Introduction
Ureteral obstruction is a common urological disease.
If not treated in a timely manner, it will progress toward renal
interstitial fibrosis, which is a common, irreversible pathological
change that is also observed in other chronic and progressive
kidney diseases (1). The
unilateral ureteral obstruction (UUO) model is a classical animal
model used to study obstructive renal tubular epithelial apoptosis
and interstitial fibrosis (2). The
major presentations of renal injury caused by UUO are renal tubular
epithelial cell apoptosis, interstitial inflammatory reaction and
progressive fibrosis, of which, cell apoptosis has a close
relationship with renal fibrosis (3). Inhibition of cell apoptosis may delay
or reverse renal tubular interstitial fibrosis to improve renal
function and the prognosis of patients with kidney diseases
(4). The Bcl-2 family members are
key factors in the regulation of apoptosis. Bcl-2 is a
proto-oncogene and can inhibit cell apoptosis, whereas Bax promotes
cell apoptosis. Up-regulation of Bcl-2 and downregulation of Bax
indicates that renal cell apoptosis is diminishing (5). During cell apoptosis, the caspase
family member, caspase-3, is the most important terminal cleavage
enzyme. It plays the role of an apoptosis executor and is also an
important component in the killing mechanism of cytotoxic T
lymphocytes (CTL).
Ulinastatin (UTI) is a type of glycoprotein isolated
from human urine. It is a typical Kunitz-type, broad-spectrum,
high-efficiency protease inhibitor (6). It functions in the clearance of
oxygen free radicals and the inhibition of inflammatory reactions
(7,8). It is applied clinically for the
treatment of acute pancreatitis and extracorporeal circulation
injury and the prevention of shock and surgical invasion. Recent
studies indicate that UTI inhibited cell apoptosis during brain
injury (9,10) and had a protective function in
various acute renal injuries (11,12).
In addition, it has been reported that UTI inhibited lung (13) and renal (14) fibrosis; however, the specific
mechanism is still not clear. This study aimed to investigate
whether UTI inhibits renal interstitial fibrosis by preventing
renal cell apoptosis in UUO rats.
Materials and methods
Ethics statement and animals
Male Wistar rats aged 8–12 weeks with body weights
of 180–200 g were provided by the Animal Experimental Center of the
Second Affiliated Hospital of Harbin Medical University (Harbin,
China). All rats were nurtured and maintained according to the
‘Care and Use of Laboratory Animals’ guidelines published by the
National Institute of Health (15). The animal use protocol was reviewed
and approved by the Institutional Animal Care and Use Committee
(IACUC) of the Second Affiliated Hospital of Harbin Medical
University.
Establishment of the animal model and
grouping
A total of 18 male Wistar rats were randomly divided
into the following 3 groups: The sham surgery group (Sham, n=6),
the UUO group (UUO, n=6) and the UTI group (UTI, n=6). The UUO
model was established according to previous literature (16). The UUO and UTI groups received a
left ureteral ligation after intraperitoneal anesthesia using 10%
chloral hydrate and received intervention using normal saline (1
ml/kg/d) and UTI (40,000 unit/kg/d; Techpool Bio-pharma Co., Ltd.,
Guangdong, China) starting on day 1 after surgery. On day 7 after
surgery, the rats were sacrificed using euthanasia. In the Sham
group, the left ureter was freed but not ligated after
intraperitoneal anesthesia using 10% chloral hydrate; after 7 days
of abdominal closure, the rats were sacrificed using
euthanasia.
Specimen collection and
processing
Before the rats were sacrificed, 5 ml of venous
blood was collected from each rat. The serum samples were separated
by centrifugation and stored in a −20°C freezer. Normal saline was
perfused through the left ventricle to wash the renal tissues. The
renal cortical tissues at the obstruction side were collected; some
tissues were placed into cryotubes, snap frozen and stored in a
−80°C freezer. The remaining tissues were divided into 2 blocks,
each with a size of approximately 4×5 mm; fixed in 4%
paraformaldehyde for 36 h; and conventionally prepared in sections
of approximately 4-µm thickness for pathology staining,
immunohistochemistry and terminal deoxynucleotidyl transferase dUTP
nick end labeling (TUNEL) staining.
Detection of biochemical
indicators
The serum blood urea nitrogen (BUN) and serum
creatinine (Scr) were detected using an automatic biochemical
analyzer.
Examination of pathological changes in
renal tissues using renal histopathology and semi-quantitative
analysis
Paraffin sections were used for routine hematoxylin
and eosin (H&E) and Masson staining. The levels of renal
tubular injury and renal interstitial fibrosis were evaluated using
a semi-quantitative scoring method (17). Twenty-five different high-power
fields were randomly selected in each specimen (magnification,
×100). Renal tubular injury included vacuolar degeneration,
necrosis and renal tubular dilation and atrophy. Renal tubular
injury classification was as follows: 0 points, normal; 1 point,
renal tubular injury in <25%; 2 points, renal tubular injury in
25–49%; and 3 points, renal tubular injury in ≥50%. Renal
interstitial fibrosis classification was as follows: 0 points,
normal; 1 point, renal interstitial fibrosis in <25% of the
field area; 2 points, renal interstitial fibrosis in 25–49% of the
field area; 3 points, renal interstitial fibrosis in 50–74% of the
field area; and 4 points, renal interstitial fibrosis in ≥75% of
the field area. The average values of 25 fields were calculated to
obtain the renal tubular injury index and renal interstitial
fibrosis scores of a specimen.
