Introduction
Balloon angioplasty, stenting and coronary arterial
bypass grafting (CABG) are common interventions for the treatment
of coronary artery disease (CAD), which is the leading cause of
death globally and is expected to account for 14.2% of all deaths
by 2030 (1). However, these
interventions are not always successful, due to the development of
stent thrombosis and excessive intimal hyperplasia formation,
narrowing the lumen (2).
Iatrogenic trauma and damage that occurs in the vascular
endothelium during angioplasty, stenting and bypass graft surgery
causes the development of remodeling, which leads to the migration
of proliferated smooth muscle cells (SMCs) from media to intima and
connective tissue accumulation in the vasculature (3).
Endothelial cell (EC) injury is considered to be the
first step towards postoperative intimal hyperplasia formation. The
injured site is less capable of producing antiproliferative
products and the regulation of vascular homeostasis in the vessel
wall is further disturbed. The damaged area is coated with
platelets and macrophages which release thrombotic factors
(fibrinogen and von Willebrand factor) and growth factors
(platelet-derived growth factor and transforming growth factor)
(4). According to the
response-to-injury theory, the mechanism that initiates intimal
thickening is that the growth factors which are released from
platelets and ECs adhering to the damaged vessel wall stimulate the
proliferation of SMCs within the first 24 h (5), and subsequently induce the
proliferated SMCs in the medial layer to migrate to the intima
during the 3rd and 4th days, ultimately leading to intimal
hyperplasia. A previous study demonstrated that acute injury to the
intima and media may produce hyperplasia and SMC proliferation,
which occur at a rate proportional to the degree of injury
(6). It has been additionally
observed that intimal hyperplasia forms around the injury sites
following balloon angioplasty (7).
Dipeptidyl peptidase 4 (DPP-4), also termed CD26, is
a widely-expressed serine peptidase that exists on the surface of
various cell types, although its expression level differs among
cells (8). However, in different
organs and tissues, including the lung, muscle and heart,
approximately all tissue DPP-4 activity is due to its presence in
the microvasculature (9). In the
immune system, DPP-4 is associated with T-cell signal transduction
as a co-stimulatory molecule.
Anagliptin, a specific DPP-4 inhibitor, is a novel
oral anti-hyperglycemic agent which has been used to treat type 2
diabetes mellitus by improving glycemic control (10). A previous study suggested that
anagliptin may exert anti-atherosclerotic effects by suppressing
inflammatory reactions of monocytes and stimulating the
mobilization of endothelial progenitor cells (EPCs) (11). An additional study demonstrated
that anagliptin may suppress plaque formation in coronary arteries
with a marked reduction in macrophage accumulation, likely via its
anti-inflammatory properties, while not affecting body weight
(12). However, whether or not
anagliptin has the effect of suppressing intimal hyperplasia
following balloon injury remains to be elucidated.
The present study aimed to investigate the effects
of anagliptin on the formation of intimal hyperplasia following
surgical procedures performed on the left carotid artery of
Sprague-Dawley rats, using the method of balloon injury.
Materials and methods
Carotid balloon injury model
Male Sprague-Dawley rats weighing 280–300 g (age, 10
weeks; n=20.) were obtained from the Animal Center of The Second
Affiliated Hospital of Harbin Medical University (Harbin, China),
and were maintained in groups of five animals per cage. Rats had
free access to water and food and were housed with a 14-h light and
10-h dark cycle under controlled conditions (23±1°C and 55±5%
humidity). Rats were randomized to the injury group (n=10) and the
anagliptin group (n=10). The experimental protocol was designed in
accordance with Institutional Laboratory Animal Care and Use
Committee standards, and all experimental procedures performed in
studies involving animals were approved by the Institutional Animal
Care and Use Committee of Harbin Medical University. Animals were
anesthetized by intraperitoneal injection of chloral hydrate (4%).
The left common carotid artery was exposed and a 2F Fogarty balloon
embolectomy catheter (Edwards Lifesciences, Irvine, CA, USA) was
inserted via an external carotid arteriotomy incision. The catheter
was advanced to the aortic arch, inflated with 0.2 ml air, and
drawn back to the arteriotomy three times. When the catheter had
been withdrawn, the proximal external carotid artery was ligated
and blood flow was restored. The surgical incision was closed and
the rats were allowed to recover from anesthesia. The right
uninjured artery was used as control tissue.
Drug administration
Anagliptin (10 mg/kg per day; MedChem Express China,
Shanghai, China) or vehicle (saline) was administered by oral
injection twice daily to the rats for 28 days. The body weight of
each group was measured at baseline. Food consumption was monitored
daily, and treatment was begun 1 day prior to surgery, and
continued for 28 days following surgery.
Tissue preparation and histological
evaluation
A total of 28 days post-injury, rats were euthanized
by a sodium pentobarbital overdose. The left and right carotid
arteries were removed. Tissues were fixed in 4% paraformaldehyde
for 30 min at 4°C, embedded in paraffin and then four sections (5
µm) were cut at multiple levels. Tissues were then dewaxed with
xylene, rehydrated with decreasing concentrations of ethanol and
washed with tap water. The sections were stained with
hematoxylin-eosin (hematoxylin, 3 min; eosin, 3 min) and elastic
van Gieson stain (Weigert, 3 min; van Gieson, 3 min; cat. no.
4033–4037; Muto Pure Chemicals Co., Ltd., Tokyo, Japan) at room
temperature. Following staining, the sections were dehydrated with
increasing concentrations of ethanol and xylene. Sections were
examined microscopically (magnification, ×200) with an optical
microscope (Olympus Corporation, Tokyo, Japan), and the
cross-sectional areas of the lumen, neointima and media were
determined using digital microscopy with SPOT Advanced software
v5.3 (SPOT Imaging; Diagnostic Instruments, Inc., Sterling Heights,
MI, USA). Intimal hyperplasia was defined as the formation of a
neointimal layer medial to the internal elastic lamina. The medial
area represents the area between the external elastic lamina and
the internal elastic lamina. The intima-to-media ratio was
calculated as the intimal area divided by the media area.
