Introduction
Polycystic ovary syndrome (PCOS) is a common
disorder that affects reproductive function in women, affecting
5–10% of women of reproductive age (1,2). It
is the primary reason for ovulation inhibition, infertility and
irregular menstruation in women. As its underlying aetiology and
pathophysiology remains to be fully understood, endocrine,
immunological and genetic factors require further investigation to
aid the identification of more effective treatments (3,4).
Recently, a study demonstrated that cross-talk between granulosa
cells (GCs) and oocytes in the follicle is a key process in oocyte
maturation and metabolism (5).
Disorders of follicular development in PCOS were closely associated
with apoptosis of GCs (6–8).
Bone morphogenetic proteins (BMPs) are functionally
involved in all stages of folliculogenesis, and are multifunctional
growth factors that belong to the transforming growth factor β
(TGFβ) superfamily. Their actions are mediated by BMP receptors
(BMPRs), which share differing degrees of affinity for their
ligands (9–11). The oocyte-secreted factor, BMP15,
is a ligand of the TGFβ superfamily that mediates Smad1 signalling
pathway when it binds to BMPR2. Following the activation of
receptor kinases by ligand binding, downstream signalling molecules
are activated, and subsequently modulate the expression of target
genes. Previous studies have demonstrated that BMP15 serves a role
in follicular growth and oocyte quality, and was downregulated in
PCOS patients (12). It has
additionally been revealed that the state of the oocyte may
reversely influence granulosa cell proliferation, and following
removal of oocytes, apoptosis of granulosa cells increased.
However, when BMP15 was added to the cultures, it significantly
reduced apoptosis of granulosa cells (10,12).
Therefore, it is of value to investigate the effect of BMP15 on
apoptosis of granulosa cells, and whether this effect is associated
with BMPs and the Smad signalling pathway in PCOS.
In the present study, the expression of B-cell
lymphoma 2 (Bcl-2), caspase-3, Smad1, BMP15 and BMPR2 was
investigated in granulosa luteinizing cells from women affected by
PCOS, compared with healthy ovulatory women. The present study
aimed to investigate the involvement of the BMP15/Smad1 signalling
pathway in granulosa cell of PCOS women, and the underlying
mechanism.
Materials and methods
Patients
The study population consisted of women who were
referred to the Reproductive Medicine Centre of Shanxi Women and
Infants Hospital (Taiyuan, China), between May 2014 and May 2015.
All patients were of Han ethnicity, from Shanxi, North China.
Informed written consent was obtained from each patient and the
study was approved by the Reproductive Ethics Committee of the
Children's Hospital of Shanxi and Women's Health Center of Shanxi
(Taiyuan, China).
Individual follicular fluid and serum samples were
collected from 138 women undergoing in vitro fertilization
(IVF)/intracytoplasmic sperm injection (ICSI) treatment. Patients
with PCOS (n=70) were selected based on the 2003 Rotterdam criteria
(13). The diagnosis of PCOS was
based on the association of two out of three of the following
criteria: i) Oligomenorrhea and/or anovulation; ii) clinical and/or
biological hyperandrogenism; iii) polycystic ovaries. Patients with
endometriosis, congenital adrenal hyperplasia, hypothyroidism,
androgen-secreting tumours, Cushing's syndrome and other diseases
that interfere with the hypothalamus-pituitary-ovary axis were
excluded from the study. The control group (n=68) were women
undergoing fertility treatment due to male infertility or tubal
infertility disorders, although they experienced regular menstrual
cycles.
All patients did not present with cardiovascular
system diseases, thyroid abnormalities or other endocrine metabolic
disorders.
Controlled ovarian hyperstimulation
protocol
The present study obtained standard operating
procedures from embryologists and physicians. Patients with PCOS
received a monophasic combined oral contraceptive pill for 28
consecutive days.