Detection of the percentage of renal
tubular epithelial cell apoptosis using TUNEL staining
After the paraffin sections of the renal tissues
were deparaffinized and rehydrated, the procedures were performed
according to the instruction manual of the TUNEL reagent kit (Roche
Applied Science, Madison, WI, USA). Sections were developed using
DAB, and the nuclei were counter stained using hematoxylin.
Sections were mounted and observed under a light microscope.
Twenty-five different fields were randomly selected under
high-power magnification (×400) to count the number of apoptotic
renal tubular epithelial cells. The average value was used as the
value of apoptosis.
Immunohistochemistry and
semi-quantitative analysis
Paraffin-embedded tissue sections of 4-µm thickness
were sequentially processed with tissue fixation, dehydration and
antigen retrieval. Changes in the distribution and expression of
Bax, Bcl-2, caspase-3 in the renal tissues were detected using
indirect immunostaining. The mouse anti-rat Bax, Bcl-2 and
caspase-3 monoclonal antibodies (1:100; BD Pharmingen, San Diego,
CA, USA) were added separately and incubated in a moisture box at
4°C overnight. PBS was used to replace the primary antibody and
used as the negative control. Horseradish peroxidase-labeled
anti-mouse secondary antibody IgG (1:100; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was added and incubated in a moisture box at
room temperature for 2 h. The results were developed using DAB, and
sections were mounted and observed under a microscope.
Classification of the Bax, Bcl-2 and caspase-3 staining was as
follows: 0 points, no renal tubular and interstitial staining; 1
point, <25% renal tubular staining with a lighter color; 2
points, 25–49% renal tubular staining with a proper color; 3
points, 50–74% renal tubular staining with a darker color; and 4
points, ≥75% renal tubular staining with a very dark color.
Twenty-five different fields were randomly selected under
high-power magnification (×200), and calculations were performed
according to the above semi-quantitative method. The average value
was the experimental value of the specimen.
Statistical analysis
All data are expressed as the mean ± standard
deviation (x ± s). SPSS 17.0 statistical software (SPSS Inc.,
Chicago, IL, USA) was used for the statistical analysis. Comparison
of the mean values between the groups was performed using one-way
analysis of variance (one-way ANOVA). Pairwise comparison between
the mean values was performed using a t test. P<0.05 indicated
the difference had statistical significance.
Results
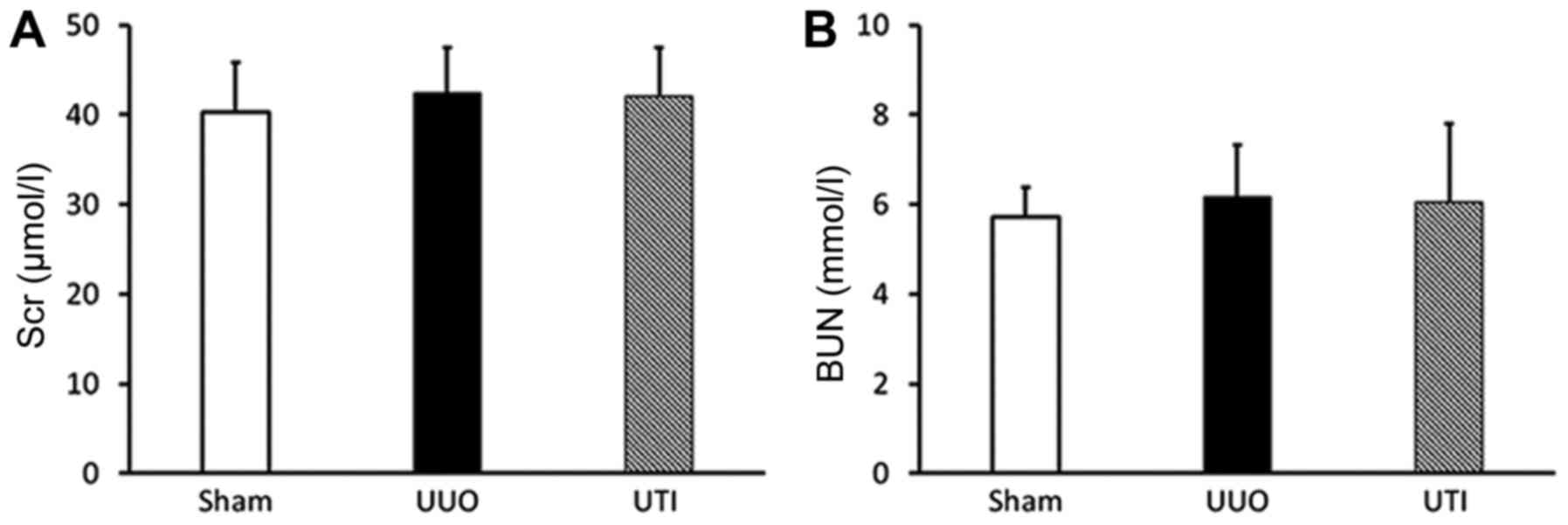
Scr and BUN results
The rat Scr and BUN levels were within the normal
ranges in all groups, and there was no significant difference among
them (P>0.05) (Fig. 1).
