Introduction
Acute pancreatitis (AP) is commonly observed in
clinics (1). Severe acute
pancreatitis (SAP) can cause necrosis of peri-pancreatic tissues,
injury and dysfunction of several organs, thus rapidly aggravating
the patient's condition. Due to the lack of effective and specific
treatment, SAP has an unfavorable prognosis and a high mortality
rate (2). SAP is frequently caused
by a secondary bacterial infection, and can cause injury to the
intestinal mucosal structure or functioning, thus disrupting the
intestinal barrier and resulting in systemic inflammatory response
syndrome (SIRS) and multiple organ dysfunction syndrome (MODS)
(3,4). During SAP, the mucosal barrier
becomes damaged, which increases the permeability of the intestinal
tract and allows the release of gut bacteria, thus resulting in
intestinal infection. Bacterial contamination can cause the body to
release large amounts of inflammatory mediators and cytokines,
causing endotoxemia and further early injuries related to MODS
(5). During the occurrence and
progression of AP, intestinal mucosal barrier injury is a critical
factor following intestinal injury; the inhibition of SAP-related
mucosal barrier injuries is a primary target for the prevention and
treatment of early MODS.
The innate immune system is the initial line of
defense against microbial invasions that cause intestinal mucosal
injury (6). During this process,
pattern recognition receptors (PRR) serve an important role in
defending against infection (7).
The extracellular PRR, Toll-like receptor (TLR) and the
intracellular PRR, nucleotide oligomerization domain (NOD)
receptor, are two important members of the innate immune system for
recognizing and fighting against microbial pathogens (8,9).
NOD-like receptors (NLRs) display highly conserved structures
(10) and participate in
recognition and defense against microbial pathogens. Furthermore,
they modulate the homeostasis of intestinal symbiotic microbes
(11), thus exhibiting a
bifunctional role. As one of the transmembrane PRRs involved in
pathogen recognition, TLRs serve important roles in signal
transduction, phagocytosis and cell apoptosis during acute
inflammation (12,13). A previous study correlated TLR4
expression with the pathogenesis of AP (14), however, TLR9 is indispensable for
inflammation, immunity and pathogen recognition (15,16).
The role of TLR9 in AP and its interaction with NOD, or other
related mechanisms, has not currently been elucidated. Therefore,
the present study aimed to investigate the role of the NOD receptor
and TLR9 in MODS-induced intestinal injury during early SAP. As NOD
receptor and TLR9 are both play an important role in microbial
pathogens recognition and inflammation, and the close association
of TLR9 with AP, the present study investigated the role of TLR9
and NOD receptor in rats with SAP through blocking TLR9 or
activating the NOD receptor.
Materials and methods
Experimental animals
A total of 40 healthy male Wistar rats (age, 2
months; body weight, 250±20 g) were purchased from Laboratory
Animal Unit of Chinese Medical Sciences University (Shenyang,
China) and were kept in a specific-pathogen-free grade facility.
The room temperature was maintained at 21±1°C and the relative
humidity was maintained at 50–70%. Animals were kept on a 12-h
light/dark cycle with free access to food and water. All procedures
were approved by the Animal Ethics Committee of China Meitan
General Hospital (Beijing, China).
Reagents and instruments
Sodium taurocholate, glutamate-meso-diaminopimelic
acid (DAP), muramic acid dipeptide (MDP) and chloroquine were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Polyvinylidene difluoride membrane was purchased from Pall Life
Sciences (Port Washington, NY, USA). Western blotting lysis buffer
was purchased from Beyotime Institute of Biotechnology (Haimen,
China). Enhanced Chemiluminescence (ECL) reagent was purchased from
Amersham (GE Healthcare Life Sciences, Little Chalfont, UK). Rabbit
anti-rat nuclear factor (NF)-κB monoclonal antibody (cat. no. 4764)
and horseradish peroxidase-labelled IgG secondary antibody (cat.
no. 7074) were purchased from Cell signaling Technology, Inc.
(Danvers, MA, USA). Tumor necrosis factor (TNF)-α (cat. no. RTA00)
and interleukin (IL)-1β (cat. no. RLB00) ELISA kits were purchased
from R&D Systems, Inc. (Minneapolis, MN, USA). The TRIzol
reagent for RNA extraction, reverse transcription (RT) cDNA first
chain synthesis kit and superoxide dismutase (SOD) assay kit were
purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). The surgical microscope was purchased from Suzhou Sunan
Zimmered Medical Instrument Co., Ltd. (Suzhou, China). The
microplate reader was purchased from BD Biosciences (Franklin
Lakes, NJ, USA). The Gene Amp PCR System 2400 DNA cycler was
purchased from PerkinElmer, Inc. (Waltham, MA, USA). Other common
reagents were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China).
