Introduction
Oral cancer is one of the major causes of
cancer-associated mortalities in the global human population and
the total number of patients with oral cancer who are younger than
40 years old has markedly increased in the last decade (1). In Taiwan, a report by the Department
of Health in 2014 (2) demonstrated
that there are 36.8 mortalities per 100,000 people associated with
oral cancer every year. During the early stages of oral cancer
patients are mainly treated with surgery, which is occasionally
coupled with radiotherapy; however, as it becomes more advanced a
combination of surgery, radiotherapy and chemotherapy is applied,
which can have adverse effects on the patients' quality of life
(3,4). Currently, there are a number of
clinically used chemotherapeutic drugs that have been approved for
the treatment of oral cancer, including cisplatin, cetuximab and
endothelial growth factor receptor (EGFR) inhibitors (5). However, the development of more
effective treatments and drugs with lower toxicities is required in
order to improve patient prognosis and reduce the number of oral
cancer-associated mortalities.
Bufalin, a small molecular compound, was isolated
from chansu, a traditional Chinese medicine (TCM) that was obtained
from the skin and parotid glands of the Chusan Island toad (Bufo
gargarizans) (6). The extract
from chansu has been used as a TCM to treat various types of
cancers in the Chinese population (7). A number of studies have revealed that
bufalin induced biological activities such as inhibiting cell
proliferation, and inducing cell differentiation and apoptosis in
various cancer cells, including human hepatocellular carcinoma
BEL-7402 cells (8), leukemia HL-60
(9) and U937 (10) cells, gastric cancer MGC803 cells
(11), prostate cancer DU145 and
PC3 cells (12), and glioma cancer
cells (13). Bufalin also induced
G0/G1 phase arrest and apoptosis in human bladder cancer T24 cells
through mitochondria signaling pathways (14). In addition, bufalin inhibited human
non-small lung cancer A549 cell proliferation via the vascular EGFR
(VEGFR)1/VEGFR2/EGFR/tyrosine-protein kinase-protein kinase
B/p44/p42/p38-nuclear factor-κB signaling pathways (15). A previous study reported that the
inhibition of the Janus kinase-signal transducer and activator of
transcription 3 signaling pathway enhances bufalin-induced
apoptosis in colon cancer SW620 cells (16).
Previous studies have demonstrated that bufalin
induced cell death through cell cycle arrest and inducing apoptosis
in many human cancer cell lines, there has yet to be a report
investigating bufalin-induced cell death via the induction of
apoptosis in human tongue cancer SCC-4 cells (2,17).
In addition, the molecular mechanism underlying bufalin-induced
cell death in SCC-4 cells is unknown. In the present study, the
SCC-4 cell line was used as an in vitro tongue cancer model
to investigate the effects of bufalin treatment. The present study
reported that bufalin induced cell cycle arrest and induced cell
apoptosis in SCC-4 cells via endoplasmic reticulum stress and
caspase- and mitochondria-dependent pathways.
Materials and methods
Chemicals and reagents
Bufalin of 99% purity,
4′,6-diamidino-2-phenylindole, dilactate (DAPI), dimethyl sulfoxide
(DMSO), propidium iodide (PI) and Trypsin-EDTA were obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). A stock solution of
bufalin (10 mM) was prepared in DMSO and further diluted in culture
medium. DMSO was used as vehicle control in all experiments.
Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1) medium, fetal
bovine serum (FBS), L-glutamine and penicillin-streptomycin were
purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Primary antibodies and peroxidase-conjugated secondary
antibodies were obtained from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Fluo-3/AM, DiOC6, H2DCF-DA
and DAF-FM were obtained by Invitrogen (Carlsbad, CA, USA).
Cell culture
The SCC-4 human tongue cancer cell line was obtained
from the Food Industry Research and Development Institute (Hsinchu,
Taiwan) and cultured in DMEM/F12 (1:1) medium supplemented with 10%
FBS, 100 µg/ml streptomycin, 100 units/ml penicillin, and 2 mM
L-Glutamine at 37°C incubator with 5% CO2 (18).
Cell morphology examinations, total
viability and cell cycle assays
SCC-4 cells (1×105 cells/well) were
cultured in 12-well plates with DMEM/F12 (1:1) medium for 24 h.
Cell were pretreated with or without inhibitor [1 µM cyclosporine
A, an inhibitor of ΔΨm or 1 mM N-acetyl cysteine (NAC), a general
ROS scavenger; both from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany] for 4 h at 37°C, and then incubated with bufalin at a
final concentration series of 100, 200, 300, 400 and 500 nM, or
0.5% DMSO only as a vehicle control for 48 h at 37°C. Plated cells
were examined and photographed under a contrast phase microscope at
×200 magnification to analyze cell morphological changes. Cells
were harvested and stained with PI (4 mg/ml) at room temperature,
followed immediately by flow cytometry (FACSCalibur™; BD
Biosciences, San Jose, CA, USA) to perform total viability assays
or cells were analyzed for cell cycle distribution as previously
described (19).
DAPI staining for chromatin
condensation examination
SCC-4 cells (2×105 cells/well) were
cultured in 6-well plates and treated with bufalin (100, 200, 300,
400 and 500 nM) or 0.5% DMSO only as a vehicle control for 24 and
48 h at 37°C. Cells were fixed in 3% methanol in PBS at room
temperature for 20 min and were then stained with DAPI solution (2
µg/ml) at 37°C for 30 min. Cells were photographed using a
fluorescence microscope as previously described (19). The ratio of nuclei condensation of
cells to total cells was calculated; 150 cells/field in at least 3
fields from each well were counted. The analysis software to
quantify the level of DNA damage was TriTek CometScore™ Freeware
version 1.5 (TriTek Corp., Sumerduck, VA, USA).
DNA fragmentation assay by Comet assay
and DNA gel electrophoresis
SCC-4 cells (2×105 cells/well) were
cultured in 6-well plates for 24 h and incubated with bufalin (100,
300 and 500 nM), 1.25 µM H2O2 or 0.5% DMSO
only as a vehicle control for 48 h at 37°C. All samples were
collected for the Comet assay as described previously (20). SCC-4 cells (1.5×106
cells/dish) were cultured in 10-cm dishes for 24 h and incubated
with bufalin (100, 300 and 500 nM) or 0.5% DMSO only as a vehicle
control for 48 h at 37°C, then cells were extracted using the
Tissue and Cell Genomic DNA Purification kit (GMbiolab Co., Ltd.,
Taichung, Taiwan) as described previously (20). A total of 2 µg DNA from each
treatment group was loaded onto 0.5% agarose gels (at 100 V for 40
min) in TBE buffer (89 mM Triseboric acid and 2 mM EDTA, pH 8.0)
for electrophoresis. Ethidium bromide was used for visualization
and gels were photographed by fluorescence microscopy.
Measurements of reactive oxygen
species (ROS), intracellular Ca2+, mitochondrial
membrane potential (ΔΨm) and nitric oxide (NO) production
The levels of ROS, Ca2+ and
ΔΨm in SCC-4 cells following exposure to bufalin were
measured by a flow cytometric assay. Briefly, SCC-4 cells
(1×105 cells/well) in 12-well plates were treated with
bufalin (300 nM) or 0.5% DMSO only as a vehicle control for various
time periods (0–9 h for ROS and NO measurements; 0–48 h for
Ca2+ and ΔΨm measurements) at 37°C. Cells
were centrifuged at 453 × g for 5 min at 25°C and resuspended with
500 µl DCFH-DA (10 µM) for ROS (H2O2)
measurements, with 500 µl DiOC6 (4 µmol/l) for
ΔΨm measurements, with 500 µl Fluo-3/AM (2.5 µg/ml) for
intracellular Ca2+ concentration measurements and with
500 µl DAF-FM (10 µM) to measure NO production. All samples were
maintained in the dark for 30 min at 37°C and then analyzed by flow
cytometry as described previously (21).