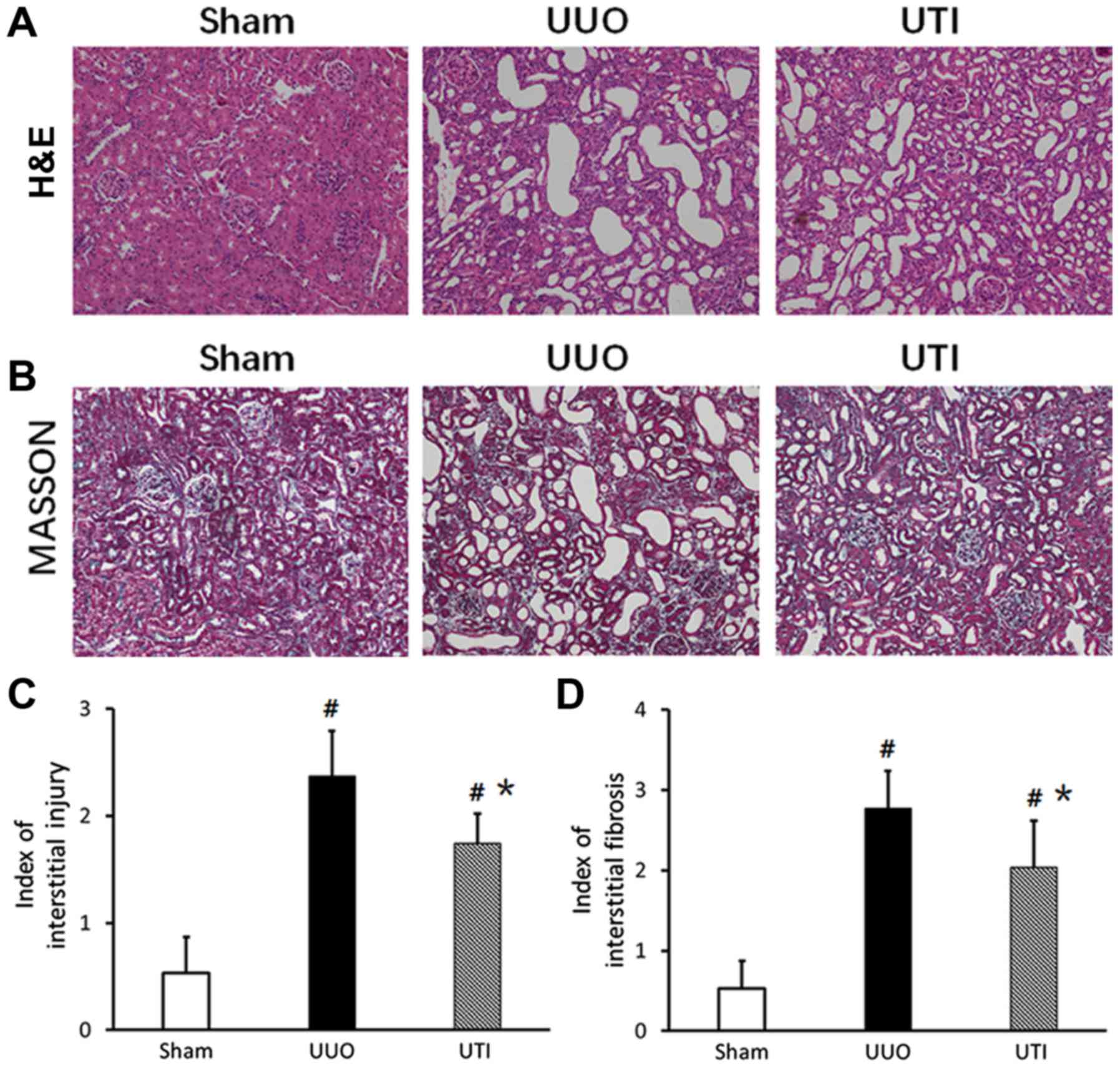
Renal pathological changes
Observation using the naked eye showed that the size
and morphology of the kidneys in the Sham group were normal and
that the color was ruddy. In the UUO group, the kidney at the
obstruction side had obvious swelling and enlargement, the renal
pelvis had a large amount of effusion, and the renal parenchyma
became thinner. In the UTI group, the kidney had slight swelling
and enlargement, there was a small amount of effusion, and the
parenchyma became slightly thinner. Under a light microscope, the
renal interstitium and renal tubular structure were basically clear
and intact, the vacuolar degeneration of renal tubular epithelial
cells was observed sporadically, and there was focal inflammatory
cell infiltration. The UUO and UTI groups had different degrees of
diffuse renal interstitial inflammatory cell infiltration, there
was interstitial widening, there were different degrees of
accompanied renal tubular dilation or atrophy, there was
interstitial fibrosis, and there were no obvious glomerular
lesions. The lesions in the UTI group were significantly decreased
compared to those in the UUO group. The kidney H&E and Masson
staining results in the 3 groups are shown in Fig. 2A and B, and the semi-quantitative
scores are shown in Fig. 2C and
D.