ELISA analysis
Blood samples were collected in anticoagulant-free
tubes pre-operatively, and at 1, 7, 14, 21 and 28 days post-surgery
in the control and anagliptin groups. Plasma was separated by
centrifugation at 1,000 × g and 4°C for 10 min and was stored at
−20°C for a maximum period of 1 month according to the
manufacturers' protocols for the ELISA kits. Glucagon-like peptide
1 receptor (GLP-1; cat. no. ZK-R3375; Shenzhen Ziker Biological
Technology Co., Ltd., Shenzhen, Guangdong, China) and stromal
cell-derived factor (SDF)-1α (cat. no. ZK-R3591, Shenzhen Ziker
Biological Technology Co., Ltd.) activity in plasma was measured
using a double-antibody sandwich ELISA method. Interleukin (IL)-1β
(cat. no. SEA563Ra), IL-6 (cat. no. SER079Ra), tumor necrosis
factor (TNF)-α (cat. no. SEA133Ra) in serum were determined using
commercial ELISA kits (Uscn Life Sciences, Inc., Wuhan, China).
Blood samples (100 µl) were added to a 96-well plate, which was
covered with an adhesive strip and incubated for 2 h at room
temperature on a horizontal orbital microplate shaker. Each well
was washed three times with wash buffer. A total of 200 µl GLP-1,
IL-1β, IL-6, TNF-α and SDF-1α conjugate was added to each well,
covered with a fresh adhesive strip, and incubated for 2 h at room
temperature on the shaker. The washing steps were repeated, and 200
µl substrate solution was added to each well and incubated for 30
min at room temperature in the dark. Stop solution (50 µl) was
added to each well and the optical density of each well was
determined within 30 min using an Infinite 200PRO microplate
spectrophotometer (Tecan Group, Ltd., Mannedorf, Switzerland) set
at 450 nm. All samples were run in duplicate.
Statistics
Obtained data are expressed as the mean ± standard
deviation. Data were assessed using SPSS 15.0 (SPSS, Inc., Chicago,
IL, USA) by one-way analysis of variance with post hoc Bonferroni
test for multiple comparisons. P<0.01 was considered to indicate
a statistically significant difference. Results are expressed as
the mean ± standard error of the mean.
Results
Neointima formation following balloon
injury
For these experiments, the right common carotid
artery did not undergo balloon injury procedures, which meant the
intimal/media ratio and the intimal area were equal to 0. Thus, the
right common carotid artery served as the loading control. Rat left
carotid artery injuries were performed and neointima formation was
evaluated at 28 days post-surgery. The left carotid artery was
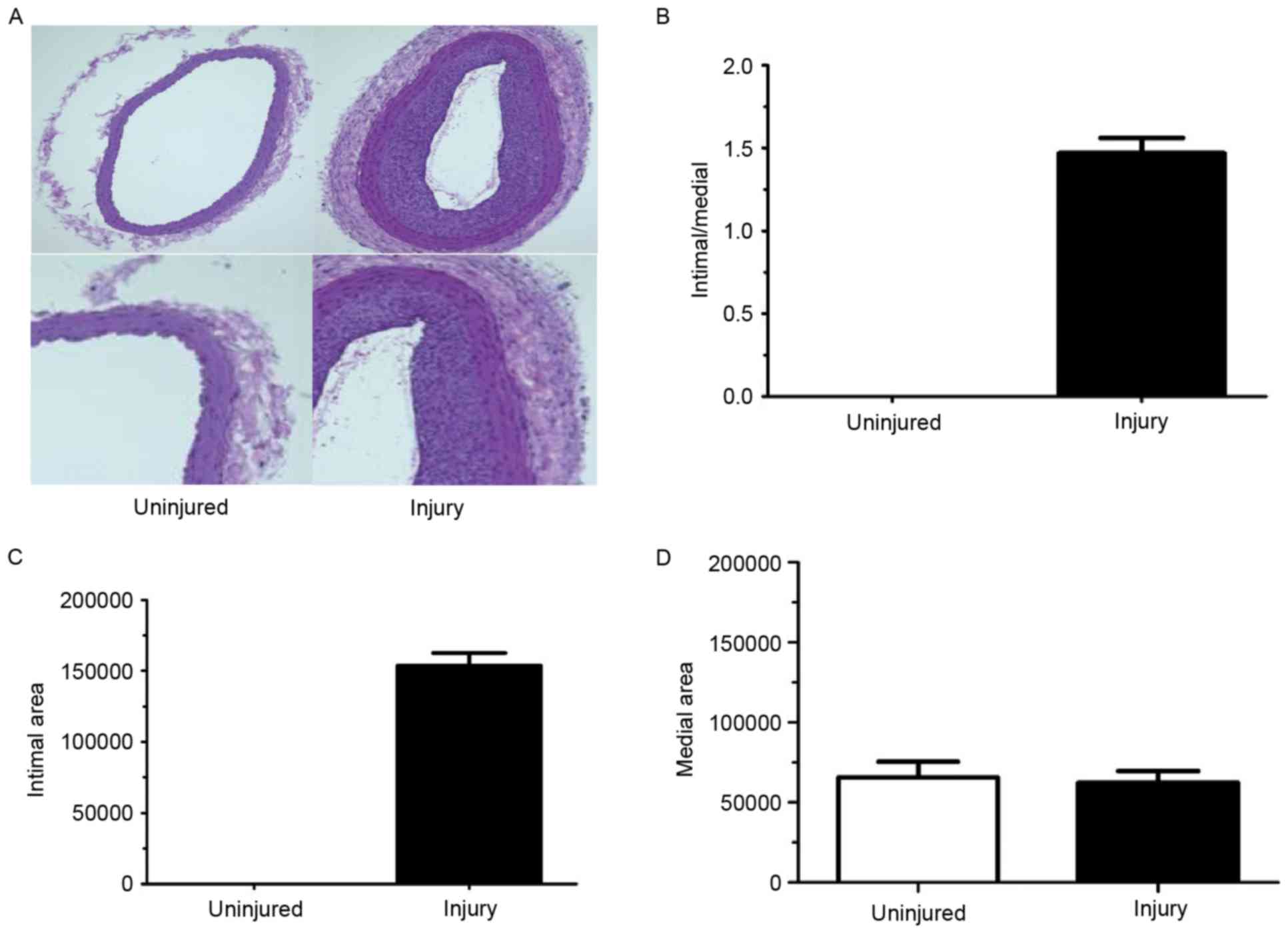
injured and exhibited a decreased lumen area (Fig. 1A and B) which corresponded to
increased intimal thickening. The intima/media ratio was also
increased in the left arteries compared with right arteries
(Fig. 1C). However, there was no
difference in the medial area between left and right carotid
arteries (Fig. 1D).
