Introduction
Bone is a tissue that is constantly being remodeled
during a human's or animal's life span through the activity or
interaction of bone generating osteoblasts and bone resorbing
osteoclasts (1). The balance
between bone resorption and bone formation is essential for
maintaining bone homeostasis. Many molecules are involved in
communication between osteoblasts and osteoclasts during bone
remodeling, such as receptor activator of nuclear factor κB ligand
(RANKL)/receptor activator of nuclear factor κB (RANK),
ephrinB2/EphB4, TGF-β, and so on (2). Recently, many studies have
demonstrated that the immune system is also involved intimately in
the regulation of bone regeneration (3). Extensive evidence suggests that the
immune system exerts powerful effects on bone cells, particularly
in chronic disease pathologies such as rheumatoid arthritis,
periodontal disease, and tumors (4,5).
As a common disease identified in patients visiting
oral clinics, the chronic inflammatory state in periodontitis
triggers bone erosion through the increased stimulation of
osteoclast formation and activity, decreasing the chance for
preservation of permanent teeth and the survival of implants
(4). Lipopolysaccharide (LPS), an
important virulence factor of periodontitis, stimulates the
accumulation of monocytes, releases many inflammatory factors, and
is the cause of maturation of osteoclasts. Furthermore, T
lymphocytes play an important role in regulation of bone mass in
inflammatory diseases. T cells can synthesize RANKL and induce
osteoclast formation and activity in vitro (4–6).
Considering the crucial effect of immune factors on the development
of periodontitis, it raises an interesting question of whether or
not clinicians can use immunosuppressive medicine to block bone
resorption.
Rapamycin is a common immunosuppressive drug that
has multiple functions such as radiation protection and prevention
of aging, inhibition of tumor growth, regulating endothelial cell
growth, inhibition of adipogenic differentiation of bone
marrow-derived mesenchymal stem cells (BMSCs), among others
(7–11). Rapamycin also plays important roles
in bone homeostasis, which regulates bone formation through the
mTOR/S6K and nuclear factor-κB (NF-κB) signaling pathway (12). mTOR is an evolutionally conserved
serine/threonine protein kinase involved in many cellular processes
such as survival and proliferation (13). Rapamycin is a specific inhibitor of
mTOR, and can downregulate expression of RANKL, M-CSF, and tumor
necrosis factor α (TNFα), and exerts suppressive effects on
proliferation and differentiation of osteoclasts (14). More importantly, rapamycin can
inhibit the release of inflammatory factors (15,16),
which further reduces differentiation of osteoclasts. The role of
rapamycin in osteogenesis, however, still remains controversial
because conflicting studies have reported both negative (17–19)
and positive (20,21) effects on osteogenesis. These
differences may depend on cell type and experimental
conditions.
Periodontitis is a chronic inflammatory disease
resulting in bone loss, and rapamycin plays a role as an
anti-inflammatory and possibly has an effect on bone formation.
Therefore, we were interested in evaluating the effects of
rapamycin on osteoblast differentiation of BMSCs in vitro,
and bone regeneration in our animal model in vivo, with or
without LPS treatment, in order to understand if rapamycin can be
used to treat periodontitis.
Materials and methods
Cell lines and cell cultures
All animals used for this study were approved by the
Jilin University (Changchun, China) Animal Care and Use Committee.
In this study, BMSCs were obtained from the femur of Wistar rats.
BMSCs were cultured/expanded in low-glucose DMEM (Thermo Fisher
Scientific, Inc., Grand Island, NY, USA)) with 10% fetal bovine
serum, 100 U/ml penicillin, and 100 mg/ml streptomycin. To perform
osteoblast differentiation, BMSCs were cultured in low-glucose DMEM
(Thermo Fisher Scientific, Inc.) supplemented with 10−8
mol/l dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 50 mg/l of
ascorbic acid (Sigma-Aldrich), and 10 mM β-glycerol phosphate
sodium (Merck, Kenilworth, NJ, USA) known as osteogenic medium with
10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml
streptomycin. Cells were incubated at 37°C in a humidified 5%
CO2 incubator.
