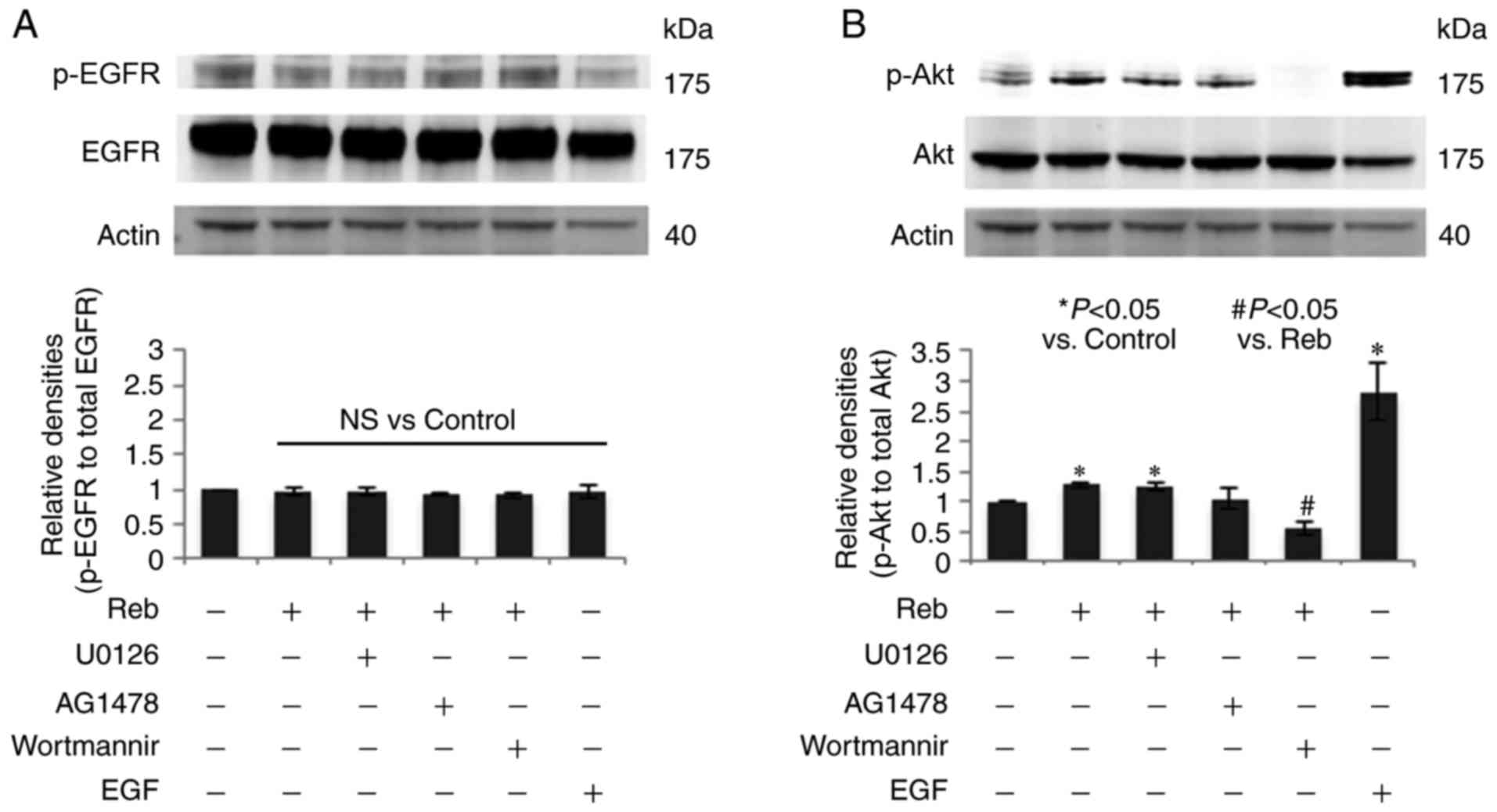 | Figure 6.Akt inhibitor suppressed Reb-induced
p-Akt. Reb (10 µM) was added to LS174T cells and the expression of
Akt and the p-Akt at 15 min were assessed by western blotting in
the presence or absence of three inhibitors; ERK1/2 kinase
inhibitor (U0126, 1 µM), EGFR kinase inhibitor (AG1478, 200 nM),
and PI3 kinase/Akt inhibitor (wortmannin, 10 µM). Three inhibitors
were added to LS174T cells 30 min prior to Reb addition. EGF (10
ng/ml, 15 min) was used as the positive control to detect (A)
p-EGFR and (B) p-Akt. Actin was used as the internal standard. A
representative image out of the three experiments was presented.