Animal grouping and treatment
Wistar rats were randomly divided into four groups
(n=20/group). The four groups were: Control group, which received
an equal volume of saline inside the bile duct; SAP model group;
TLR inhibitor group, which received an intraperitoneal injection of
the TLR9 inhibitor chloroquine, following SAP model induction; and
NOD receptor activation group, which received an intraperitoneal
injection of the NOD receptor agonists MDP and DAP, following SAP
model induction.
Rat SAP model preparation
Animals were fasted for 12 h, and subsequently
anesthetized using 10% chloral hydrate via intraperitoneal
injection. The animals were secured onto the stage, and a median
incision was made in the upper abdomen to expose the duodenum and
biliary pancreatic duct, which was doubly clipped on the proximal
site of the hepatic portal vein using a non-invasive artery clip. A
retrograde puncture was made on the biliary pancreatic duct via the
duodenal papilla, followed by fixation using non-invasive artery
clips. Freshly prepared 5% sodium taurocholate solution was applied
into the biliary pancreatic duct at 0.1 ml/min velocity to reach an
internal concentration of 0.1 mg/100 g body weight. The duct was
then clipped for 5 min to completely immerse the pancreatic lobes
in sodium taurocholate solution. The artery clip was then released
to reperfuse the duodenum, followed by abdominal suture. In the
control group, an equal volume of saline was applied instead of
sodium taurocholate. The TLR9 inhibitor group was treated with an
intraperitoneal injection of 10 mM/kg TLR9 inhibitor chloroquine.
The NOD receptor activation group was treated with an
intraperitoneal injection of 10 mM/kg MDP and 10 mM/kg DAP. The
injections were administered immediately after the SAP
operation.
Sample collection
Rats were anaesthetized with ketamine-zylazine and
blood samples were collected from the abdominal aorta using vacuum
tubes at 12 h after SAP. Blood was incubated at room temperature
for 30 min, followed by 4°C centrifugation at 1,200 × g for 10 min
to collect the supernatant. Serum was frozen at −20°C for further
use. Injured intestinal tissues were collected from all groups and
stored at −80°C.
Serology indexes assay
An automatic biochemical analyzer (AU680, Beckman
Coulter) was used to test the serum amylase (AMY), creatinine (Cr)
and alanine aminotransferase (ALT) levels according to
manufacturer's protocol.
ELISA test for serum levels of
inflammatory factors TNF-α and IL-1β
Serum samples were tested for inflammatory factors
including TNF-α and IL-1β levels using ELISA kits, following the
manufacturer's protocol. In brief, 50 µl serially diluted standards
were added into a 96-well plate, and test samples (50 µl) were
applied in triplicates and incubated for 2 h. Following gentle
washing (5 times) with washing buffer and a 30 sec vortex, 50 µl
enzyme labeling reagent was added into each well and incubated at
37°C for 30 min. Following a further 5 washes, chromogenic
substrates A and B (50 µl each) were added and developed in the
dark at 37°C for 10 min. The reaction was quenched with 50 µl
stopping buffer. A microplate reader was used to measure absorbance
values at 450 nm. A standard curve was plotted based on the
standard concentrations and respective optical density (OD) values,
followed by calculation of the sample concentrations using the
sample OD values.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) for TLR9, NOD1 and NOD2 mRNA
expression in SAP intestines
Intestinal tissues were collected and rinsed in PBS.
Tissues were homogenized in liquid nitrogen and total RNA was
extracted using TRIzol reagent. cDNA was synthesized using cDNA
first chain synthesis kit. A fluorescent qPCR kit (Verso 1-Step
RT-qPCR SYBR Green kit; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) was used to collect data and determine the Cq value, with
reference to GAPDH. PCR amplification was performed in a total
volume of 20 µl, including 10 µl SYBR Green qPCR Super Mix, 0.5 µl
forward primer (10 µM), 0.5 µl reverse primer (10 µM), 5 µl cDNA
and 4 µl sterile water under the following conditions: 52°C for 1
min, followed by 35 cycles of 90°C denaturation for 30 sec, 58°C
annealing for 50 sec and 72°C elongation for 35 sec. Primers are
presented in Table I. The relative
expression level was determined using the 2− DDCq method
(17).
 | Table I.Primer sequences used for quantitative
polymerase chain reaction. |
Table I.