Western blot analysis
Western blotting was performed to detect the levels
of apoptotic proteins. SCC-4 cells (5×106 cells/dish)
were cultured in a 10 cm dish for 24 h and were then incubated with
0.5% DMSO only as a vehicle control or 300 nM bufalin for 0, 12,
24, 36 and 48 h at 37°C. The protein was lysed by PRO-PREP Protein
Extraction Solution (iNtRON Biotechnology, Sungnam, Korea), and
total protein was measured using a Bio-Rad protein assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) as described
previously (21). Proteins (20 µg)
were separated by electrophoresis 12% (v/v) SDS-PAGE and then
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked for 1 h at
room temperature with 5% non-fat milk in PBST (0.1% Tween-20 in 1X
PBS), then hybridized with the following primary antibodies at 4°C
overnight. The primary antibodies supplied by Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) were used at a 1:1,000
dilution; endonuclease G (Endo G; sc-365359), apoptosis-inducing
factor (AIF; sc-13116), cytochrome (Cyt) c (sc-13560), X-box
binding protein (XBP)-1 (sc-7160), DNA damage inducible transcript
3 (GADD153; sc-7351), inositol-requiring enzyme (IRE)-1α
(sc-25563), activating transcription factor (ATF)-6α (sc-58323),
ATF-6β (sc-30596), glucose-regulated protein, 78 kDa (GRP78;
sc-13968), calpain-1 (sc-58323) and tumor necrosis factor
(TNF)-related apoptosis-inducing ligand (TRAIL; sc-80393). The
primary antibody supplied by EMD Millipore was used at a 1:500
dilution for BH3 interacting-domain death agonist (Bid; AB1730).
The primary antibodies supplied by Cell Signaling Technology, Inc.
(Danvers, MA, USA) were used at a 1:1,000 dilution for B-cell
lymphoma 2 (Bcl-2; 2870), Bcl-2-associated X protein (Bax; 2772),
death receptor (DR) 5 (8074), DR4 (42533), PARP (9532), caspase-3
(9669) and caspase-9 (9508). The primary antibody supplied by
Sigma-Aldrich (Merck KGaA) was used at a 1:10,000 dilution for
β-actin (A5316). This was followed by incubation with the secondary
antibody at room temperature (25°C) for 1 h. The secondary
antibodies supplied by GeneTex (Irvine, CA, USA) were used at a
1:10,000 dilution for horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (GTX213110), HRP-conjugated donkey anti-goat IgG
(GTX26885) and HRP-conjugated rabbit anti-mouse IgG (GTX213112).
Protein bands were visualized by chemiluminescence signals, which
were enhanced using electrochemiluminescence detection (Amersham
ECL™; GE Healthcare, Chicago, IL, USA) (21,22).
ImageJ version 1.49o software (National Institutes of Health,
Bethesda, MD, USA) was used to quantify changes in protein
expression by densitometry analysis and using β-actin as the
loading control.
Statistical analysis
All values are presented as the mean ± standard
deviation of three independent experiments. Differences between
groups were analyzed by one-way analysis of variance and Dunnett or
Tukey test for multiple comparisons (SigmaPlot for Windows version
12.0; Systat Software, Inc., San Jose, CA). P<0.05 was
considered to indicate a statistically significant difference.
Results
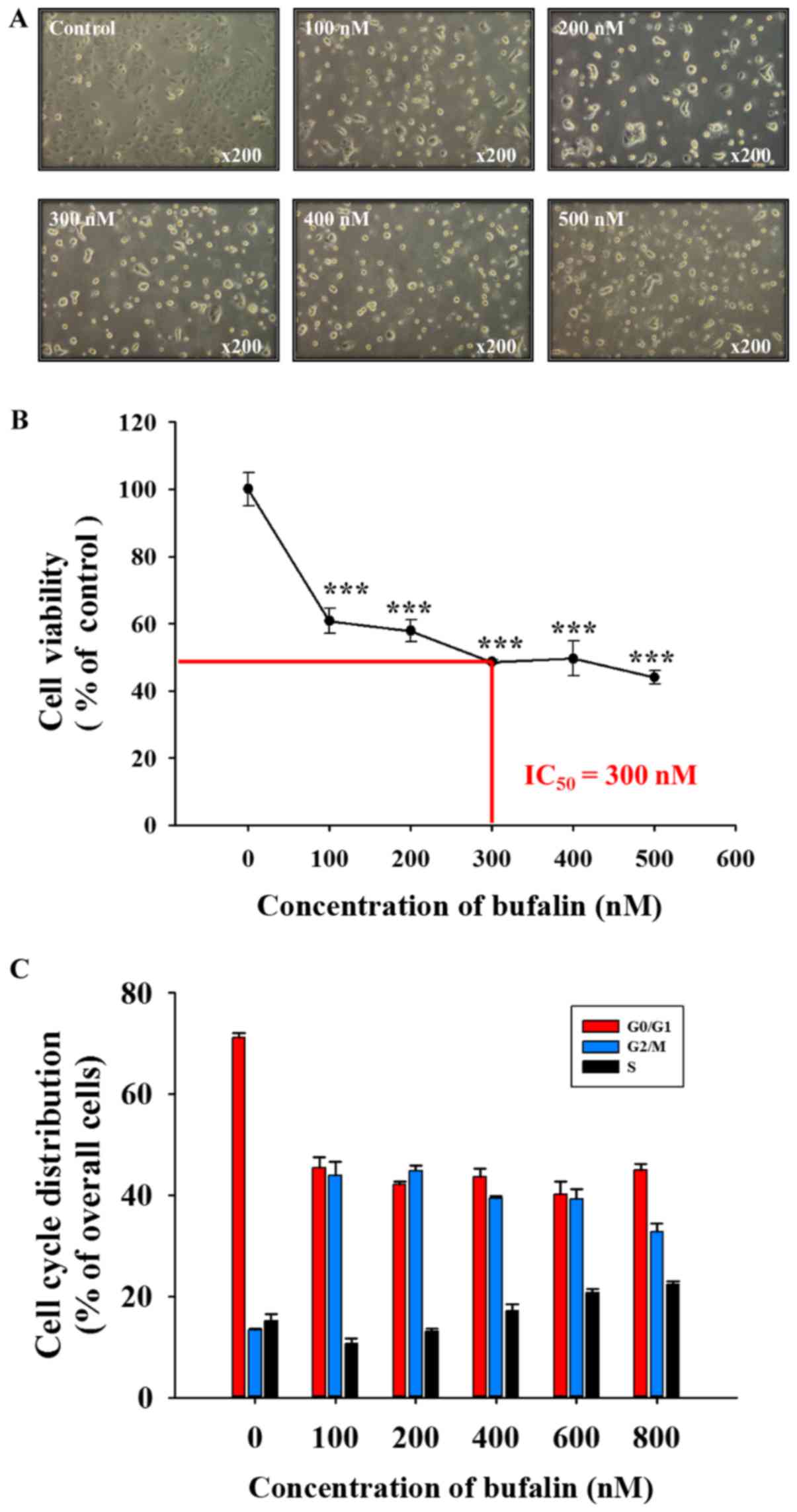
Bufalin induces cell morphological
changes, decreases total viability and induces G2/M phase arrest in
SCC-4 cells
Following exposure to various concentrations of
bufalin for 48 h, SCC-4 cells were examined to determine any
cytotoxic effects (Fig. 1).