The relative band density (p-EGFR/EGFR and p-Akt/Akt) was presented
in the lower panel. Data were presented in mean ± SE out of the
three experiments. *P<0.05 vs. the control;
#P<0.05 vs. Reb, NS vs. the control. Reb, rebamipide;
p-Akt, phosphorylated Akt; EGFR, epidermal growth factor receptor;
EGF, epidermal growth factor; p-EGFR, phosphorylated EGFR; NS; not
significant. |
Akt inhibitor suppressed Reb-induced
p-Akt
To further evaluate the Reb-activated signaling
pathway, we used three inhibitors; ERK1/2 kinase inhibitor (U0126,
1 µM), EGFR kinase inhibitor (AG1478, 200 nM), and PI3 kinase/Akt
inhibitor (wortmannin, 10 µM). We found that Reb did not increase
p-EGFR, and the three inhibitors did not affect p-EGFR (Fig. 6A). Among the three inhibitors, only
wortmannin significantly suppressed Reb-increased p-Akt (Fig. 6B). Although MK-2206 would be a more
specific inhibitor of Akt than wortmannin, the use of MK-2206 in
LS174T cell has not been reported. Since several papers have
reported the inhibitory effect of wortmannin in Akt signaling, we
used wortmannin in this experiment.
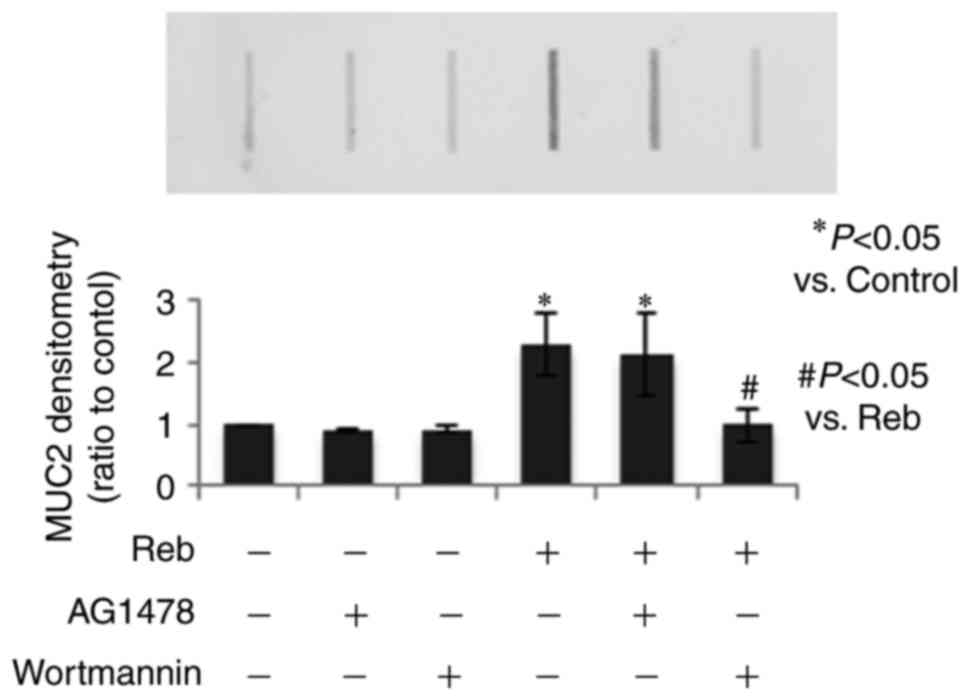
Reb significantly increased MUC2
secretion via p-Akt
To confirm the involvement of p-Akt in Reb-induced
mucin secretion, we used two inhibitors, EGFR kinase inhibitor
(AG1478, 200 nM) and PI3 kinase/Akt inhibitor (wortmannin, 10 µM)
for the dot blot analysis. We found that Reb-increased MUC2
secretion was significantly reduced by wortmannin but not by AG1478
(Fig. 7), thereby suggesting that
Reb specifically increased MUC2 secretion via p-Akt.