Patients with PCOS were pretreated with protection
of the endometrium, adjustment of the menstrual cycle, and
downregulation of hyperandrogenism prior to the downregulation
protocol using oral ethinylestradiol [Diane®-35 (Bayer
HealthCare Pharmaceuticals; Bayer AG, Leverkusen, Germany) or
Marvelon® (Organon Pharmaceuticals; Merck KGaA,
Darmstadt, Germany)]. All women underwent controlled ovarian
hyper-stimulation with the gonadotropin releasing hormone agonist
long protocol (14), and pituitary
suppression was commenced with leuprolide acetate
(Diphereline®; GeneScience Pharmaceuticals Co., Ltd.,
Changchun, China) at a dose of 0.05 mg/day, during the mid-luteal
phase of the preceding cycle. Complete pituitary suppression was
confirmed by serum follicle-stimulating hormone (FSH) levels of
<5 mIU/ml, luteinising hormone (LH) ≤5 mIU/ml, estradiol
(E2) <50 pg/ml, bilateral antral follicle diameter
<5 mm and endometrial thickness ≤5 mm. Urofollitropin (Lizhu
Pharmaceutical Trading Co., Ltd., Nanjing, China) was used at doses
ranging between 75 and 300 IU/day in accordance with patient age,
body mass index (BMI), size and number of antral follicles and
serum FSH levels. The dosage of urofollitropin was adjusted
according to ovarian response, which was assessed by ultrasound and
serum E2 levels. Treatment with urofollitropin was
continued until ≥2 follicles had reached 18 mm in diameter.
Treatment with 250 µg subcutaneous human chorionic gonadotropin
(HCG; Lizhu Pharmaceutical Trading Co., Ltd.) was subsequently
administered to stimulate follicle maturation. Oocyte retrieval was
performed with the guidance of ultrasound 34–36 h after HCG
injection. Routine IVF or ICSI procedures were performed, and two
embryos were transferred 3–5 days after oocyte retrieval using
ultrasound guidance. Pregnancy was diagnosed by a rising
concentration of serum or urine β-HCG, which was measured 14 days
after embryo transfer. The fertilization rate was defined as the
percentage of fertilized embryos (2 pronuclei) in the mature
oocytes. Clinical pregnancy was defined as the presence of a
gestational sac or heartbeat during vaginal ultrasound examination
4 weeks after embryos transfer. Miscarriage was defined as
pregnancy loss before 28 weeks.
Clinical measurements
Medical information was collected and recorded,
including age, BMI, menarche age, serum basic sexual hormone
levels, retrieved oocyte number, mature oocyte rate, fertilization
rate, portable embryo rate, high-quality embryo rate and clinical
pregnancy rate.
Collection of follicular fluid and
granular cells
After oocyte retrieval, the follicular fluids from
each patient were pooled and stored in a tube at 37°C. Cells and
supernatant were separated by centrifugation for 30 min at 2,200 ×
g at room temperature. Supernatants were collected in a tube for
analysis of BMP15. The cell pellet was resuspended in 1 ml PBS. The
suspension was overlayed on 1 ml Ficoll (Tianjin Haoyang Biological
Products Technology Co., Ltd., Tianjin, China) and was centrifuged
at 1,000 × g at room temperature for 15 min. Granular cells were
aspirated from the interface, washed with PBS three times and
counted on a Neubauer haemocytometer. Cells were either used for
the terminal deoxynucleotide transferase dUTP nick-end labelling
(TUNEL) assay or stored at −80°C for mRNA, protein and cell cycle
analysis.
Serum collection and analysis
Blood samples were taken between 8:00 and 9:00 am
after a 12 h overnight fast, and collected into a tube containing
EDTA. The samples were centrifuged at 2,200 × g at room temperature
for 3 min and the serum and buffy coat were separated. Serum was
either used for testing sex hormone levels or was stored at
−80°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to assess the mRNA expression
levels of BMPR2, BCL2, CASP3 and SMAD1.
Total RNA was extracted from granular cells using the RNAsimple
Total RNA kit (Tiangen Biotech Co., Ltd., Beijing, China), and was
reverse-transcribed using a kit from Takara Bio, Inc. (Otsu,
Japan). A SYBR Green kit (Takara Bio, Inc.) was used as described
previously (15). The ACTB
gene that encodes the β-actin protein was used as the endogenous
housekeeping gene for normalization. The PCR was performed at 93°C
for 2 min, followed by a total of 40 cycles at 93°C for 1 min and
55°C for 2 min. Primers sequences were as follows: Forward,
5′-AATACTCGCACTTCCTCAGAACC-3′ and reverse
5′-AGCATAGCAAGGCTTCAGACAG-3′ for BMPR2; forward,
5′-GTCATCGTTGGGCAGAAGTTT-3′ and reverse,
5′-GAAGACTCAACATGGGCTCTAAA-3′ for SMAD1; forward,
5′-GGTGGGGTCATGTGTGTGG-3′ and reverse, 5′-CGGTTCAGGTACTCAGTCATCC-3′
for BCL2; forward, 5′-CATGGAAGCGAATCAATGGACT-3′ and reverse,
5′-CTGTACCAGACCGAGATGTCA-3′ for CASP3; forward,
5′-TGTACGTTGCTATCCAGGCT-3′ and reverse, 5′-CTCCTTAATGTCACGCACGA-3′
for ACTB. The 2−∆∆Cq method was used for
quantification (16).