Primer sequences used for quantitative
polymerase chain reaction.
| Target gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| GADPH |
AGTGCCAGCCTCGTCTCATAG |
ACTTGCAACTTGCCGTGGGTAG |
| TLR9 |
CTCATCTAAGCGGAACAATGG |
GCACATTCTCTCCGTAGCG |
| NOD1 |
TAAGCATCTAAGGAACGGAATG | ACATTCTCTTCATCTA |
| NOD2 | TCATAGCCTCCATCT | ACTTGCACTTGCGGG |
Western blotting for NF-κB protein
expression
Total proteins were extracted from intestinal
tissues after homogenization on liquid nitrogen, mixed with lysis
buffer (Beyotime Institute of Biotechnology) for 15–30 min and
incubated on ice. Using ultrasonic rupture (5 sec, 4 times) and
centrifugation (10,000 × g for 15 min) at 4°C, proteins were
collected and stored at −20°C for subsequent western blotting.
Proteins (20 mg/lane) were separated by 10% SDS-PAGE, and were
transferred to PVDF membranes using the semi-dry method.
Non-specific binding sites were blocked by 5% non-fat milk powder
for 2 h. The membrane was incubated with anti-NK-κB monoclonal
antibody (1:1,000) at 4°C overnight. Goat anti-rabbit IgG (1:2,000)
was subsequently added for 30 min at room temperature. Following
0.1% (v/v) PBS-Tween washing and ECL development for 1 min, the
membrane was exposed to X-ray film. An image analyzing system
(ImageQuant LAS 500, GE Healthcare Life Sciences) and Quantity One
software version 4.3.0 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used to scan the X-ray films and to detect the density of
bands, from repeated experiments (n=4).
SOD activity assay
SOD activity was tested in intestinal tissues using
a SOD activity assay kit, according to the manufacturer's protocol.
In brief, tissue homogenate samples prepared as aforementioned were
denatured at 95°C for 40 min, and centrifuged at 1,500 × g for 10
min at 4°C. An ethanol-chloroform mixture (5:3, v/v) was used to
extract the ethanol phase in the homogenate, to determine the total
SOD activity.
Reactive oxygen species (ROS) content
assay
Intestinal tissue homogenates were denatured at 95°C
for 40 min, cooled in tap water, and centrifuged at 1,500 × g for
10 min at 4°C. Homogenates were incubated at 37°C in
2′,7′-dichlorofluorescein diacetate for 15 min. Following
centrifugation at 4,000 × g for 15 min at room temperature, the
precipitates were re-suspended in sterilized PBS buffer, and
incubated at 37°C for 60 min. Spectrometry was used to detect the
ROS levels at a wavelength of 520 nm, and data were expressed as a
ROS production percentage.
Statistical analysis
SPSS v16.0 software (SPSS, Inc., Chicago, IL, USA)
was used to analyze all data. Measurements were expressed as the
mean ± standard deviation. One-way analysis of variance with
Newman-Keuls multiple comparison post-hoc analysis was used to
compare the means across groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
TLR9 expression in rat intestinal
tissues
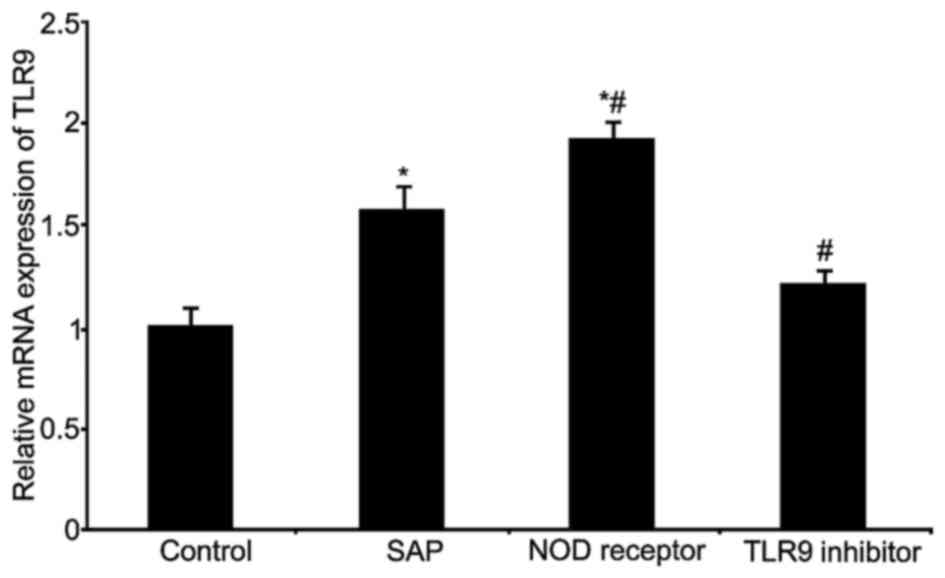
RT-qPCR was used to measure TLR9 mRNA expression
levels in rat intestinal tissues from all treatment groups
(Fig. 1). The results indicated
significantly elevated levels of TLR9 mRNA in the SAP and NOD
receptor activation groups (P<0.05, compared with the control
group); the NOD receptor activation group exhibited the greatest
increase of TLR9 mRNA (P<0.05, compared with the SAP group). The
TLR9 inhibitor group significantly inhibited TLR9 mRNA expression
(P<0.05, compared with the SAP group).
