Introduction
For severe hip joint degeneration, post-traumatic
and other types of end-stage osteoarthritis, the safest and most
effective method of treatment is total hip arthroplasty (THA)
(1). Since 1960, THA has become a
cost-effective surgery to reduce joint pain and recover movement
capabilities, which also makes it one of the most successful
surgeries in the history of joint reconstruction (2). However, periprosthetic osteolysis
remains a frequent complication following total hip replacement due
to inflammatory responses to the microscopic particles from the
bone-implant interface membrane (3,4).
Monocytes/macrophages, fibroblast and mesenchymal stem cells are
major cell types and effector cells involved in inflammatory
osteolysis. At the interface membrane of prostheses, macrophages
phagocytose foreign particles into a larger cell containing
inclusions forming the osteoclast precursor cells, which can also
be isolated from a number of macrophages (5). Currently available evidence suggests
that macrophage phagocytose wear particles by stimulating cellular
responses, including the expansion of the cytoplasm and secretion
of a variety of pro-inflammatory cytokines. These cells are capable
of secreting a large number of inflammatory cytokines, including
interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α) and IL-6 that
stimulate osteoclast-induced bone resorption (6,7).
These inflammatory factors further increase the number of monocytes
in the peripheral system and induce the transformation of
macrophages into osteoclasts. The osteoclasts are terminal cells
leads to osteolysis and bone resorption and are arthroplasty
prosthesis loosening occurred the end effector cells and root
cause. Osteoclasts cause osteolysis and bone resorption around the
joint prosthesis, which is the main cause of joint prosthesis
loosening.
The molecular mechanisms governing osteolysis and
detailed mechanism of the formation of osteoclasts remain unknown.
At present, the generally accepted process of osteolysis is that
following stimulation of wear particles, they can activate a
variety of cellular signaling pathways, regulate the expression of
a number of signaling proteins and ultimately induce osteoclast
differentiation and formation (8).
Osteoclasts are large multinucleated cells characteristic of bone
tissue, differentiated from hematopoietic stem cells and are
involved in regulating physiological functions, bone homeostasis,
and hematopoiesis. Osteoclasts differentiate under the control of
two major cytokines, namely receptor activator of nuclear factor-κB
ligand (RANKL) and macrophage colony-stimulating factor (M-CSF)
(9). Without these factors,
osteoclast differentiation is delayed, inefficient and leads to
early apoptosis (10). M-CSF
binding to colony stimulating factor 1 receptor (c-fms) is a key
signal for the proliferation and survival of osteoclast precursor
cells. RANKL binding to RANK is a stimulating signal for osteoclast
differentiation and bone resorption, and maturation of osteoclasts
(11,12). RANK interacts with RANKL when
co-stimulatory signals including M-CSF are present. The
encapsulated fraction of transmembrane receptor RANK first induces
TNF receptor-associated factor (TRAF), which then activates
multiple signal transduction pathways. Finally, through
proto-oncogene c-Src, tartrate-resistant acid phosphatase (TRAP),
cathepsin K, carbonic anhydrase 2 and a series of
osteoclast-specific gene expression, osteoclast differentiation,
function and apoptosis is regulated (13).
RANKL is a member of the TNF receptor ligand
superfamily and is located in cells in a membrane-bound form with a
soluble carboxyl terminal. Osteoblasts, bone marrow stromal cells
and activated T lymphocytes express RANKL (14). RANK binds to RANKL, and is mainly
expressed in monocytes/macrophages and in osteoclast precursor
cells or on the surface of mature osteoclasts. RANK promotes
osteoclast differentiation and activation, and prevents apoptosis
of osteoclasts. Osteoblasts and bone marrow stromal cells also
express another member of the tumor necrosis factor superfamily,
osteoprotegerin (OPG). OPG is present in the form of soluble
secretory proteins, which bind to RANK, thus competing with RANKL.
Inhibiting the interaction between RANK and RANKL can lead to
inhibition of osteoclast differentiation and function. Therefore,
in the process of dynamic balance between bone remodeling and bone
resorption, RANKL/OPG interaction is an important regulating
mechanism (15,16). RANKL binds to RANK and activates
the downstream signaling pathway. RANKL lacks the enzyme active
site and it binds to RANK through recruitment of linker molecules,
including TRAF6, which in turn activates nuclear factor κB (NF-κB),
calcineurin/nuclear factor of activated T cells (CN/NFAT) and
phosphatidylinositol signaling pathways.
M-CSF is a lineage specific cytokine located
primarily in the bone marrow cavity. M-CSF is a ligand for colony
stimulating factor 1 receptor membrane-bound form, and serves an
important role in the proliferation, differentiation and
maintenance activity of the mononuclear cells. Previous experiments
(17) demonstrated that in the
presence of bone resorption-stimulating factor, co-culturing of
normal mouse skull bone marrow cells with other bone marrow cells
generates osteoclasts. However, M-CSF-deficient mice failed to
produce the osteoclast precursor cells and osteoclasts. The
osteoclast formation potential was restored by adding exogenous
M-CSF to M-CSF-deficient mice, which emphasizes the importance of
these cytokines in osteoclast formation. Relevant studies have also
demonstrated that M-CSF serves a critical role in the regulation of
multiple steps involved in the regulation of human osteoclasts,
including proliferation, differentiation and the fusion of their
precursor cells (18,19,20).
