Introduction
Endometriosis is a common, and multifactorial
gynaecological disorder that affects 10–15% of reproductive-age
women (1). The development of this
condition is endometrial tissue grows outside of the uterine
cavity, which can induce varying degrees of painful symptoms and
infertility in infected individuals (2). Endometriosis also has a propensity to
recur and may be associated with ovarian cancer (3). The impact of endometriosis is great,
likely exceeding our expectations, as it can dramatically impair
the quality of life.
The pathogenesis of endometriosis was initially
defined as the presence of endometrial tissues in extra-uterine
cavity, including pelvic peritoneum, bladder and ureters (4). There are several hypotheses have been
proposed, including lymphatic and vascular metastasis, iatrogenic
direct implantation, coelomic metaplasia, embryonic rest and
mesenchymal cell induction (5).
Moreover, the development of endometriosis was also involved in
stem cells (6). This theory
postulates that the endometrial stem cells are abnormally shed
during menses, where they gain access to the peritoneal cavity by
retrograde menstruation and ectopic implantation. The presence of
mesenchymal stem cells (MSCs) in stromal cells of ectopic
endometrial tissues was reported (7). These results indicated that ectopic
endometrial stem cells (EnSCs) are regarded as responsible for the
pathogenesis of endometriosis.
Endometriosis has also been defined as an angiogenic
disease, since the ectopic survival of endometrium requires the
formation of new blood vessels. Angiogenesis, therefore, is a
critical step in developing endometriotic lesions (8). Vascular endothelial growth factor
(VEGF) is a member of VEGFA family, coding by a 28 kb-long gene
which is located on chromosome 6p21.3. It is known as a crucial
regulator of angiogenesis, endothelial cells growth and migration
(9). Several studies have reported
that VEGFA play an important role in the angiogenesis of
endometriosis (10). miRNAs such
as miR-126, Let7-f, miR-27b, miR-17-92 cluster and miR-130a were
identified as proangiogenic miRNAs that regulate the translation of
angiogenic factors (VEGF-A) (11).
miRNAs have recently emerged as an important factor in
endometriosis (12).
Toloubeydokhti et al (13)
assessed the expression of miR-17-5p, miR-23a, miR-23b and
miR-542-3p, and found that miR-23b and miR-542-3p are
downregulated, whereas miR-17-5p is upregulated in ectopic
endometrium, affecting the stability of their target genes'
expression, and playing an important role in the pathogenesis of
endometriosis. Moreover, miRNAs are aberrantly expressed in
endometriotic stromal cells play an important role in the
pathogenesis of endometriosis (14). miR-34a-5p is highly expressed in
multiple types of cancer, which inhibited tumor angiogenesis by
blocking VEGF production by directly inhibiting endothelial cell
functions (15).
In this study, the aim was to investigate the
expression of miR-34a-5p and VEGFA in endometriosis tissues, and
analyzed the function and mechanism of miR-34a on the endometriosis
stem cells (EnSCs).
Materials and methods
Endometriosis tissues
Ten endometriosis patients were diagnosed by
laparoscopic surgical examination, and undergone surgical excision
of endometriosis tissues. The control tissue samples were collected
from 10 premenopausal patients without endometriosis. All these
patients had not received any preoperative hormonal therapy or
taken any medicine for at least three months. The resected tissues
were minced and part of samples was stored at −80°C before the
total RNA and protein extraction. Written consent was obtained from
each patient before the study. This study was approved by the
Research Ethics Committee of the Zhujiang Hospital of Southern
Medical University (Guangzhou, China).
Endometrial stem cell isolation,
culture and transfection
Human endometriosis tissue was washed in PBS (Gibco,
USA), minced, and digested in collagenase (1 mg/ml, Gibco, USA) for
30–45 min at 37°C with agitation. Then, resultant cell solutions
were filtered and centrifuged, and mononuclear cells were separated
by Ficoll (Sigma, St. Louis, MO, USA) and washed in PBS. The
isolated cells were cultured in Dulbecco's modified Eagle's medium
(DMEM)/F-12 medium containing 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin antibiotic (all Gibco, Grand Island, NY,
USA) and then incubated at 37°C in 5% CO2. When the
cells were approximately 80% confluent, they were trypsinized
(Gibco) and halved for characterization by flow cytometry and
expansion. EnSCs were seeded into a 24-well plate and transfected
with the miR-34a-5p mimics or control miRNAs by
Lipofectamine® 2000 Transfection Reagent (Invitrogen,
Carlsbad, CA, USA).