Bufalin induced cell morphological changes (cell shrinkage,
cytoplasmic and nuclear condensation or pyknosis) and reduced the
total cell viability in a dose-dependent manner; a half-maximal
inhibitory concentration (IC50) of 300 nM was observed
at 48 h (Fig. 1A and B). In
addition, bufalin induced G2/M phase arrest in SCC-4 cells
(Fig. 1C). Therefore, the findings
of the present study indicated that bufalin may induce cytotoxic
effects on SCC-4 cells via cell morphological changes and by
decreasing the number of viable SCC-4 cells in vitro.
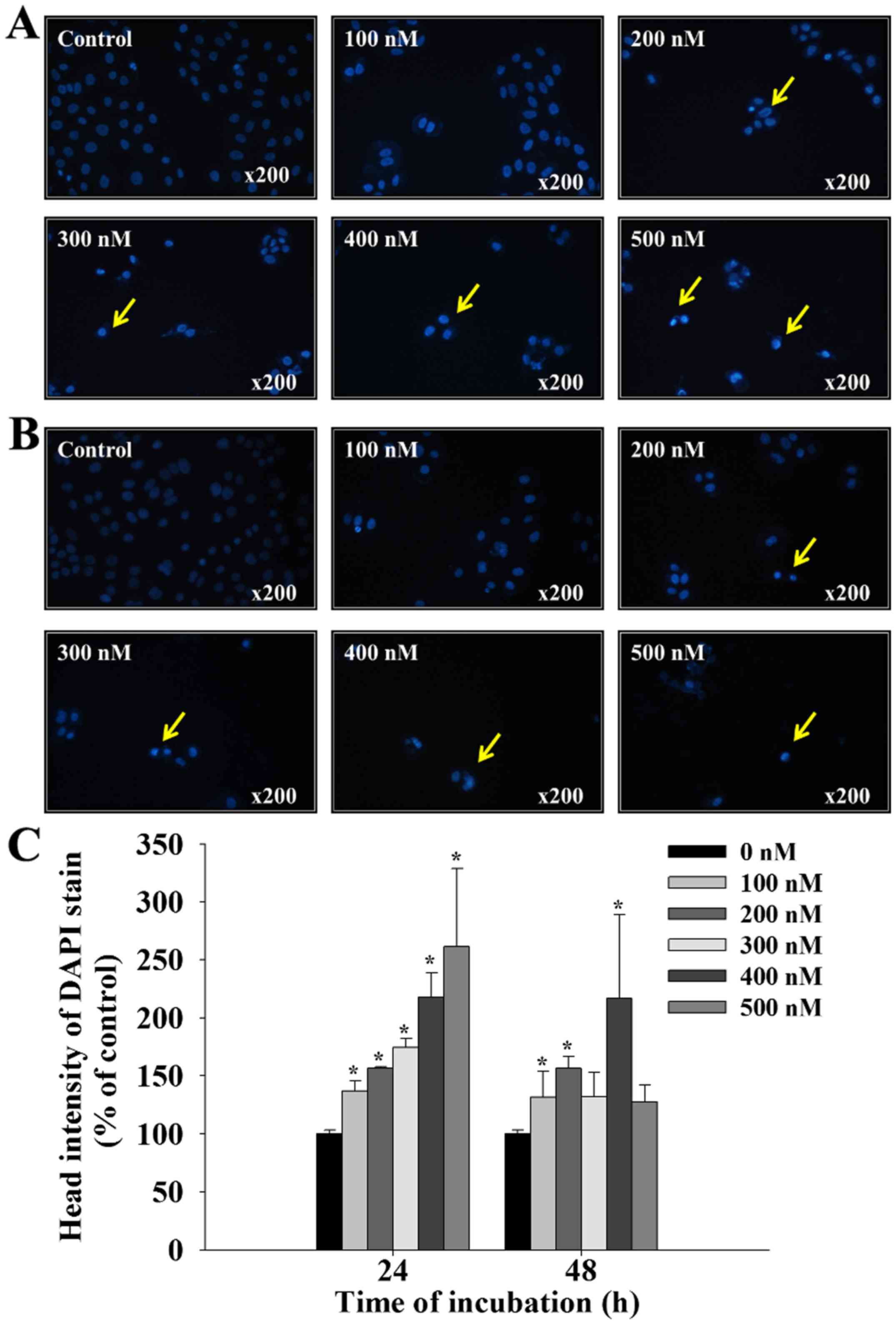
Bufalin induces chromatin condensation
in SCC-4 cells
Following treatment with bufalin (0, 100, 200, 300,
400 and 500 nM) for 24 and 48 h, SCC-4 cells were stained with DAPI
and photographed using fluorescence microscopy; the results are
presented in Fig. 2. Treatment
with bufalin at 100–500 nM for 24 and 48 h, induced brighter DAPI
fluorescence in SCC-4 cells when compared with control cells
(Fig. 2A and B). Brighter
fluorescence indicates nicked DNA and chromatin condensation, which
were observed in SSC-4 cells in a dose-dependent manner (Fig. 2C). These findings indicate that
bufalin may induce early and late apoptosis in SCC-4 cells,
characterized by the presence of irregular and fragmented nuclei,
and shrunken cells.