Discussion
In the present study, we found for the first time
that a gastro-mucoprotective drug, Reb, increases mucin secretion
in intestinal goblet cells. The most significant finding of the
present study was that Reb strongly increased intracellular mucin
production by PAS staining method and that the increase of MUC2
secretion in the cell culture supernatant was confirmed by dot blot
analysis using a MUC2 specific antibody. Similar to previous
studies that indicate Reb-increased mucin secretion in the
conjunctiva (14) and stomach
(15), Reb can increase mucin
secretion by intestinal goblet cells, thereby suggesting that even
in the intestine Reb can act as a prophylactic agent or a treatment
drug for various intestinal diseases.
In this study, Reb significantly increased MUC2 mRNA
expression in LS174T cells using real time PCR method; however,
this did not lead to an upregulation in MUC2 protein expression as
assessed by western blotting (data not shown). To confirm the
Reb-induced MUC2 secretion in the cell culture supernatant, we
performed ELISA; however, Reb-induced MUC2 secretion by LS174T was
lower than the detection limit. Therefore, we used the dot blot
method in order to detect small amounts of MUC2 protein; in this
method all proteins in the cell culture supernatant are forced to
bind directly to the membranes due to creation of a vacuum and MUC2
proteins can be detected by a specific antibody. This method
revealed that Reb significantly increased MUC2 secretion. Taken
together, we concluded that Reb increases MUC2 mRNA expression and
synthesized MUC2 will be directly secreted into the cell culture
supernatant.
In the next series of experiments, we investigated
the mechanism by which Reb increases mucin secretion. It has been
reported that Reb increases mucin secretion in the stomach
(15,21) and eyes (22). However, only two studies have
investigated the signal transduction mechanism by which Reb induces
mucin secretion. In the human corneal epithelia, Reb upregulates
MUC1, MUC4, and MUC16 expression via the activation of EGFR
(23) and in rat conjunctival
goblet cells, Reb induces mucin secretion by the activation of the
EGFR and the MAPK pathway (14).
In addition, in intestinal goblet cell-like cells, LS174T, EGF, and
Lactobacillus-derived protein, p40, has been shown to
enhance mucin secretion via trans-activation of EGFR/Akt pathway
(1). Therefore, we assessed the
phosphorylation status of EGFR and Akt after Reb treatment and
found that Reb significantly upregulated p-Akt for 15 min without
affecting p-EGFR. The difference between the results of our study
and those of a previous study may be due to the difference in the
methodology. However, almost all methodologies employed in our
study, especially antibodies, are same as those used in the
previous study. The antibodies (for EGFR, p-EGFR, Akt and p-Akt)
were purchased from the same company and were used at the
recommended concentration. The antibody for p-EGFR recognises only
Tyr1068, therefore we also tried a different antibody to detect
p-EGFR that recognises Tyr1148 (rabbit monoclonal anto-phopho-EGFR
Tyr1148, cat. no. 4404; 1:1,000 dilution); however, Reb did not
upregulate p-EGFR as assessed by two different antibodies. The
other possibility is that LS174T cells used in this study might be
a different clone of LS174T. In some reports, the expression of
EGFR is very low and the phosphorylation is hardly detected in
LS174T (24,25), thereby suggesting the heterogeneity
of the LS174T cell line. In the LS174T cells used in this study,
Reb specifically activated Akt signaling without affecting
p-EGFR.
To confirm the active involvement of p-Akt on
Reb-increased mucin secretion, we used two inhibitors in dot blot
analysis to evaluate MUC2 secretion: EGFR kinase inhibitor (AG1478)
and PI3 kinase/Akt inhibitor (wortmannin). In this study,
wortmannin, but not AG1478, significantly suppressed Reb-increased
MUC2 secretion. This result strongly supported our hypothesis that
Reb directly induces p-Akt bypassing the EGFR pathway; however, the
mechanism through which Reb induces p-Akt is yet to be
elucidated.
The limitation of this study is that we did not
prove the protective effect of Reb-increased mucin on intestinal
mucosal injury. Since we reported that aspirin-increased
para-cellular permeability might be one of the mechanisms of
aspirin-induced small intestinal mucosal injury (26), the in vitro experiment that
tried to determine whether mucin secreted from goblet cells can
suppress aspirin-induced increase of para-cellular permeability
might clarify the protective effect of Reb. Animal experiments to
elucidate whether Reb can increase mucin secretion in the intestine
are also important.