The role of CN/NFAT signaling pathways in bone
metabolism, immunity and other systems has been extensively studied
over previous years. Studies have indicated that the osteoclast
differentiation begins with RANKL-mediated activation of the
CN/NFAT signaling pathway. Upon this activation, transcription
factor NFATc1 enters the nucleus to initiate osteoclast specific
gene transcription (21,22). NFATc1 is an essential transcription
factor in the process of osteoclast differentiation and maturation
as it regulates osteoclast specific gene expression (10). A previous study demonstrated that
early in osteoclast differentiation, c-Fos is recruited to the
NFATc1 promoter region and the interaction with RANKL signal
induces the normal expression of NFATc1, which can promote
osteoclast precursor cells to differentiate into mature osteoclasts
(23). Gene chip studies have
demonstrated that RANKL induces p50 and p65 subunit aggregation to
the NFATc1 promoter region as a part of the NF-κB signaling pathway
during osteoclast differentiation and maturation (24). The immunoreceptors on the surface
of the cell membrane, including osteoclast-associated
immunoglobulin-like receptor, pirin-A, surface triggering receptor
expressed on myeloid cells 2 and signal regulatory protein-β1, are
essential for the reception of costimulatory signals during
osteoclast differentiation and activation. Immunoreceptors, through
their allosteric intracellular immunoreceptor tyrosine-based
activation motif, detect changes in Ca2+ concentration
and patina, activate CN and promote the expression of NFATc1, which
induces osteoclast generation. In addition, LIM homeobox 2,
interferon regulatory factor 8, B-cell lymphoma 6 and other NFATc1
suppressor genes were significantly downregulated in osteoclast
progenitor cells when RANKL was present, whereas RANKL
gene-knockout mice demonstrated significantly increased expression
of NFATc1 and osteoporosis (25,26).
Therefore, in the process of osteoclast differentiation and
maturation, NFATc1 is a vital component of the signal transduction
pathway leading to osteoclast formation. Previous studies have
demonstrated that wear particles can enhance the activity of the
CN/NFAT signaling pathway during osteoclast differentiation and
promote osteoclast differentiation and function through this
pathway (27,28).
Wear particles can induce various types of
inflammatory factors and periprosthetic macrophages, which, via a
series of biological reactions, induce osteolysis (29). A previous study from the United
States indicates that the proportion of metal interfaces in joint
prostheses remains high (30), and
therefore the incidence of osteolysis induced by metal wear
particles remains relatively high. Therefore, titanium particles
were chosen as the research subject of this paper. The authors
previously demonstrated that lentiviruses can be successfully used
to induce the release of inflammatory cytokines around a prosthesis
(31). In the present study a
lentiviral vector containing M-CSF-shRNA and RANKL-shRNA was
constructed to inhibit the upstream regulatory factors of
osteoclast formation, further proving the key role of NFATc1 in the
CN/NFAT signaling pathway. The aim of the present study was to
identify a target for the inhibition of osteolysis around the
prosthesis.
Materials and methods
Cell culture
Murine osteoclast progenitor macrophage/monocyte
RAW264.7 cells (American Type Culture Collection, Manassas, VA,
USA) suitable for host transfection were used. Cells were cultured
in α-minimum essential medium (α-MEM; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) containing 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences), 100 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 g/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37.6°C
under 5% CO2 and 95% humidity. Cells at the prosthesis
interface were plated with α-MEM containing FBS and treated or
untreated with M-CSF-shRNA-RANKL-shRNA depending on the
experimental design. Cells were cultured in differentiation medium
with or without the Ti particles (0.1 mg/ml). The RAW264.7 cultures
were divided into three groups: i) Ti particles only (Ti group);
ii) Ti particles and lentiviral vector (lenti) -M-CSF-RANKL
(lenti-M-CSF-RANKL group); and iii) neither wear particles nor
lenti-M-CSF-RANKL (control group).
Lentiviral vector construction and
recombinant lentivirus production
The shRNA expression vector PLV-shRNA-green
fluoresecent protein (GFP; Shanghai GeneChem Co., Ltd., Shanghai,
China) was used in the present study. This vector contained two U6
promoters, which were independent of each other, so that two
interfering genes could be activated at the same time and the
interference efficiency was independent of each other. The RANKL
interference sequence was cloned into the PLV-shRNA-GFP vector by
restriction endonucleases HpaI and XhoI (Shanghai
GeneChem Co., Ltd.) to form a lentiviral vector PLV-shRNA-GFP
(RANKL-shRNA). The U6-shRNA expression cassette was cloned into the
lentiviral vector PLV-shRNA-GFP (RANKL-shRNA) using NotI and
NsiI (Shanghai GeneChem Co., Ltd.) sites. The interference
sequence for the second gene was cloned into the lentiviral vector
using the restriction endonuclease sites of HpaI and
XhoI to construct a double RNA vector
PLV-M-CSF-shRNA-RANKL-shRNA-GFP. This construct was transfected
into cells to induce interference with M-CSF and RANKL genes
simultaneously. Following construction, the recombined lentiviral
vector and pPACK Packaging Plasmid mix (Invitrogen; Thermo Fisher
Scientific, Inc.) were cotransfected into 293T cells (GeneChem,
Inc., Daejeon, Korea). The pGag/Pol, pRev and pVSV-G packaging
vectors, and recombinant lentivirus were packaged into plasmid
vectors, and the recombinant lentivirus was amplified by
transforming 293T cells with the packaging plasmids (0.5 µg/µl)
using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). At 48 h post-transfection, the lentiviruses were harvested
and centrifuged at 4,000 × g for 10 min at 4°C to remove cell
debris. Condensation was performed by filtration of the supernatant
into a filtrate collection tube through a filter cup, followed by
centrifugation at 4,000 × g for 13 min at 4°C. The filter cup was
removed, a sample collection cup was inserted into the filtrate
collection tube and the solution was centrifuged at 1,000 × g for 2
min at 4°C. Finally, a concentrated lentivirus solution was
obtained, with a final titer of 1.5×109 transducing
units/l.
Titanium particle preparation
In the present study, commercial pure titanium
particles were obtained from Johnson Matthey Pharma Services (Ward
Hill, MA, USA) and the average particle diameter was 4.5 mm (range,
1–10 mm). The titanium particles were dissolved in 75% ethanol,
washed in PBS (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) at room temperature 4 times, 1 h/wash, and then washed in
PBS (Wuhan Boster Biological Technology, Ltd., Wuhan, China) and
incubated in 100% ethanol overnight. Finally, the treated titanium
particles were soaked in 4°C α-MEM for storage. Titanium particles
used in the experiment had endotoxin levels <0.2 endotoxin
units/ml verified using a Limulus Amebocyte Lysate assay (Xiamen
Houshiji, Ltd., Xiamen, China), according to the manufacturer's
protocol. It is reported from the literature that these particles
are similar to particles produced in the surrounding tissue of the
prosthesis (32).