Western blotting analysis
Follicular fluids were prepared from all patients
for western blotting analysis to determine the expression levels of
BMP15 (17). Cell lysates were
prepared in lysis buffer (Nanjing KeyGen Bio Tech Co., Ltd.,
Nanjing, China) for the measurement of Smad1, Bcl-2 and caspase-3
expression. Protein concentration was determined using a
bicinchoninic acid assay kit. A total of 50 µg protein/lane was
separated by SDS-PAGE on a 10% gel and blotted onto polyvinylidene
difluoride membranes (Merck KGaA). The membranes were blocked with
5% bovine serum albumin (Beyotime Institute of Biotechnology,
Haimen, China) for 1 h at room temperature and incubated with
rabbit anti-BMP15 (catalog no. 18982-1-AP; 1:500; ProteinTech
Group, Inc., Chicago, IL, USA) Smad-1 (catalog no. 10429-1-AP;
1:500; ProteinTech Group, Inc.), caspase-3 (catalog no. 19677-1-AP;
1:500; ProteinTech Group, Inc.), Bcl-2 (catalog no. 12789-1-AP;
1:1,000; ProteinTech Group, Inc.) and β-actin (catalog no.
60008-1-Ig, 1:2,000; ProteinTech Group, Inc.) in TBS with Tween-20
(TBST) with 5% non-fat milk at 4°C overnight. Membranes were
subsequently probed with a horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:2,000; Protein Tech Group Inc.;
catalog no. SA00001-2) at room temperature for 1 h. The proteins of
interest were detected using enhanced chemiluminescence (Beyotime
Institute of Biotechnology) and analysed using Image-Pro Plus
software (version 5.1; Media Cybernetics, Inc., Rockville, MD,
USA).
Cell cycle analysis
The cell cycle distribution was analysed using a
flow cytometer (FACSAria II; BD Biosciences, Franklin Lakes, NJ,
USA). Granular cells were pooled and stored in tubes at a density
of 1.6×105 cells per tube. Cells were fixed in 70%
ethanol for 24 h at 4°C and washed three times with PBS. Finally,
the cell pellets were stained with RNase (1 mg/ml, Thermo Fisher
Scientific, Inc. Waltham, MA, USA) and 400 µl propidium iodide
solution (100 µl/ml) for 30 min in the dark and analysed by flow
cytometry using ModFit LT software (version 3.2; Verity Software
House, Inc., Topsham, ME, USA). Each experiment was repeated at
least three times.
TUNEL assay
Granular cells (1×105 cells/well) were
fixed in 4% paraformaldehyde solution for 20 min and permeabilized
in 0.1% Triton X-100 in 0.1% citrate solution for 5 min at room
temperature in 24-well plates. Subsequently, granular cells were
incubated in TUNEL reaction medium (Nanjing KeyGen Biotech Co.,
Ltd.) for 1 h at 37°C in the dark. After the reaction was stopped,
granular cells were washed three times in PBS and the cell nuclei
were labelled with streptavidin-horseradish peroxidase (Nanjing
KeyGen Bio Tech Co., Ltd.) for 30 min at room temperature in the
dark. A total of 1–5 drops diaminobenzidine chromogen solution was
added to the cells and incubated for 5 min at room temperature.
Cells were visualized under a brightfield microscope at ×200
magnification. TUNEL-positive cells appeared to be brown or buffy.
The total number of cells and the number of apoptotic cells were
counted in a randomly-selected visual field in each group. Negative
control cells were subjected to the TUNEL assay without the
addition of terminal deoxynucleotidyl transferase in the reaction
mixture. Positive control cells were incubated with 100 µl DNase I
solution prior to the TUNEL assay, in order to induce DNA strand
degradation.
Statistical analysis
Statistical analysis was performed using the SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Normally
distributed data were expressed as the mean ± standard deviation.
To check the normality of the distribution, the Shapiro-Wilk test
was performed. Two-tailed Student's t-test was used to assess
differences in values between each group. P<0.05 was considered
to indicate a statistically significant difference.
