Introduction
Cervical cancer is the second most commonly
diagnosed cancer and third leading cause of cancer death among
females in developing countries, accounting for an estimated 3.7%
(527,600) new cancer cases and 3.2% (265,700) deaths worldwide in
2012 (1). Patients with squamous
cell carcinoma of cervical cancer (CSCC) who have locally advanced
and recurrent and/or metastatic disease are mostly treated with
concurrent chemoradiotherapy (CRT) (2–4).
Cisplatin-paclitaxel combination is still the standard regimen in
CRT compared with other cisplatin-based chemotherapy (5,6).
However, the widespread adoption of platinum-based
chemoradiotherapy protocols for CSCC patients would make these
drugs less effective when acquired resistance occurs.
Fructose-1,6-bisphosphatase-1 (FBP1) is a
gluconeogenesis rate-limiting enzyme used to catalyzes the
hydrolysis of fructose-1,6-bisphosphate to fructose-6-phosphate and
inorganic phosphate. There are two isoenzymes of FBP in mammalian
cells, FBP1 and FBP2. FBP1 is widely expressed in different tissues
while the expression of FBP2 is limited to muscle (7,8).
FBP1 deficiency is associated with hypoglycemia and metabolic
acidosis. Interestingly, recent studies have shown that decreased
level of FBP1 expression was observed in several solid tumors such
as liver, colon, gastric and renal tumors, and was associated with
poor prognosis in patients (8–10).
Promoter hypermethylation may probably contribute to this
phenomenon even though the exact mechanism is still unclear
(11). In addition, FBP1 appears
to be a functional tumor suppressor involved in the carcinogenesis
through inhibiting a potential ‘Warburg effect’ that cancer cells
have a higher rate of aerobic glycolysis compared with oxidative
phosphorylation (12,13).
However, the role of FBP1 in regulating the
carcinogenesis and chemoresistance in cervical cancer has not been
well validated. In the present study, we detected the expression of
FBP1 in CSCC patients, and investigated the associations between
FBP1 expression and the carcinogenesis as well as chemosensitivity
in cervical cancer cell lines.
Materials and methods
Compliance with ethical standards
All procedures performed in this study involving
human participants were in accordance with the ethical standards of
the Institutional Review Board of FUSCC and with the 1964 Helsinki
declaration and its later amendments or comparable ethical
standards.
Tissue samples and cell lines
We recruited 140 consecutive CSCC patients with
International Federation of Gynecology and Obstetrics (FIGO, 2009)
stages IB, IIA or IIB, who had radical hysterectomy and pelvic
lymphadenectomy with histopathologic confirmed high-risk factors.
All patients were treated with adjuvant concurrent chemoradiation
therapy at Fudan University Shanghai Cancer Center (FUSCC) between
March 2008 and March 2009. The cases were histopathologically
confirmed to be squamous cell carcinoma by two gynecologic
pathologists. Detailed clinical information has been described
elsewhere (14). Patients were
followed every 3 months for the first 2 years, every 6 months for
the next 2 years, and annually for the following years thereafter.
Disease-free survival (DFS) and overall survival (OS) duration were
calculated from the date of first surgery to the date of disease
recurrence and death or the last follow-up visit, respectively.
Patients without progression, lost to follow-up or died from other
causes, were censored at their last date of record. The project was
approved by the Institutional Review Board of FUSCC. Written
informed consents were obtained from all recruited individuals, and
each clinical investigation was conducted according to the
principles expressed in the Declaration of Helsinki
consent.
The established human cervical cancer cell lines
HeLa and CaSki were obtained from American Type Culture Collection
(ATCC). All cells were maintained in Dulbecco's modified Eagle's
medium (DMEM; Hyclone; Thermo Scientific, Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco Life Technologies,
Grand Island, NY, USA), 100 U/ml penicillin, and 100 U/ml
streptomycin (both from Biowest, Nuaillé, France) and incubated at
37°C in a humidified atmosphere with 5% CO2.
Immunohistochemistry (IHC) assay
The 10×12 (120 cores) tissue microarray (TMA) was
made by FUSCC Tissue Bank, as described previously (15). Each case has two cores made from
separate sources. IHC was performed on 5-µm-thick TMA sections from
formalin-fixed, paraffin-embedded tissue using the antibody against
FBP1 (ab196556, mouse polyclonal antibody, 1:200 dilution; Abcam,
Cambridge, MA, USA). The positive control is a known positive case
sample. And non-immune goat serum takes the place of primary
antibody serving as the negative control. The IHC staining results
were scored independently by two gynecologic pathologists, who were
blinded to clinical information of the patients. Scoring system is
on the basis of both percentage of positive tumor cells and
staining intensity, as described previously (16). Briefly, staining intensity was
graded as 0 (none), 1+ (weak), 2+ (intermediate) and 3+ (strong),
and percentage of positive tumor cells was graded as 1+ (<10% of
the cells), 2+ (10–50% of the cells) or 3+ (≥50% of the cells). The
combination of intensity and distribution was graded as 0 (<1+),
1 (1+ to 2+), 2 (>2+ to 4+) or 3 (>4+ to 6+). Finally, the
assessment of the protein expression was defined as negative (grade
0 or 1) and positive (grade 2 or 3).
RT-PCR and real-time PCR
Total RNA was isolated from fresh frozen tumor
tissues and normal tissues of 20 CSCC patients using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and reversely
transcribed into cDNA using PrimeScript™ RT Reagent kit (Takara
Bio, Inc., Otsu, Japan). PCR products were amplified with Takara
Taq™ with reactions of 30 cycles of (94°C, 30 sec; 58°C, 30 sec and
72°C, 1 min) using the Master Cycler 1 Eprealplex (Eppendorf AG,
Hamburg, Germany). PCR products (5 µl) were analyzed by
electrophoresis on 1.5% agarose gel containing ethidium bromide and
visualized under UV illumination. Real-time PCR was carried out in
the Applied Biosystems Prism 7900 system (Applied Biosystems, Life
Technologies, Foster City, CA, USA) using ExScipt SYBER-Green QPCR
kit (Takara Bio, Inc.) in the following conditions: An initial
denaturation of 95°C for 30 sec, 1 cycle; 95°C for 5 sec; 55°C for
30 sec; and 72°C for 30 sec, 40 cycles followed by a melting curve
analysis to check the specificity of amplification. Each sample was
tested in triplicate, and primers to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) were used in parallel reactions as internal
control. Three independent experiments were done for final analyses
using the 2−ΔΔCT relative quantification method. The
primer pairs of FBP1 used were CTA CGC CAG GGA CTT TGA CC and GGC
CCC ATA AGG AGC TGA AT. The primer pairs of GAPDH were
5′-GGCCTCCAAGGAGTAAGACC-3′ (forward primer) and
5′-CAAGGGGTCTACATGGCAAC-3′ (reverse primer).