MTT assay
The cytotoxicity of Ti particles in RAW264.7 cells
transfected with M-CSF-shRNA-RANKL-shRNA was examined using the MTT
assay. The cells (5×103 cells/well) were cultured in
96-well tissue culture plates for 24 h and incubated with 0.5 mg/ml
MTT at 37°C for 4 h. Following the removal of the supernatant, the
insoluble formazan crystals were dissolved in 200 µl dimethyl
sulfoxide and the absorbance was measured using a Synergy HT
microtiter plate reader (BioTek Instruments, Inc., Winooski,
Vermont, USA) at a wavelength of 570 nm.
TRAP staining of osteoclasts
TRAP osteoclast-specific staining can be used to
observe the formation of osteoclasts. A total of three osteoclast
specimens were taken for analysis from an osteolytic area. After 7
days RAW264.7 cells were fixed with 4% paraformaldehyde (at 37°C
for 20 min) and stained at 37°C for 4 h for TRAP using a leukocyte
acid phosphatase staining kit (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) according to the manufacturer's protocol, for
the analysis of the formation of osteoclasts. TRAP-positive cells
with >3 nuclei were considered differentiated osteoclast-like
cells (33).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was used to assess the expression of RANKL,
M-CSF, NFATc1, TNF-α, IL-1β and IL-6 in RAW264.7 cells under
different conditions. Total RNA was extracted from RAW264.7
cultures using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. RNA purity was determined
using the 260/280 nm absorbance ratio (NanoDrop; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). Each first-strand cDNA was
synthesized with 2 mg total RNA using the TaqMan Real-Time PCR
Master Mixes (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) and 1/10th of the total cDNA was used for each
PCR mixture containing EXPRESS SYBR-Green (Takara Bio, Inc., Otsu,
Japan) and PCR Supermix (Fermentas; Thermo Fisher Scientific,
Inc.). The primers and thermal cycling conditions for RANKL, M-CSF,
TNF-α, IL-1β, IL-6 and NFATc1 are listed in Table I. The cycling conditions were: 95°C
for 15 sec, 95°C for 5 sec and 60°C for 30 sec. A total of 45
cycles were run. Relative mRNA expression of selected genes was
normalized to the GAPDH reference gene and quantified using the
2−∆∆Cq method (34).
 | Table I.Primers for the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for the reverse
transcription-quantitative polymerase chain reaction.
| Target | Forward primer
(5′-3′) | Reverse primer
(3′-5′) |
|---|
| TNF-α |
TCCTCACCCACACCGTCAG |
GCTGAGTTGGTCCCCCTTC |
| IL-1β |
TTCTCGCAGCAGCACATC |
CAGCAGGTTATCATCATCATCC |
| IL-6 |
TCCATCCAGTTGCCTTCTTG |
TTTCTCATTTCCACGATTTCCC |
| NFATc1 |
CAACGCCCTGACCACCGATAG |
GGCTGCCTTCCGTCTCATAGT |
| RANKL |
AGATTTGCAGGACTCGACTC | CCCACA
ATGTGTTGCAGTTC |
| M-CSF |
CCACTTGTAGAACAGGAGGCCC |
GCTTGAGGGCAAGAGAAGTACC |
| GAPDH |
TGGTGAAGGTCGGTGTGAAC |
GCTCCTGGAAGATGGTGATGG |
ELISA
Following 48 h of incubation with recombinant
lentivirus (produced as described above), the RAW264.7 cells were
incubated with or without Ti particles in the presence or absence
of M-CSF-shRNA-RANKL-shRNA for 24 h and the cell supernatants were
collected and centrifuged at 1,200 × g for 8 min at room
temperature to remove the cell particles. Aliquots were stored at
−20°C for TNF-α measurement. Mouse-specific ELISA kits were used to
analyze the amount of TNF-α. ELISA kit (Mouse TNF-α Tumor Necrosis
Factor-α ELISA kit; cat. no. E-EL-M0049; R&D Systems, Inc.,
Minneapolis, MN, USA) was used for quantitative measurement,
according to the manufacturer's protocol.
Statistical analysis
All data were expressed as the mean ± standard
deviation of the mean. Differences between groups were analyzed
using one-way analysis of variance followed by Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS software
(version 17.0; SPSS, Inc., Chicago, IL, USA).
Results
Effects of Ti particles and
lenti-M-CSF-RANKL on cell viability
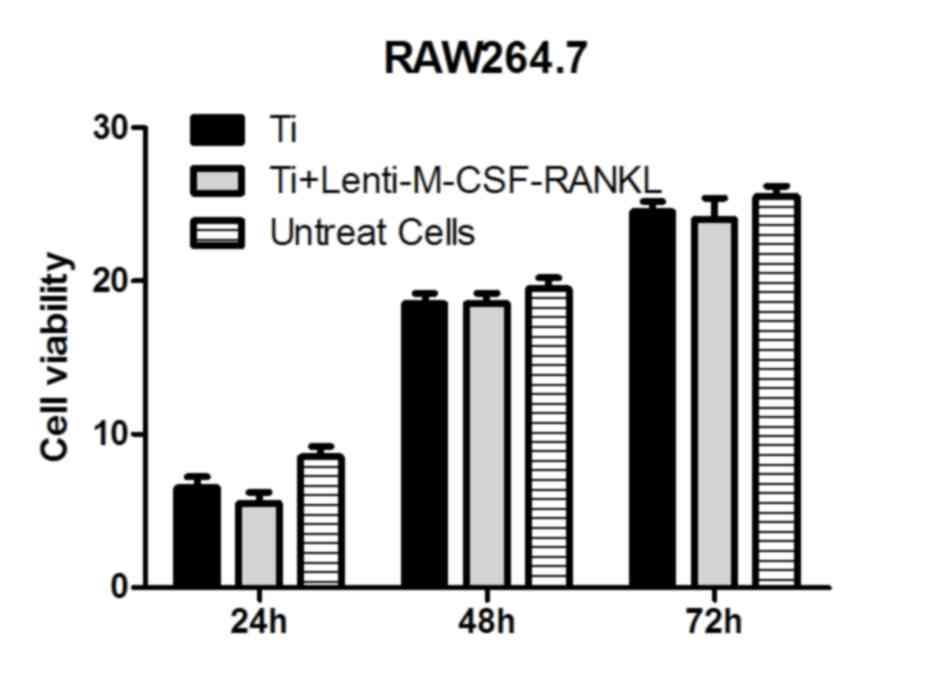
In the presence of titanium particles, RAW264.7
cells cultured for 24–72 h and analyzed using the MTT assay,
revealed no significant differences between the cells transfected
with lenti-M-CSF-RANKL and the untransfected cells (Fig. 1). Therefore, it was observed that
the treatments had no significant effects on the activity of the
cells in Ti group and Lenti-M-CSF-RANKL group.