NOD1 and NOD2 expression change in rat
intestinal tissues
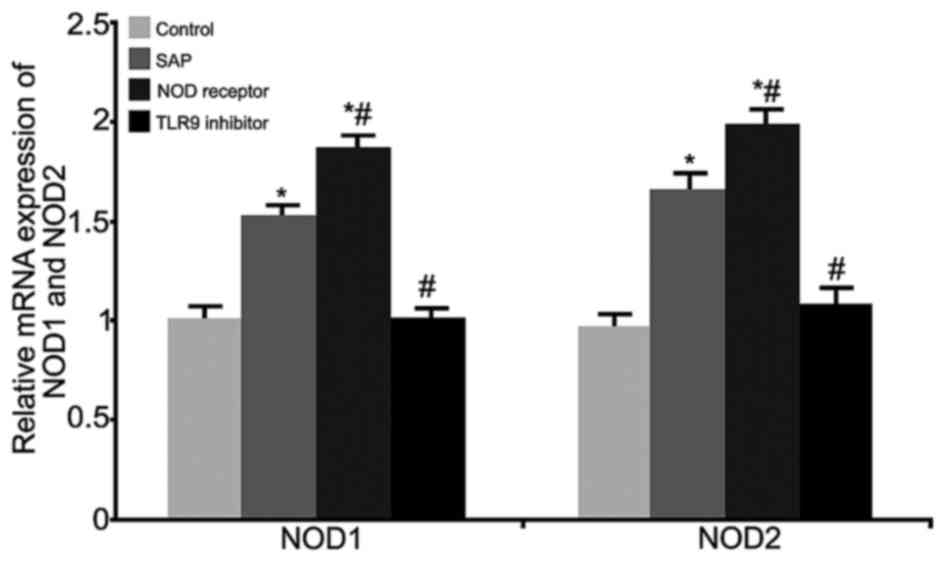
RT-qPCR was performed to measure the mRNA levels of
NOD1 and NOD2 in rat intestinal tissues from all treatment groups
(Fig. 2). The results demonstrated
significantly elevated NOD1 and NOD2 mRNA expression levels in the
SAP model and NOD receptor activation groups (P<0.05, compared
with the control group); the NOD receptor activation group
exhibited a stronger increase of NOD1 and NOD2 mRNA (P<0.05,
compared with the SAP group). The TLR9 inhibitor group
significantly inhibited NOD1 and NOD2 mRNA expression (P<0.05,
compared with the SAP group). These results indicated an
inter-regulation between TLR9 and NOD in SAP-induced intestinal
injury.
Serology index analysis
Serology indices were measured 12 h following SAP in
all treatment groups (Table II).
The results indicated significantly elevated AMY, Cr and ALT in the
SAP and NOD receptor activation groups (P<0.05, compared with
the control group); the NOD receptor activation group exhibited the
greatest increase of the measured indices (P<0.05, compared with
the SAP group). Treatment with the TLR9 inhibitor significantly
inhibited the elevation of these serology indices (P<0.05,
compared with the SAP group), however, the measured levels were
higher than the control group. These results demonstrated that
modulation of the NOD receptor and TLR9 may improve the serology
indices in the early phase of MODS related with SAP.
 | Table II.Serology indices of SAP rats. |
Table II.
Serology indices of SAP rats.