Plasmid construction and cell
transfection
To selectively overexpress FBP1, the plasmid
pENTER-FBP1, containing human full cDNA sequence of FBP1, was
purchased from Vigene Biosciences (Jinan, China). The recombinant
plasmid PCDH/FBP1 cDNA was generated by subcloning the cDNA
sequence of FBP1 into lentivirus vector PCDHCMV- MCS-EF1-PURO. Both
of HeLa and CaSki cells were transfected with the PCDH/FBP1 cDNA or
the PCDH/negative control for a total of 4 days (2 days for each
infection) and the positive clones were selected with puromycin
(200 ng/ml) for 7–10 days.
Western blot analysis
Western blot analysis was performed to determine the
expression levels of FBP1 and glycolysis-related protein GLUT1,
GLUT4, LDHB in cervical cancer cell lines. Cells were harvested,
washed with cold 1X PBS, and lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) for 30 min on ice, then
centrifuged at 12,000 g for 15 min at 4°C. The total protein
concentration was determined by BCA Protein Assay kit (Beyotime
Institute of Biotechnology). Equal amounts (30 µg per load) of
protein samples were subjected to SDS-PAGE electrophoresis and
transferred on to polyvinylidene fluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). The blots were blocked in 8%
non-fat milk, and incubated with primary antibodies, followed by
incubation with secondary antibodies conjugated with horseradish
peroxidase (HRP). The protein bands were developed with the
chemiluminescent reagents (Millipore). Antibody to FBP1 was from
Abcam, GLUT1, GLUT4, LDHB were from ProteinTech Group, Inc.
(Chicago, IL, USA), and antibody to β-actin was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Colony formation assay
Cells were seeded in 6-well plates at a density of
500 per well. The fresh medium was added to allow cell growth for
at least one week. The colonies with more than 50 cells were
counted after staining with gentian violet (Beijing Solarbio
Science and Technology Co., Ltd., Beijing, China). Three
independent experiments were done in triplicate wells.
Cell proliferation assay
To evaluate cell proliferation rate, we plated
1×103 cells per well in 96-wells plates with 100 µl
maintenance medium. Cell Counting Kit-8 (CCK-8) (Dojindo
Laboratories, Kumamoto, Japan) was used to monitor cell growth at
0–7 day and the number of viable cells was assessed by measurement
of absorbance at 450 nm by a Microplate Reader (BioTek Instruments,
Inc., Winooski, VT, USA). The proliferation index was calculated as
experimental OD value/control OD value. Cell numbers were
calculated with the following equation: Cell number = proliferation
index × 1,000.
Cell viability assay
Cell viability was also evaluated by CCK-8. We
plated 8×103 cervical cancer cells per well in 96-well
plates. The next day, the cells were treated with various
concentrations of cisplatin purchased from Sigma-Aldrich. Cell
viability was then measured as described above. Three independent
experiments were done in triplicate wells.
We used the 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-diphenytetrazoliumromide
(MTT) assay to detect in vitro inhibition rates of four
platinum agents (cisplatin, carboplatin, nedaplatin and
oxaliplatin), as described previously (15). Optical densities (OD570
nm) were measured by Microplate Reader (Bio-Rad 550; Bio-Rad
Laboratories, Hercules, CA, USA). The inhibition rate = (1 -
ODplatinum/ODcontrol) × 100%.
Statistical methods
We performed the Pearson's χ2-test to
evaluate differences in the distributions of FBP1 expression with
clinical and pathological variables. Kruskal-Wallis test was used
to compare tumor inhibition rates among different groups.
Kaplan-Meier curve and multivariate Cox proportional hazards
regression analysis (including enter, back and forward Wald tests)
were conducted for survival estimate. All statistical analyses were
performed with SPSS version 19.0 (SPSS, Inc., Chicago, IL, USA).
Each reported P-value was two sided with a significance level of
P<0.05.
Results
Loss of FBP1 is a negative prognostic
factor in CSCC patients
Clinical and pathological characteristics of the 140
CSCC patients enrolled in the study are summarized in Table I (the status of HPV was not
included for the reason that most of the patients had not done HPV
tests before radical surgery). The median follow-up time was 34.6
months (range, 20.0–40.0 months). There were 24 (17.1%) recurrences
and 10 (7.1%) deaths. The median time to recurrence was 17.1
months. According to the scoring criteria, we observed 70 (50%)
patients with FBP1-positive (Fig.
1A) and 70 (50%) with FBP1-negative (Fig. 1B) expression, respectively.
Interestingly, FBP1 protein levels exhibited a prognostic value, as
OS (P=0.011) and DFS (P=0.026) time were markedly shortened in
patients whose tumors exhibited low FBP1 protein levels (Fig. 1C and D). By using log-rank test and
multivariate Cox proportional hazards regression model (including
enter, back and forward Wald tests), we evaluated potentially
prognostic factors in CSCC patients (Fig. 1). Univariate analysis showed that
patients with lympho-vascular space invasion (LVSI) (P=0.023;
Table IA) and low expression of
FBP1 (P=0.011; Table IA) had
shorter OS time. In multivariate analysis, there was no significant
associations in enter mode (Table
IA); however, both of back and forward Wald tests revealed that
low expression of FBP1 was significantly related to prognosis of
CSCC patients (Wald=4.470, HR=9.287, 95% CI=9.287 (1.177–73.312),
P=0.034; Table II). We further
explored the association of clinicopathological characteristics
with recurrence in CSCC patients and found that FBP1 expression
(χ2-test, P=0.025) was significantly associated with the
recurrent status of cervical cancer patients (Table IA). In addition, the expression
level of FBP1 had a negative correlation with tumor stage among
various prognostic factors of CSCC (χ2-test, P=0.000)
(Table III).