Effect of anagliptin on the degree of
neointimal thickness following balloon injury
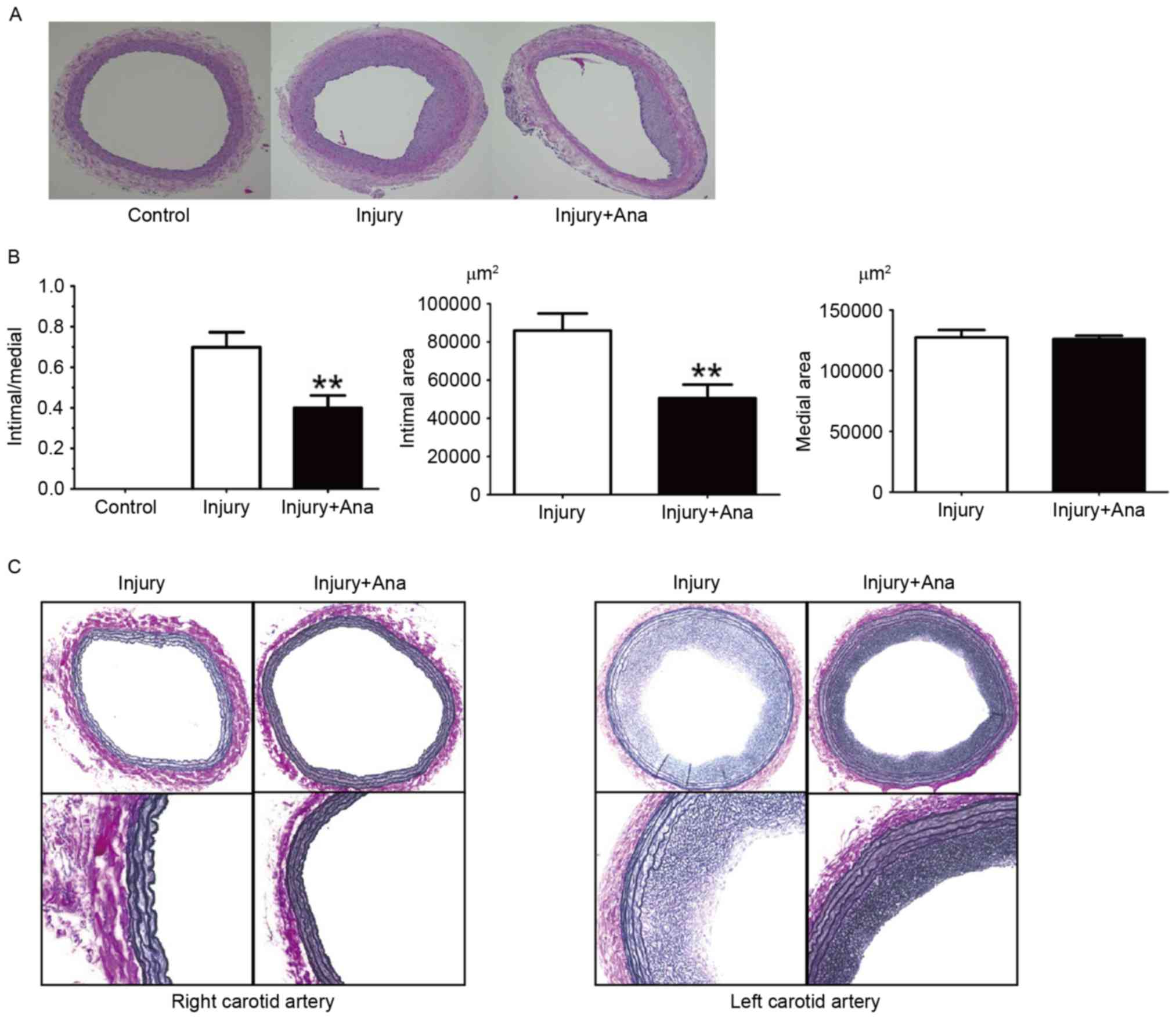
In the histological sections obtained from the rats
which were given anagliptin (10 mg/kg) twice daily, compared with
the injury group, no significant differences between the right
carotid arteries were observed. It was observed that the lumen
area, intimal area and intima/media ratio was decreased at 28 days
compared with the injury group (Fig.
2A). Notably, the medial area in the left carotid artery of
both groups was same (Fig.
2B).
It has been hypothesized that the structural
integrity of the IEL may be essential in the internal elastic
lamina (IEL) rupture mechanism, in order to minimize intimal
hyperplasia following vascular injury. The right carotid artery did
not undergo vascular injury, and hence no intimal hyperplasia or
IEL rupture was detected in the right carotid arteries of the
injury and anagliptin groups. IEL rupture was subsequently analyzed
in rat left carotid arteries, which were subjected to balloon
injury in each group. Notably, balloon injury using the 2F Fogarty
catheter did not result in any fractures in the whole IEL
circumference, although intimal hyperplasia was still observed in
the two groups (Fig. 2C). Intimal
hyperplasia was decreased in the anagliptin group (Fig. 2C). The results of the present study
indicated that there was no association between IEL rupture and
intimal hyperplasia following balloon injury.