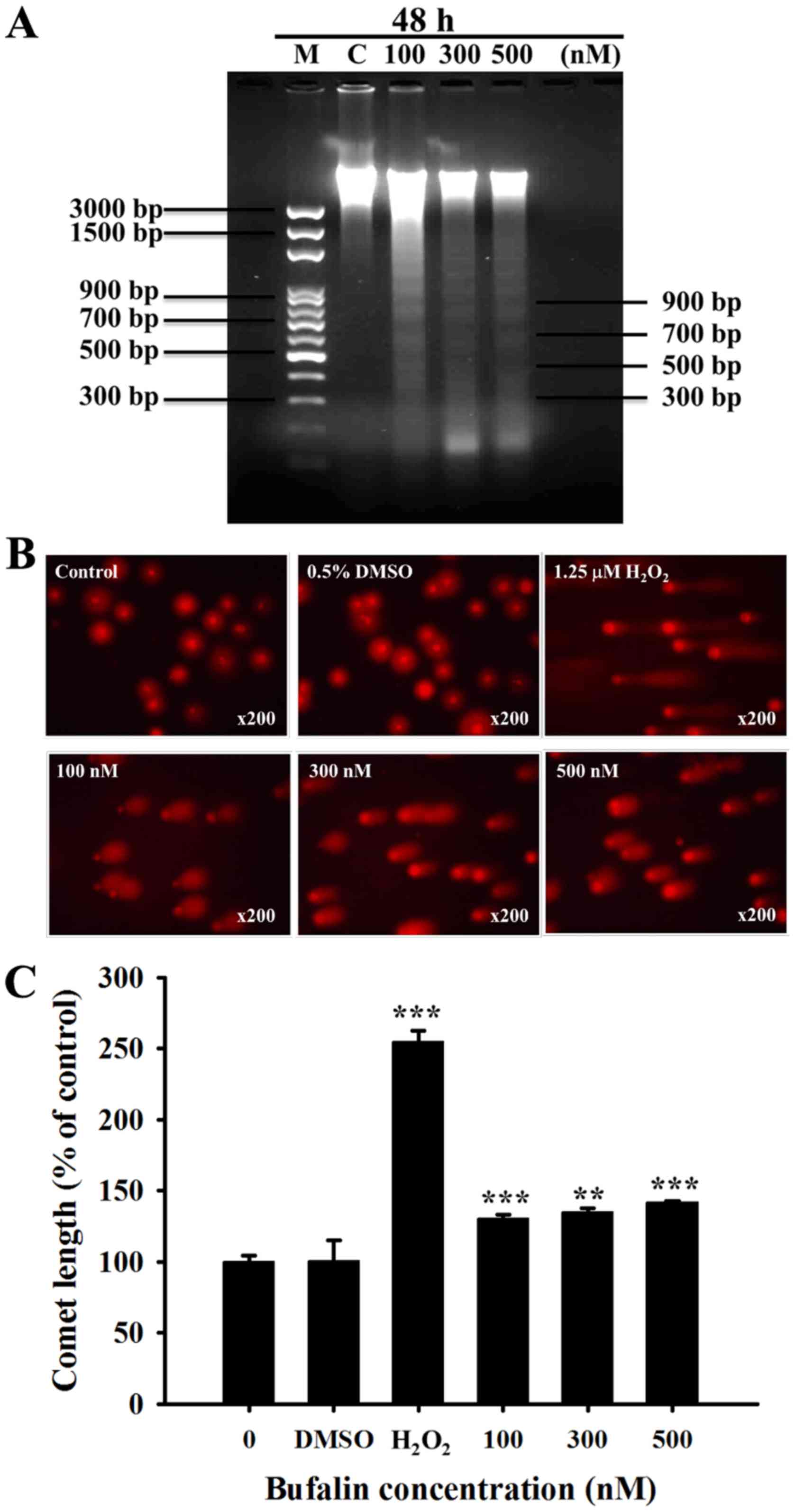
Bufalin induces DNA fragmentation and
DNA damage in SCC-4 cells
It has been previously revealed that DNA
fragmentation (DNA ladder) is one of the hallmarks of apoptotic
cell death (23). Following SCC-4
cell treatment with bufalin (0, 100, 300 and 500 nM) for 48 h,
total DNA was isolated from each treatment group and run on DNA
agarose gel electrophoresis (Fig.
3A). Bufalin induced a typical DNA ladder for all 3 examined
doses in SCC-4 cells. In addition, the results of the Comet assay
indicated that bufalin-induced DNA damage based on the production
of Comet tails (Fig. 3B); these
effects were observed in a dose-dependent manner (Fig. 3C).
Bufalin induces the production of ROS
and Ca2+, and decreases the levels of ΔΨm and NO in
SCC-4 cells
In order to further investigate whether
bufalin-induced cell apoptosis is involved in the production of
ROS, Ca2+ and NO, or the dysfunction of the ΔΨm, SCC-4
cells were treated with 300 nM bufalin for various time periods.
All samples were collected and analyzed by a flow cytometric assay
(Fig. 4). The results revealed
that bufalin decreased the production of ROS following 1–9 h of
treatment (Fig. 4A) and the ΔΨm
following 6–48 h of treatment (Fig.
4B). However, 6–9 h of bufalin treatment increased NO
production (Fig. 4C) and increased
Ca2+ production following 12–48 h of treatment (Fig. 4D). Cells were also pretreated with
1 µM cyclosporine A, an inhibitor of ΔΨm, for 4 h at 37°C and then
co-treated with or without 300 nM bufalin to analyze cell
viability. The results demonstrated that pretreatment with
cyclosporine A increased cell viability when compared with bufalin
only treatment (Fig. 4E). These
findings indicate that ROS, NO, Ca2+ and ΔΨm may be
involved in bufalin-induced apoptotic cell death in SCC-4 cells
in vitro.
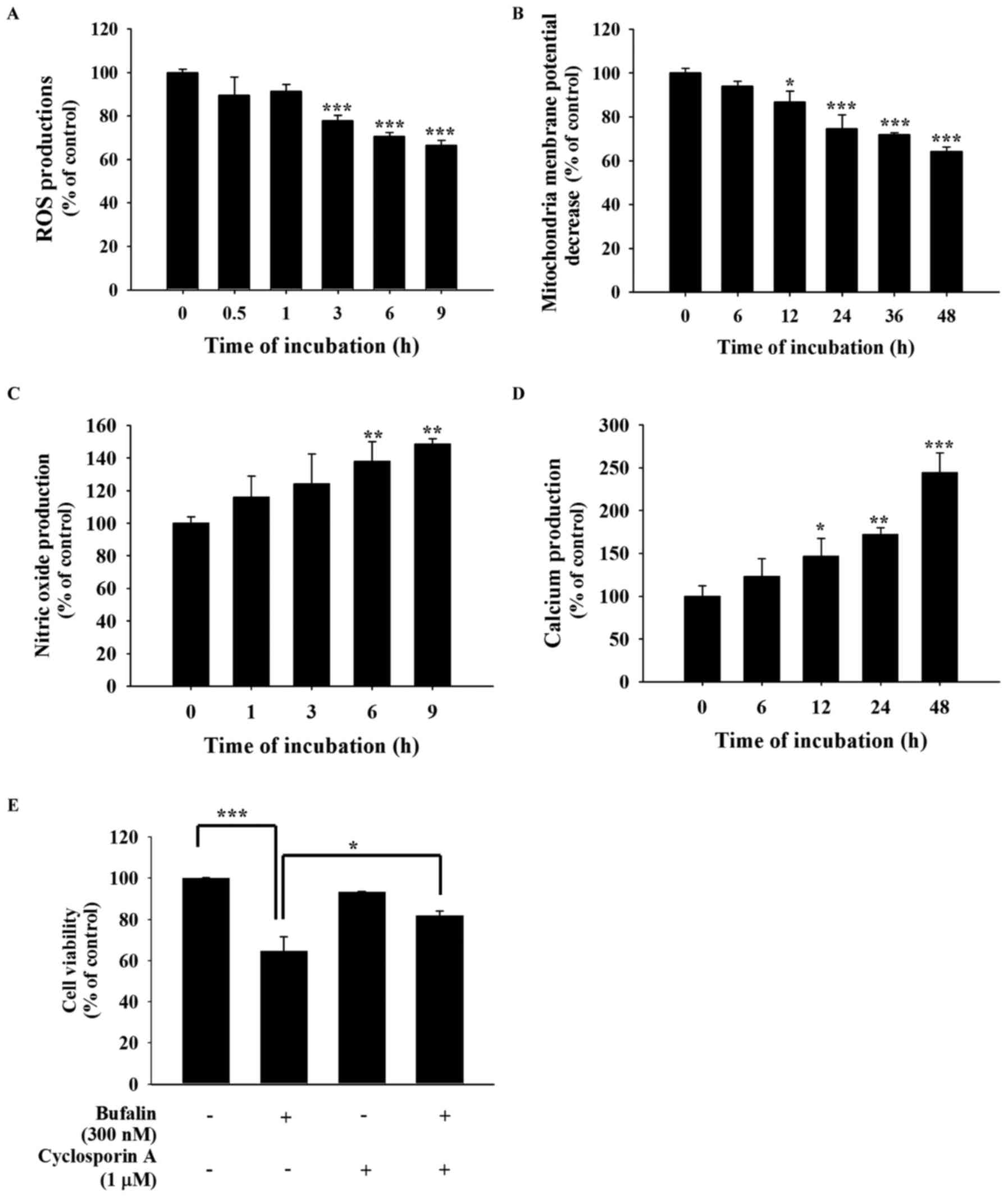 | Figure 4.Bufalin affected the production of
ROS, NO and Ca2+ and the ΔΨm in SCC-4 cells. Cells were
isolated and resuspended in different reagents to analyze the
levels of (A) ROS (in 500 µl of 10 µM DCFH-DA), (B) ΔΨm
(in 500 µl of 4 µmol/l DiOC6), (C) NO (in 500 µl of 10
µM DAF-FM) and (D) intracellular Ca2+ concentrations
(500 µl of 2.5 µg/ml Fluo-3/AM), and these results are presented as
the mean ± standard deviation (n=3). *P<0.05; **P<0.01;