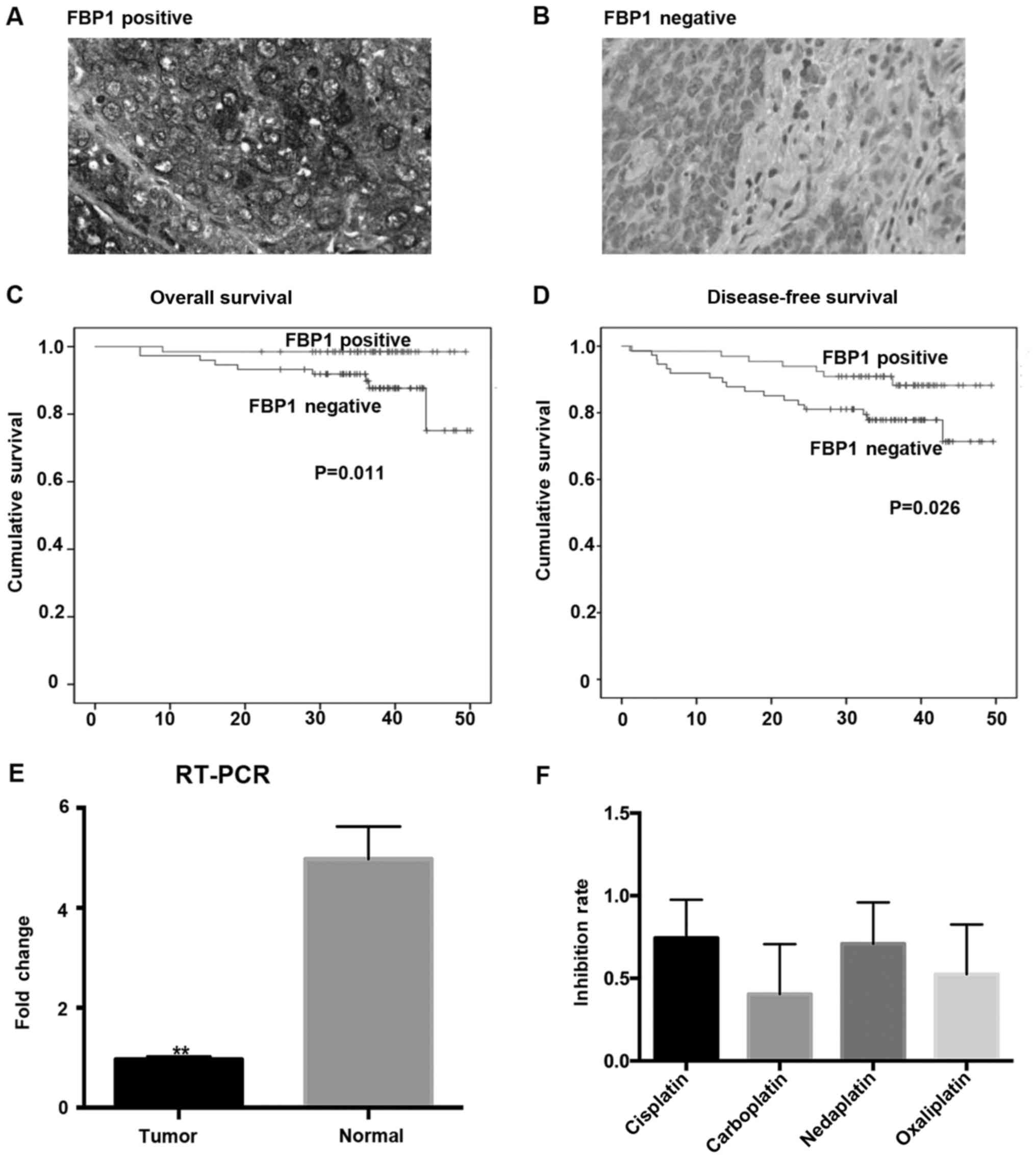 | Figure 1.Decreased level of FBP1 is a negative
prognostic factor in CSCC patients. (A) FBP1-positive expression
and (B) FBP1-negative expression of tumor tissues were detected by
immunohistochemistry (×400). Kaplan-Meier survival estimates showed
that patients with FBP1-negative expression had a shorter (C)
overall survival and (D) disease-free survival compared to those
with FBP1-positive expression (P=0.011 and P=0.026, respectively).
(E) FBP1 mRNA expression level is lower in human CSCC tumor tissues
than normal cervical tissues (n=20, P=0.0005). (F) Inhibition rates
of four platinum agents in CSCC patients. The median in
vitro inhibition rates by cisplatin, carboplatin, nedaplatin,
and oxaliplatin were 62, 47, 58, and 52% respectively. FBP1,
fructose-1,6-bisphosphatase-1; CSCC, squamous cell carcinoma of
cervical cancer; RT-PCR, reverse transcription-polymerase chain
reaction. |
 | Table I.Association of clinicopathological
characteristics with recurrence in CSCC patients. |
Table I.