Effect of food consumption and body
weight following balloon injury
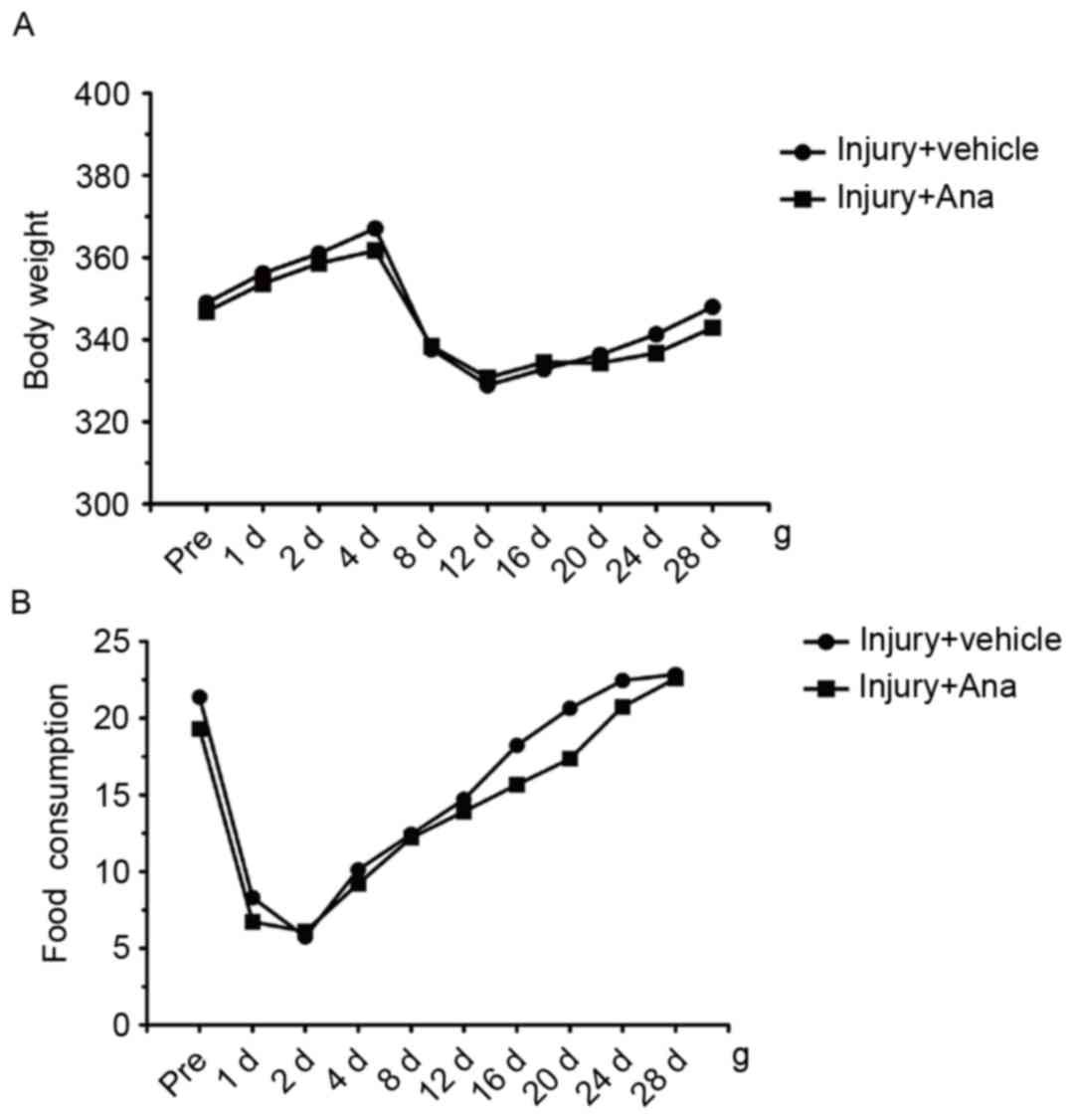
In order to examine the systemic influence of
anagliptin on metabolism, the food consumption and body weight of
all the animals were determined at different time points. Following
surgery, the body weight of both groups decreased at 4 days, and
gradually recovered from 12 days until normal body weight was
attained 28 days in the two groups (Fig. 3A). Daily analyses of food
consumption illustrated decreased food intake at 4 days subsequent
to balloon injury and recovery at 12 days, and this phenomenon was
accordance with the loss of body weight observed in the groups
(Fig. 3B). The results of the
present study indicated that anagliptin attenuated neointima
formation, independent of the glucose-lowering effect and body
weight reduction among the groups.
Detection of the activity of plasma
GLP-1 and SDF-1α in α balloon injury model following treatment with
anagliptin
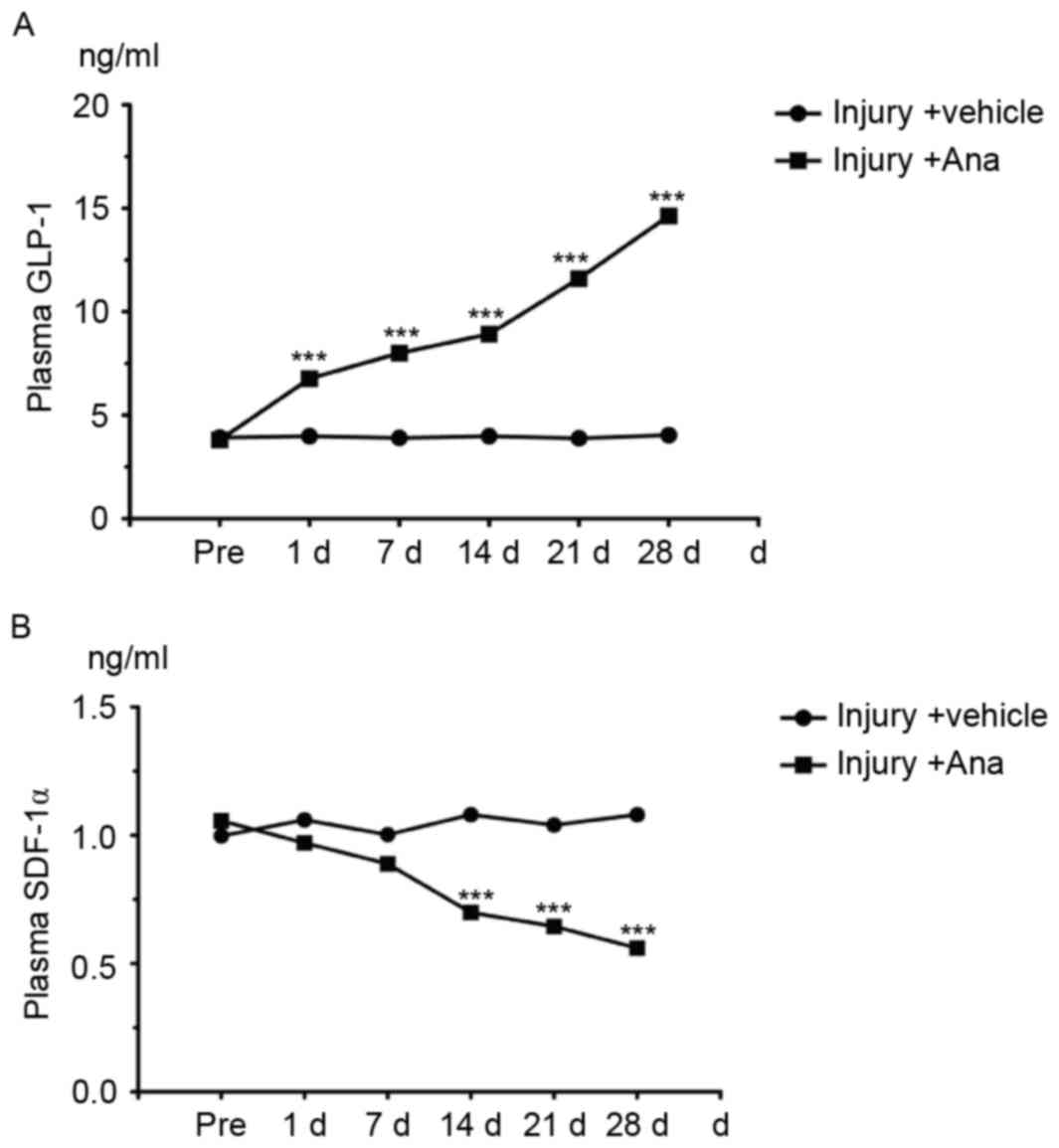
GLP-1 is one of the substrates of DPP-4 in
vivo which is rapidly degraded by the enzyme DPP-4 under normal
conditions. Therefore, the concentration of GLP-1 may reflect the
activity of DPP-4. ELISA analysis was employed for the detection of
serum GLP-1 content which was collected from injury and anagliptin
rats at the chosen time points (pre-operatively, and at 1, 7, 14,
21 and 28 days). The results demonstrated that serum GLP-1 content
in anagliptin group was significantly increased compared with the
control group and peaked at 28 days (Fig. 4A) which suggested the activity of
DPP-4 was decreased.