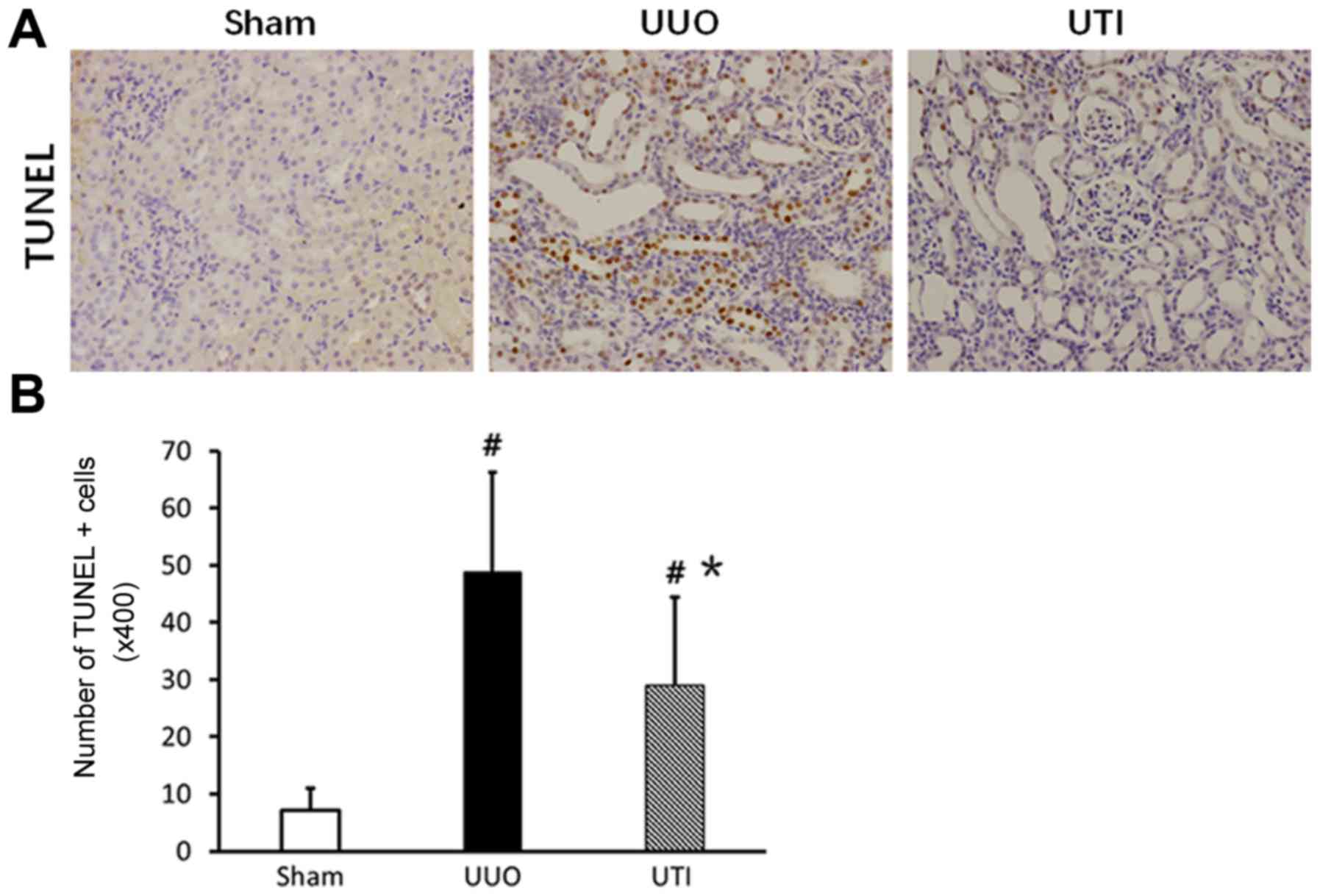
Changes in renal tubular epithelial
cell apoptosis
The TUNEL staining results showed that renal tubular
epithelial cell apoptosis was sporadically observed in the Sham
group, while apoptotic cells in the UUO group significantly
increased and were mainly distributed in dilated or atrophic renal
tubules. However, compared to that in the UUO group, the number of
apoptotic cells in the UTI group significantly decreased. The
corresponding results are shown in Fig. 3.