Alkaline phosphatase (ALP) and
alizarin red assays
BMSCs cells were seeded/cultured in a 6-well plate
at 1×104 cells/well with low-glucose DMEM (Thermo Fisher
Scientific, Inc.) for 24 h. Then, plates divided into four groups,
control phosphate-buffered saline (PBS)], LPS at 1 µg/ml
(Sigma-Aldrich), rapamycin at 20 ng/ml (Rapa, Sigma-Aldrich), and
LPS at 1 µg/ml plus rapamycin at 20 ng/ml (LPS + Rapa), and
continue to culture with osteogenic medium. The osteogenic medium
with or without LPS or rapamycin was replaced every 3 days. On days
3, 7, and 14, ALP activity assay was performed with ALP substrate
(Sigma-Aldrich), and optical density (OD) was measured at 520 nm
with a spectrometer according to the manufacturer's instructions.
On days 14, 21, and 28 cells from the same experiments were fixed
with 95% ethanol and stained with 2% alizarin red (Sigma-Aldrich).
The stained cells were then eluted with 10% cetylpyridinium
chloride. The absorbance was measured at 550 nm with a
spectrometer.
Reverse transcription quantitative
polyermerase chain reaction (RT-qPCR)
BMSCs were seeded in a 6-well plate at
1×104 cells/well and cultured with osteogenic medium for
24 h. Then, plates divided into four groups, control (PBS), LPS at
1 µg/ml (Sigma-Aldrich), rapamycin at 20 ng/ml (Rapa,
Sigma-Aldrich), and LPS at 1 µg/ml plus Rapa at 20 ng/ml (LPS +
Rapa). On days 3, 7, and 14, total RNA was extracted with EASYspin
(Takara, Dalian, China), and reverse transcription was carried out
using 1 µg total RNA with PrimerScript RT Reagent kit (Takara), The
qPCRs were performed using SYBR Premix Ex Taq TM (Takara).
Sequences of primers are listed in Table I. The β-actin was used as the
internal control. TNFα and interleukin (IL)-1β are important
mediators of the inflammatory response. Runt related transcription
factor 2 (Runx2) is a necessary protein in the early and middle
stages of osteogenic differentiation (22) and acts as an essential bone
specific transcription factor (23). Collagen I (Col I) is an important
protein for bone formation and repair (24). ALP is a very common bone formation
marker (25). Sp7 is a
transcription factor and indicator for osteogenic differentiation
(26) and is involved in
osteoblast differentiation (27).
 | Table I.Quantitative polymerase chain
reaction primers used in this study. |
Table I.
Quantitative polymerase chain
reaction primers used in this study.
| Gene | Forward primer
(5′-3′) | Reverse orimer
(5′-3′) | Size (base pair,
bp) |
|---|
| ALP |
CTCAACACCAATGTAGCCAAGAATG | GGCAGCGGTT
ACTGTGGAGA | 75 |
| Runx2 |
GCACAAACATGGCCAGATTCA |
AAGCCATGGTGCCCGTTAG | 126 |
| Sp7 |
GCACAAACATGGCCAGATTCA |
AGAAATCTACGAGCAAGGTCTCCAC | 129 |
| Col I |
GACATGTTCAGCTTTGTGGACCTC |
GGGACCCTTAGGCCATTGTGTA | 119 |
Animal model
A classical animal model, alveolar bone regeneration
model was used to evaluate effects of rapamycin on bone
regeneration in vivo (28).
To successfully create this model, Wistar rats were anesthetized
with ketamine (60 mg/kg) and xylazine (8 mg/kg) intramuscularly,
and the crown of the left mandibular incisor was repeatedly cut at
the gingival level with a high-speed turbine drill on days 8, 5,
and 2 prior to the mandibular incisor being fully extracted on day
0. The mandibular incisor of rodents keep erupting over their life
span. Once the crown of the mandibular incisor is removed, the
tooth will erupt more quickly, resulting in edema of the
periodontal ligaments (28), which
can facilitate the extraction of the mandibular incisor.