Association of clinicopathological
characteristics with recurrence in CSCC patients.
| A, Association of
clinicopathological characteristics with recurrence in CSCC
patients (in situ) |
|---|
|
|---|
|
| Multivariate
(enter) |
|---|
|
|
|
|---|
| Prognostic
factors | All patients N
(%) | Non recurrence N
(%) | Recurrence N (%) | Univariate
P-valuea | HR (95%
CI)b | P-valueb |
|---|
| All patients | 140 (100) | 116 (82.9) | 24 (17.1) |
|
|
|
| Age, years |
|
|
| 0.370c |
|
|
| ≤46
(median) | 70 (50) | 60 (85.7) | 10 (14.3) |
| 1.000 |
|
| >46
(median) | 70 (50) | 56 (80) | 14 (20) | 0.935 | 0.943
(0.224–3.967) | 0.936 |
| FIGO stage |
|
|
| 0.106c |
|
|
| IB | 70 (50) | 61 (87.1) | 9 (12.9) |
| 1.000 |
|
|
IIA | 64 (45.7) | 52 (81.3) | 12 (18.8) |
| 1.000 |
|
|
IIB | 6 (4.3) | 3 (50) | 3 (50) | 0.496 | 0.353
(0.030–4.200) | 0.410 |
| Tumor
sized, cm |
|
|
| 0.575c |
|
|
| ≤4 | 83 (59.3) | 70 (84.3) | 13 (15.7) |
| 1.000 |
|
|
>4 | 57 (40.7) | 46 (80.7) | 11 (19.3) | 0.550 | 1.669
(0.427–6.524) | 0.461 |
| Pelvic LN |
|
|
| 0.595c |
|
|
|
Negative | 94 (67.1) | 79 (84) | 15 (16) |
| 1.000 |
|
|
Positive | 46 (32.9) | 37 (80.4) | 9 (19.6) | 0.227 | 0.415
(0.070–2.446) | 0.331 |
| LVSI |
|
|
| 0.058c |
|
|
|
Negative | 88 (62.9) | 77 (87.5) | 11 (12.5) |
| 1.000 |
|
|
Positive | 52 (37.1) | 39 (75) | 13 (25) | 0.023e | 6.979
(0.933–52.189) | 0.058 |
| Depth of cervical
stromal invasion |
|
|
| 0.309c |
|
|
|
≤2/3 | 83 (59.3) | 71 (85.5) | 12 (14.5) |
| 1.000 |
|
|
>2/3 | 57 (40.7) | 45 (78.9) | 12 (21.1) | 0.157 | 1.679
(0.321–8.777) | 0.539 |
| P16 expression
status |
|
|
| 0.585c |
|
|
|
Negative | 29 (20.7) | 23 (79.3) | 6 (20.7) |
| 1.000 |
|
|
Positive | 111 (79.3) | 93 (83.8) | 18 (16.2) | 0.932 | 1.077
(0.183–6.329) | 0.934 |
| FBP1 expression
status |
|
|
| 0.025c,e |
|
|
|
Negative | 70 (50) | 53 (75.7) | 17 (24.3) | 0.011e | 8.850
(0.957–81.834) | 0.055 |
|
Positive | 70 (50) | 63 (90) | 7 (10) |
| 1.000 |
|
|
| B, Association of
clinicopathological characteristics with recurrence in CSCC
patients (in vitro) |
|
|
| Multivariate
(enter) |
|
|
|
| Prognostic
factors | All patients N
(%) | Non recurrence N
(%) | Recurrence N
(%) | Univariate
P-valuea | HR (95%
CI)b |
P-valueb |
|
| All patients | 140 (100) | 116 (82.9) | 24 (17.1) |
|
|
|
| Cisplatin inhibiton
rates |
|
|
| 0.335c |
|
|
| ≤0.74
(median) | 53 (37.9) | 46 (86.8) | 7 (13.2) |
| 0.269
(0.052–1.398) |
|
|
>0.74 (median) | 87 (62.1) | 70 (80.5) | 17 (19.5) | 0.234 | 1.000 | 0.118 |
|
Carboplatin-inhibition rates |
|
|
| 0.758c |
|
|
| ≤0.40
(median) | 74 (52.9) | 62 (83.8) | 12 (16.2) |
| 0.282
(0.049–1.635) |
|
|
>0.40 (median) | 66 (47.1) | 54 (81.8) | 12 (18.2) | 0.675 | 1.000 | 0.158 |
|
Nedaplatin-inhibition rates |
|
|
| 0.613c |
|
|
|
≤0.71(median) | 59 (42.1) | 50 (84.7) | 9 (15.3) |
| 2.525
(0.420–15.178) |
|
|
>0.71(median) | 81 (57.9) | 66 (81.5) | 15 (18.5) |
| 1.000 | 0.311 |
|
Oxaliplatin-inhibition rates |
|
|
| 0.264c |
|
|
| ≤0.52
(median) | 67 (47.9) | 58 (86.6) | 9 (13.4) |
| 4.438
(0.716–27.509) |
|
|
>0.52 (median) | 73 (52.1) | 58 (79.5) | 15 (20.5) | 0.759 | 1.000 | 0.109 |
 | Table II.Cox proportional hazards regression
analysis of CSCC patients (back and forward Wald tests). |
Table II.
Cox proportional hazards regression
analysis of CSCC patients (back and forward Wald tests).
| Prognostic factors
(with significance) | B | SE | Wald | HR | 95% CI | P-value |
|---|
| FBP1 expression
status |
|
Negative | 2.229 | 1.054 | 4.470 | 9.287 | 1.177–73.312 | 0.034 |
|
Positive |
|
|
| 1.000 |
|
|
 | Table III.Various prognostic factors of
CSCC. |
Table III.
Various prognostic factors of
CSCC.
| Prognostic
factors | FBP1-negative
(%) | FBP1-positive
(%) |
P-valuea |
|---|
| Age, years |
|
| 0.176 |
| ≤46
(median) | 31 | 39 |
|
| >46
(median) | 39 | 31 |
|
| FIGO stage |
|
| 0.000c |
| IB | 25 | 45 |
|
|
IIA | 39 | 25 |
|
|
IIB | 6 | 0 |
|
| Tumor
sizeb, cm |
|
| 0.390 |
| ≤4 | 44 | 39 |
|
|
>4 | 26 | 31 |
|
| Pelvic LN |
|
| 0.150 |
|
Negative | 43 | 51 |
|
|
Positive | 27 | 19 |
|
| LVSI |
|
| 0.080 |
|
Negative | 39 | 49 |
|
|
Positive | 31 | 21 |
|
| Depth of cervical
stromal invasion |
|
| 0.390 |
|
≤2/3 | 39 | 44 |
|
|
>2/3 | 31 | 26 |
|
| Recurrence |
|
| 0.10 |
|
Local | 9 | 5 |
|
|
Distant | 3 | 7 |
|
| P16 expression
status |
|
| 1.000 |
|
Negative | 14 | 15 |
|
|
Positive | 56 | 55 |
|
FBP1 regulates carcinogenesis in
cervical cancer
As the loss of FBP1 is a critical oncogenic event in
many cancers (9,11,17),
we thus investigated the role of FBP1 in human CSCC. We first
tested the messenger RNA (mRNA) levels in 20 human CSCC tissues and
normal cervical tissues (clinical pathological features; Table IV). The result showed that mRNA
expression of FBP1 is lower in human CSCC tumor tissues than normal
cervical tissues (P=0.0005; Fig.