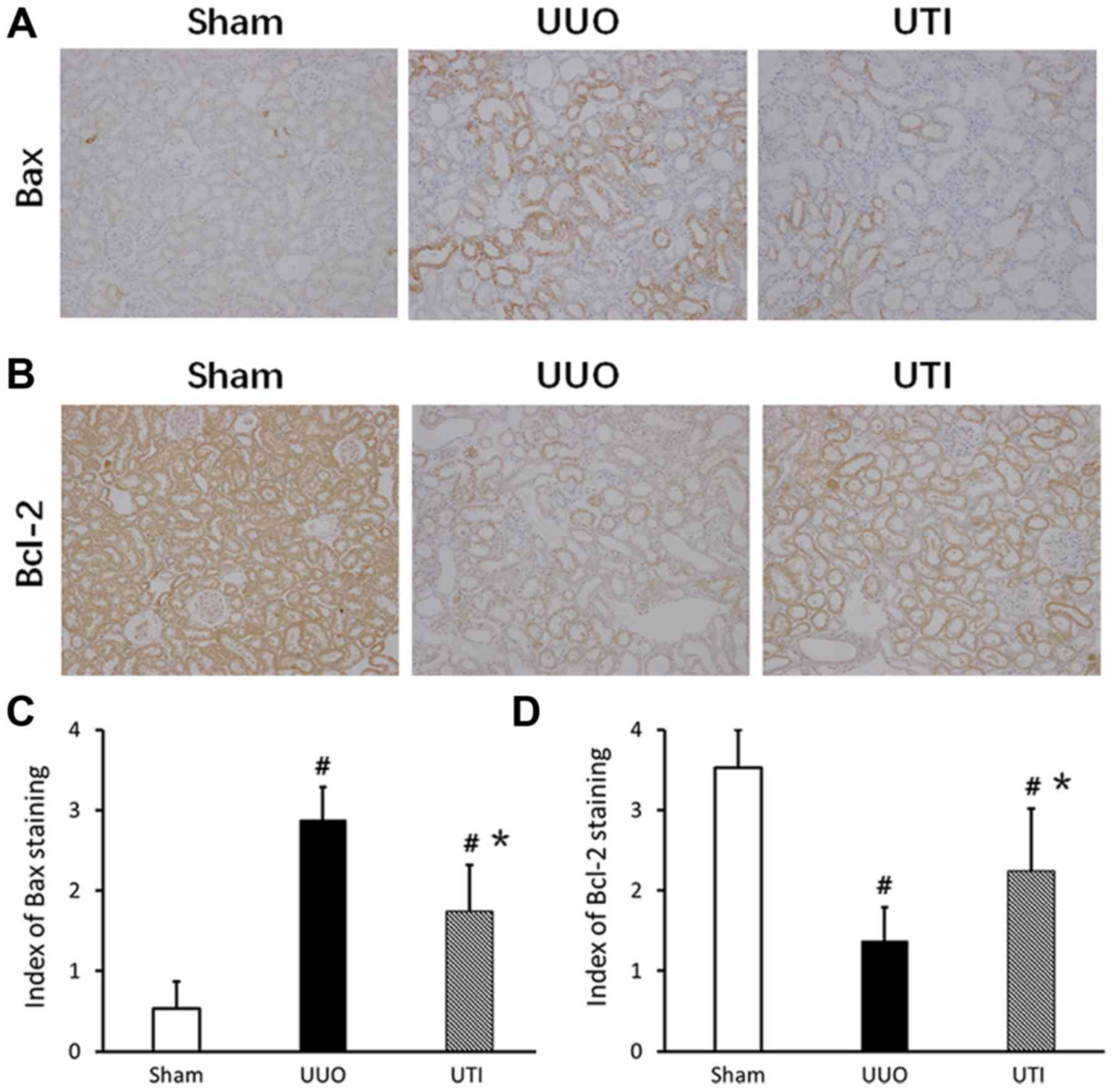
Expression of Bcl-2/Bax in renal
tubular epithelial cells
There was more Bcl-2 expression in normal kidney,
especially in the cytoplasm of renal tubular epithelial cells.
After the UUO injury, the Bcl-2 expression significantly decreased.
After the UTI intervention, the expression increased. In the Sham
group, the expression of Bax was lower. After UUO, Bax was mainly
expressed in dilated or atrophic renal tubules; compared to that in
the Sham group, the expression significantly increased. After the
UTI, Bax expression was significantly downregulated. The staining
results are shown in Fig. 4A and
B, and the semi-quantitative scores are shown in Fig. 4C and D.