Effect of lenti-M-CSF-RANKL on Ti
particle-induced expression of inflammatory factors in RAW264.7
cells
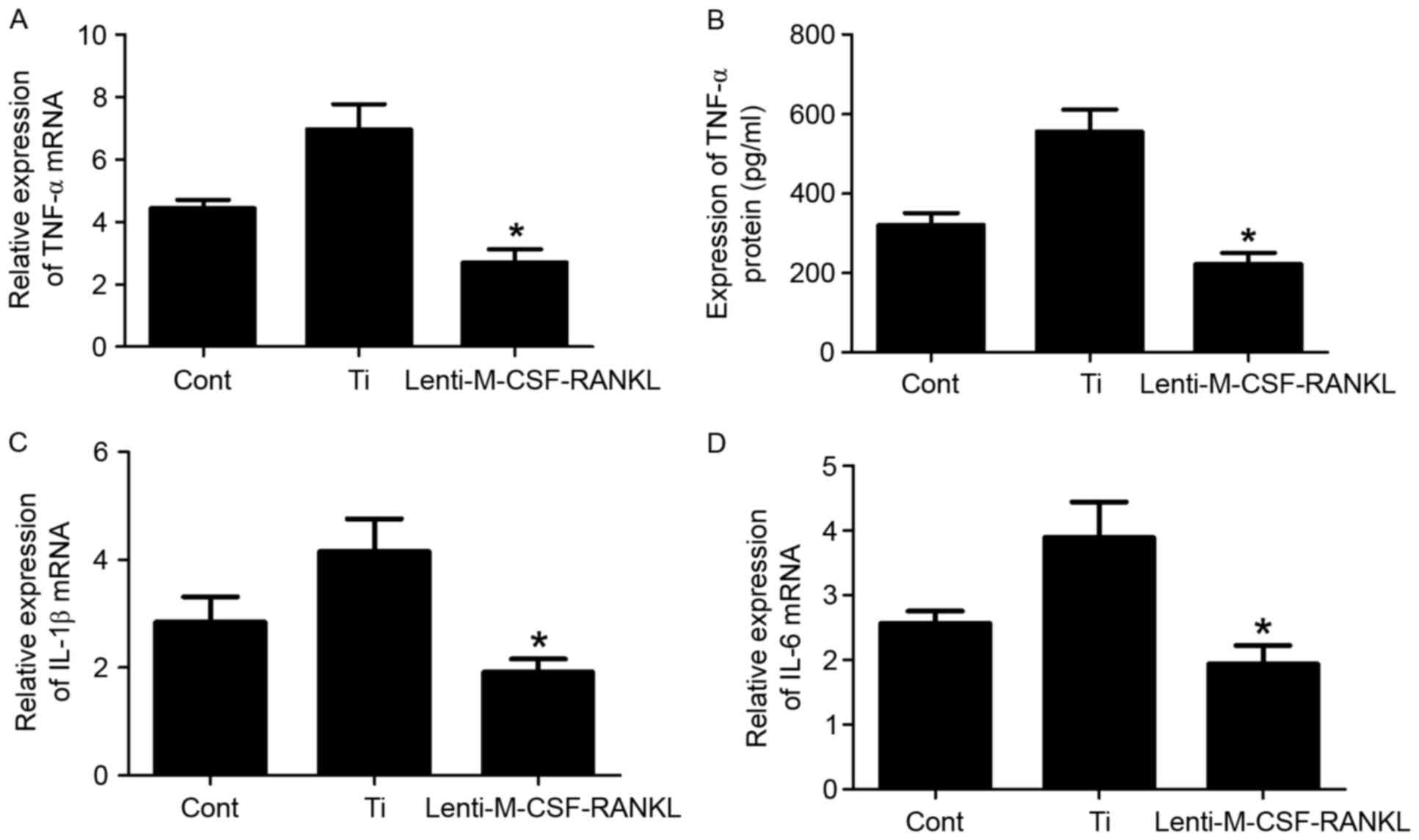
The murine macrophage/monocyte cell line RAW264.7
was treated with Ti or lenti-M-CSF-RANKL, or left untreated.
Transduced RAW264.7 cells were cultured in the presence of 0.1
mg/ml Ti particles to investigate the capacity of M-CSF and RANKL
mediated inhibition to reduce wear debris-induced inflammation. To
evaluate the particle-induced inflammatory response, the present
study examined the expression levels of proinflammatory cytokines,
including TNF-α, IL-1β and IL-6 (Fig.
2). Following a 24 h incubation with a combination of Ti
particles and lenti-M-CSF-RANKL, the TNF-α expression was inhibited
in the RAW264.7 cells but not in the Ti group cells, as determined
using ELISA analysis (Fig. 2B).
The mRNA levels of TNF-α, IL-1β and IL-6 were also assessed using
RT-qPCR (Fig. 2A, C and D).
Following 24 h of culture, the concentration of
TNF-α, IL-1β and IL-6 was significantly increased in the cell
lysate of cultures containing wear particles compared with those
without the particles (Fig. 2A, C and
D). Contrastingly (from the experimental results), TNF-α, L-1β
and IL-6 mRNA levels were significantly decreased in cultures
containing RAW264.7 cells infected with lenti-M-CSF-RANKL compared
with the Ti group (Fig. 2; all
P<0.05). Downregulation of M-CSF and RANKL resulted in a
decrease in the mRNA expression levels of IL-1β (Fig. 2C) and IL-6 (Fig. 2D), compared with the Ti group,
which indicates that M-CSF controls the mRNA expression levels of
IL-6 and IL-1β.