| Index | Control | SAP | NOD receptor | TLR9 inhibitor |
|---|
| AMY(U/l) |
1,520±216 |
6,659±232a |
7,617±378a,b |
3,159±345a,b |
| ALT (U/l) |
118±13.2 |
342±31.2a |
451±12.1a,b |
186±22.4a,b |
| Cr (U/l) |
32±2.1 |
97±3.6a |
121±6.6a,b |
51±4.3a,b |
Effects of TLR9 inhibition and NOD
receptor activation on the levels of serum inflammatory factors
TNF-α and IL-1β
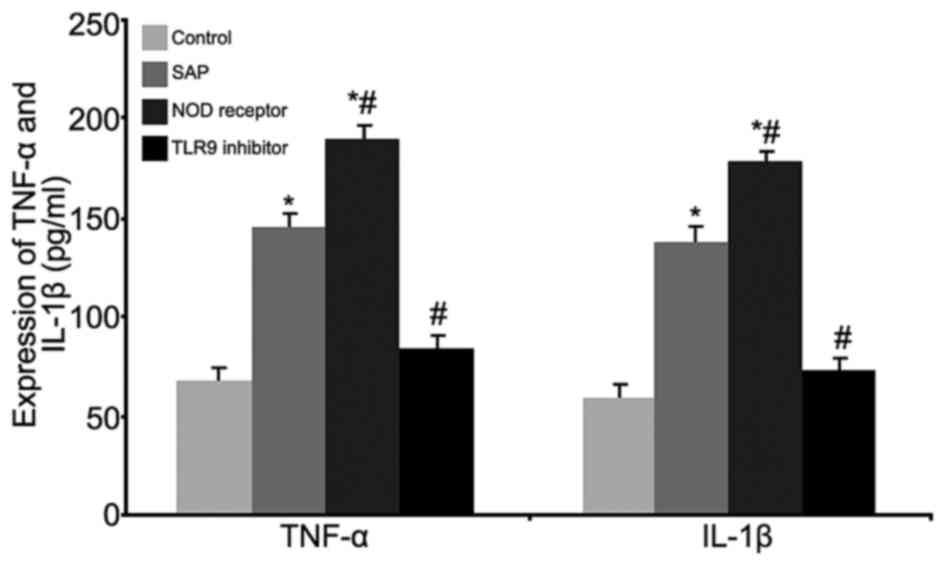
ELISA tests were used to investigate the effects of
TLR9 inhibition and NOD receptor activation on the serum levels of
the inflammatory factors TNF-α and IL-1β (Fig. 3). The results indicated
significantly elevated levels of the serum inflammatory factors
TNF-α and IL-1β in the SAP and NOD receptor activation groups
(P<0.05, compared with the control group), and the NOD receptor
activation group exhibited the greatest increase of these factors
(P<0.05, compared with the SAP group). The TLR9 inhibitor group
significantly inhibited secretion of these inflammatory factors
(P<0.05, compared with the SAP group). These results
demonstrated that modulation of the NOD receptor and TLR9 may
ameliorate SAP-induced intestinal injury by altering the secretion
of serum inflammatory factors.
Effects of TLR9 inhibition and NOD
receptor activation on the intestinal expression of NF-κB
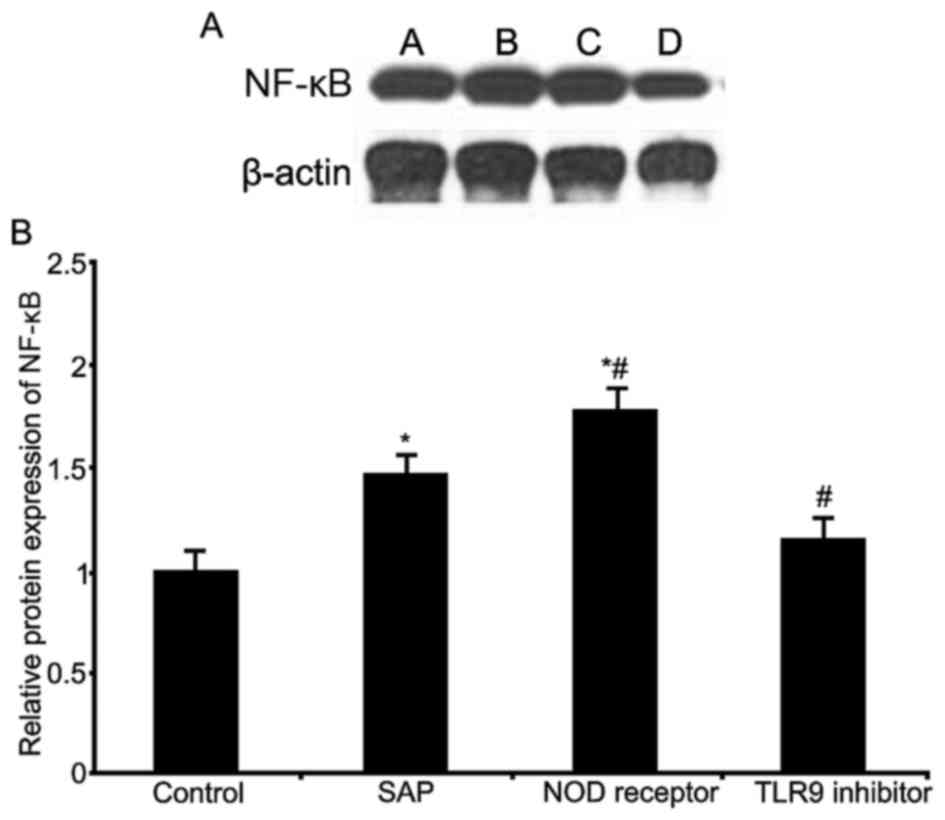
Western blot analysis was used to investigate the
effect of TLR9 inhibition and NOD receptor activation on intestinal
NF-κB expression (Fig. 4). The
results indicated significantly elevated NF-κB expression in the
SAP and NOD receptor activation groups (P<0.05, compared with
the control group); the NOD receptor activation group exhibited a
stronger increase of NF-κB expression (P<0.05, compared with the
SAP group). The TLR9 inhibitor group significantly inhibited NF-κB
expression levels (P<0.05, compared with the SAP group). These
results indicated that the NOD receptors and TLR9 may modulate
SAP-induced intestinal injury, via the regulation of intestinal
NF-κB expression.