***P<0.001 vs. 0 nM bufalin (Dunnett multiple comparisons test).
(E) Cells were also pretreated with 1 µM cyclosporine A, then
incubated with 300 nM bufalin for 48 h to determine the percentage
of viable cells, and the result is presented as the mean ± standard
deviation (n=3). *P<0.05; ***P<0.001, denotes statistically
significant difference (Tukey multiple comparisons test). ROS,
reactive oxygen species; NO, nitric oxide; ΔΨm, mitochondrial
membrane potential. |
Bufalin alters apoptosis-associated
protein expression in SCC-4 cells
The present study also investigated whether
bufalin-induced cell apoptosis alters the expression of
apoptosis-associated proteins in SCC-4 cells. Cells were treated
with bufalin (300 nM) for 0, 12, 24, 36 and 48 h, then proteins
were measured and quantitated by western blotting (Fig. 5). The results demonstrated that
following 48 h of incubation, bufalin markedly increased the
expression of Endo G, Bcl-2, Bax, poly(ADP-ribose) polymerase, AIF,
Cyt c (Fig. 5A), TRAIL,
DR4, caspase-3 (Fig. 5B), XBP1,
GADD153, IRE-1α, ATF-6α and GRP78 (Fig. 5C). Bufalin treatment for 36 h
increased the expression of DR5 and caspase-9 (Fig. 5B); however, following 48 h their
expression decreased when compared with 0 h of treatment. In
addition, bufalin treatment markedly reduced the expression of
Bcl-2, Bid (Fig. 5A), calpain 1
and ATF-6β (Fig. 5C). These
results indicated that bufalin may induce apoptosis in SCC-4 cells
via mitochondria-dependent signaling pathways.
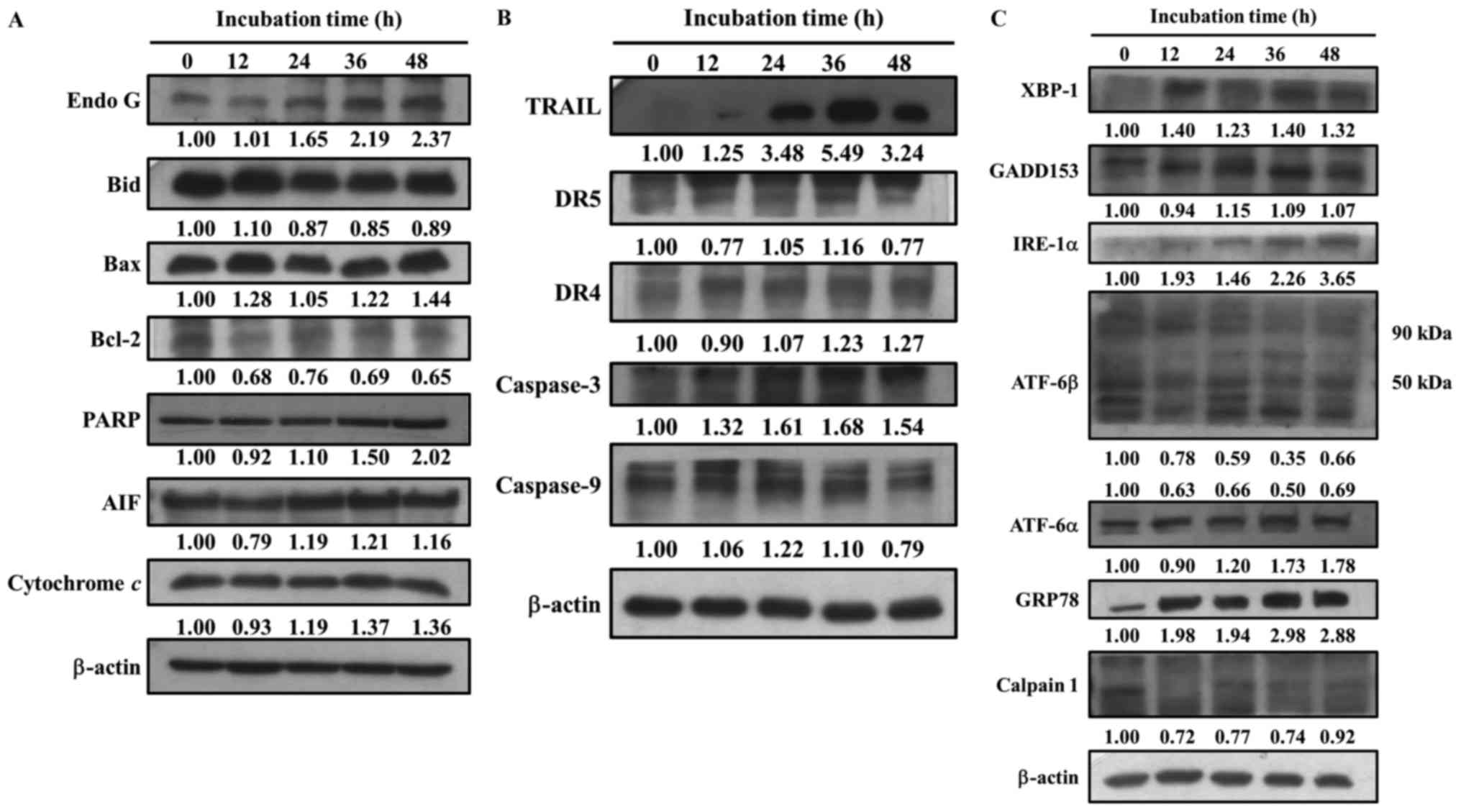 | Figure 5.Bufalin affects apoptosis-associated
protein expression in SCC-4 cells. Cells were treated with 300 nM
of bufalin for 0, 12, 24, 36 and 48 h and then total proteins were
quantified by western blotting. The expression of the following
apoptosis-associated proteins was analyzed and normalized to
β-actin: (A) Endo G, Bid, Bax, Bcl-2, PARP, AIF and cytochrome
c; (B) TRAIL, DR5, DR4, caspase-3 and −9; (C) XBP-1,
GADD153, IRE-1α, ATF-6β, ATF-6α, GRP78 and Calpain 1. Endo G,
endonuclease G; Bid, BH3 interacting-domain death agonist; Bcl-2,
B-cell lymphoma 2; Bax, Bcl-2-associated X protein; PARP,
poly(ADP-ribose) polymerase; AIF, apoptosis-inducing factor; TRAIL,
tumor necrosis factor-related apoptosis-inducing ligand; DR, death
receptor; XBP-1, X-box binding protein 1; GADD153, DNA damage
inducible transcript 3; IRE-1α, inositol-requiring enzyme 1α; ATF,
activating transcription factor; GRP78, heat shock 70 kDa protein 5
(glucose-regulated protein, 78 kDa). |
Discussion
Previous studies have demonstrated that bufalin
induces cell death via cell cycle arrest and inducing apoptosis in
many human cancer cells (2,24,25);
however, currently there is a lack of information available on
bufalin-induced apoptosis in human tongue cancer SCC-4 cells in
vitro. Thus, the present study investigated the cytotoxic
effects of bufalin on human tongue cancer SCC-4 cells. The results
indicated that: i) Bufalin induced cell morphological changes and
reduced the total number of viable cells (Fig. 1); ii) bufalin induced chromatin
condensation (Fig. 2), DNA
fragmentation (apoptotic cell death) and DNA damage (Fig. 3); iii) bufalin increased NO and
Ca2+ levels; however, treatment decreased the levels of
ROS and ΔΨm (Fig. 4); and iv)
bufalin increased the expression of pro-apoptotic proteins,
including Bax, Endo G and AIF, and decreased the expression
anti-apoptotic proteins such as Bcl-2 and calpain 1 (Fig. 5).