SDF-1α, one of the DPP-4 substrates that is degraded
by DPP-4 through its cleavage, is a chemokine which induces EPCs to
differentiate into ECs in order to protect the injured artery.
Therefore, the serum SDF-1α concentration was measured. Anagliptin
decreased the serum SDF-1α level (Fig.
4B). These data suggested that anagliptin increased the serum
active GLP-1 concentration, and altered serum factors associated
with EPC migration.
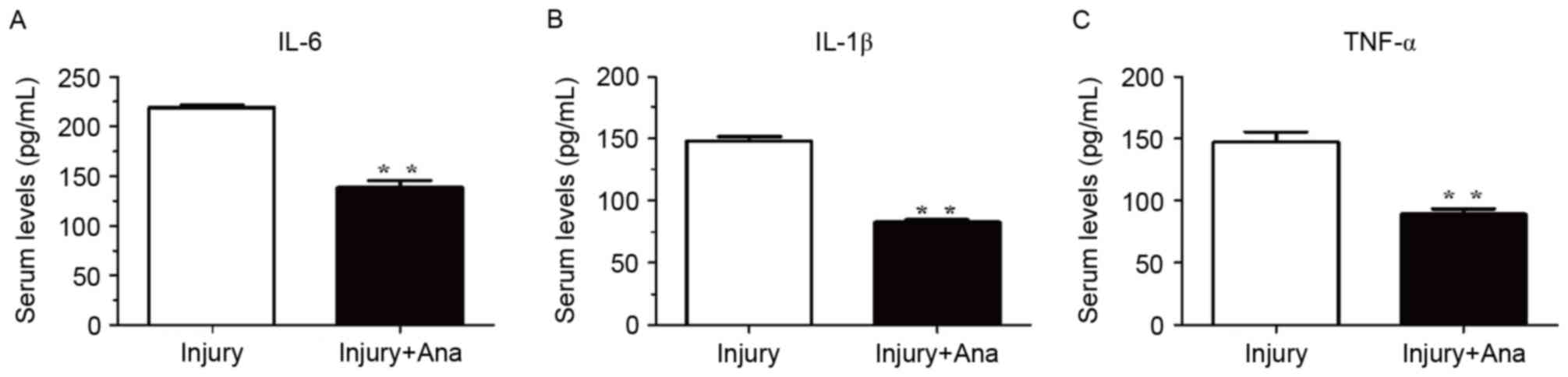
Detection of the serum activity of
inflammatory cytokines following administration of anagliptin
Inflammatory cytokines are among the most important
accelerators of intimal hyperplasia. Therefore, the present study
assessed the inflammatory response following balloon injury. The
serum levels of IL-1β, IL-6 and TNF-α were measured using ELISA
analysis. The serum levels of IL-6, IL-1β, and TNF-α in the injury
group were significantly increased compared with the group treated
with anagliptin at 7 days (Fig.
5).
Discussion
CAD is the leading cause of mortality and morbidity
in the developed world (13).
Numerous improvements have been made in coronary angioplasty,
including balloon angioplasty, stenting and CABG, although a
metallic scaffold device, alone or in combination with drug eluting
stents (DESs) is considered to be the most effective method of
treating CAD (14). However,
studies have suggested that DESs delay re-endothelialization, which
is important for the prevention of restenosis and stent thrombosis.
Intimal hyperplasia develops as a result of SMC migration and
proliferation, and the accumulation of the extracellular matrix,
which is a normal adaptive response in arteries against hemodynamic
stress and is a characteristic feature of arterial injury healing
(15,16).
ECs regulate vascular physiology by producing a
variety of small molecules which have been identified, and regulate
vascular homeostasis, including inhibiting the formation of
thrombosis, coagulation, vasomotor tone and blood flow (17). It has been demonstrated that
defects in endothelial integrity and dysfunction may be the initial
steps leading to the adhesion of phagocytic cells (18) and proliferation of SMCs (19) in an injured artery, and may further
induce atherosclerotic lesion and neointima formation (20). Therefore, EC loss is a factor that
is a primary contributor to remodeling (21), and strategies to protect the
endothelium and/or stimulate its repair following injury have been
sought to reduce intimal hyperplasia formation.
Excessive intimal hyperplasia causes a decrease in
blood flow, narrowing of the lumen and thrombosis formation by
impairing the release of anticoagulants from ECs. A number of
studies have been conducted using drugs to prevent intimal
hyperplasia following balloon injury or stenting (22,23).
Various mechanisms, including growth factors, inflammation,
metabolic disorders and blood flow disturbances may cause intimal
hyperplasia (24). Studies about
the use of anagliptin for preventing intimal hyperplasia are novel
and have not been published previously, however, positive results
were obtained in the present study by using anagliptin in the
prevention of intimal hyperplasia.