The other limitation of this study is that the
involvement of prostaglandin, which is reported to be a mucin
secretagogue, was not investigated. Many studies have reported the
importance of prostaglandin in promoting gastric epithelial mucin
secretion in rat stomach (27,28).
However, only two studies have clearly stated the relationship
between prostaglandin and intestinal mucin secretion in the
intestine (2,29). Moreover, McElroy et al
(2) have shown that the addition
of prostaglandin alone did not increase mucin secretion in rat
experiments. However, this should be confirmed under experimental
conditions in a future study.
Taken together, we concluded that Reb increased
mucin secretion directly via p-Akt. Further, Reb-increased mucin
could be a strong non-specific barrier against pathogenic
stimulants in various intestinal diseases.
Acknowledgements
This study was supported by Grants-in-Aid for
Scientific Research (KAKENHI) (C) to Y.N. (no. 25460958) from the
Japan Society for the Promotion of Science (JSPS), and by an
Adaptable and Seamless Technology Transfer Program through target
driven R&D (to Y.N.) from the Japan Agency for Medical Research
and Development (AMED).
References
|
1
|
Wang L, Cao H, Liu L, Wang B, Walker WA,
Acra SA and Yan F: Activation of epidermal growth factor receptor
mediates mucin production stimulated by p40, a Lactobacillus
rhamnosus GG-derived protein. J Biol Chem. 289:20234–20244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McElroy SJ, Prince LS, Weitkamp JH, Reese
J, Slaughter JC and Polk DB: Tumor necrosis factor receptor
1-dependent depletion of mucus in immature small intestine: A
potential role in neonatal necrotizing enterocolitis. Am J Physiol
Gastrointest Liver Physiol. 301:G656–G666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwashita J, Sato Y, Sugaya H, Takahashi N,
Sasaki H and Abe T: mRNA of MUC2 is stimulated by IL-4, IL-13 or
TNF-alpha through a mitogen-activated protein kinase pathway in
human colon cancer cells. Immunol Cell Biol. 81:275–282. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corfield AP: Mucins: A biologically
relevant glycan barrier in mucosal protection. Biochim Biophys
Acta. 1850:236–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deplancke B and Gaskins HR: Microbial
modulation of innate defense: Goblet cells and the intestinal mucus
layer. Am J Clin Nutr. 73:1131S–1141S. 2001.PubMed/NCBI
|
|
6
|
Elamin E, Masclee A, Troost F, Dekker J
and Jonkers D: Cytotoxicity and metabolic stress induced by
acetaldehyde in human intestinal LS174T goblet-like cells. Am J
Physiol Gastrointest Liver Physiol. 307:G286–G294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satoh H, Amagase K and Takeuchi K: Mucosal
protective agents prevent exacerbation of NSAID-induced small
intestinal lesions caused by antisecretory drugs in rats. J
Pharmacol Exp Ther. 348:227–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takeuchi T, Ensrud ER and Steggerda FR:
The effects of large doses of aspirin and cortisone on the goblet
cells and the mucosal membranes in the small and large intestine.
Am J Dig Dis. 17:49–53. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YS and Ho SB: Intestinal goblet cells
and mucins in health and disease: Recent insights and progress.
Curr Gastroenterol Rep. 12:319–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacobs LR and Huber PW: Regional
distribution and alterations of lectin binding to colorectal mucin
in mucosal biopsies from controls and subjects with inflammatory
bowel diseases. J Clin Invest. 75:112–118. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Theodossi A, Spiegelhalter DJ, Jass J,
Firth J, Dixon M, Leader M, Levison DA, Lindley R, Filipe I, Price
A, et al: Observer variation and discriminatory value of biopsy
features in inflammatory bowel disease. Gut. 35:961–968. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naito Y and Yoshikawa T: Rebamipide: A
gastrointestinal protective drug with pleiotropic activities.
Expert Rev Gastroenterol Hepatol. 4:261–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH, Park SH, Cho CS, Lee ST, Yoo WH,
Kim SK, Kang YM, Rew JS, Park YW, Lee SK, et al: Preventive
efficacy and safety of rebamipide in nonsteroidal anti-inflammatory
drug-induced mucosal toxicity. Gut Liver. 8:371–379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ríos JD, Shatos MA, Urashima H and Dartt
DA: Effect of OPC-12759 on EGF receptor activation, p44/p42 MAPK
activity, and secretion in conjunctival goblet cells. Exp Eye Res.