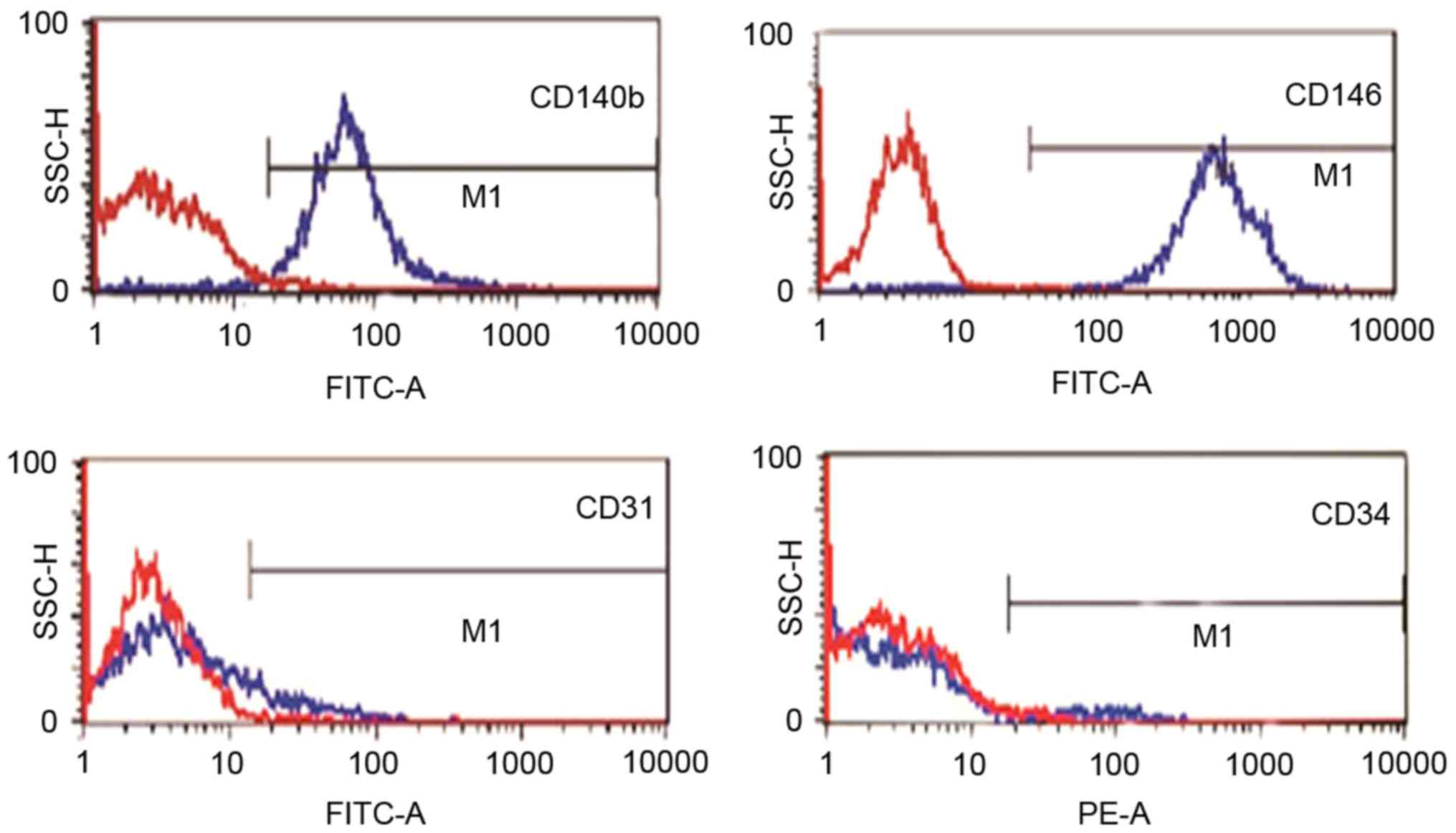
Flow cytometry
The EnSCs were characterized by flow cytometry for
cell surface markers. Cells were incubated with the specific
antibody at concentrations recommended by the respective
manufacturers for 1 h and analyzed by flow cytometry. The
antibodies used were: FITC-conjugated anti-CD146 (eBioScience Inc.,
San Diego, CA, USA; endometrial stem cell markers), FITC-conjugated
anti-CD140b (Abcam, Cambridge, MA, USA; endometrial stem cell
markers), FITC-conjugated anti-CD34 (Abcam; hemato-poieticmarker),
FITC-conjugated anti-CD31 (Novus Biologicals, Littleton, CO, USA;
endothelial marker).
RNA extraction and real-time PCR
To investigate the expression level of VEGFA mRNA
and miR-34a-5p, we extracted the total RNA from endometriosis
tissues or EnSCs. Tissues or cells were lysed with the Trizol
reagent (Life Technologies, Grand Island, NY, USA) according to the
manufacturer's specification. mRNAs were dissolved in RNase-free
water and stored at −80°C before utilize. The quantitative analysis
of RNA was performed by RT-PCR using One Step SYBR PrimeScript PLUS
RT-PCT kit (Takara, Tokyo, Japan) for each sample according to the
manufacturer's manual. After the reaction, the VEGFA mRNA level and
the miR-34a-5p level was calculated and presented as the relative
level of VEGFA to β-actin (as control) by ∆∆Ct method, each sample
was measured for three independent experiments.
Western blot analysis
Western blot analysis was performed to determine the
expression of VEGFA on the protein level. Harvested the EnSCs post
transfecting, and lysed the cells into cell lysis buffer (Bio-Rad,
Hercules, CA, USA). Centrifuged the samples at 12,000 × g for 15
min at 4°C and collected the supernatant. Protein extracts were
boiled in SDS/β-mercaptoethanol and separated in a 10% SDS-PAGE
(sodium dodecyl sulphate-polya-crylamide gel electrophoresis).
Then, transferred the protein sample to a nitrocellulose membrane
(Millipore, Bedford, MA, USA), blocked with 5% skimmed milk powder
overnight at 4°C. The membrane was incubated with VEGFA-spe-cific
antibody in TBST (mouse monoclonal antibody, Abcam, Cambridge, UK,
1:500) at 37°C for 1 h, washed with TBST, next incubated with the
HRP-linked secondary anti-mouse antibody (New England Biolabs,
Ipswich, UK) for 30 min at 37°C. ECL kit (Life Science, Woodland
Hills, CA, USA) was used to carry out the chemiluminescence
reaction. The membrane was scanned by a Smart Chemi-TM lamp
Analysis System (Thermo Scientific, Rockford, IL, USA) and
quantified according to the band density by Quantity One software
with β-actin as loading control.