1E). To further confirm the role of FBP1 in cancer cell growth
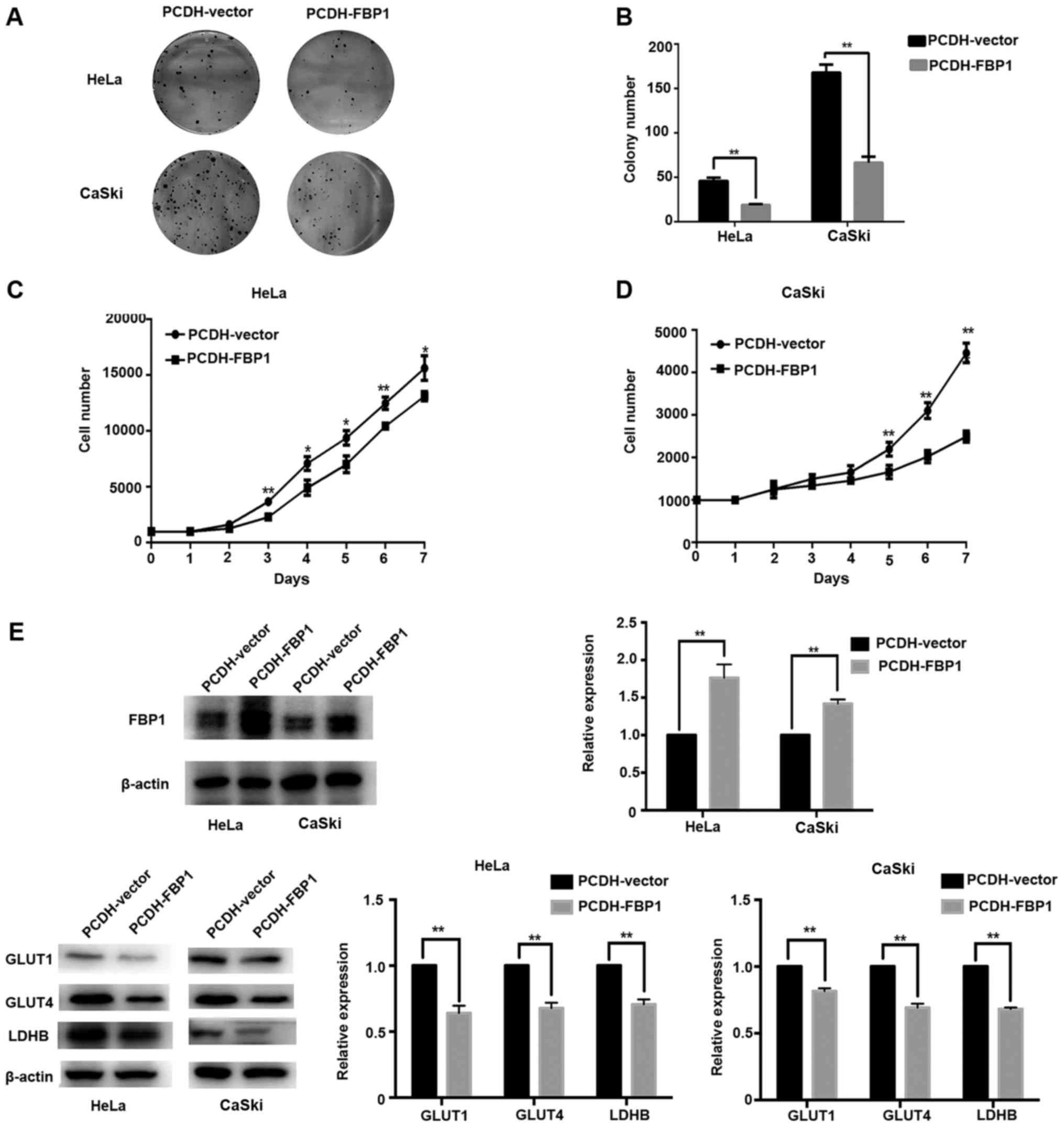
inhibition, we established HeLa/FBP1 and CaSki/FBP1 cells stably
expressing FBP1 cDNA and then performed cell colony formation assay
and proliferation assay. The results of CCK-8 and colony formation
assay exhibited that the ability of cervical cancer cell growth and
proliferation was obviously weakened by the induction of FBP1 when
compared with controls (P<0.05 or P<0.01; Fig. 2A-D).
 | Table IV.Clinical pathological features of 15
CSCC patients in reverse transcription-polymerase chain reaction
analysis. |
Table IV.
Clinical pathological features of 15
CSCC patients in reverse transcription-polymerase chain reaction
analysis.
| Prognostic
factors | Cases, N |
|---|
| Age (years) |
|
| ≤43
(median) | 10 |
| >43
(median) | 10 |
| FIGO stage |
| IB | 8 |
|
IIA | 8 |
|
IIB | 4 |
| Tumor size
(cm) |
| ≤4 | 14 |
|
>4 | 6 |
| Pelvic LN |
|
Negative | 15 |
|
Positive | 5 |
| LVSI |
|
Negative | 16 |
|
Positive | 4 |
| Depth of cervical
stromal invasion |
|
≤2/3 | 15 |
|
>2/3 | 5 |
| P16 expression
status |
|
Negative | 3 |
|
Positive | 17 |
Then, we detected the glycolysis-related protein
expression in HeLa and CaSki cells with or without FBP1 by western
blot analysis. The results exhibited that protein level of GLUT1,
GLUT4 and LDHB were downregulated compared with their controls
(P<0.01; Fig. 2E), indicating
FBP1 was involved in cancer metabolism.