In vivo animal experiment
All animals used in this study were approved by the
Jilin University Animal Care and Use Committee. Eighty male Wistar
rats (210±10 g, and 7 to 8 weeks old) were randomly divided into
three groups on day 0 of alveolar bone regeneration model: 20 rats
for the control group administered with 10 µl of PBS, 20 rats for
the Rapa group administered with 10 µl of rapamycin at 1 µg/µl into
the tooth socket, 20 rats for the LPS group administered with 10 µl
of LPS at 5 µg/µl into the tooth socket, and 20 rats for the LPS +
Rapa group administered with 10 µl of LPS at 5 µg/µl with rapamycin
at 1 µg/µl into the tooth socket. Defects were closed by
periodontal dressing. Rats were anesthetized and euthanized by
heart perfused fixation with 4% paraformaldehyde solution after 30
and 60 days post-treatment. Mandible samples were examined with
micro-computed tomography (micro-CT) for bone mass. Tooth
extraction sockets from different treated groups were immersed in
10% EDTA for decalcification for two months, 10% EDTA was refreshed
every two days. After decalcification, samples were embedded in
paraffin and tissues were sectioned at 4 µm for hematoxylin and
eosin (H&E) staining to evaluate pathological changes.
Micro-CT
The left mandible was placed in a tube and scanned
over the entire mandible using a micro-CT system (µCT50; Scanco
Medical, Bassersdorf, Switzerland). The same condition was used to
scan all samples. Three rats from each group were scanned by
micro-CT. A series of two-dimensional image data was collected to
reconstruct three-dimensional images. Regions of interest (ROI)
were confined to the anterior area of the molar region of the
mandible near the inferior border. This ROI was further analyzed
using a fixed global threshold of 22% (220 on a grayscale of
0–1,000). Trabecular bone volume fraction (BV/TV), trabecular
thickness (Tb.Th), trabecular number (Tb.N), trabecular separation
(Tb.Sp), bone mineral density (BMD), and tissue mineral density
(TMD) were analyzed using the manufacturer's evaluation
software.
Statistical analysis
Results were presented as mean ± standard deviation