Dual luciferase assay
The sequence of 3′UTR of VEGFA from hs-miR-34a-5p
were download from Genebank (NC-BI), aligned by Megalign (DNASTAR;
GATC Biotech, Konstanz, Germany). The sequence of mutant 3′UTR of
VEGFA was synthesized by Sangon Biotech (Shanghai, China). All the
sequences in this study were amplified by PCR (polymerase chain
reaction) with Phusion polymerase (New England Biolabs). 3′UTR of
VEGFA and the mutant 3′UTR of VEGFA were cloned into the upstream
of firefly luciferase (Fluc) gene behind the Cytomegalovirus
promoter in pmirGLO (Promega, Madison, WI, USA) with restriction
endonuclease and DNA ligase (New England Biolabs), received
VEGFA-Fluc and mutant-VEGFA-Fluc recombined plasmid. The ESCs
seeded in a 24-well plate were co-transfected with the miR-34a-5p
mimics/miR-Ctrl and VEGFA-Fluc/mutant-VEGFA-Fluc by
Lipofectamine® 2000 Transfection Reagent (Invitrogen).
48 h after transfecting, collected the cells and assayed with the
Dual-Luciferase Assay kit (Promega) used GLOMAX (Promega) and
recorded data, the relative luciferase activity (%) represented the
expression level of VEGFA.
MTT assay
Transfected EnSCs were diluted to a certain
concentration and then were placed over a 96-well plate, at a
density of 5×103 cells/well. Each well was inoculated
with 100 µl cell suspension, apart from one blank well (instead
with 100 µl medium containing 10% fetal bovine serum). Then they
were cultured in a 5% CO2 incubator at 37°C. After 0,
12, 24 and 48 h of culture, the proliferation of ESCs was detected
by MTT assay. With an addition of 20 µl
3-(4,5-Dimethylthiazol-2-yl)-5-(3-Carboxymethoxyphenyl)-2-
(4-Sulfophenyl)-2H-Tetrazolium, Inner Salt (MTS) reagent (Promega)
to each well, cells were then cultured in an incubator for 1–4 h.
After that, the optical density (OD) at 580 nm of each well was
obtained with a microplate reader (Biotek Instruments, Inc.,
Vermont, USA). The cell proliferation was evaluated by OD value.
All of these experiments were repeated three times.
Statistical analysis
All the analyses were performed using SPSS software,