The intimal/medial ratio of the left carotid artery
and the intimal area where the artery was injured were thicker
compared with the right carotid artery in the injury group,
although the medial area was the same. These results indicated that
the model system used in the present study was successful and
stable. In a previous study, it was observed that intimal
hyperplasia began to form within 5 days following balloon injury
and continued to thicken for 8 weeks until a maximum intima/media
ratio was attained (25). From
these observations, it is likely that the time course of intimal
hyperplasia formation following balloon injury is complex and may
involve a number of signaling pathways, although the beginning of
intimal hyperplasia formation following injury may be reproducible.
In the group treated with anagliptin, the intimal/medial ratio and
intimal area were decreased compared with the group in which saline
was used. Anagliptin alleviated the intimal hyperplasia on the
injured side and decreased the intimal area. However, in the
present study it was observed that anagliptin exerted no effect on
medial area compared with the control group. The results of the
present study only exhibited the intimal/medial ratio at 28 days
following balloon injury whether.
A previous study indicated that the IEL may act as a
physical barrier to SMC migration or inhibit paracrine
communication between cells of the intima and media, and that IEL
may disrupt the development of intimal hyperplasia (26). In support of this, elastic van
Gieson staining was used in the present study to detect the rupture
of the IEL in the model and anagliptin groups. Notably, the IEL
remained intact in the two groups, meaning that the effect of
anagliptin inhibiting intimal hyperplasia following balloon injury
may not be mediated by maintenance of the IEL.
DPP-4 inhibitors, including anagliptin, are a novel
class of drug which were introduced in the treatment of type 2
diabetes mellitus. DPP-4 inhibitors decrease blood glucose levels
through inhibition of the degradation of GLP-1, which results in
reduced secretion of glucagon and increased secretion of insulin
(27). A previous study
demonstrated that sitagliptin, a DPP-4 inhibitor, has protective
properties against restenosis following carotid injury in an animal
model of type 2 diabetes and in vascular cell lines, indicating
that sitagliptin may be a novel agent for the treatment of
macrovascular-related complications in patients with type 2
diabetes (28). With the results
of the histological analysis performed in the present study, it may
be demonstrated that anagliptin inhibited intimal hyperplasia in
vivo. However, the present study sought to elucidate whether
the effect of anagliptin on neointimal hyperplasia was mediated by
a decrease in the activity of DPP-4. Therefore, the plasma
concentration of GLP-1, which may reflect the plasma activity of
DPP-4, was measured. Serum GLP-1 concentrations rose with
anagliptin treatment at the different time points indicating that
anagliptin may inhibit the plasma activity of DPP-4 in vivo,
with no difference being observed in the model group. Although the
plasma levels of DPP-4 and GLP-1 were altered, body weight and food
intake were not markedly different in the two groups. Notably, the
active GLP-1 levels tended to increase in the anagliptin group
compared with the injury group, although there was no significant
difference preoperatively. A previous report indicated that the
daily glucose levels of rats that were fasted for 4 h were similar
in a group treated with vildagliptin compared with the control
group, although they significantly decreased between week 2 and
week 5 (29); it was hypothesized
that this may be associated with tissue or circulating DPP-4 enzyme
activity (30). The DPP-4
expression and enzyme activity in other tissues remain unknown and
its role requires future investigation. It may be that there exist
other pathways which control the process of intimal hyperplasia
apart from the metabolic pathway.
The C-X-C chemokine SDF-1α, which is important for
cell mobilization/homing (31), is
highly expressed in human atherosclerotic plaques and effectively
activates platelets in vitro (32). There are two SDF-1 isoforms which
are derived from a single SDF-1 gene that is encoded and produced
by alternative splicing (33). The
two SDF-1 isoforms are cleaved by soluble or cellular DPP-4, which
has been demonstrated to inactivate their antiviral and chemotactic
properties in cell-based assays in vitro (34). SDF-1α is constitutively expressed
in a number of tissues and has been observed to be a
chemoattractant for many types of cell. In the present study, it
was observed that serum levels of SDF-1α did not notably fluctuate
in the model group. However, SDF-1α expression significantly
decreased at 1 day post-balloon injury and peaked at 28 days
following treatment with anagliptin. The results of the present
study were in contrast to those of a previous study which reported
an increase in plasma SDF-1α within 24 h following vascular injury
in mice (35). A previous study
demonstrated that anagliptin did not increase the serum SDF-1α
level within atherosclerotic lesions in apolipoprotein E-deficient
mice (36). DPP-4 is a
membrane-bound exopeptidase that rapidly degrades GLP-1. A previous
study demonstrated that a soluble form of DPP-4 (s-DPP-4) that
lacks the short intracellular tail and the transmembrane regions is
present in serum and other bodily fluids which exhibit DPP-4 enzyme
activity (37). High serum levels
of s-DPP-4 have been described in various conditions (38). It may therefore be hypothesized
that anagliptin may only inhibit the activity of s-DPP-4, whilst
preserving the function of DPP-4 in the membrane. However, the
underlying mechanism of this action remains unknown. A previous
study demonstrated that systemic treatment of mice with a SDF-1α
blocking antibody reduced injury-induced neointima formation
(39). Therefore, neutralizing
SDF-1α may have the effect of diminishing plaque formation and
reducing smooth muscle progenitor cell recruitment and neointima
formation following vascular injury (40). In the present study, following
treatment of the balloon injury model with anagliptin, the intimal
area and intimal/medial ratio was reduced. Notably, the plasma
level of SDF-1α was decreased. This indicated that SDF-1α may serve
an important role in the process of vascular remodeling,
particularly the formation of intimal hyperplasia. The major new
insight provided by the results of the present study in terms of
mechanism is that administering anagliptin may reduce the level of
SDF-1α, which is associated with inhibition of the formation of
intimal hyperplasia. It may be hypothesized that reducing the
plasma level of SDF-1α may promote leukocyte adhesion and
migration, and hence stimulate migration and proliferation of ECs
from the adjacent non-injured endothelium via additional factors
released from the site of injury. These factors may drive vascular
re-endothelialization, which may inhibit platelet aggregation and
adhesion, and recovery of the function of the injured artery.