The present study indicated that bufalin reduced the
total percentage of viable cells in a dose-dependent manner, which
is similar to findings reported previously in other cell lines
(26–28); the results of the present study
also identified an IC50 of 300 nM following 48 h
treatment in SCC-4 cells. In addition, bufalin induced G2/M phase
arrest in SCC-4 cells, which is also in agreement with previous
reports (11,29–31).
It has been previously established that anticancer drugs, such as
paclitaxel have been used for treating patients with cancer, also
known to induce G2/M phase arrest (32). Apoptosis has been demonstrated to
be one of the potential mechanisms of anticancer activity and it is
also a fundamental process in the development of various cell types
(33,34). Therefore, the present study further
investigated the induction of apoptosis in SCC-4 cells in
vitro. DAPI staining and DNA gel electrophoresis were performed
to confirm that bufalin induced cell apoptosis in SCC-4 cells. The
results demonstrated that bufalin significantly induced cell
apoptosis in SCC-4 cells in dose-dependent manner.
Apoptosis pathways can be divided into two types:
The death receptor (extrinsic) pathway and the mitochondrial
(intrinsic) pathway (35). The
death receptor pathway may function via receptors including the Fas
cell surface death receptor, DR5 and DR4, which then activate
caspase-8, followed by caspase-9 and −3 in order to induce
apoptosis or it may trigger mitochondrial dysfunction to induce the
intrinsic cell apoptotic pathway (36,37).
The western blotting results revealed that bufalin promoted the
expression of TRAIL and DR4 following 48 h, and DR5 following 36 h
of treatment, indicating that bufalin may have induced cell
apoptosis via the death (extrinsic) receptor pathway. DR4 and DR5
proteins are involved in apoptosis and are the membrane receptors
for TRAIL (38). In addition,
other membrane receptors for TRAIL are also involved in inducing
apoptosis (39). Bufalin was also
observed to increase the protein expression of caspase-3 following
48 h, and caspase-9 following 36 h, which supports the involvement
of both pathways.
Flow cytometric analysis indicated that bufalin
increased the production of Ca2+ and NO, and decreased
the levels of ΔΨm and ROS in SCC-4 cells in a time-dependent
manner. SCC-4 cells were also pretreated with 1 mM NAC for 4 h at
37°C and then co-treated with or without bufalin. As expected, the
general ROS scavenger NAC did not significantly decrease
bufalin-induced ROS generation and did not increase the total
number of viable cells (data not shown). ROS have an important role
in cancer cell death; under starvation or stress conditions, the
levels of ROS are increased for the induction of autophagy
(40). These findings indicate
that bufalin did not induce cell apoptosis via the ROS signaling
pathway. Ca2+ uptake into the mitochondrial matrix has
been demonstrated to be critical for cellular function (41). The present study revealed that
bufalin promoted Ca2+ release. In addition, bufalin
significantly reduced the ΔΨm, and pretreatment with cyclosporine
A, followed by bufalin treatment increased the total number of
viable SCC-4 cells when compared with bufalin treatment only.
Anticancer drugs may induce cell apoptosis through the dysfunction
of mitochondria or by decreasing ΔΨm (42,43),
and mitochondria are involved in the stimulation of apoptosis in
the intrinsic signaling pathway (44). Thus, the results of the present
study suggest that bufalin may induce cell apoptosis via a
mitochondria-dependent signaling pathway in SCC-4 cells. Western
blot analysis revealed that bufalin increased the expression of the
pro-apoptotic protein Bax and reduced the expression of the
anti-apoptotic protein Bcl-2. Previous studies determined that the
ratio of Bax/Bcl-2 affects the ΔΨm, and in turn the initiation of
apoptosis (45,46). Mitochondrial control of apoptosis
is thought to primarily involve the ΔΨm and membrane permeability
(47). Thus, if the levels of ΔΨm
are reduced, cytochrome c, AIF or Endo G may release from
the mitochondria, triggering apoptosis.
In conclusion, to the best of our knowledge, the
present study demonstrated for the first time that bufalin
treatment inhibits the progression of SCC-4 human tongue cancer
cells in vitro. Bufalin induced cell morphological changes,
reduced the number of viable cells, and induced G2/M phase arrest
and apoptosis. Bufalin may have triggered cell apoptosis via a
mitochondria-dependent signaling pathway in SCC-4 cells, which is
summarized in Fig. 6. Apoptosis
may also have been induced via the extrinsic pathway, which
involves DR4, DR5, TRAIL and various caspases, or via intrinsic
pathways that lead to the release of AIF and Endo G in order to
induce apoptosis.