86:629–636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iijima K, Ichikawa T, Okada S, Ogawa M,
Koike T, Ohara S and Shimosegawa T: Rebamipide, a cytoprotective
drug, increases gastric mucus secretion in human: Evaluations with
endoscopic gastrin test. Dig Dis Sci. 54:1500–1507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizukami K, Murakami K, Abe T, Inoue K,
Uchida M, Okimoto T, Kodama M and Fujioka T: Aspirin-induced small
bowel injuries and the preventive effect of rebamipide. World J
Gastroenterol. 17:5117–5122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe T, Takeuchi T, Handa O, Sakata Y,
Tanigawa T, Shiba M, Naito Y, Higuchi K, Fujimoto K, Yoshikawa T
and Arakawa T: A multicenter, randomized, double-blind,
placebo-controlled trial of high-dose rebamipide treatment for
low-dose aspirin-induced moderate-to-severe small intestinal
damage. PLoS One. 10:e01223302015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai Y, Zhong W, Yu T, Xia ZS, Li JY,
Ouyang H, Shan TD, Yang HS and Chen QK: Rebamipide promotes the
regeneration of aspirin-induced small-intestine mucosal injury
through accumulation of β-catenin. PLoS One. 10:e01320312015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Klinken BJ, Oussoren E, Weenink JJ,
Strous GJ, Büller HA, Dekker J and Einerhand AW: The human
intestinal cell lines Caco-2 and LS174T as models to study
cell-type specific mucin expression. Glycoconj J. 13:757–768. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akamatsu T, Nagaya T, Ichikawa S, Sudo T,
Takeda R, Takenaka K, Kodama R, Ito T, Arakura N and Tanaka E:
Small bowel tissue concentration of rebamipide: Study of two
dosages in healthy subjects. J Clin Biochem Nutr. 47:256–260. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ishihara K, Komuro Y, Nishiyama N,
Yamasaki K and Hotta K: Effect of rebamipide on mucus secretion by
endogenous prostaglandin-independent mechanism in rat gastric
mucosa. Arzneimittelforschung. 42:1462–1466. 1992.PubMed/NCBI
|
|
22
|
Urashima H, Okamoto T, Takeji Y, Shinohara
H and Fujisawa S: Rebamipide increases the amount of mucin-like
substances on the conjunctiva and cornea in the
N-acetylcysteine-treated in vivo model. Cornea. 23:613–619. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itoh S, Itoh K and Shinohara H: Regulation
of human corneal epithelial mucins by rebamipide. Curr Eye Res.
39:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Nagahara H, Mimori K, Inoue H,
Sawada T, Ohira M, Hirakawa K and Mori M: Mutations of epidermal
growth factor receptor in colon cancer indicate susceptibility or
resistance to gefitinib. Oncol Rep. 19:1541–1544. 2008.PubMed/NCBI
|
|
25
|
Liu Z, Tabakman S, Sherlock S, Li X, Chen
Z, Jiang K, Fan S and Dai H: Multiplexed five-color molecular
imaging of cancer cells and tumor tissues with carbon nanotube
raman tags in the near-infrared. Nano Res. 3:222–233. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukui A, Naito Y, Handa O, Kugai M, Tsuji
T, Yoriki H, Qin Y, Adachi S, Higashimura Y, Mizushima K, et al:
Acetyl salicylic acid induces damage to intestinal epithelial cells
by oxidation-related modifications of ZO-1. Am J Physiol
Gastrointest Liver Physiol. 303:G927–G936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamasaki K, Kanbe T, Chijiwa T, Ishiyama H
and Morita S: Gastric mucosal protection by OPC-12759, a novel
antiulcer compound, in the rat. Eur J Pharmacol. 142:23–29. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kleine A, Kluge S and Peskar BM:
Stimulation of prostaglandin biosynthesis mediates gastroprotective
effect of rebamipide in rats. Dig Dis Sci. 38:1441–1449. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cassidy MM and Lightfoot FG: Effects of
prostaglandin E1, administered by gastric intubation, on mucus
secretory patterns in rat small intestine. Adv Prostaglandin
Thromboxane Res. 8:1589–1593. 1980.PubMed/NCBI
|