Effects of TLR9 inhibition and NOD
receptor activation on oxidative stress
ROS levels and total SOD activity were measured in
rat intestines following TLR9 inhibition and NOD receptor
activation (Table III). The
results indicated significantly elevated ROS production and reduced
SOD activity in the SAP and NOD receptor activation groups
(P<0.05, compared with the control group), and the NOD receptor
activation group exhibited the greatest change in ROS and SOD
levels (P<0.05, compared with the SAP group). The TLR9 inhibitor
group significantly inhibited ROS production and elevated SOD
activity (P<0.05, compared with the SAP group). These results
indicated that the NOD receptors and TLR9 may modulate SAP-induced
intestinal injury via alteration of the oxidation/antioxidation
balance.
 | Table III.Effects of TLR9 and NOD receptor on
oxidative stress indices of pancreatic tissues. |
Table III.
Effects of TLR9 and NOD receptor on
oxidative stress indices of pancreatic tissues.
| Parameter (relative
value) | Control | SAP | NOD receptor | TLR9 inhibitor |
|---|
| ROS |
56±14 |
259±31a |
289±67a,b |
162±42a–c |
| SOD |
137±23 |
85±12a |
58±6a,b |
117±21a–c |
Discussion
TLRs can recognize molecular markers from a wide
range of pathogens. There are currently 11 known members of the TLR
family with unique ligands. The endotoxin lipopolysaccharide from
gram-negative bacteria can be recognized by TLR3, however, the
major ligand of TLR9 is CpG-DNA (18,19).
Upon activation, TLR9 transduces signals via Toll-interleukin
receptor structural domains to activate NF-κB, thereby regulating
gene transcription, inducing the release of inflammatory factors,
such as TNF-α and IL-1β, and leading to an increased inflammatory
response (20). The NOD receptor
family serves a similar function to the TLR family. Amongst these,
NOD1 and NOD2 are associated with the induction of inflammation,
with DAP and MDP as their ligands, respectively (21,22).
A recent study has revealed a correlation between NOD receptors and
TLR or pancreatitis (23). The
role and mechanism of the NOD receptor and TLR9 in SAP-induced
intestinal injury, however, has not been elucidated. The present
study established a SAP rat model, following by treatments with
either NOD ligand agonists or a TLR9 inhibitor in order to
investigate the impact of their activity modulation on serological
and inflammatory factors. TLR9, NOD1 and NOD2 expression in the SAP
and NOD receptor activation groups were significantly elevated,
with the greatest effect observed in the NOD receptor activation
group. The TLR9 inhibitor group exhibited decreased TLR9, NOD1 and
NOD2 expression. These results suggested that TLR9 and NOD may have
inter-regulatory effects on intestinal injury during SAP.
SAP commonly occurs in early MODS and late
infectious necrosis (24), and the
pathogenesis can facilitate the abundant release of inflammatory
factors by lymphocytes, neutrophils and macrophages. The
upregulation of anti-inflammatory factors further interferes with
the pro-inflammatory/anti-inflammatory balance, eventually causing
mortality as a result of SIRS and multiple organ failure (25). Therefore, a core explanation for
SAP-related intestinal injury is induction of the inflammatory
response. Furthermore, SAP can damage the liver, resulting in the
release of enzymes synthesized by liver cells into the hepatic
portal vein, from which they are distributed to the tissues and
organs via the circulation, causing elevated serum AMY, Cr and ALT,
and aggravating intestinal injury (26). The present study demonstrated that
TLR9 and NOD receptor modulation may modify the inflammatory
response via alterations to the serum inflammatory factor release
in SAP. Regulation of these receptors may improve the serology
index in early MODS of SAP, and potentially limit SAP pathogenesis
and SAP-related intestinal injury.