version 17.0 (SPSS Inc., Chicago, IL, USA). SPSS was used to
compare miR-34a-5p expression levels between samples taken from
patients with or without endometriosis. Data are represented as
mean ± standard deviation (SD) unless indicated otherwise.
Statistical significance was tested using Student's t-test, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of isolated human
EnSCs
EnSCs isolated from endometriosis tissue were
positive for CD140b, CD146 and negative for CD31 and CD34, as
demonstrated by flow cytometry analysis (Fig. 1).
Expression of miR-34a-5p and VEGFA in
endometriosis
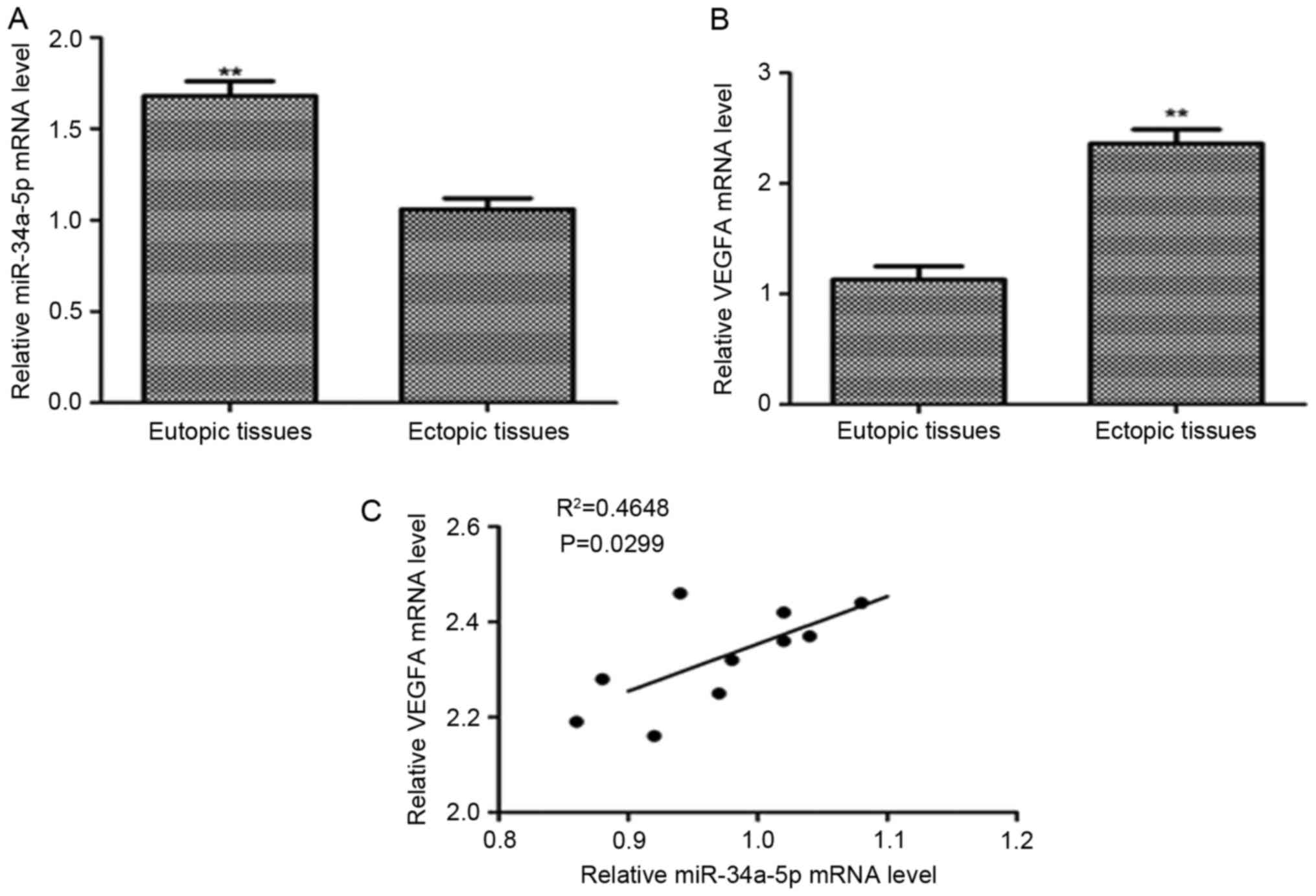
To investigate the expression of VEGFA in
endometriosis, we examined the mRNA level of VEGFA and miR-34a-5p
level by quantitative RT-PCR in 10 eutopic endometrial tissues and
10 ectopic endometrial tissues. We focused on miR-34a-5p because it
was significantly downregulated miRNA in ectopic endometrial
tissues by microarray analysis. It had shown that the relative mRNA
level of VEGFA was significantly upregulated in ectopic endometrial
tissues than in eutopic endometrial tissues (P<0.01) (Fig. 2A). The relative level of miR-34a-5p
was significantly lower in endometriosis tissues than that in
eutopic endometrial tissues (P<0.01) (Fig. 2B). By analyzing the correlation of
relative VEGFA mRNA level with the miR-34a-5p level in ectopic
endometrial tissues, we found an inverse correlation between them.
The high mRNA level of VEGFA accompanied a low expression level of
miR-34a-5p (Pearson correlation, R2=0.4648, P=0.0299 (Fig. 2C).
Expression of VEGFA in EnSCs after
transfection with miR-34a-5p mimics
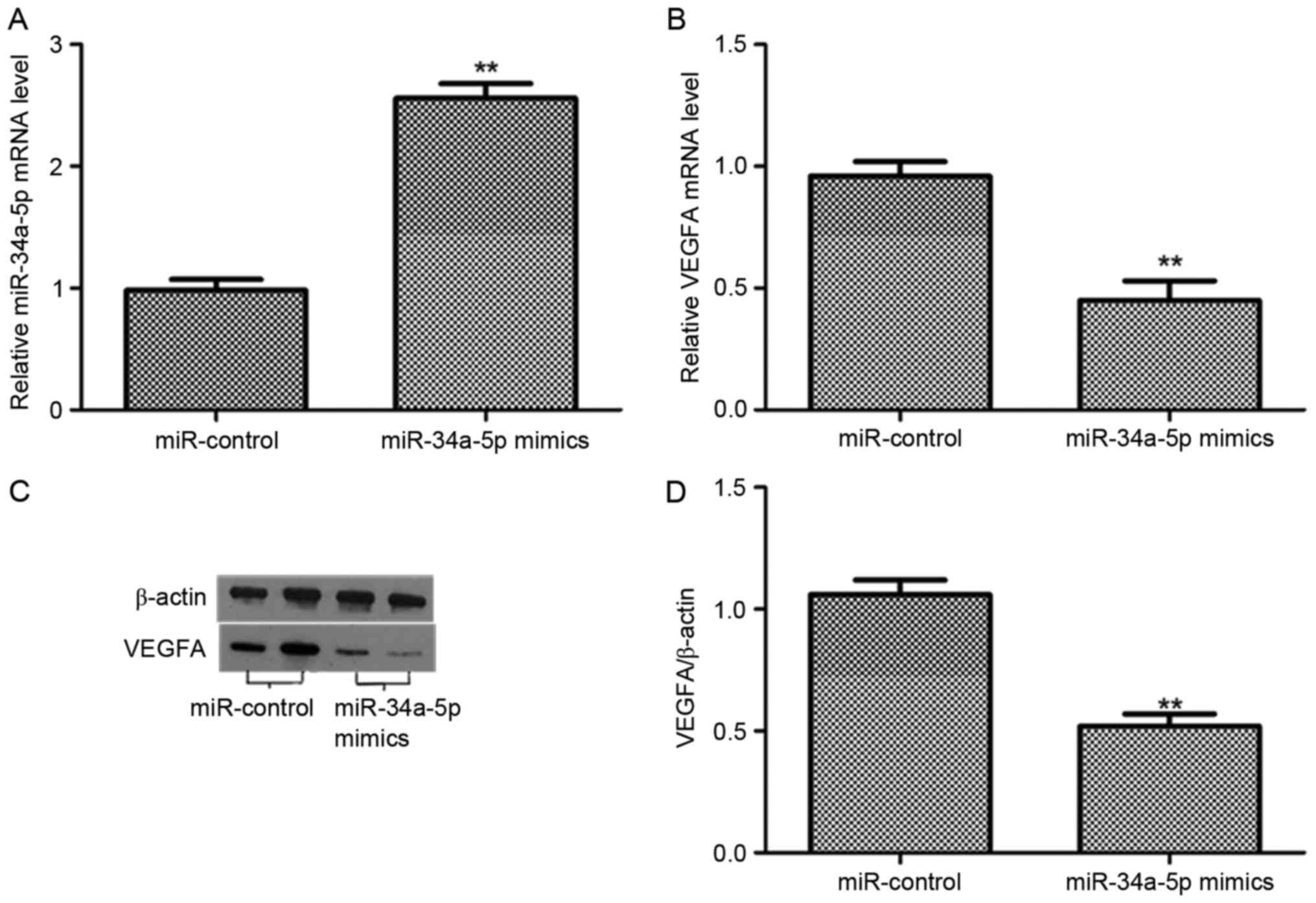
To further determined whether the expression of
VEGFA was downregulated by miR-34a-5p in EnSCs. We examined the
relative level of miR-34a-5p and the expression of VEGFA in both
mRNA and protein levels after 48 h transfection. The level of
miR-34a-5p was significantly increased after the transfection with
miR-34a-5p mimics (P<0.01), compared with the miR-control group.