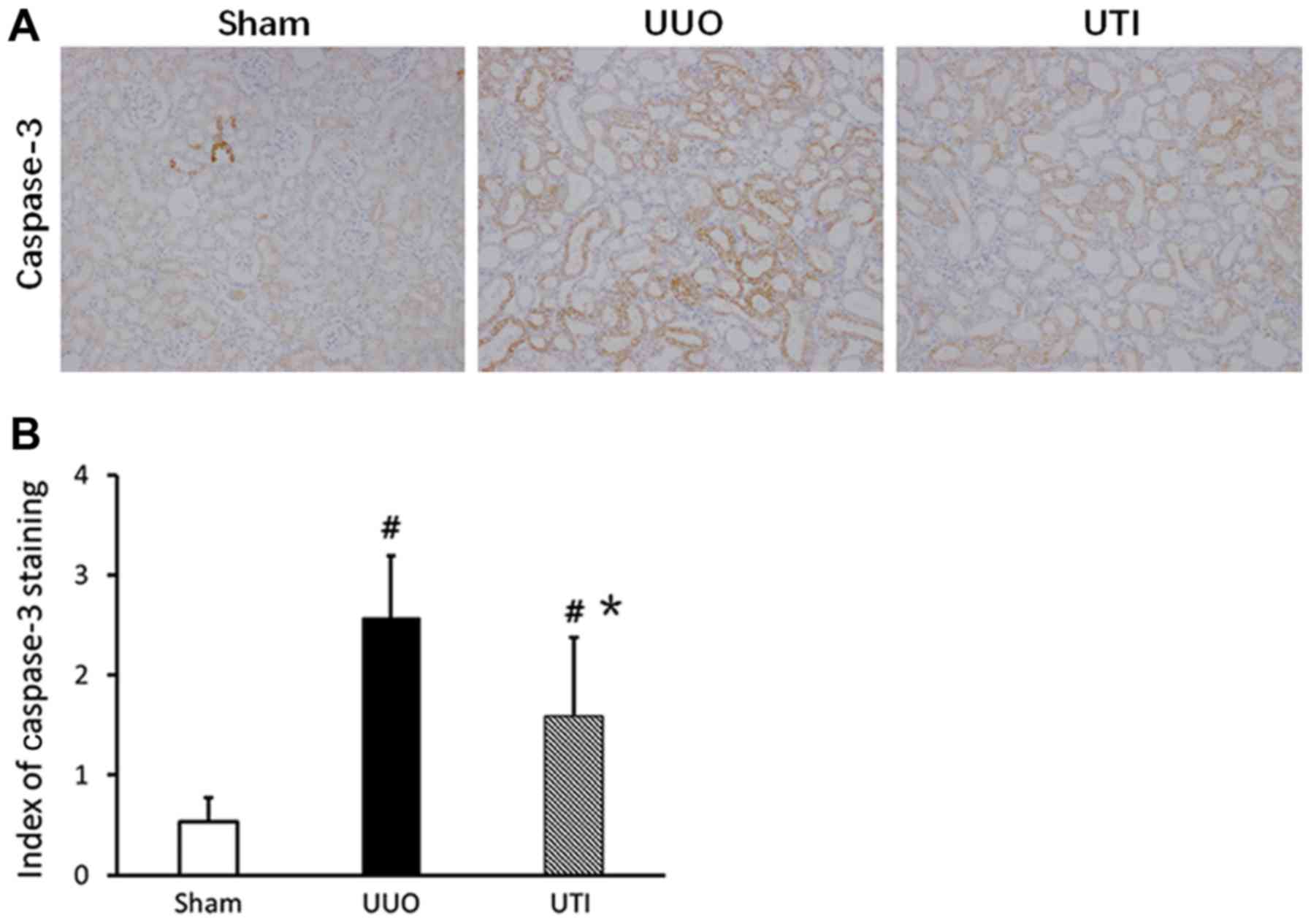
Caspase-3 expression in renal tubular
epithelial cells
Positive caspase-3 expressing renal tubular
epithelial cells mainly localized in dilated or atrophic renal
tubules. The cytoplasm of positive cells were yellow or brown, and
the cell nuclei presented with partial yellow or brown-yellow
color. In the Sham group, the caspase-3 expression level was lower;
in comparison, the expression in the UUO group significantly
increased. After UTI treatment, the expression was downregulated.
The staining results are shown in Fig.
5A, and the semi-quantitative scores are shown in Fig. 5B.
Discussion
Renal interstitial fibrosis is a common presentation
in all types of kidney diseases of end-stage disease progression.
Ureteral obstruction is also an important factor that causes renal
interstitial fibrosis. The UUO rat model features include that it
does not influence the blood pressure and blood lipid levels of
model animals, there is no proteinuria, there is no obvious immune
and toxic renal injury, and it does not cause renal impairment
(compensated by the contralateral kidney) (18). It is a classical animal model used
to study renal interstitial fibrosis (2). Its renal injury process is the result
of the common action of many factors, such as various types of
cells and cytokines. However, the question of how to block and/or
reverse the renal interstitial fibrosis process is of significant
clinical concern.
UTI is a glycoprotein isolated and purified from
human urine. It has inhibitory functions on many types of enzymes,
including serine proteases such as trypsin and α-chymotrypsin,
granulocyte elastase, hyaluronidase, thiolase and plasmin (6), playing a role in the clearance of
oxygen free radicals and inhibition of release of inflammatory
mediators and neutrophil activation (7). It is used clinically for patients
with acute pancreatitis, ischemia/reperfusion injury, multiple
organ dysfunction syndrome (MODS), acute respiratory distress
syndrome (ARDS), organ transplantation, post-cardiopulmonary bypass
surgery, inhibition of apoptosis, inhibition of inflammation,
protection of cells and improvement of circulation and coagulation.