Results
Comparison of conditions and IVF
outcomes between PCOS and control patients
Tables I and
II summarize the medical
information and IVF outcomes of patients. Compared with the control
group, the BMI and the levels of LH and testosterone (TES) were
significantly increased, whereas the portable embryo rate was
significantly decreased (P<0.05).
 | Table I.Comparison of clinicopathological
factors in PCOS and control patients. |
Table I.
Comparison of clinicopathological
factors in PCOS and control patients.
| Parameters | PCOS group | Control group | P-value |
|---|
| Age | 29.01±3.56 | 28.47±3.52 | 0.37 |
| BMI | 25.37±6.73 | 22.62±5.81 | 0.01a |
| Menarche age | 13.11±1.25 | 13.58±1.47 | 0.81 |
| FSH (mIU/ml) | 6.70±1.35 | 6.88±1.67 | 0.49 |
| LH (mIU/ml) | 10.95±7.12 | 4.73±1.54 |
<0.0001a |
| E2
(pg/ml) | 57.32±14.74 | 55.28±18.31 | 0.47 |
| PRL (ng/ml) | 13.77±5.89 | 14.23±5.62 | 0.64 |
| TES (ng/dl) | 92.80±15.53 | 45.91±18.20 |
<0.0001a |
 | Table II.Comparison of in vitro
fertilization outcomes in PCOS and control patients. |
Table II.
Comparison of in vitro
fertilization outcomes in PCOS and control patients.
| Parameters | PCOS group | Control group | P-value |
|---|
| Retrieved oocyte
number (n) | 23.61±2.77 | 17.35±1.93 | 1.65 |
| Mature oocytes rate
(%) | 78.71
(1301/1653) | 78.98
(932/1180) | 0.86 |
| Fertilization rate
(%) | 66.24
(1095/1653) | 67.29
(794/1180) | 0.56 |
| Portable embryo
rate (%) | 86.39
(946/1095) | 90.05
(715/794) | 0.02a |
| High-quality embryo
rate (%) | 56.98
(539/956) | 60.62
(440/715) | 0.06 |
| Clinical pregnancy
rate (%) | 47.14 (33/70) | 52.94 (36/68) | 0.50 |
Comparison of apoptosis status in
granulosa cells between the PCOS and control groups
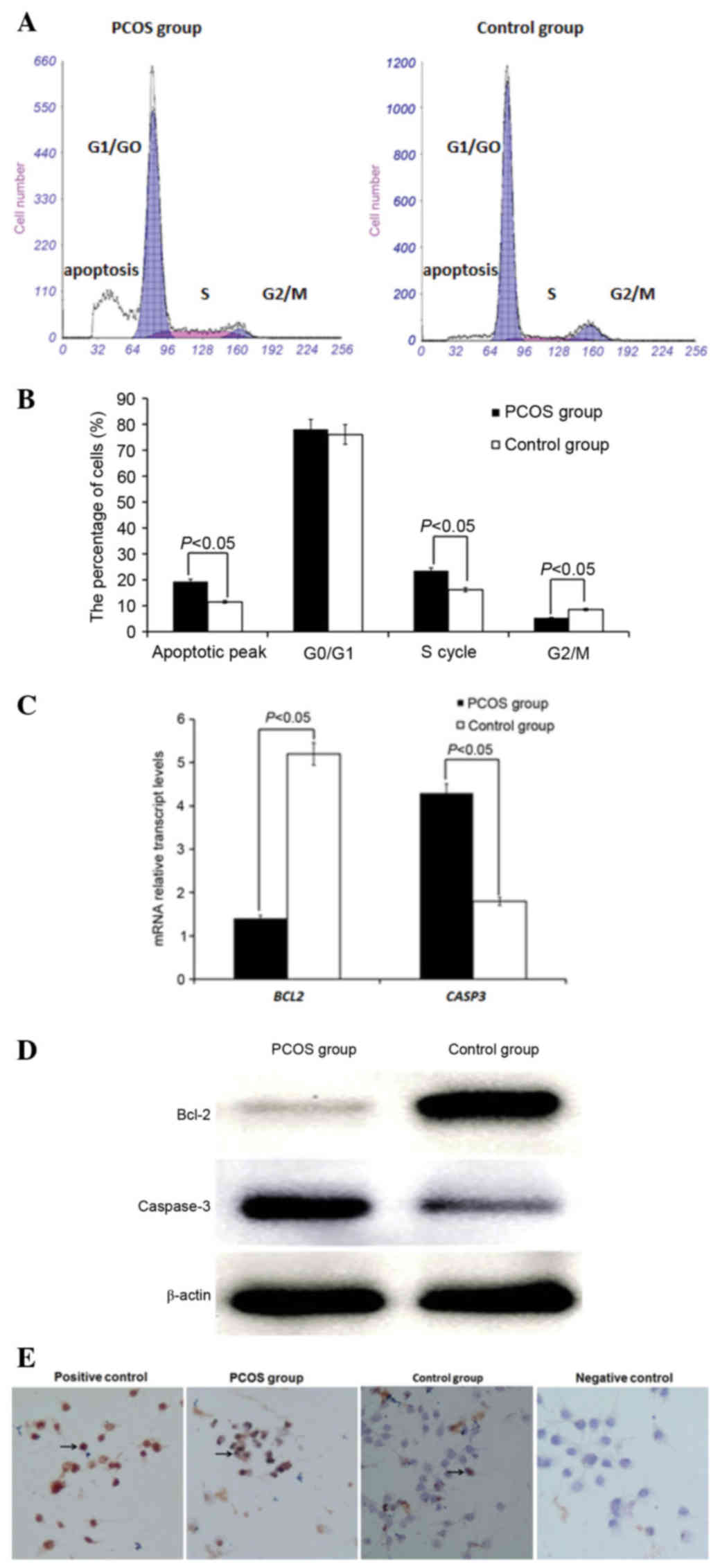
As demonstrated in Fig.
1A and B, there was no significant difference in the percentage
of cells in G0/G1 between the PCOS and control groups (P>0.05).
In the PCOS group, the percentage of cells in S phase was
significantly higher, the percentage of cells in G2/M phase was
significantly lower and the apoptosis peak was significantly