Inhibition of osteoclastogenesis by
transduction of RAW264.7 cells with lenti-M-CSF-RANKL
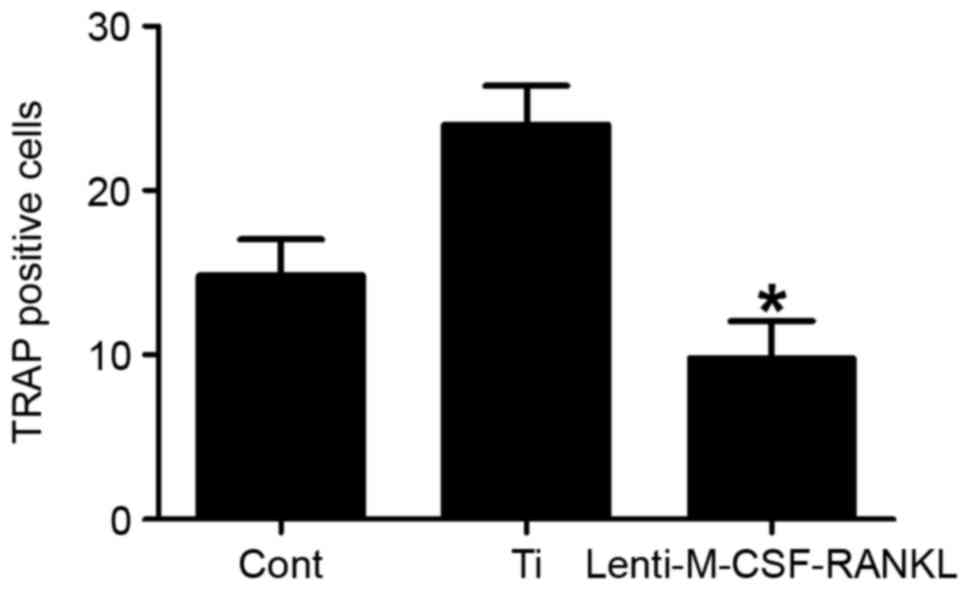
To determine the effect of each treatment on the
stimulation of osteoclastogenesis, TRAP-positive multinucleated
RAW264.7 cells (osteoclasts) were examined. A total of 7 days post
Ti-particle or lenti-M-CSF-RANKL stimulation, RAW264.7 cells were
fixed and stained for TRAP. The number of TRAP positive cells was
significantly decreased (P<0.05) in cultures infected with
lenti-M-CSF-RANKL compared with the Ti only group (Fig. 3). This data therefore suggests that
the lentiviral treatment was able to inhibit the Ti
particle-induced osteoclastogenesis.
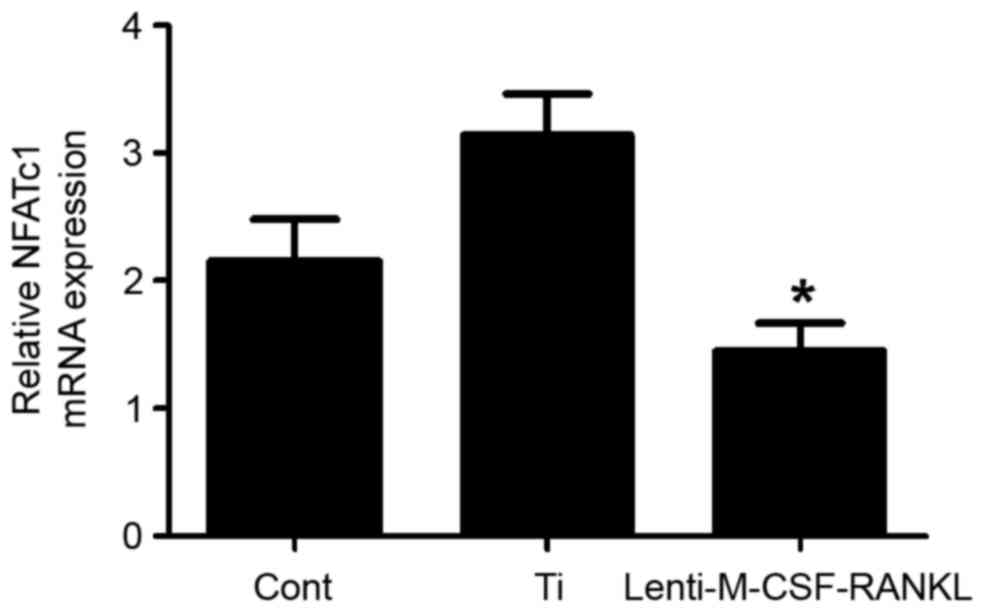
Expression of NFATc1 following
treatment with Ti particles and lenti-M-CSF-RANKL
A previous study demonstrated that upon inhibition
of RANK activity, osteoclast function in bone resorption decreases
significantly (25). In the
present study, the levels of NFATc1 mRNA in RAW264.7 cells when
cultured in with or without Ti particles or lenti-M-CSF-RANKL were
determined. At 48 h post transduction, in the lenti-M-CSF-RANKL
group, the expression of NFATc1 in RAW264.7 cells was reduced
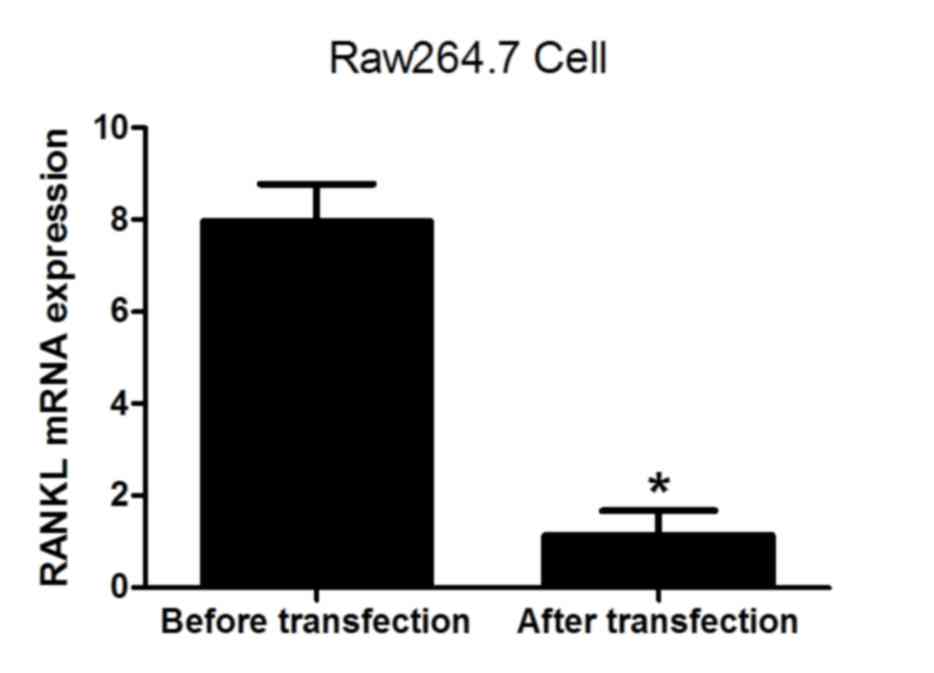
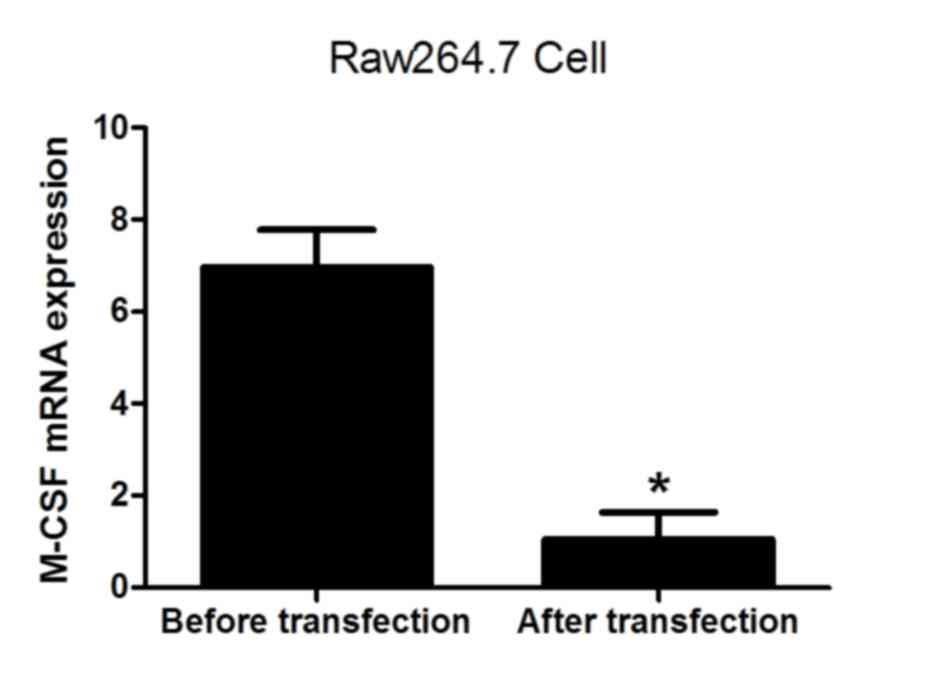
(Fig. 4). The levels of RANKL and
M-CSF mRNA were also analyzed prior to and following transfection.
The results demonstrated that the expression of RANKL and M-CSF was
significantly decreased following lentiviral transfection (both
P<0.05; Figs. 5 and 6), indicating that the lentivirus was
effective. The results suggest that the expression of NFATc1
positively correlates with the formation of osteoclasts.
Discussion
At present, aseptic loosening of joint replacement
prostheses is the most prevalent reason for joint revision
surgeries (35). Due to the
increasing median age of the population and a rise in the use of
prosthetics at a younger age, the number of joint replacement
procedures is increasing and the number of joint renovations is
also predicted to continue to rise in the future (36). At present, the best strategy for
the treatment of aseptic loosening is joint revision. Therefore,
investigation of the process of pathogenesis of aseptic loosening