UTI also has immune regulation functions; it can attenuate
excessive inflammatory reactions and protect lung injury induced by
lipopolysaccharide (LPS) and severe burns (7). Animal studies have already indicated
that UTI significantly increased superoxide dismutase (SOD) in the
renal tissues of renal injury rats induced by severe burns and
decreased Scr and BUN (11). UTI
attenuated renal tubular epithelial cell necrosis, inflammatory
cell infiltration and BUN increase in yeast polysaccharide-induced
multiple organ dysfunction in rats (12). The biochemical detection results in
this study showed that neither UUO nor UTI significantly affected
renal function in experimental rats. The H&E and Masson
staining results showed that after UTI treatment, the renal
interstitial injury index of the UUO rats significantly decreased
and the area of fibrosis significantly decreased, which was similar
to previous results (14). These
results indicated that UTI had a protective function in obstructive
renal tissue injury.
UTI has been shown to inhibit lung fibrosis
(13) and renal fibrosis through
blocking the TGF-β/Smad signaling pathway (14). Excessive proliferation of myoblasts
and excessive accumulation of extracellular matrix (including
COL-I, CIL-III and fibronectin) are the major pathological changes
in renal interstitial fibrosis. α-SMA is a marker of myoblasts.
There are studies showing that after UUO, the rat renal tissues had
significantly increased COL-I and α-SMA expression and exhibited
renal interstitial fibrosis. Compared to the UUO group, COL-I and
α-SMA significantly decreased after UTI treatment, renal
interstitial extracellular matrix decreased, and renal interstitial
fibrosis significantly decreased (14,19).
These results indicate that UTI had a specific function in the
resistance to fibrosis in obstructive renal tissue injury.
There are many factors causing apoptosis in ureteral
obstruction, such as ischemia and hypoxia, cytokines, growth
factors, release of reactive oxygen and mechanical stretch
(20). A large number of studies
have indicated that excessive apoptosis decreased the number of
renal tubular epithelial cells to cause renal tubular atrophy, thus
accelerating the progression of renal interstitial fibrosis
(21). Inhibition of cell
apoptosis may delay or reverse renal tubular interstitial fibrosis,
thus improving the renal function and prognosis of patients with
kidney diseases (4). In addition,
recent studies indicated that apoptosis in the hippocampus of
gerbils increased after cerebral ischemia, while UTI treatment
inhibited Bax molecule expression and increased Bcl-2 expression in
ischemia-induced apoptosis in hippocampus and decreased the TUNEL+
and caspase-3+ cells in the hippocampal CA1 region induced by
cerebral ischemia, thus improving ischemia-induced short-term
memory impairment (9). UTI reduced
the heatstroke-induced Bax/Bcl-2 ratio and caspase-3 levels in
brain cells and inhibited cell apoptosis during cerebral injury
(10). In this study, the TUNEL
results showed that obvious renal tubular epithelial cell apoptosis
occurred after 7 days of UUO surgery. Compared to the UUO group,
renal interstitial cell apoptosis significantly decreased after UTI
treatment.
The Bcl-2 family and the caspase family members are
major signaling regulatory proteins in cell apoptosis pathways.
Currently, 25 Bcl-2 family homologous proteins have been
discovered. Some members, such as Bad, Bid and Bax, have apoptosis
promotion functions, and some members, such as Bcl-2, Bcl-x and
Bcl-w, can block the release of cytochrome C from mitochondria to
cytoplasm to inhibit cell apoptosis (22). The caspase family member caspase-3
is the most important terminal (PARP) cleavage enzyme. It is an
apoptosis executor and is also an important component in the
killing mechanism of CTL.
The immunohistochemistry results in this study
indicated that compared to those in the Sham group, Bax and
caspase-3 expression increased and Bcl-2 expression decreased in
the renal tubular epithelium of rats in the UUO group. UUO
increased the Bax/Bcl-2 ratio and caspase-3 levels to induce cell
apoptosis. Compared to that in the UUO group, Bax and caspase-3
expression significantly decreased, and Bcl-2 expression was
upregulated in the UTI treatment group. These results indicated
that UTI reduced cell apoptosis through the regulation of their
expression levels.
In summary, UTI inhibited renal tubular epithelial
cell apoptosis and fibrosis in UUO rats. These results suggest that
UTI may play a role in preventing renal interstitial fibrosis
through the inhibition of renal tubular epithelial cell apoptosis;
therefore, UTI demonstrated a protective function against renal
injury on the obstruction side. As an endogenous protease
inhibitor, UTI has complicated biological functions. Its protective
mechanism in UUO renal injury merits further study.