A previous study demonstrated that another DPP-4
inhibitor, alogliptin, was able to inhibit intimal hyperplasia in
rats by inhibiting inflammation (20 mg/kg/day for 14 days; oral
injection) (41). Although this
previous study and the present study used the same source drug
(alogliptin vs. anagliptin) and obtained the same results
(inhibition of intimal hyperplasia), there remain certain
differences: i) The two types of animal model may reflect the
different pathological processes of intimal hyperplasia, although
the balloon injury model is considered to be a priority in the
study of arterial restenosis following mechanical injury [which is
the primary complication of percutaneous luminal coronary
angioplasty (PTCA)]; and ii) the balloon injury model may be useful
for the discovery of drugs which have the ability to inhibit the
formation of intimal hyperplasia. However, it is thought that
inhibiting the activity of DPP-4 may be a potential therapy to
inhibit the process of intimal hyperplasia, and thus, this may also
be a novel therapy for inhibiting restenosis following PTCA. A
previous study reported that DPP-4 is released as an adipokine from
visceral fat, and stimulates vascular (V) SMC proliferation through
extracellular signal-regulated kinase/mitogen-activated protein
kinase phosphorylation in VSMCs (40). Other DPP-4 substrates, including
BNP (42), were not measured in
the present study due to sample limitation. It was not possible to
investigate all the mechanisms through which anagliptin may
attenuate neointima formation following vascular injury. Further
studies are required to clarify the vasculoprotective effect of
anagliptin and to detect other DPP-4 substrates.
In conclusion, the results of the present study
demonstrated that anagliptin was able to inhibit intimal
hyperplasia by reducing the plasma level of SDF-1α, rather than by
altering the process of metabolism. It was hypothesized that the
potential mechanism involves decreasing the plasma level of SDF-1α
to inhibit leukocyte adhesion and the migration of SMCs, and hence
stimulate the migration and proliferation of ECs from the adjacent
non-injured endothelium via additional factors released from the
site of balloon injury. Future studies in the field of vascular
biology following injury may positively affect the quality of life
of patients who have undergone cardiovascular surgery by providing
an improved understanding of endothelial functioning and recovery
from injury, which is primarily responsible for intimal
hyperplasia. The prevention of the intimal hyperplasia response may
be effective in increasing lifespan following vascular
reconstructive interventions, including surgical graft bypass or
balloon angioplasty.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81500209), the
University Nursing Program for Young Scholars with Creative Talents
in Heilongjiang Province (grant no. 2016050) and Heilongjiang
Postdoctoral Financial Assistance (grant no. LBH-Z14215).
References
|
1
|
Molina JA and Heng BH: Global trends in
cardiology and cardiothoracic surgery-an opportunity or a threat?
Ann Acad Med Singapore. 38:541–545. 2009.PubMed/NCBI
|
|
2
|
Uchida Y, Uchida Y, Matsuyama A, Koga A,
Kanai M and Sakurai T: Formation of web- and membrane-like
structures on the edges of bare-metal coronary stents. Circ J.
74:1830–1836. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pauletto P, Sartore S and Pessina AC:
Smooth-muscle-cell proliferation and differentiation in neointima
formation and vascular restenosis. Clin Sci (Lond). 87:467–479.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delafontaine P: Growth factors and
vascular smooth muscle cell growth responses. Eur Heart J. 19 Suppl
G:G18–G22. 1998.PubMed/NCBI
|
|
5
|
Ross R: Cell biology of atherosclerosis.
Annu Rev Physiol. 57:791–804. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chervu A and Moore WS: An overview of
intimal hyperplasia. Surg Gynecol Obstet. 171:433–447.
1990.PubMed/NCBI
|
|
7
|
Allaire E and Clowes AW: Endothelial cell
injury in cardiovascular surgery: The intimal hyperplastic
response. Ann Thorac Surg. 63:582–591. 1997.PubMed/NCBI
|
|
8
|
Hong WJ, Petell JK, Swank D, Sanford J,
Hixson DC and Doyle D: Expression of dipeptidyl peptidase IV in rat
tissues is mainly regulated at the mRNA levels. Exp Cell Res.
182:256–266. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matheeussen V, Baerts L, de Meyer G, de
Keulenaer G, van der Veken P, Augustyns K, Dubois V, Scharpé S and
De Meestre I: Expression and spatial heterogeneity of dipeptidyl
peptidases in endothelial cells of conduct vessels and capillaries.
Biol Chem. 392:189–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shinjo T, Nakatsu Y, Iwashita M, Sano T,
Sakoda H, Ishihara H, Kushiyama A, Fujishiro M, Nishimura F and
Asano T: High-fat diet feeding significantly attenuates
anagliptin-induced regeneration of islets of Langerhans in
streptozotocin-induced diabetic mice. Diabetol Metab Syndr.
7:502015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ervinna N, Mita T, Yasunari E, Azuma K,
Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori
R, et al: Anagliptin, a DPP-4 inhibitor, suppresses proliferation
of vascular smooth muscles and monocyte inflammatory reaction and
attenuates atherosclerosis in male apo E-deficient mice.
Endocrinology. 154:1260–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirano T, Yamashita S, Takahashi M,
Hashimoto H, Mori Y and Goto M: Anagliptin, a dipeptidyl
peptidase-4 inhibitor, decreases macrophage infiltration and
suppresses atherosclerosis in aortic and coronary arteries in
cholesterol-fed rabbits. Metabolism. 65:893–903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Indolfi C, Pavia M and Angelillo IF:
Drug-eluting stents versus bare metal stents in percutaneous
coronary interventions (a meta-analysis). Am J Cardiol.
95:1146–1152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cassess S, Hoppmann P, Kufner S, Byrne RA,
Wiebe J, Colleran R, Giacoppo D, Harada Y, Laugwitz KL, Schunkert
H, et al: Intraindividual comparison of everolimus eluting
bioresorbable vascular scaffolds versus drug eluting metallic
stent. Circ Cardiovasc Interv. 9:pii: e0036982016. View Article : Google Scholar
|
|
15
|
Allagnat F, Dubuis C, Lambelet M, Le Gal
L, Alonso F, Corpataux JM, Déglise S and Haefliger JA: Connexin37
reduces smooth muscle cell proliferation and intimal hyperplasia in
a mouse model of carotid artery ligation. Cardiovasc Res.
113:805–816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, He X, Chen X, Ma H, Liu D, Luo J,
Du Z, Jin Y, Xiong Y, He J, et al: Enhanced external
counterpulsation inhibits intimal hyperplasia by modifing shear
stress responsive gene expression in hypercholesterolemic pigs.