The present study also investigated related
inflammatory mechanisms, and demonstrated elevated ROS and
decreased SOD levels during SAP pathogenesis. Under normal
functioning of the cellular antioxidant system, ROS is continuously
cleared, thus preventing and alleviating tissue injury. SOD is an
important antioxidant enzyme involved in the clearance of free
oxygen radicals, and serves an important role in maintaining the
oxidation and antioxidation balance (27). Modulation of TLR9 and NOD receptor
activity may impact upon the oxidation/antioxidation balance, as
SOD upregulation will accelerate ROS clearance; therefore,
regulation of TLR9 and NOD may potentially decrease SAP-related
intestinal tissue injury. TLR and NOD receptor function as
important innate immune receptors, and can recruit innate immune
cells under pathogenic invasion, thus participating in the immune
response. NF-κB, as a target gene for facilitating expression and
transcription, is a critical mediator (28). The present study demonstrated
significantly elevated NF-κB expression in rat intestinal tissues
in SAP and NOD receptor groups, whilst TLR9 inhibition
significantly depressed the intestinal expression of NF-κB,
suggesting that TLR9 and NOD may modulate SAP-related intestinal
injury by regulating NF-κB expression in SAP.
In conclusion, the NOD1 and NOD2 receptors and TLR9
demonstrated an ability to regulate NF-κB expression and the
oxidation/antioxidation balance to modulate the inflammatory
response, which may affect SAP-related intestinal injury. The NOD
receptors and TLR9 may function synergistically to accomplish this
effect. The present study investigated the SAP-related intestinal
injury at a molecular level, thus providing a molecular mechanism
for the investigation of novel clinical treatments for SAP-related
intestinal injury.
References
|
1
|
Gooshe M, Abdolghaffari AH, Nikfar S,
Mahdaviani P and Abdollahi M: Antioxidant therapy in acute, chronic
and post-endoscopic retrograde cholangiopancreatography
pancreatitis: An updated systematic review and meta-analysis. World
J Gastroenterol. 21:9189–9208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang DL, Yang J, Jiang SY, Yuan FL, Gu
YL, Li JP and Pei ZJ: Modified Da Chengqi granules improvement in
immune function in early severe acute pancreatitis patients. Genet
Mol Res. 15:2016. View Article : Google Scholar
|
|
3
|
Zhu Y, Yin H, Zhang R, Ye X and Wei J:
Nasogastric nutrition versus nasojejunal nutrition in patients with
severe acute pancreatitis: A meta-analysis of randomized controlled
trials. Gastroenterol Res Pract. 2016:64306322016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herath HM and Kulatunga A: Acute
pancreatitis complicated with deep vein thrombosis and pulmonary
embolism: A case report. J Med Case Rep. 10:1822016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi T, Miura K, Ishikawa H, Soma D,
Zhang Z, Yuza K, Hirose Y, Takizawa K, Nagahashi M, Sakata J, et
al: Successful endoscopic management of acute necrotic pancreatitis
and walled off necrosis after auxiliary partial orthotopic
living-donor liver transplantation: A case report. Transplant Proc.
48:pp. 1212–1214. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gorsky VA, Agapov MA, Khoreva MV and
Leonenko IV: The effect of lornoxicam on TLR2 and TLR4 messenger
RNA expression and tumor necrosis factor-α, interleukin-6 and
interleukin-8 secretion in patients with systemic complications of
acute pancreatitis. Pancreas. 44:824–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matas-Cobos AM, Redondo-Cerezo E,
Alegría-Motte C, Martínez-Chamorro A, Saenz-López P, Jiménez P,
Jiménez MR, González-Calvín JL, de Teresa J and Osuna FR: The role
of Toll-like receptor polymorphisms in acute pancreatitis
occurrence and severity. Pancreas. 44:429–433. 2015.PubMed/NCBI
|
|
8
|
Takagi Y, Masamune A, Kume K, Satoh A,
Kikuta K, Watanabe T, Satoh K, Hirota M and Shimosegawa T:
Microsatellite polymorphism in intron 2 of human Toll-like receptor
2 gene is associated with susceptibility to acute pancreatitis in
Japan. Hum Immunol. 70:200–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caruso R and Núñez G: Innate immunity: ER
stress recruits NOD1 and NOD2 for delivery of inflammation. Curr
Biol. 26:R508–R511. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He X, Wei Z, Wang J, Kou J, Liu W, Fu Y
and Yang Z: Alpinetin attenuates inflammatory responses by
suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute
colitis. Sci Rep. 6:283702016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou Y, Lei W, He Z and Li Z: The role of
NOD1 and NOD2 in host defense against chlamydial infection. FEMS
Microbiol Lett. 363:pii: fnw1702016. View Article : Google Scholar
|
|
12
|
Paria A, Deepika A, Sreedharan K, Makesh
M, Chaudhari A, Purushothaman CS, Thirunavukkarasu AR and Rajendran
KV: Identification of Nod like receptor C3 (NLRC3) in Asian
seabass, lates calcarifer: Characterisation, ontogeny and
expression analysis after experimental infection and ligand
stimulation. Fish Shellfish Immunol. 55:602–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albayrak S, Zengin K, Tanik S, Atar M,
Unal SH, Imamoglu MA and Gurdal M: Can the neutrophil-to-lymphocyte
ratio be used to predict recurrence and progression of
non-muscle-invasive bladder cancer? Kaohsiung J Med Sci.