Acknowledgements
The present study was supported by the Scientific
Research Project of the Department of Health of Heilongjiang
Province of China (2010–099) and the Youth Fund Project of the
Second Affiliated Hospital of Harbin Medical University
(QN2011-21).
References
|
1
|
Liu Y: Renal fibrosis: New insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chevalier RL, Thornhill BA, Forbes MS and
Kiley SC: Mechanisms of renal injury and progression of renal
disease in congenital obstructive nephropathy. Pediatr Nephrol.
25:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Docherty NG, O'Sullivan OE, Healy DA,
Fitzpatrick JM and Watson RW: Evidence that inhibition of tubular
cell apoptosis protects against renal damage and development of
fibrosis following ureteric obstruction. Am J Physiol Renal
Physiol. 290:F4–F13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Letai A: Pharmacological manipulation of
Bcl-2 family members to control cell death. J Clin Invest.
115:2648–2655. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fries E and Blom AM: Bikunin-not just a
plasma proteinase inhibitor. Int J Biochem Cell Biol. 32:125–137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang Y, Xu P, Gu C, Wang Y, Fu XJ, Yu WR
and Yao M: Ulinastatin improves pulmonary function in severe
burn-induced acute lung injury by attenuating inflammatory
response. J Trauma. 71:1297–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu M, Wen XH, Chen SP, An XX and Xu HY:
Addition of ulinastatin to preservation solution promotes
protection against ischemia-reperfusion injury in rabbit lung. Chin
Med J (Engl). 124:2179–2183. 2011.PubMed/NCBI
|
|
9
|
Cho YS, Shin MS, Ko IG, Kim SE, Kim CJ,
Sung YH, Yoon HS and Lee BJ: Ulinastatin inhibits cerebral
ischemia-induced apoptosis in the hippocampus of gerbils. Mol Med
Rep. 12:1796–1802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao Z, Hu FQ, Li CF, Zhang T, Cao BZ and
Cui LQ: Effect of ulinastatin, a human urinary protease inhibitor,
on heatstroke-induced apoptosis and inflammatory responses in rats.
Exp Ther Med. 13:335–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao C, Huan J, Li W and Tang J: Protective
effects of ulinastatin on pancreatic and renal damage in rats
following early scald injury. Burns. 35:547–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Q, Liu X, Liu M, Zhang L and Guan Y:
Ulinastatin-mediated protection against zymosan-induced multiple
organ dysfunction in rats. Biologicals. 38:552–556. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katoh H, Ishikawa H, Hasegawa M, Yoshida
Y, Suzuki Y, Ohno T, Takahashi T and Nakano T: Protective effect of
urinary trypsin inhibitor on the development of radiation-induced
lung fibrosis in mice. J Radiat Res. 51:325–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ning XH, Ge XF, Cui Y and An HX:
Ulinastatin inhibits unilateral ureteral obstruction-induced renal
interstitial fibrosis in rats via transforming growth factor β
(TGF-β)/Smad signalling pathways. Int Immunopharmacol. 15:406–413.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (NRC), . Guide
for the Care and Use of Laboratory Animals. 8th edition. National
Academies Press; Washington, DC: 2011, PubMed/NCBI
|
|
16
|
Satoh M, Kashihara N, Yamasaki Y, Maruyama
K, Okamoto K, Maeshima Y, Sugiyama H, Sugaya T, Murakami K and
Makino H: Renal interstitial fibrosis is reduced in angiotensin II
type 1a receptor-deficient mice. J Am Soc Nephrol. 12:317–325.
2001.PubMed/NCBI
|
|
17
|
Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N,
Ikeda K, Yanase T and Fujimi S: Glomerular score as a
prognosticator in IgA nephropathy: Its usefulness and limitation.
Clin Nephrol. 49:1–8. 1998.PubMed/NCBI
|
|
18
|
Grande MT, Pérez-Barriocanal F and
López-Novoa JM: Role of inflammation in túbulo-interstitial damage
associated to obstructive nephropathy. J Inflamm (Lond). 7:192010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang GT, Chen X, Dong L, An HX and Jiao
JD: Ulinastatin attenuates renal interstitial inflammation and
inhibits fibrosis progression in rats under unilateral ureteral
obstruction. Mol Med Report. 10:1501–1508. 2014. View Article : Google Scholar
|
|
20
|
Misseri R and Meldrum KK: Mediators of
fibrosis and apoptosis in obstructive uropathies. Curr Urol Rep.
6:140–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maoka T, Tokuda H, Suzuki N, Kato H and
Etoh H: Anti-oxidative, anti-tumor-promoting, and
anti-carcinogensis activities of nitroastaxanthin and nitrolutein,
the reaction products of astaxanthin and lutein with peroxynitrite.
Mar Drugs. 10:1391–1399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dolka I, Król M and Sapierzyński R:
Evaluation of apoptosis-associated protein (Bcl-2, Bax, cleaved
caspase-3 and p53) expression in canine mammary tumors: An
immunohistochemical and prognostic study. Res Vet Sci. 105:124–133.
2016. View Article : Google Scholar : PubMed/NCBI
|