However, the VEGFA mRNA was reduced post the transfection with
miR-34a-5p mimics (P<0.01). The expression level of VEGFA
protein was decreased in EnSCs transfected with miR-34a-5p mimics
rather than miR-control group. The band intensity of VEGFA was
downregulated in EnSCs transfected with miR-34a-5p mimics
(P<0.01). Therefore, miR-34a-5p suppressed the expression of
VEGFA in both mRNA and protein levels in ESCs (Fig. 3).
miR-34a-5p targets the 3′UTR of VEGFA
gene
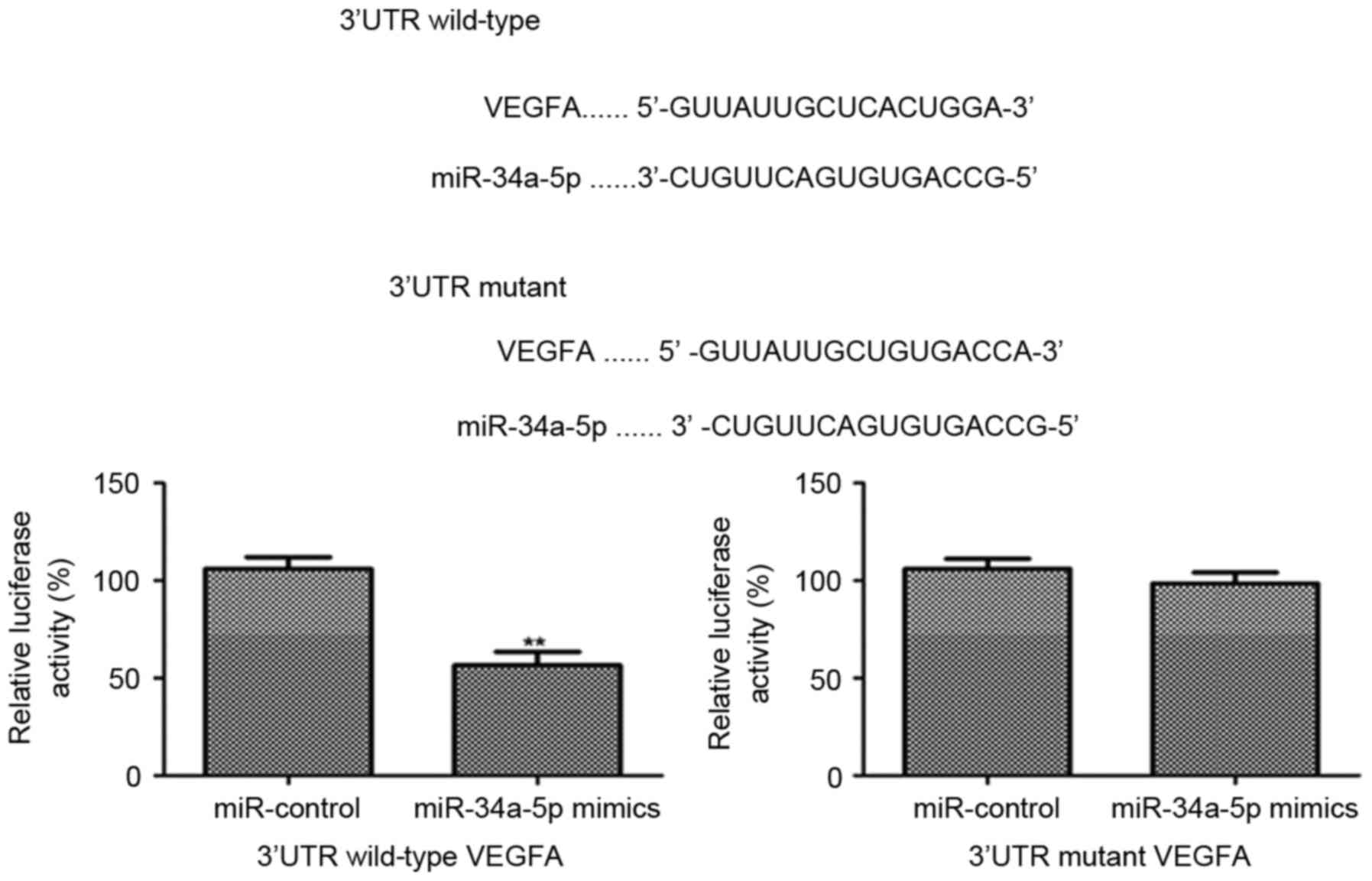
To verify miR-34a-5p inhibited the expression of
VEGFA by targeting the 3′UTR of VEGFA, miR-34a-5p mimics and
miR-control were transfected into EnSCs which already had been
transfected with the 3′UTR of VEGFA linked a luciferase reporter.