Above all, FBP1 inhibited cancer growth and
proliferation in cervical cancer cells by regulating the expression
of glycolysis related protein such as GLUT1, GLUT4 and LDHB.
FBP1 overexpression restores
chemosensitivity of cervical cancer cells
The MTT assay aims to detect in vitro
inhibition rates of four platinum agents (cisplatin, carboplatin,
nedaplatin, and oxaliplatin), as described previously (12). The median in vitro
inhibition rate of tumor cell growth by cisplatin, carboplatin,
nedaplatin, and oxaliplatin was 62, 47, 58, and 52%, respectively.
Four platinum agents showed a significantly difference in
inhibiting CSCC cells (Kruskal-Wallis test, P<0.0001). Cisplatin
and nedaplatin group had larger effects on the inhibition of tumor
cells, and no significant difference in inhibition rates was
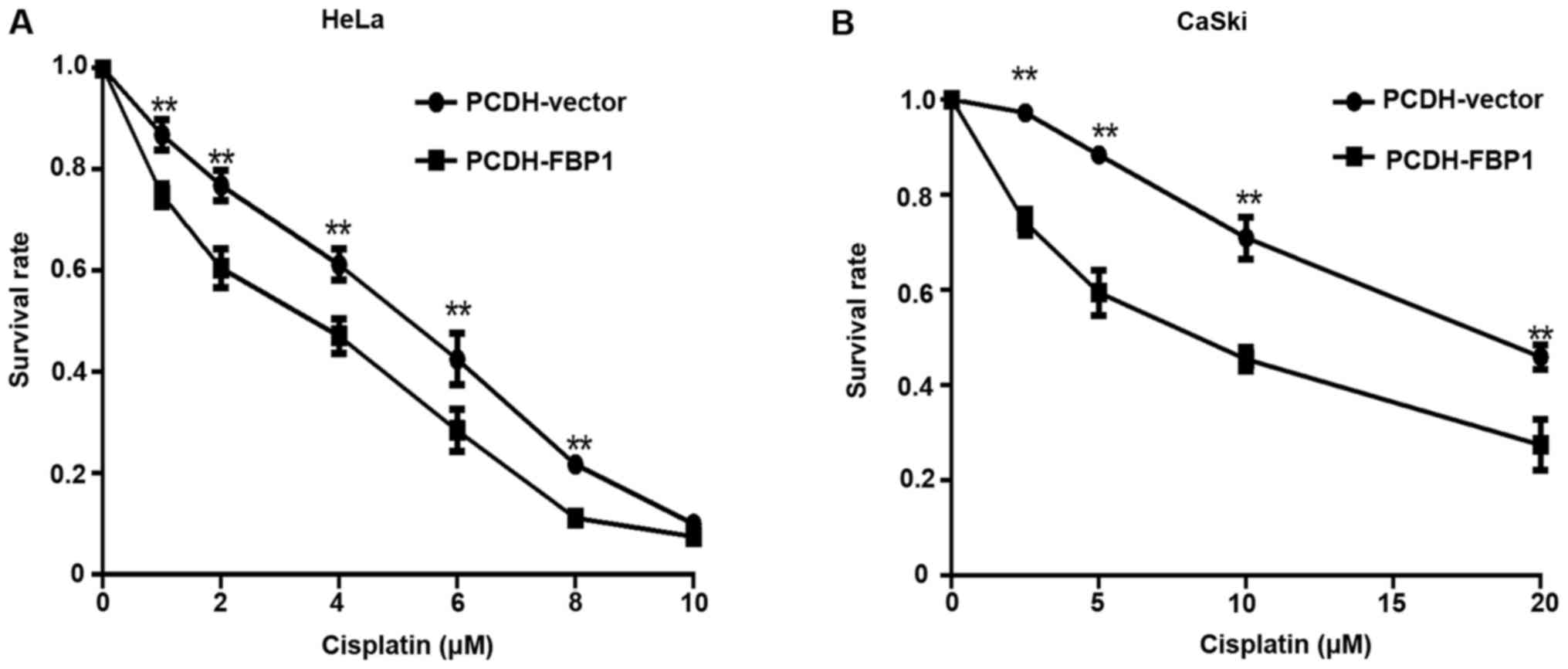
observed between cisplatin and nedaplatin group (Fig. 1F). To further validate the effect
of FBP1 on the chemosensitivity of cervical cancer cells, we
examined the inhibition rate of HeLa and CaSki cells after
cisplatin treatment when overexpressing FBP1 compared with negative
control. The results showed that overexpression of FBP1 restored
the cisplatin sensitivity of the cervical cancer cells (Fig. 3). However, by using the
Kruskal-Wallis test, the FBP1 protein expression level showed no
significant association with resistance to the four platinum agents
in vitro (Table V).
 | Table V.Association of FBP1 expression with
resistance to the four platinum agents in vitro. |
Table V.
Association of FBP1 expression with
resistance to the four platinum agents in vitro.
|
| In vitro tumor
inhibition rates (median ± SE) |
|
|---|
|
|
|
|
|---|
| Platium agents | FBP1-negative | FBP1-positive |
P-valuea |
|---|
| Cisplatin |
0.63±0.058 |
0.61±0.059 | 0.863 |
| Carboplatin |
0.49±0.060 |
0.46±0.060 | 0.737 |
| Nedaplatin |
0.57±0.059 |
0.59±0.059 | 0.865 |
| Oxaliplatin |
0.61±0.059 |
0.43±0.060 | 0.186 |
Discussion
Aerobic glycolysis, rather than oxidative
phosphorylation, often occurs in tumor cells, which is called
‘Warburg effect’. In this pathway, many metabolic intermediates
were produced serving as biosynthesis molecules which are necessary
for active cell proliferation. Moreover, the enhanced production of
lactic acid from tumor cells will lower the pH of the
microenvironment surrounding tumor cells, which can induce
apoptosis for normal cells but not for tumor cells (17). In this study, the role of FBP1
which functions to antagonize glycolysis (8) in tumor tissues from CSCC patients was
investigated. Our study revealed that the expression level of FBP1
was negatively correlated with the OS and DFS of cervical cancer
patients. In addition, we found that the tumor tissues collected
from CSCC patients expressed significantly lower level of FBP1 as
compared with the normal cervical tissues. Our data clearly
indicated that FBP1 appears to be functional tumor suppressor
involved in cervical carcinogenesis, which could be used as a
biomarker for CSCC. Fella et al (18) also investigated that FBPase protein
is very early downregulated and postulated it as an early
predictive biomarker for liver carcinogenicity.