of the prosthesis, and seeking effective prevention and treatment
measures can prolong the durability of the prosthesis. This project
studied the formation and differentiation of osteoclasts with an
emphasis on two critical factors: M-CSF and RANKL. The use of gene
silencing technology allowed for synergistic inhibition of
periprosthetical osteolysis induced by wear particles. The present
study investigated the role of CN/NFAT signaling pathway and RANKL
receptor in the differentiation of osteoclasts. Inhibition of M-CSF
and RANKL allowed for determination of the molecular mechanisms
underlying osteoclast differentiation and maturation. The gathered
evidence provides experimental basis for the treatment of
periprosthetic osteolysis induced by wear particles.
In the present study, a lentiviral vector containing
M-CSF-shRNA and RANKL-shRNA double sequences was constructed, which
can effectively inhibit the inflammatory reaction and osteoclast
formation, and confirmed the role of these genes in the wear
particle-induced osteolysis around the prostheses. The levels of
RANKL and M-CSF mRNA were also analyzed prior to and following
transfection. The results demonstrated that the expression of RANKL
and M-CSF was significantly decreased following lentiviral
transfection, indicating that the lentivirus was successfully
constructed and met the requirements of the study. Cells around the
prosthesis (osteoclasts, macrophages, fibroblasts and osteoblasts)
engulfed the wear particles, which could not have been otherwise
degraded by the bodily enzymes. Wear particles stimulate the
release of IL-1, IL-6, TNF-α, putative prostaglandin E2 synthase,
OPG, RANKL, M-CSF, vascular endothelial growth factor A and other
chemokines, leading to decreased osteoblast activity, increased
osteoclast differentiation, cell damage, cell apoptosis and
necrosis. As a result, the particles are re-released into the
extracellular matrix to become repeatedly phagocytosed leading to
repeated activation of phagocytes which secrete more inflammatory
cytokines and protease enzymes, to induce, maintain and exacerbate
wear particles induced chronic inflammatory and apoptotic
responses. This effectively leads to a decrease in the number of
osteoblasts and increased osteoclast differentiation (18).