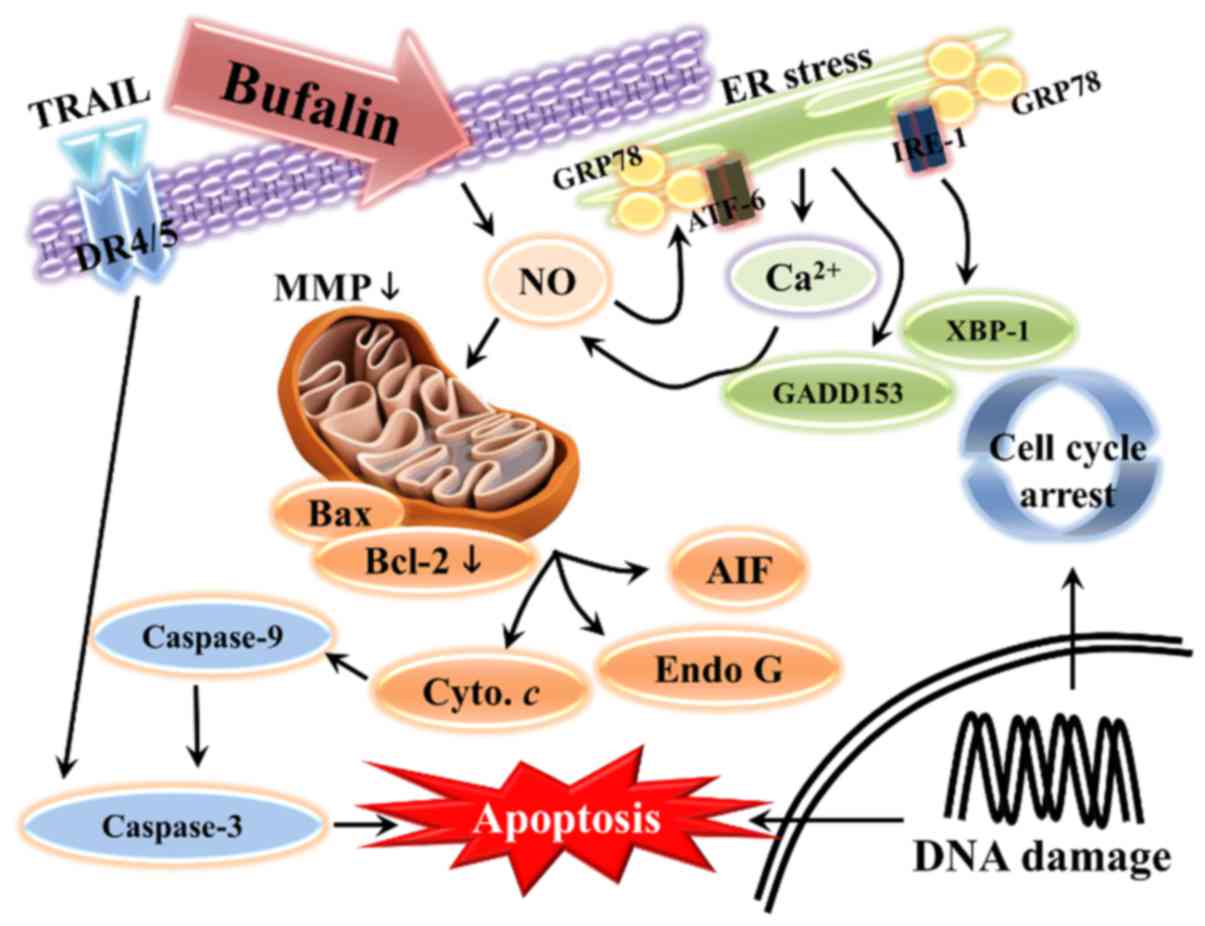 | Figure 6.Schematic of the potential signaling
pathways used by bufalin to induce apoptosis in SCC-4 human tongue
cancer cells. Endo G, endonuclease G; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; AIF, apoptosis-inducing factor;
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; DR,
death receptor; XBP-1, X-box binding protein 1; GADD153, DNA damage
inducible transcript 3; IRE-1α, inositol-requiring enzyme 1α; ATF,
activating transcription factor; GRP78, heat shock 70 kDa protein 5
(glucose-regulated protein, 78 kDa); NO, nitric oxide; ER,
endoplasmic reticulum; MMP, mitochondrial membrane potential. |
Acknowledgements
The present study was supported by China Medical
University, Taichung, Taiwan, R.O.C. (grant no.
CMU101-ASIA-09).
References
|
1
|
Khalili J: Oral cancer: Risk factors,
prevention and diagnostic. Exp Oncol. 30:259–264. 2008.PubMed/NCBI
|
|
2
|
Lee CH, Shih YL, Lee MH, Au MK, Chen YL,
Lu HF and Chung JG: Bufalin induces apoptosis of human osteosarcoma
U-2 os cells through endoplasmic reticulum stress, caspase- and
mitochondria-dependent signaling pathways. Molecules. 22:E4372017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gomez DR, Zhung JE, Gomez J, Chan K, Wu
AJ, Wolden SL, Pfister DG, Shaha A, Shah JP, Kraus DH, et al:
Intensity-modulated radiotherapy in postoperative treatment of oral
cavity cancers. Int J Radiat Oncol Biol Phys. 73:1096–1103. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Major AG, Pitty LP and Farah CS: Cancer
stem cell markers in head and neck squamous cell carcinoma. Stem
Cells Int. 2013:3194892013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Urban D, Corry J and Rischin D: What is
the best treatment for patients with human papillomavirus-positive
and -negative oropharyngeal cancer? Cancer. 120:1462–1470. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krenn L and Kopp B: Bufadienolides from
animal and plant sources. Phytochemistry. 48:1–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge
Y, Newman RA, Cohen L, Liu L, Thornton B, et al: Pilot study of
huachansu in patients with hepatocellular carcinoma, nonsmall-cell
lung cancer, or pancreatic cancer. Cancer. 115:5309–5318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han KQ, Huang G, Gu W, Su YH, Huang XQ and
Ling CQ: Anti-tumor activities and apoptosis-regulated mechanisms
of bufalin on the orthotopic transplantation tumor model of human
hepatocellular carcinoma in nude mice. World J Gastroenterol.
13:3374–3379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashimoto S, Jing Y, Kawazoe N, Masuda Y,
Nakajo S, Yoshida T, Kuroiwa Y and Nakaya K: Bufalin reduces the
level of topoisomerase II in human leukemia cells and affects the
cytotoxicity of anticancer drugs. Leuk Res. 21:875–883. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watabe M, Ito K, Masuda Y, Nakajo S and
Nakaya K: Activation of AP-1 is required for bufalin-induced
apoptosis in human leukemia U937 cells. Oncogene. 16:779–787. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3 K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen S, Zhang Y, Wang Z, Liu R and Gong X:
Bufalin induces the interplay between apoptosis and autophagy in
glioma cells through endoplasmic reticulum stress. Int J Biol Sci.
10:212–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang WW, Yang JS, Pai SJ, Wu PP, Chang
SJ, Chueh FS, Fan MJ, Chiou SM, Kuo HM, Yeh CC, et al: Bufalin
induces G0/G1 phase arrest through inhibiting the levels of cyclin
D cyclin E, CDK2 and CDK4 and triggers apoptosis via mitochondrial
signaling pathway in T24 human bladder cancer cells. Mutat Res.
732:26–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang Y, Zhang Y, Luan J, Duan H, Zhang F,
Yagasaki K and Zhang G: Effects of bufalin on the proliferation of
human lung cancer cells and its molecular mechanisms of action.
Cytotechnology. 62:573–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Z, Li E, Liu Y, Gao Y, Sun H, Ma G,
Wang Z, Liu X, Wang Q, Qu X, et al: Inhibition of Jak-STAT3 pathway
enhances bufalin-induced apoptosis in colon cancer SW620 cells.
World J Surg Oncol. 10:2282012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu SH, Wu TY, Hsiao YT, Lin JH, Hsu SC,
Hsia TC, Yang ST, Hsu WH and Chung JG: Bufalin induces cell death
in human lung cancer cells through disruption of DNA damage
response pathways. Am J Chin Med. 42:729–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang Y-M, Velmurugan BK, Kuo W-W, Chen
Y-S, Ho T-J, Tsai C-T, Ye C-X, Tsai C-H, Tsai F-J and Huang C-Y:
Inhibitory effect of alpinate Oxyphyllae fructus extracts on Ang
II-induced cardiac pathological remodeling-related pathways in H9c2
cardiomyoblast cells. Biomedicine. 3:148–152. 2013. View Article : Google Scholar
|
|
19
|
Yu FS, Huang AC, Yang JS, Yu CS, Lu CC,
Chiang JH, Chiu CF and Chung JG: Safrole induces cell death in
human tongue squamous cancer SCC-4 cells through
mitochondria-dependent caspase activation cascade apoptotic
signaling pathways. Environ Toxicol. 27:433–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chueh FS, Chen YL, Hsu SC, Yang JS, Hsueh
SC, Ji BC, Lu HF and Chung JG: Triptolide induced DNA damage in
A375.S2 human malignant melanoma cells is mediated via reduction of
DNA repair genes. Oncol Rep. 29:613–618. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu KC, Yen CY, Wu RS, Yang JS, Lu HF, Lu
KW, Lo C, Chen HY, Tang NY, Wu CC and Chung JG: The roles of
endoplasmic reticulum stress and mitochondrial apoptotic signaling
pathway in quercetin-mediated cell death of human prostate cancer
PC-3 cells. Environ Toxicol. 29:428–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin M-C, Tsai S-Y, Wang F-Y, Liu F-H, Syu
J-N and Tang F-Y: Leptin induces cell invasion and the upregulation
of matrilysin in human colon cancer cells. Biomedicine. 3:174–180.