The results showed that the relative luciferase activity was
significantly decreased post transfecting miR-34a-5p mimics
(P<0.001). However, there was no significant difference in the
relative luciferase activity between miR-34a-5p mimics and
miR-control in the EnSCs, which were transfected with the mutant
3′UTR of VEGFA. All these data demonstrates that miR-34a-5p targets
the 3′UTR of VEGFA gene and inhibits the VEGFA expression
effectively (Fig. 4).
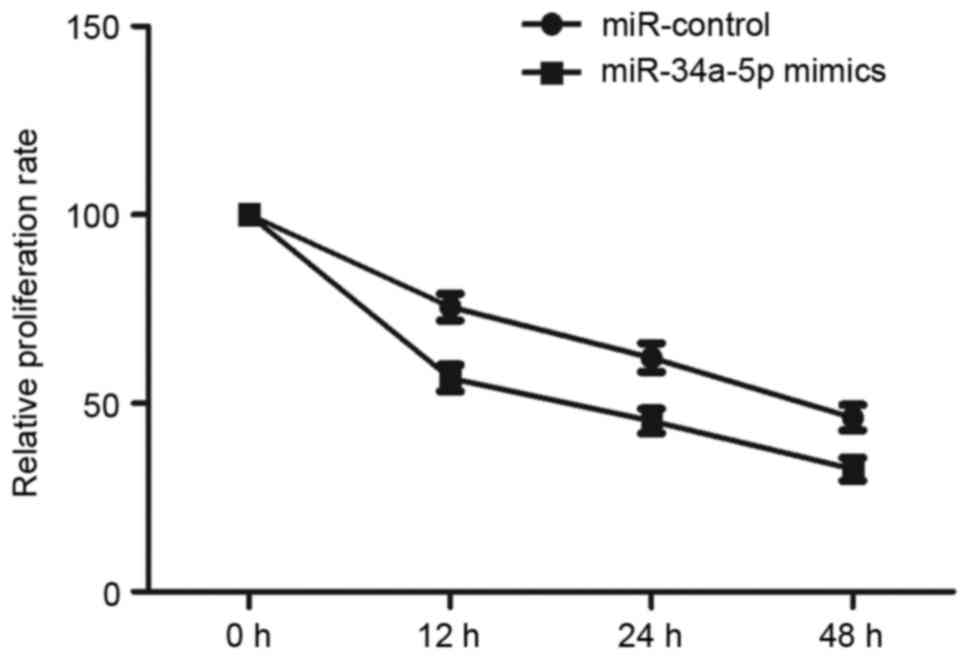
Effect of miR-34a-5p mimics on the
proliferation of EnSCs
miR-34a-5p could modulate the level of VEGFA, which
is a regulator of cell growth, so miR-34a-5p may also influence
proliferation of EnSCs. Then, we curved the ESCs number at 0, 12,
24 and 48 h after transfecting with miR-control and miR-34a-5p
mimics. Fig. 5 showed that the
proliferation ability of EnSCs was significantly reduced post the
transfection with miR-34a-5p mimics (P<0.01).
Discussion
The present study is the first to investigate the
expression of miR-34a-5p and VEGFA in endometriosis. The results
showed that miR-34a-5p expression levels significantly decreased in
endometriosis compared with that in without endometriosis patients,
but the VEGFA expression levels significantly increased in
endometriosis compared with without endometriosis patients.
Negative correlation was observed between relative VEGFA mRNA level
and miR-34a-5p level in ectopic endometrial tissues. Moreover,
over-expression of miR-34a-5p can significantly inhibit the
expression and proliferation of VEGFA in EnSCs.
miR-34a belongs to a miRNA family, and its as a
strong antitumor regulator of cell growth in multiple myeloma was
observed (16,17). miR-34a is, to date, one of the most
characterized tumor suppressor miRNAs in a variety of tumors
(18). miR-34a-5p expression has
been shown to be directly regulates the expression of proteins
involved in cell cycle, differentiation, apoptosis, and antagonizes
processes, including cervical, ovarian and testicular cancer
(19,20). Ma et al conducted a series
of studies on miR-34a-5p, and were the first to confirm its
pro-apoptotic function (21).
Moreover, miR-34a-5p has been found to inhibit cell proliferation
and invasion in vitro, which suggested that miR-34a-5p might
play a role in inhibiting tumor recurrence (22). miR-34a-5p has been reported to be a
direct transcriptional target of p53 and is downregulated in
various types of tumors (23). We
measured miR-34a-5p expression levels in endometrial tissue and
ESCs. Regardless of the sample types, miR-34a-5p expression levels
were consistently downregulated in patients with endometriosis. The
results support previous reports that miR-34a-5p suppresses disease
progression, including diseases associated with the
endometrium.