M-CSF and NF-κB are involved in the differentiation
of osteoclasts, which is the only multinucleated cell derived from
monocyte-macrophage precursors with bone resorption activity. The
binding of M-CSF and RANKL to cell surface receptors provides
signals for osteoclast survival and proliferation. The binding
activates the corresponding signal transduction pathways allowing
differentiating osteoclasts to express specific genes to eventually
form mature osteoclasts and perform bone resorption functions
(37). It has been observed that
various cytokines (M-CSF, RANKL, TNF-α and IL-1) that mediate
osteoclast differentiation and maturation, can directly and
indirectly regulate the expression of key nuclear genes through
complex signaling pathways, to promote or inhibit the
differentiation of osteoclasts. NF-κB-associated activator protein
1 (AP-1) transcription factors, mainly c-Fos, c-Jun and NFAT
(13,16,19)
induce transcription of TRAP, calcitonin receptor, histone K,
carbonylase II and other genes promoting osteoclast
differentiation. Multiple signaling pathways interact forming a
complex regulatory network. TRAF6 is an essential upstream effector
in RANKL signaling pathway, Inhibitor of κB kinase activates NF-κB
causing disassociation of the inhibitor of κB subunit and rapid
translocation of the transcription factor into the nucleus. The
transcription factor interacts with promoter regions of a series of
genes, including IL-1, IL-6, TNF-α and M-CSF, to initiate their
transcription. However, the inflammatory cytokines can also
activate osteoclast differentiation (20,38).
Research has demonstrated that TNF-α is positively
regulated in osteoblasts by expression of RANKL, M-CSF and IL-6,
and enhances the activity of osteoclast precursor cells to promote
osteoclast differentiation. IL-1 is induced by M-CSF and IL-6
production, and is expressed in a paracrine manner to activate jun
N-terminal kinase (JNK) and promote osteoclast precursor cell
differentiation into mature osteoclasts. In addition, TNF-α, IL-1
and IL-6 can also activate the NF-κB signaling pathway (39,40)
causing secretion of more inflammatory factors, reinforcing the
inflammatory response. Sabokbar et al (41) also observed that inhibiting
RANKL-induced formation of osteoclasts was more evident in the
presence of a small amount of wear particles but when a large
number of wear particles accumulated around the prosthesis,
cytokines M-CSF and TNF-α were sufficient to induce osteoclast
differentiation. TNF-α and IL-1 can also promote synergistic
osteolysis, suggesting that when numerous wear particles accumulate
around the prosthesis, M-CSF and other inflammatory cytokines may
also also represent a reasonable system to directly induce
osteolysis. The results of the present study demonstrated that the
inhibition of M-CSF and RANKL perturbs osteoclast formation and
leads to osteolysis, which supports the results obtained by
Sabokbar et al (41).
Inhibiting M-CSF and RANKL can completely inhibit
osteoclastogenesis and the release of TNF-α. These results provided
grounds for the gene therapy of chronic osteolytic diseases. The
above experiment confirmed that titanium particles can induce
osteoclastogenesis and promote the release of inflammatory
factors.
NFAT is a Ca2+ regulatory transcription
factor family including five members, NFATc1-4 and NFAT5, which
have been described in osteoclasts. NFATcl is a key target gene
downstream of the RANK signal and is a fundamental effector
molecule responsible for osteoclast differentiation. Previous
studies demonstrated that RANKL could activate TRAF6/NF-κB,
JNK/AP-1 and Ca2+/CN pathways and activate NFATc1 to
induce differentiation of osteoclast (13,16).
Contrastingly, specific inhibitor CN can inhibit osteoclast
differentiation. In osteoclast progenitor Fos double-negative
cells, the expression level of NFATc1 decreased in the presence of
RANKL. NFATc1 gene silencing can effectively inhibit wear
particle-induced osteoclast differentiation (24), indicating that NFATc1 is
indispensable in the activation of osteoclasts. As NFATc1 is
induced by RANKL and regulates osteoclast differentiation (18,19),
wear particles promote osteoclast differentiation and inhibit
osteoblast differentiation and function by activating CN/NFAT
signaling pathway (24,25). The present study demonstrated that
the expression of NFATc1 in the presence of titanium particles is
significantly increased, but it decreases significantly in the
lentiviral group, indicating that the titanium particle-induced
activation of the NFAT pathway is implicated in the
osteoclastogenesis process.
In conclusion, the present study confirmed that the
technology used is reasonable and effective, and may provide a
novel effective way to prevent and treat the osteoclast osteolysis
induced by the abrasive particles. At the same time, inhibition of
wear particle-induced osteolysis around the prosthesis by gene
silencing, can contribute to gene-associated drug research and
development.
Acknowledgements
All experiments were completed in the Laboratory of
Cardiology of The First Affiliated Hospital of Harbin Medical
University (Harbin, China). The authors of the present study would
like to thank the managers and staff for their hospitality, time
and opinions. The present study was supported by funding from the
National Natural Science Foundation of China (grant no.
81270635).
References
|
1
|
Gallo J, Goodman SB, Konttinen YT and
Raska M: Particle disease: Biologic mechanisms of periprosthetic
osteolysis in total hip arthroplasty. Innate Immun. 19:213–224.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mellon SJ, Liddle AD and Pandit H: Hip
replacement: Landmark surgery in modern medical history. Maturitas.
75:221–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abu-Amer Y, Darwech I and Clohisy JC:
Aseptic loosening of total joint replacements: Mechanisms
underlying osteolysis and potential therapies. Arthritis Res Ther.
9 Suppl 1:S62007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobs JJ, Hallab NJ, Urban RM and Wimmer
MA: Wear particles. J Bone Joint Surg. 88 Supple 2:S99–S102. 2006.
View Article : Google Scholar
|
|
5
|
Ollivere B, Wimhurst JA, Clark IM and
Donell ST: Current concepts in osteolysis. J Bone Joint Surg Br.
94:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamanaka Y, Clohisy JC, Ito H, Matsuno T
and Abu-Amer Y: Blockade of JNK and NFAT pathways attenuates
orthopedic particle-stimulated osteoclastogenesis of human
osteoclast precursors and murine calvarial osteolysis. J Orthop
Res. 31:67–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones LC, Frondoza C and Hungerford DS:
Immunohistochemical evaluation of interface membranes from failed
cemented and uncemented acetabular components. J Biomed Mater Res.