higher, compared with the control group (P<0.05). RT-qPCR
(Fig. 1C) and western blotting
(Fig. 1D) demonstrated that the
expression levels of the gene encoding Bcl-2 and its corresponding
protein were significantly decreased in granulosa cells of PCOS
group compared to control group (P<0.05), whereas the expression
of gene encoding caspase-3 and its corresponding protein was higher
than control group (P<0.05). As demonstrated in Fig. 1E, the level of apoptosis in
granulosa cells was measured by a TUNEL assay. The level of
apoptosis in granulosa cells was 21.47±6.81 in the PCOS group,
which was significantly greater than the control group (P<0.05;
14.78±4.58) (data not shown). Cells in 20 randomly-selected fields
(magnification, ×200) were counted by eye.
Comparison of BMP15 in follicular
fluid and BMPR2 and Smad1 in granulosa cells of the PCOS and
control groups
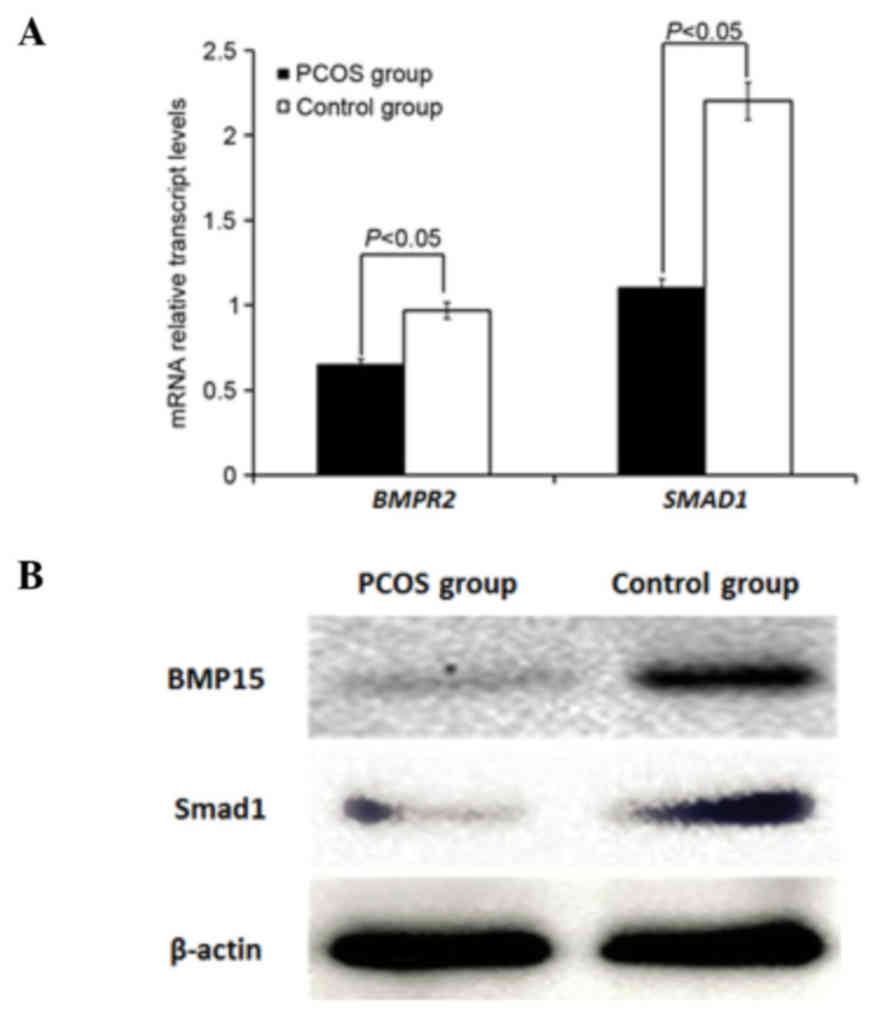
As demonstrated in Fig.
2A, the relative mRNA expression levels of BMPR2 and
SMAD1 were significantly decreased in granulosa cells of the
PCOS group compared with the control group (P<0.05). Western
blot analysis (Fig. 2B)
demonstrated that the expression of BMP15 in follicular fluid and
Smad1 in granulosa cells was reduced in the PCOS group compared
with the control group.
Discussion
PCOS is the most prevalent female endocrinopathy and
is the largest single cause of anovulatory infertility. It is
classically characterized by chronic anovulation, hyperandrogenism
and a polycystic ovarian morphology, as revealed by ultrasonography
(16,18,19).
PCOS has been defined as a metabolic syndrome associated with
obesity, insulin resistance, type 2 diabetes, dyslipidaemia,
hypertension and other cardiovascular diseases (20–22).
The present study demonstrated that BMI, LH and TES were
significantly increased, whereas portable embryo rate was
significantly decreased in PCOS patients compared with healthy
controls. However, the pathogenesis of PCOS remains unclear.
Recently, researchers have hypothesised that the