VEGFA is a dimeric glycoprotein that plays an
important role in vasculogenesis, and its overexpression often
occurred in various cancers (24).
VEGF is involved in the pathogenesis of endometriosis. Blocking
VEGF to treat endometriosis decreases vascular density and cell
proliferation and increases cell apoptosis (25). In this study, we compared the
expression level of VEGFA in eutopic and ectopic endometrial
tissues. The results showed that the VEGFA was overexpressed in
ectopic endometrial tissues compared with eutopic endometrial
tissues. It had reported that miRNAs could regulate the expression
of VEGFA (26). miRNA-34a
modulates the phosphorylation of FAK by negatively regulating VEGF
in colorectal cancer (27).
miR-638 expression was inversely correlated with VEGF expression in
human hepatocellular carcinoma samples (28). In addition, miR-3072-5p inhibited
VEGF expression in ischemic preconditioning was also observed
(29). Here we demonstrated that
miR-34a-5p regulates cell proliferation and angiogenesis by
regulating VEGFA levels in EnSCs, and VEGFA mRNA level was
significantly increased while the level of miR-34a-5p was markedly
decreased in endometriosis tissues. The correlation analysis showed
that the VEGFA mRNA level was inversely correlated with miR-34a-5p.
All these data indicated a potential role of miR-34a-5p in
inhibiting expression of VEGFA in endometriosis.
Angiogenesis is a crucial determinant in tumor
initiation, progression, and metastasis. Angiogenesis plays an
important role in endometriosis progression (30). VEGFA is thought to be the primary
stimulator of angiogenesis, during the course of development and in
a variety of pathological conditions. A large number of researches
have been done showing that miRNAs play important roles in vascular
development and angiogenesis (31). It is reported that miRNAs (eg
miR-17-5p and miR-199a-5p) regulate angiogenesis (32). In addition, miR-203 suppresses
tumor growth and angiogenesis by targeting VEGFA in cervical cancer
(33). We found an inverse
correlation between levels of VEGFA and miR-34a-5p. To our
knowledge, this is the first demonstration that VEGFA is a
functional target of miR-34a-5p. As such, miR-34a-5p regulates cell
proliferation and angiogenesis through its effect on VEGFA.
In summary, VEGFA was overexpressed in endometriosis
tissues, and we identified miR-34a-5p is able to target the VEGF
gene, preventing the latter to function as an inhibitor of
angiogenesis. These results imply that miR-34a-5p might regulate
VEGFA in ESCs and might contribute to the pathogenesis of
endometriosis. This study may provide a potential biomarker for
endometriosis therapeutics.
Acknowledgements
The present study was supported by the Science and
Technology Planning Project of Guangdong Province (grant no.
2014A020212667) and the National Natural Science Fundation of China
(grant no. 81701418).
References
|
1
|
Yoder N, Tal R and Martin JR: Abdominal
ectopic pregnancy after in vitro fertilization and single embryo
transfer: A case report and systematic review. Reprod Biol
Endocrinol. 14:692016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinulescu DM, Ince TA, Quade BJ, Shafer
SA, Crowley D and Jacks T: Role of K-ras and Pten in the
development of mouse models of endometriosis and endometrioid
ovarian cancer. Nat Med. 11:63–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greene AD, Lang SA, Kendziorski JA,
Sroga-Rios JM, Herzog TJ and Burns KA: Endometriosis: Where are we
and where are we going? Reproduction. 152:R63–R78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maruyama T and Yoshimura Y: Stem cell
theory for the pathogenesis of endometriosis. Front Biosci.
4:2754–2763. 2012. View
Article : Google Scholar
|
|
6
|
Sasson IE and Taylor HS: Stem cells and
the pathogenesis of endometriosis. Ann N Y Acad Sci. 1127:106–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kao AP, Wang KH, Chang CC, Lee JN, Long
CY, Chen HS, Tsai CF, Hsieh TH and Tsai EM: Comparative study of
human eutopic and ectopic endometrial mesenchymal stem cells and
the development of an in vivo endometriotic invasion model. Fertil
Steril. 95:1308–1315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Groothuis PG, Nap AW, Winterhager E and
Grümmer R: Vascular development in endometriosis. Angiogenesis.
8:147–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teague EM, Print CG and Hull ML: The role
of microRNAs in endometriosis and associated reproductive
conditions. Hum Reprod Update. 16:142–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou Y, Guo CG and Zhang MM: Inhibition of
human hepatocellular carcinoma tumor angiogenesis by siRNA
silencing of VEGF via hepatic artery perfusion. Eur Rev Med
Pharmacol Sci. 19:4751–4761. 2015.PubMed/NCBI
|
|
11
|
Liu B, Ding JF, Luo J, Lu L, Yang F and
Tan XD: Seven protective miRNA signatures for prognosis of cervical
cancer. Oncotarget. 7:56690–56698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho S, Mutlu L, Grechukhina O and Taylor
HS: Circulating microRNAs as potential biomarkers for
endometriosis. Fertil Steril. 103:1252–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toloubeydokhti T, Pan Q, Luo X, Bukulmez O
and Chegini N: The expression and ovarian steroid regulation of
endometrial micro-RNAs. Reprod Sci. 15:993–1001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Braza-Boïls A, Salloum-Asfar S,
Marí-Alexandre J, Arroyo AB, González-Conejero R, Barceló-Molina M,
García-Oms J, Vicente V, Estellés A, Gilabert-Estellés and Martínez
C: Peritoneal fluid modifies the microRNA expression profile in
endometrial and endometriotic cells from women with endometriosis.