48:889–898. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rao AJ, Gibon E, Ma T, Yao Z, Smith RL and
Goodman SB: Revision joint replacement, wear particles, and
macrophage polarization. Acta Biomater. 8:2815–2823. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teitelbaum SL and Ross FP: Genetic
regulation of osteoclast development and function. Nat Rev Genet.
4:638–649. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikeda K and Takeshita S: The role of
osteoclast differentiation and function in skeletal homeostasis. J
Biochem. 159:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suda T, Takahashi N, Udagawa N, Jimi E,
Gillespie MT and Martin TJ: Modulation of osteoclast
differentiation and function by the new members of the tumor
necrosis factor receptor and ligand families. Endocr Rev.
20:345–357. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH and Kim N: Signaling pathways in
osteoclast differentiation. Chonnam Med J. 52:12–17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka H, Mine T, Ogasa H, Taguchi T and
Liang CT: Expression of RANKL/OPG during bone remodeling in vivo.
Biochem Biophys Res Commun. 411:690–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakashima T and Takayanagi H: New
regulation mechanisms of osteoclast differentiation. Ann N Y Acad
Sci. 1240:E13–E18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsurukai T, Udagawa N, Matsuzaki K,
Takahashi N and Suda T: Roles of macrophage-colony stimulating
factor and osteoclast differentiation factor in osteoclastogenesis.
J Bone Miner Metab. 18:177–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Y, Jia T, Gong W, Wooley PH and Yang
SY: Titanium particle-challenged osteoblasts promote
osteoclastogenesis and osteolysis in a murine model of
periprosthestic osteolysis. Acta Biomater. 9:7564–7572. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takayanagi H: Osteoclast differentiation
and activation. Clin Calcium. 17:484–492. 2007.PubMed/NCBI
|
|
20
|
Yamashita T, Takahashi N and Udagawa N:
New roles of osteoblasts involved in osteoclast differentiation.
World J Orthop. 3:175–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Negishi-Koga T and Takayanagi H:
Ca2+-NFATc1 signaling is an essential axis of osteoclast
differentiation. Immunol Rev. 231:241–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aliprantis AO, Ueki Y, Sulyanto R, Park A,
Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR and Glimcher LH:
NFATc1 in mice represses osteoprotegerin during osteoclastogenesis
and dissociates systemic osteopenia from inflammation in cherubism.
J Clin Invest. 118:3775–3789. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Asagiri M, Sato K, Usami T, Ochi S,
Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E and
Takayanagi H: Autoamplification of NFATc1 expression determines its
essential role in bone homeostasis. J Exp Med. 202:1261–1269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JH, Youn BU, Kim K, Moon JB, Lee J,
Nam KI, Park YW, O'Leary DD, Kim KK and Kim N: Lhx2 regulates bone
remodeling in mice by modulating RANKL signaling in osteoclasts.
Cell Death Differ. 21:1613–1621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao B, Takami M, Yamada A, Wang X, Koga
T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, et al: Interferon
regulatory factor-8 regulates bone metabolism by suppressing
osteoclastogenesis. Nat Med. 15:1066–1071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu F, Zhu Z, Mao Y, Liu M, Tang T and Qiu
S: Inhibition of titanium particle-induced osteoclastogenesis
through inactivation of NFATc1 by VIVIT peptide. Biomaterials.
30:1756–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maoqiang L, Zhenan Z, Fengxiang L, Gang W,
Yuanqing M, Ming L, Xin Z and Tingting T: Enhancement of osteoblast
differentiation that is inhibited by titanium particles through
inactivation of NFATc1 by VIVIT peptide. J Biomed Mater Res A.
95:727–734. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Howie DW, Neale SD, Haynes DR, Holubowycz
OT, McGee MA, Solomon LB, Callary SA, Atkins GJ and Findlay DM:
Periprosthetic osteolysis after total hip replacement: Molecular
pathology and clinical management. Inflammopharmacology.
21:389–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lehil MS and Bozic KJ: Trends in total hip
arthroplasty implant utilization in the United States. J
Arthroplasty. 29:1915–1918. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peng L, Wang H, Song K, Wang H and Liu P:
Lentivirus-mediated TNF-α gene silencing and overexpression of
osteoprotegerin inhibit titanium particle-induced inflammatory
response and osteoclastogenesis in vitro. Mol Med Rep.
13:1010–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clohisy JC, Hirayama T, Frazier E, Han SK
and Abu-Amer Y: NF-kB signaling blockade abolishes implant
particle-induced osteoclastogenesis. J Orthop Res. 22:13–20. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng H, Yu X, Collin-Osdoby P and Osdoby
P: RANKL stimulates inducible nitric-oxide synthase expression and
nitric oxide production in developing osteoclasts. An autocrine
negative feedback mechanism triggered by RANKL-induced
interferon-beta via NF-kappaB that restrains osteoclastogenesis and
bone resorption. J Biol Chem. 281:15809–15820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Del Buono A, Denaro V and Maffulli N:
Genetic susceptibility to aseptic loosening following total hip
arthroplasty: A systematic review. Br Med Bull. 101:39–55. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh JA and Sloan JA: Health-related
quality of life in veterans with prevalent total knee arthroplasty
and total hip arthroplasty. Rheumatology (Oxford). 47:1826–1831.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH and Kim N: Regulation of NFATc1 in
Osteoclast Differentiation. J Bone Metab. 21:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zaidi M, Blair HC, Moonga BS, Abe E and
Huang CL: Osteoclastogenesis, bone resorption, and osteoclast-based
therapeutics. J Bone Miner Res. 18:599–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hotamisligil GS: Endoplasmic reticulum
stress and the inflammatory basis of metabolic disease. Cell.
140:900–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sabokbar A, Itonaga I, Sun SG, Kudo O and
Athanasou NA: Arthroplasty membrane-derived fibroblasts directly
induce osteoclast formation and osteolysis in aseptic loosening. J
Orthop Res. 23:511–519. 2005. View Article : Google Scholar : PubMed/NCBI
|