Although the exact mechanisms of FBP1 down
regulation in carcinogenesis is unclear, studies of several solid
tumors, such as liver, colon, gastric as well as breast cancer
cells showed an increased methylation of FBP1 promoter (8,9,19).
Since epigenetic modification are known to contribute to the
multitude of metabolic changes in cancer cells (20), during carcinogenesis the genome
simultaneously undergoes genome-wide hypomethylation and regional
hypermethylation of CpG islands, which may be of selective
advantage for the incipient tumor cells (21). Inhibition of NF-κB restored FBP1
expression in gastric cancer cells, partially through demethylation
of FBP1 promoter (8). In breast
cancer, Snail-G9a-Dnmt1 complex, which is critical for E-cadherin
promoter silencing, is also required for the promoter methylation
of FBP1 in basal-like breast cancer (BLBC) (22). With promoter methylation,
downregulated expression of FBP1 confers to enhancing ‘Warburg
effect’ by increasing glucose uptake, lactate secretion and
glycolytic intermediates for biosynthesis in mitochondria of tumor
cells. And this glycolytic switch could help tumor cells, rather
than normal cells acquire more energy in the process of
carcinogenesis and progression. Besides, loss of FBP1 might promote
carcinogenesis in a catalytic activity independent way, by
decreasing interaction with hypoxia inducible factor (HIF) domain
directly to promote cell proliferation or by increasing the number
of cancer stem cells which contribute to tumor recurrence and
therapeutic resistance (10,17,19,22).
Our study showed that glycolysis-related protein expression was
significantly inhibited by enhancing FBP1 expression. And
overexpression of FBP1 could further inhibit the colony formation
and proliferation ability of cervical cancer cells. These results
indicated that FBP1 might inhibit the growth of cervical cancer
cells partially through suppressing glycolytic pathway.
Chemotherapy has been widely used in the treatment
of various cancers, including advanced or recurrent and/or
metastatic cervical cancer (2–4,23,24).
Platinum especially cisplatin is still the basic agent in the
chemotherapeutic regimen for CSCC patients (25,26).
However, frequent resistance to platinum agents in cervical cancer
cells obstructs the progress in satisfying treatment. In this
study, we detected in vitro inhibition rate of tumor cells
of four platinum agents including cisplatin, carboplatin,
nedaplatin, and oxaliplatin. We found the inhibition rates of
nedaplatin and cisplatin were much higher than that of carboplatin
and oxaliplatin, which explained why cisplatin drugs are most
commonly used in chemotherapy of CSCC patients (27). Although we did not detect the
statistical significant association of in vitro tumor
inhibition rates with FBP1 expression, FBP1-negative tumor cells
had a lower inhibition rates in most of the platinum treated
groups, compared with FBP1-negative tumor cells. Several studies
revealed that increased expression of glycolytic pathway associated
genes, such as HIF-1α, GLUT1, and lactate dehydrogenase (LDH) were
also associated with drug resistance in cancer (28,29),
and our data showed that overexpression of FBP1 restored the
sensitivity to cisplatin and suppressed glycolysis-related protein
GLUT1, GLUT4 and LDHB in cervical cancer cells. Collectively, these
results suggested that FBP1 might block the glucose metabolism to
further impact the chemosensitivity of cervical cancer cells.
However, further studies are necessary to reveal the mechanisms of
this phenomenon.
In conclusion, we have found for the first time that
FBP1 expression had an inverse correlation with prognosis of CSCC
patients. And decreased level of FBP1 could promote carcinogenesis
of CSCC patients. Furthermore, FBP1 overexpression could inhibit
proliferation ability and restore chemosensitivity of cervical
cancer cells probably by suppressing glycolysis pathway. These
findings suggested that FBP1 could be a useful biomarker for
predicting prognosis of CSCC patients, and restoring expression of
FBP1 could be a potential therapeutic target to suppress tumor
progression. However, there are some limitations in our study.
Firstly, we did not perform in vivo experiments to further
confirm our hypothesis. Secondly, a deeper investigation is needed
to clarify potential mechanisms through which decreased level of
FBP1 promotes carcinogenesis and chemoresistance in CSCC. Indeed,
our study is exploratory and descriptive, we would try our best to
solve these problems in the next future.
Acknowledgements
The present study was supported by National Nature
Science Foundation of China (NSFC1212) (grant no. 81202050) for Xi
Cheng. We would like to thank Guangqi Qin and Xu Cai of Fudan
University Shanghai Cancer Center for performing tissue microarray
and immunohistochemistry techniques, respectively.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lissoni AA, Colombo N, Pellegrino A, Parma
G, Zola P, Katsaros D, Chiari S, Buda A, Landoni F, Peiretti M, et
al: A phase II, randomized trial of neo-adjuvant chemotherapy
comparing a three-drug combination of paclitaxel, ifosfamide and
cisplatin (TIP) versus paclitaxel and cisplatin (TP) followed by
radical surgery in patients with locally advanced squamous cell
cervical carcinoma: The snap-02 Italian collaborative study. Ann
Oncol. 20:660–665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katanyoo K, Tangjitgamol S, Chongthanakorn
M, Tantivatana T, Manusirivithaya S, Rongsriyam K and Cholpaisal A:
Treatment outcomes of concurrent weekly carboplatin with radiation
therapy in locally advanced cervical cancer patients. Gynecol
Oncol. 123:571–576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Symonds RP, Gourley C, Davidson S, Carty
K, McCartney E, Rai D, Banerjee S, Jackson D, Lord R, McCormack M,
et al: Cediranib combined with carboplatin and paclitaxel in
patients with metastatic or recurrent cervical cancer (CIRCCa): A
randomised, double-blind, placebo-controlled phase 2 trial. Lancet
Oncol. 16:1515–1524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Monk BJ and Tewari KS: Evidence-based
therapy for recurrent cervical cancer. J Clin Oncol. 32:2687–2690.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Monk BJ, Sill MW, McMeekin DS, Cohn DE,
Ramondetta LM, Boardman CH, Benda J and Cella D: Phase III trial of
four cisplatin-containing doublet combinations in stage IVB,
recurrent, or persistent cervical carcinoma: A Gynecologic Oncology
Group Study. J Clin Oncol. 27:4649–4655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tillmann H and Eschrich K: Isolation and
characterization of an allelic cDNA for human muscle
fructose-1,6-bisphosphatase. Gene. 212:295–304. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu X, Wang X, Zhang J, Lam EK, Shin VY,
Cheng AS, Yu J, Chan FK, Sung JJ and Jin HC: Warburg effect
revisited: An epigenetic link between glycolysis and gastric
carcinogenesis. Oncogene. 29:442–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen M, Zhang J, Li N, Qian Z, Zhu M, Li
Q, Zheng J, Wang X and Shi G: Promoter hypermethylation mediated
downregulation of FBP1 in human hepatocellular carcinoma and colon
cancer. PLoS One. 6:e255642011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li B, Qiu B, Lee DS, Walton ZE, Ochocki
JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I and Simon MC:
Fructose-1,6-bisphosphatase opposes renal carcinoma progression.