pathogenesis of PCOS may be due to a combination of genetic and
environmental factors, where the follicle microenvironment affects
the quality of oocytes and cleavage quality (23–25).
Granulosa cells are key somatic cells in the follicle
microenvironment, and serve an important role in oocyte maturation
and follicular development. Follicular development is accompanied
by growth, proliferation, differentiation and maturation of
granulosa cells. Studies have demonstrated that intrinsic
follicular dysplasia may be associated with regulation disorders of
ovarian granulosa cell apoptosis (6,26,27).
Research on ovarian granulosa cell apoptosis in patients with PCOS
may provide an insight into the pathological mechanisms and
generate novel clinical treatments. Therefore, the present study
investigated apoptosis of granulosa cells. There was no significant
difference in the percentage of cells in the G0/G1 phase between
the PCOS and control groups. The percentage of cells in S phase was
significantly higher, cells in G2/M phase was significantly lower
and the degree of apoptosis was significantly higher in the PCOS
group compared with the control group. Bcl-2 is an anti-apoptotic
protein and caspase-3 serves a central role in the apoptotic
execution pathway. Therefore, the present study measured the
expression levels of these proteins. Expression of Bcl-2 was
significantly decreased in granulosa cells of the PCOS, whereas
expression of caspase-3 was higher compared to the control group.
This suggested that there was a greater degree of apoptosis in the
PCOS group.
BMPs belong to the TGFβ superfamily and have been
implicated in the control and regulation of follicular development
and female fertility. As an oocyte-secreted factor, BMP15 serves a
crucial role in follicular growth and oocyte quality, which is
underexpressed in PCOS patients. Adding BMP15 to cultures
significantly reduced the apoptosis of granulosa cells (9,28–30).
Therefore, it is of value to investigate the effect of BMP15 on the
apoptosis of granulosa cells in PCOS (11,12,31).