Circulation. 116:526–534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davies MG and Hagen PO: The vascular
endothelium. A new horizon. Ann Surg. 218:593–609. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kibos A, Campeanu A and Tintoiu I:
Pathophysiology of coronary artery in-stent restenosis. Acute Card
Care. 9:111–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoneda S, Abe S, Kanaya T, Oda K, Nishino
S, Kageyama M, Taguchi I, Masawa N and Inoue T: Late-phase
inflammatory response as a feature of in-stent restenosis after
drug-eluting stent implantation. Coron Artery Dis. 24:368–373.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McDonald RA, Halliday CA, Miller AM, Diver
LA, Dakin RS, Montgomery J, McBride MW, Kennedy S, McClure JD,
Robertson KE, et al: Reducing in-stent restenosis: Therapeutic
manipulation of miRNA in vascular remodeling and inflammation. J Am
Coll Cardiol. 65:2314–2327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paneghetti L and Ng YS: A novel
endothelial-derived anti-inflammatory activity significantly
inhibits spontaneous choroidal neovascularisation in a mouse model.
Vasc Cell. 8:22016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang W, Zheng B, Zhang XH, Yue LY, Liu C,
Ma D, Yang Z and Wen JK: Tongxinluo inhibits neointimal formation
by regulationg the expression and post-translational modification
of KLF5 in macrophages. Am J Transl Res. 8:4778–4790.
2016.PubMed/NCBI
|
|
23
|
Zhang YQ, Tian F, Zhou Y, Chen YD, Li B,
Ma Q and Zhang Y: Nicorandil attenuates carotid intimal hyperplasia
after balloon catheter injury in diabetic rats. Cardiovasc
Diabetol. 15:622016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grus T, Lambert L, Matěcha J, Grusová G,
Špaček M and Mlček M: The ratio of diameters between the target
artery and the bypass modifies hemodynamic parameters related to
intimal hyperplasia in the distal end to side anastomosis. Physiol
Res. 65:901–908. 2016.PubMed/NCBI
|
|
25
|
Honda Y, Kitano T, Fukuya F, Sato Y, Iwama
S, Morie T and Notake M: A novel alphavbeta3 integrin antagonist
suppresses neointima formation for more than 4 weeks after balloon
injury in rats. Arterioscler Thromb Vasc Biol. 25:1376–1382. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Touchard AG and Schwartz RS: Preclinical
restenosis models: Challenges and successes. Toxicol Pathol.
34:11–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shinjo T, Nakatus Y, Iwashita M, Sano T,
Sakoda H, Ishihara H, Kushiyama A, Fujishiro M, Nishimura F and
Asano T: High-fat diet feeding significantly attenuates
anagliptin-induced regeneration of islets of Langerhans in
streptozotocin-induced diabetic mice. Diabetol Metab Syndr.
7:502015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lim S, Choi SH, Shin H, Cho BJ, Park HS,
Ahn BY, Kang SM, Yoon JW, Jang HC, Kim YB and Park KS: Effect of a
dipeptidyl peptidase-IV inhibitor, des-fluoro-sitagliptin, on
neointimal formation after balloon injury in rats. PLoS One.
7:e350072012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eom YS, Gwon AR, Kwak KM, Kim JY, Yu SH,
Lee S, Kim YS, Park IB, Kim KW, Lee K and Kim BJ: Protective
effects of vildagliptin against pioglitazone induced bone loss in
type 2 diabetic rat. PLoS One. 11:e01685692016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mentlein R: Dipeptidyl peptidase IV
(CD26)-role in the inactivation of regulatory peptides. Regul Pept.
85:9–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tachibana K, Hirota S, Iizasa H, Yoshida
H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N,
Nishikawa S, et al: The chemokine receptor CXCR4 is essential for
vascularization of the gastrointestinal tract. Nature. 393:591–594.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abi-Younes S, Sauty A, Mach F, Sukhova GK,
Libby P and Luster AD: The stromal cell-derived factor-1 chemokine
is a potent platelet agonist highly expressed in atherosclerotic
plaques. Circ Res. 86:131–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shirozu M, Nakano T, Inazawa J, Tashiro K,
Tada H, Shinohara T and Honji T: Structure and chromosomal
localization of the human stromal cell-derived factor 1 (SDF1)
gene. Genomics. 28:495–500. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shioda T, Kato H, Ohnishi Y, Tashiro K,
Ikegawa M, Nakayama EE, Hu H, Kato A, Sakai Y, Liu H, et al:
Anti-HIV-1 and chemotactic activities of human stromal cell-derived
factor 1alpha (SDF-1alpha) and SDF-1beta are abolished by
CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci
USA. 95:pp. 6331–6336. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schober A, Knarren S, Lietz M, Lin EA and
Weber C: Crucial role of stromal cell-derived factor-1alpha in
neointima formation after vascular injury in apolipoprotein
E-deficient mice. Circulation. 108:2491–2497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ervinna N, Mita T, Yasunari E, Azuma K,
Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori
R, et al: Anagliptin, a DPP-4 inhibitor, suppresses proliferation
of vascular smooth muscles and monocyte inflammatory reaction and
attenuates atherosclerosis in male apo E-deficient mice.
Endocrinology. 153:1260–1270. 2013. View Article : Google Scholar
|
|
37
|
Lambeir AM, Durinx C, Scharpe S and De
Meester I: Dipeptidyl-peptidase IV from bench to bedside: An update
on structural properties, functions, and clinical aspects of the
enzyme DPP IV. Crit Rev Clin Lab Sci. 40:209–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gorrell MD, Gysbers V and McCaughan GW:
CD26: A multifunctional integral membrane and secreted protein of
activated lymphocytes. Scand J Immunol. 54:249–264. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zernecke A, Schober A, Bot I, Von
Hundelshausen P, Liehn EA, Möpps B, Mericskay M, Gierschik P,
Biessen EA and Weber C: SDF-1alpha/CXCR4 axis is instrumental in
neointimal hyperplasia and recruitment of smooth muscle progenitor
cells. Circ Res. 96:784–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akita K, Isoda K, Shimada K and Daida H:
Dipeptidyl-Peptidase-4 inhibitor, alogliptin, attenuates arterial
inflammation and neointimal formation after injury in low-density
lipoprotein (LDL) receptor-deficient mice. J Am Heart Assoc.
4:e0014692015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lamers D, Famulla S, Wronkowitz N, Hartwig
S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Müller S, et
al: Dipeptidyl peptidase 4 is a novel adipokine potentially linking
obesity to the metabolic syndrome. Diabetes. 60:1917–1925. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Murohara T: Dipeptidyl peptidase-4
inhibitor: Another player for cardiovascular protection. J Am Coll
Cardiol. 59:277–279. 2012. View Article : Google Scholar : PubMed/NCBI
|