Nature. 513:251–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bigl M, Jandrig B, Horn LC and Eschrich K:
Aberrant methylation of human L- and M-fructose 1,6-bisphosphatase
genes in cancer. Biochem Biophys Res Commun. 377:720–724. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heiden MG Vander, Cantley LC and Thompson
CB: Understanding the warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DeBerardinis RJ and Thompson CB: Cellular
metabolism and disease: What do metabolic outliers teach us? Cell.
148:1132–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng X, Cai SM, Li ZT, Wu XH, Ding YQ,
Wang XE and Zang RY: Concurrent chemotherapy and adjuvant extended
field irradiation after radical surgery for cervical cancer
patients with lymph node metastases. Int J Gynecol Cancer.
18:779–784. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi TY, Yang G, Tu XY, Yang JM, Qian J, Wu
XH, Zhou XY, Cheng X and Wei Q: RAD52 variants predict platinum
resistance and prognosis of cervical cancer. PLoS One.
7:e504612012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng X, Yang G, Schmeler KM, Coleman RL,
Tu X, Liu J and Kavanagh JJ: Recurrence patterns and prognosis of
endometrial stromal sarcoma and the potential of tyrosine
kinase-inhibiting therapy. Gynecol Oncol. 121:323–327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: A review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
18
|
Fella K, Glückmann M, Hellmann J, Karas M,
Kramer PJ and Kröger M: Use of two-dimensional gel electrophoresis
in predictive toxicology: Identification of potential early protein
biomarkers in chemically induced hepatocarcinogenesis. Proteomics.
5:1914–1927. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirata H, Sugimachi K, Komatsu H, Ueda M,
Masuda T, Uchi R, Sakimura S, Nambara S, Saito T, Shinden Y, et al:
Decreased expression of fructose-1,6-bisphosphatase associates with
glucose metabolism and tumor progression in hepatocellular
carcinoma. Cancer Res. 76:3265–3276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito Y, Liang G, Egger G, Friedman JM,
Chuang JC, Coetzee GA and Jones PA: Specific activation of
microRNA-127 with downregulation of the proto-oncogene BCL6 by
chromatin-modifying drugs in human cancer cells. Cancer Cell.
9:435–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones PA and Baylin SB: The fundamental
role of epigenetic events in cancer. Nat Rev Genet. 3:415–428.
2002.PubMed/NCBI
|
|
22
|
Dong C, Yuan T, Wu Y, Wang Y, Fan TW,
Miriyala S, Lin Y, Yao J, Shi J, Kang T, et al: Loss of FBP1 by
snail-mediated repression provides metabolic advantages in
basal-like breast cancer. Cancer Cell. 23:316–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Green JA, Kirwan JM, Tierney JF, Symonds
P, Fresco L, Collingwood M and Williams CJ: Survival and recurrence
after concomitant chemotherapy and radiotherapy for cancer of the
uterine cervix: A systematic review and meta-analysis. Lancet.
358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuliani AC, Esteves SC, Teixeira LC,
Teixeira JC, de Souza GA and Sarian LO: Concomitant cisplatin plus
radiotherapy and high-dose-rate brachytherapy versus radiotherapy
alone for stage IIIB epidermoid cervical cancer: A randomized
controlled trial. J Clin Oncol. 32:542–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitagawa R, Katsumata N, Shibata T, Kamura
T, Kasamatsu T, Nakanishi T, Nishimura S, Ushijima K, Takano M,
Satoh T and Yoshikawa H: Paclitaxel plus carboplatin versus
paclitaxel plus cisplatin in metastatic or recurrent cervical
cancer: The open-label randomized phase III trial JCOG0505. J Clin
Oncol. 33:2129–2135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mabuchi S, Isohashi F, Yokoi T, Takemura
M, Yoshino K, Shiki Y, Ito K, Enomoto T, Ogawa K and Kimura T: A
phase II study of postoperative concurrent carboplatin and
paclitaxel combined with intensity-modulated pelvic radiotherapy
followed by consolidation chemotherapy in surgically treated
cervical cancer patients with positive pelvic lymph nodes. Gynecol
Oncol. 141:240–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dunst J and Haensgen G: Simultaneous
radiochemotherapy in cervical cancer: Recommendations for
chemotherapy. Strahlenther Onkol. 177:635–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sowa T, Menju T, Chen-Yoshikawa TF,
Takahashi K, Nishikawa S, Nakanishi T, Shikuma K, Motoyama H,
Hijiya K, Aoyama A, et al: Hypoxia-inducible factor 1 promotes
chemoresistance of lung cancer by inducing carbonic anhydrase IX
expression. Cancer Med. 6:288–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song K, Li M, Xu X, Xuan Li, Huang G and
Liu Q: Resistance to chemotherapy is associated with altered
glucose metabolism in acute myeloid leukemia. Oncol Lett.
12:334–342. 2016.PubMed/NCBI
|