In the present study, the expression of BMP15 in follicular fluid,
and BMPR2 and Smad1 expression levels were measured in granulosa
cells of women with PCOS undergoing ovarian stimulation for IVF.
The mRNA expression levels of BMPR2 and SMAD1 were
significantly decreased in granulosa cells of the PCOS group
compared to the control group and the expression of BMP15 in
follicular fluid was significantly decreased in PCOS group.
Therefore, this suggested that the BMP15/Smad1 signalling pathway
may be associated with apoptosis of granulosa cells in PCOS.
In the present study, oocyte-secreted factor BMP15
was detected in follicular fluid of mature follicles, and the
expression of BMP15 was significantly decreased in the PCOS group.
Aberrations in the BMP15 signalling pathway may affect oocyte
maturation disorders and reduce the developmental potential. In
addition, there was increased apoptosis of granulosa cells and
alterations in the expression levels of apoptotic proteins in PCOS,
suggesting that this may be associated with abnormal alterations in
the BMP15 signalling pathway; however, this requires further
investigation.
References
|
1
|
Ganie MA, Marwaha RK, Dhingra A, Nisar S,
Mani K, Masoodi S, Chakraborty S and Rashid A: Observation of
phenotypic variation among Indian women with polycystic ovary
syndrome (PCOS) from Delhi and Srinagar. Gynecol Endocrinol.
32:566–570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kondo M, Osuka S, Iwase A, Nakahara T,
Saito A, Bayasula, Nakamura T, Goto M, Kotani T and Kikkawa F:
Increase of kisspeptin-positive cells in the hypothalamus of a rat
model of polycystic ovary syndrome. Metab Brain Dis. 31:673–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rissanen AP, Koskela-Koivisto T, Hägglund
H, Koponen AS, Aho JM, Pöyhönen-Alho M, Tiitinen A, Tikkanen HO and
Peltonen JE: Altered cardiorespiratory response to exercise in
overweight and obese women with polycystic ovary syndrome. Physiol
Rep. 4:pii: e127192016. View Article : Google Scholar
|
|
4
|
Casarini L, Simoni M and Brigante G: Is
polycystic ovary syndrome a sexual conflict? A review. Reprod
Biomed Online. 32:350–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lei X, Cui K, Li Z, Su J, Jiang J, Zhang
H, Liu Q and Shi D: BMP-1 participates in the selection and
dominance of buffalo follicles by regulating the proliferation and
apoptosis of granulosa cells. Theriogenology. 85:999–1012. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Q, Liu D, Zhang M, Li N, Lu S, Du Y
and Chen ZJ: Effects of brain-derived neurotrophic factor on oocyte
maturation and embryonic development in a rat model of polycystic
ovary syndrome. Reprod Fertil Dev. Jun 25–2015.(Epub ahead of
print).
|
|
7
|
Kim E, Seok HH, Lee SY, Lee DR, Moon J,
Yoon TK, Lee WS and Lee KA: Correlation between expression of
glucose transporters in granulosa cells and oocyte quality in women
with polycystic ovary syndrome. Endocrinol Metab (Seoul). 29:40–47.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang X, Hao C, Shen X, Zhang Y and Liu X:
RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are
reflective oocyte/embryo competence and potentially reliable
predictors of embryo developmental competence in PCOS patients.
Reprod Biol Endocrinol. 11:1092013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei LN, Huang R, Li LL, Fang C, Li Y and
Liang XY: Reduced and delayed expression of GDF9 and BMP15 in
ovarian tissues from women with polycystic ovary syndrome. J Assist
Reprod Genet. 31:1483–1490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Houten EL, Laven JS, Louwers YV,
McLuskey A, Themmen AP and Visser JA: Bone morphogenetic proteins
and the polycystic ovary syndrome. J Ovarian Res. 6:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khalaf M, Morera J, Bourret A, Reznik Y,
Denoual C, Herlicoviez M, Mittre H and Benhaim A: BMP system
expression in GCs from polycystic ovary syndrome women and the in
vitro effects of BMP4, BMP6, and BMP7 on GC steroidogenesis. Eur J
Endocrinol. 168:437–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao SY, Qiao J, Chen YJ, Liu P, Li J and
Yan J: Expression of growth differentiation factor-9 and bone
morphogenetic protein-15 in oocytes and cumulus granulosa cells of
patients with polycystic ovary syndrome. Fertil Steril. 94:261–267.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
consensus workshop group, . Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome. Hum Reprod. 19:41–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarhan A, Harira M, Elshazly S and Nouh A:
Comparing stimulation requirements and final outcome between early
follicular and mid luteal pituitary suppression in the long
gonadotropin releasing hormone agonist protocol. JBRA Assist
Reprod. 20:59–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui X, Jing X, Wu X, Wang Z and Li Q:
Potential effect of smoking on semen quality through DNA damage and
the downregulation of Chk1 in sperm. Mol Med Rep. 14:753–761. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Krulewitch CJ: Reproductive Health of
Active Duty Women in Medically Austere Environments. Mil Med. 181(1
Suppl): S63–S69. 2016. View Article : Google Scholar
|
|
18
|
Copp T, McCaffery K, Azizi L, Doust J, Mol
BWJ and Jansen J: Influence of the disease label ‘polycystic ovary
syndrome’ on intention to have an ultrasound and psychosocial
outcomes: A randomised online study in young women. Hum Reprod.