2013. View Article : Google Scholar
|
|
23
|
Iglesias-Guimarais V, Gil-Guinon E,
Sanchez-Osuna M, Casanelles E, Garcia-Belinchon M, Comella JX and
Yuste VJ: Chromatin collapse during caspase-dependent apoptotic
cell death requires DNA fragmentation factor, 40-kDa
subunit-/caspase-activated deoxyribonuclease-mediated 3′-OH
single-strand DNA breaks. J Biol Chem. 288:9200–9215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang L, Zhao MN, Liu TY, Wu XS, Weng H,
Ding Q, Shu YJ, Bao RF, Li ML, Mu JS, et al: Bufalin induces cell
cycle arrest and apoptosis in gallbladder carcinoma cells. Tumour
Biol. 35:10931–10941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang DM, Liu JS, Tang MK, Yiu A, Cao HH,
Jiang L, Chan JY, Tian HY, Fung KP and Ye WC: Bufotalin from
venenum bufonis inhibits growth of multidrug resistant HepG2 cells
through G2/M cell cycle arrest and apoptosis. Eur J Pharmacol.
692:19–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu SH, Bau DT, Hsiao YT, Lu KW, Hsia TC,
Lien JC, Ko YC, Hsu WH, Yang ST, Huang YP and Chung JG: Bufalin
induces apoptosis in vitro and has Antitumor activity against human
lung cancer xenografts in vivo. Environ Toxicol. 32:1305–1317.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: new hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao H, Zhao D, Tan G, Liu Y, Zhuang L and
Liu T: Bufalin promotes apoptosis of gastric cancer by
down-regulation of miR-298 targeting bax. Int J Clin Exp Med.
8:3420–3428. 2015.PubMed/NCBI
|
|
29
|
Hong SH and Choi YH: Bufalin induces
apoptosis through activation of both the intrinsic and extrinsic
pathways in human bladder cancer cells. Oncol Rep. 27:114–120.
2012.PubMed/NCBI
|
|
30
|
Jing Y, Watabe M, Hashimoto S, Nakajo S
and Nakaya K: Cell cycle arrest and protein kinase modulating
effect of bufalin on human leukemia ML1 cells. Anticancer Res.
14:1193–1198. 1994.PubMed/NCBI
|
|
31
|
Numazawa S, Shinoki MA, Ito H, Yoshida T
and Kuroiwa Y: Involvement of Na+, K(+)-ATPase
inhibition in K562 cell differentiation induced by bufalin. J Cell
Physiol. 160:113–120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu K, Cang S, Ma Y and Chiao JW:
Synergistic effect of paclitaxel and epigenetic agent phenethyl
isothiocyanate on growth inhibition, cell cycle arrest and
apoptosis in breast cancer cells. Cancer Cell Int. 13:102013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chu YL, Ho CT, Chung JG, Raghu R, Lo YC
and Sheen LY: Allicin induces anti-human liver cancer cells through
the p53 gene modulating apoptosis and autophagy. J Agric Food Chem.
61:9839–9848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Green DR and Fitzgerald P: Just so stories
about the evolution of apoptosis. Curr Biol. 26:R620–R627. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Zhao L, Wei T, Zhao Y and Chen C:
The inhibition of death receptor mediated apoptosis through
lysosome stabilization following internalization of
carboxyfullerene nanoparticles. Biomaterials. 32:4030–4041. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grunnet LG, Aikin R, Tonnesen MF,
Paraskevas S, Blaabjerg L, Storling J, Rosenberg L, Billestrup N,
Maysinger D and Mandrup-Poulsen T: Proinflammatory cytokines
activate the intrinsic apoptotic pathway in beta-cells. Diabetes.
58:1807–1815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Santin I, Moore F, Colli ML, Gurzov EN,
Marselli L, Marchetti P and Eizirik DL: PTPN2, a candidate gene for
type 1 diabetes, modulates pancreatic beta-cell apoptosis via
regulation of the BH3-only protein Bim. Diabetes. 60:3279–3288.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Milutinovic S, Kashyap AK, Yanagi T, Wimer
C, Zhou S, O'Neil R, Kurtzman AL, Faynboym A, Xu L, Hannum CH, et
al: Dual agonist surrobody simultaneously activates death receptors
dr4 and dr5 to induce cancer cell death. Mol Cancer Ther.
15:114–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin FL, Hsu JL, Chou CH, Wu WJ, Chang CI
and Liu HJ: Activation of p38 MAPK by damnacanthal mediates
apoptosis in SKHep 1 cells through the DR5/TRAIL and
TNFR1/TNF-alpha and p53 pathways. Eur J Pharmacol. 650:120–129.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Azad MB and Gibson SB: Superoxide
is the major reactive oxygen species regulating autophagy. Cell
Death Differ. 16:1040–1052. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ip SW, Chu YL, Yu CS, Chen PY, Ho HC, Yang
JS, Huang HY, Chueh FS, Lai TY and Chung JG: Bee venom induces
apoptosis through intracellular Ca2+ -modulated
intrinsic death pathway in human bladder cancer cells. Int J Urol.
19:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chiu TH, Lan KY, Yang MD, Lin JJ, Hsia TC,
Wu CT, Yang JS, Chueh FS and Chung JG: Diallyl sulfide promotes
cell-cycle arrest through the p53 expression and triggers induction
of apoptosis via caspase- and mitochondria-dependent signaling
pathways in human cervical cancer Ca Ski cells. Nutr Cancer.
65:505–514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsai CW, Yang MD, Hsia TC, Chang WS, Hsu
CM, Hsieh YH, Chung JG and Bau DT: Dithiothreitol enhanced
arsenic-trioxide-induced cell apoptosis in cultured oral cancer
cells via mitochondrial dysfunction and endoplasmic reticulum
stress. Environ Toxicol. 32:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mohan S, Abdelwahab SI, Kamalidehghan B,
Syam S, May KS, Harmal NS, Shafifiyaz N, Hadi AH, Hashim NM,
Rahmani M, et al: Involvement of NF-kappaB and Bcl2/Bax signaling
pathways in the apoptosis of MCF7 cells induced by a xanthone
compound Pyranocycloartobiloxanthone A. Phytomedicine.
19:1007–1015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma YS, Hsu SC, Weng SW, Yu CC, Yang JS,
Lai KC, Lin JP, Lin JG and Chung JG: Crude extract of Rheum
palmatum L induced cell death in LS1034 human colon cancer cells
acts through the caspase-dependent and -independent pathways.
Environ Toxicol. 29:969–980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu J, Tan Z, Chen J and Dong C:
Cyclovirobuxine D inhibits cell proliferation and induces
mitochondria-mediated apoptosis in human gastric cancer cells.
Molecules. 20:20659–20668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chiang JH, Yang JS, Ma CY, Yang MD, Huang
HY, Hsia TC, Kuo HM, Wu PP, Lee TH and Chung JG: Danthron, an
anthraquinone derivative, induces DNA damage and caspase
cascades-mediated apoptosis in SNU-1 human gastric cancer cells
through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar : PubMed/NCBI
|