Hum Reprod. 30:2292–2302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao J, Li N, Dong Y, Li S, Xu L, Li X, Li
Y, Li Z, Ng SS, Sung JJ, et al: miR-34a-5p suppresses colorectal
cancer metastasis and predicts recurrence in patients with stage
II/III colorectal cancer. Oncogene. 34:4142–4152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scognamiglio I, Di Martino MT, Campani V,
Virgilio A, Galeone A, Gullà A, Cantafio ME Gallo, Misso G,
Tagliaferri P, Tassone P, et al: Transferrin-conjugated SNALPs
encapsulating 2′-O-methylated miR-34a for the treatment of multiple
myeloma. Biomed Res Int. Biomed Res Int. 2014:2173652014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Martino MT, Campani V, Misso G,
Cantafio ME Gallo, Gullà A, Foresta U, Guzzi PH, Castellano M,
Grimaldi A, Gigantino V, et al: In vivo activity of miR-34a mimics
delivered by stable nucleic acid lipid particles (SNALPs) against
multiple myeloma. PLoS One. 9:e900052014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Misso G, Di Martino MT, De Rosa G, Farooqi
AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P,
Tassone P and Caraglia M: Mir-34: A new weapon against cancer? Mol
Ther Nucleic Acids. 3:e1942014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song P, Ye LF, Zhang C, Peng T and Zhou
XH: Long non-coding RNA XIST exerts oncogenic functions in human
nasopharyngeal carcinoma by targeting miR-34a-5p. Gene. 592:8–14.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li XY, Wen JY, Jia CC, Wang TT, Li X, Dong
M, Lin QU, Chen ZH, Ma XK, Wei LI, et al: MicroRNA-34a-5p enhances
sensitivity to chemotherapy by targeting AXL in hepatocellular
carcinoma MHCC-97 L cells. Oncol Lett. 10:2691–2698.
2015.PubMed/NCBI
|
|
21
|
Ma ZB, Kong XL, Cui G, Ren CC, Zhang YJ,
Fan SJ and Li YH: Expression and clinical significance of miRNA-34a
in colorectal cancer. Asian Pac J Cancer Prev. 15:9265–9270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walton SJ, Lewis A, Jeffery R, Thompson H,
Feakins R, Giannoulatou E, Yau C, Lindsay JO, Clark SK and Silver
A: Familial adenomatous patients with desmoid tumours show
increased expression of miR-34a in serum and high levels in
tumours. Oncoscience. 3:173–185. 2016.PubMed/NCBI
|
|
23
|
Lu H, Hao L, Li S, Lin S, Lv L, Chen Y,
Cui H, Zi T, Chu X, Na L and Sun C: Elevated circulating stearic
acid leads to a major lipotoxic effect on mouse pancreatic beta
cells in hyperlipidaemia via a miR-34a-5p-mediated
PERK/p53-dependent pathway. Diabetologia. 59:1247–1257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YZ, Wang LJ, Li X, Li SL, Wang JL, Wu
ZH, Gong L and Zhang XD: Vascular endothelial growth factor gene
polymorphisms contribute to the risk of endometriosis: An updated
systematic review and meta-analysis of 14 case-control studies.
Genet Mol Res. 12:1035–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramón LA, Braza-Boïls A, Gilabert-Estellés
J, Gilabert J, España F, Chirivella M and Estellés A: MicroRNAs
expression in endometriosis and their relation to angiogenic
factors. Hum Reprod. 26:1082–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang D, Zhou J and Dong M: Dysregulation
of microRNA-34a expression in colorectal cancer inhibits the
phosphorylation of FAK via VEGF. Dig Dis Sci. 59:958–967. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng J, Chen Y, Zhao P, Liu X, Dong J, Li
J, Huang C, Wu R and Lv Y: Downregulation of miRNA-638 promotes
angiogenesis and growth of hepatocellular carcinoma by targeting
VEGF. Oncotarget. 7:30702–30711. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ueno K, Samura M, Nakamura T, Tanaka Y,
Takeuchi Y, Kawamura D, Takahashi M, Hosoyama T, Morikage N and
Hamano K: Increased plasma VEGF levels following ischemic
preconditioning are associated with downregulation of miRNA-762 and
miR-3072-5p. Sci Rep. 6:367582016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SH, Choi YM, Chae HD, Kim CH and Kang
BM: Decreased expression of angiogenin in the eutopic endometrium
from women with advanced stage endometriosis. J Korean Med Sci.
23:802–807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Finn NA and Searles CD: Intracellular and
extracellular miRNAs in regulation of angiogenesis signaling. Curr
Angiogenes. 4:299–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu CY, Hsieh TH, Tsai CF, Tsai HP, Chen
HS, Chang Y, Chuang HY, Lee JN, Hsu YL and Tsai EM: miRNA-199a-5p
regulates VEGFA in endometrial mesenchymal stem cells and
contributes to the pathogenesis of endometriosis. J Pathol.
232:330–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y,
Zhu J, Cui F, Zhao W and Shi H: miR-203 suppresses tumor growth and
angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol
Biochem. 32:64–73. 2013. View Article : Google Scholar : PubMed/NCBI
|