32:876–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng J, Yin Q, Cao J and Zhang B: Obesity
contributes more to increasing ApoB/ApoA1 ratio than
hyperandrogenism in PCOS women aged 20–38 years in China. Exp Ther
Med. 13:1337–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Wu X, Ding M, Yu X, Liu G and Shi
Y: Case-control based study between polymorphisms in the
adiponectin gene and polycystic ovary syndrome. Zhonghua Fu Chan Ke
Za Zhi. 50:825–829. 2015.(In Chinese). PubMed/NCBI
|
|
21
|
Adeniji AA, Essah PA, Nestler JE and
Cheang KI: Metabolic effects of a commonly used combined hormonal
oral contraceptive in Women with and without polycystic ovary
syndrome. J Womens Health (Larchmt). 25:638–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xi W, Yang Y, Mao H, Zhao X, Liu M and Fu
S: Circulating anti-mullerian hormone as predictor of ovarian
response to clomiphene citrate in women with polycystic ovary
syndrome. J Ovarian Res. 9:32016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Timur H, Yimaz N, Kahyaoglu I, Inal HA and
Erkaya S: The effect of serum and follicular fluid
amyloid-associated protein levels on in vitro fertilization outcome
in patients with polycystic ovary syndrome. J Assist Reprod Genet.
32:1637–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi L, Liu S, Zhao W and Shi J: miR-483-5p
and miR-486-5p are down-regulated in cumulus cells of metaphase II
oocytes from women with polycystic ovary syndrome. Reprod Biomed
Online. 31:565–572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang BZ, Cui W and Li J: Effects of
electroacupuncture intervention on changes of quality of ovum and
pregnancy out- come in patients with polycystic ovarian syndrome.
Zhen Ci Yan Jiu. 40:151–156. 2015.(In Chinese). PubMed/NCBI
|
|
26
|
Coskun S, Otu HH, Awartani KA, Al-Alwan
LA, Al-Hassan S, Al-Mayman H, Kaya N and Inan MS: Gene expression
profiling of granulosa cells from PCOS patients following varying
doses of human chorionic gonadotropin. J Assist Reprod Genet.
30:341–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palin MF, Bordignon VV and Murphy BD:
Adiponectin and the control of female reproductive functions. Vitam
Horm. 90:239–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Persani L, Rossetti R, Di Pasquale E,
Cacciatore C and Fabre S: The fundamental role of bone
morphogenetic protein 15 in ovarian function and its involvement in
female fertility disorders. Hum Reprod Update. 20:869–883. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhai B, Liu H, Li X, Dai L, Gao Y, Li C,
Zhang L, Ding Y, Yu X and Zhang J: BMP15 prevents cumulus cell
apoptosis through CCL2 and FBN1 in porcine ovaries. Cell Physiol
Biochem. 32:264–278. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hussein TS, Froiland DA, Amato F, Thompson
JG and Gilchrist RB: Oocytes prevent cumulus cell apoptosis by
maintaining a morphogenic paracrine gradient of bone morphogenetic
proteins. J Cell Sci. 118:5257–5268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu XQ, Wang YQ, Xu SM, Liu JF, Bi XY, Wang
ZQ and Zhang JP: The WNT/β-catenin signaling pathway may be
involved in granulosa cell apoptosis from patients with PCOS in
North China. J Gynecol Obstet Biol Reprod (Paris). Oct
16–2015.(Epub ahead of print). PubMed/NCBI
|