32:327–333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bobbala D, Orkhis S, Kandhi R, Ramanathan
S and Ilangumaran S: Interleukin-21-dependent modulation of T cell
antigen receptor reactivity towards low affinity peptide ligands in
autoreactive CD8(+) T lymphocytes. Cytokine. 85:83–91. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patin EC, Jones AV, Thompson A, Clement M,
Liao CT, Griffiths JS, Wallace LE, Bryant CE, Lang R, Rosenstiel P,
et al: IL-27 induced by select candida spp. via TLR7/NOD2 signaling
and IFN-β production inhibits fungal clearance. J Immunol.
197:208–221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang LL, Zhang DM, Ma CH, Zhang JH, Jia
KK, Liu JH, Wang R and Kong LD: Cinnamaldehyde and allopurinol
reduce fructose-induced cardiac inflammation and fibrosis by
attenuating CD36-mediated TLR4/6-IRAK4/1 signaling to suppress
NLRP3 inflammasome activation. Sci Rep. 6:274602016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suppiah A, Malde D, Arab T, Hamed M,
Allgar V, Smith AM and Morris-Stiff G: The prognostic value of the
neutrophil-lymphocyte ratio (NLR) in acute pancreatitis:
Identification of an optimal NLR. J Gastrointest Surg. 17:675–681.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cen Y, Liu C, Li X, Yan Z, Kuang M, Su Y,
Pan X, Qin R, Liu X, Zheng J and Zhou H: Artesunate ameliorates
severe acute pancreatitis (SAP) in rats by inhibiting expression of
pro-inflammatory cytokines and Toll-like receptor 4. Int
Immunopharmacol. 38:252–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong K: Curcumin mediates a protective
effect via TLR-4/NF-κB signaling pathway in rat model of
severe acute pancreatitis. Cell Biochem Biophys. 73:175–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou PF, Chang MX, Li Y, Xue NN, Li JH,
Chen SN and Nie P: NOD2 in zebrafish functions in antibacterial and
also antiviral responses via NF-κB and also MDA5, RIG-I
and MAVS. Fish Shellfish Immunol. 55:173–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khare S, Radian AD, Dorfleutner A and
Stehlik C: Measuring NLR oligomerization I: Size exclusion
chromatography, co-immunoprecipitation and cross-linking. Methods
Mol Biol. 1417:131–143. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vaz J and Andersson R: Intervention on
toll-like receptors in pancreatic cancer. World J Gastroenterol.
20:5808–5817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al Mofleh IA: Severe acute pancreatitis:
Pathogenetic aspects and prognostic factors. World J Gastroenterol.
14:675–684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jaffer U, Wade RG and Gourlay T: Cytokines
in the systemic inflammatory response syndrome: A review. HSR Proc
Intensive Care Cardiovasc Anesth. 2:pp. 161–175. 2010; PubMed/NCBI
|
|
26
|
El-Sayedel SM, Mansour AM and Nady ME:
Protective effects of pterostilbene against acetaminophen-induced
hepatotoxicity in rats. J Biochem Mol Toxicol. 29:35–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Ding XQ, Gu TT, Song L, Li JM, Xue
QC and Kong LD: Pterostilbene and allopurinol reduce
fructose-induced podocyte oxidative stress and inflammation via
microRNA-377. Free Radic Biol Med. 83:214–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang C, Sun H, Song Y, Ma Z, Zhang G, Gu X
and Zhao L: Pterostilbene attenuates inflammation in rat heart
subjected to ischemia-reperfusion: Role of TLR4/NF-κB
signaling pathway. Int J Clin Exp Med. 8:1737–1746. 2015.PubMed/NCBI
|