(SD). Statistical analysis was performed using the one-way ANOVA
and multiple comparison method. A difference with a P-value of
<0.05 was deemed as statistically significant. All in
vitro experiments were repeated three times; in vivo
experiments were repeated twice.
Results
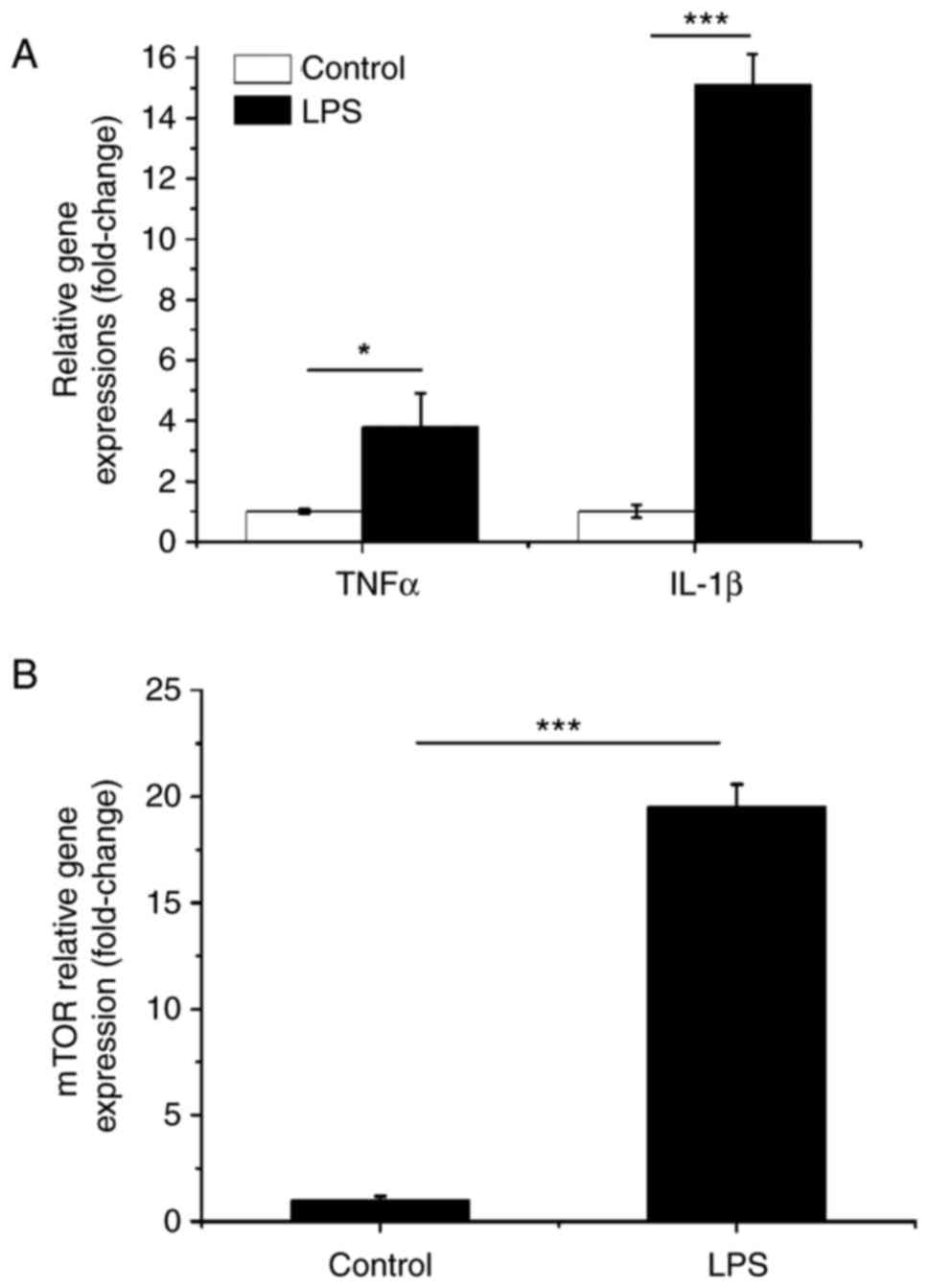
Effects of LPS on inflammatory
cytokines and mTOR
To confirm the LPS used here has the expected
biological effects, the gene expressions of TNFα, IL-1β and mTOR
were measured after BMSCs were cultured with LPS at 1 µg/ml
(Sigma-Aldrich) for 24 h. Fig. 1
showed that the LPS dramatically induced the gene expressions of
TNFα, IL-1β and mTOR compared to the control group. These indicate
that the LPS has expected biological effects.
Effects of rapamycin on
osteoblast-related gene expression
To evaluate if rapamycin can induce osteogenic
differentiation at the gene expression level, four marker genes,
Runx2, Sp7, Col I, and ALP for osteogenic differentiation, were
measured after BMSCs were treated with rapamycin, LPS, or rapamycin
plus LPS by RT-qPCR on days 3, 7, and 14. Rapamycin alone
significantly decreased gene expression of Runx2, but not Sp7, Col
I, or ALP compared to the control group on day 3 (Fig. 2A). LPS alone decreased gene
expressions of Runx2 and Col I, but not ALP and Sp7 compared to the
control group on day 3 (Fig. 2A).
LPS plus rapamycin also decreased gene expressions of Runx2 and Col
I, but increased ALP and Sp7 compared to the control group on day 3
(Fig. 2A). On day 7, rapamycin
alone only increased Col I gene expression while LPS alone
increased gene expressions of Runx2, Col I, and ALP compared to the
control (Fig. 2B). Interestingly,
LPS plus rapamycin significantly increased gene expressions of
Runx2, Col I, ALP, and Sp7 compared to the control on day 7
(Fig. 2B). Rapamycin downregulated
gene expressions of Runx2, Col I, and Sp7 while LPS decreased gene
expressions of Runx2 and Sp7 compared to the control on day 14
(Fig. 2C). Both LPS and LPS plus
rapamycin increased gene expressions of Col I and ALP compared to
the control on day 14 (Fig. 2C).
These data indicate that rapamycin plus LPS can induce osteoblast
differentiation.
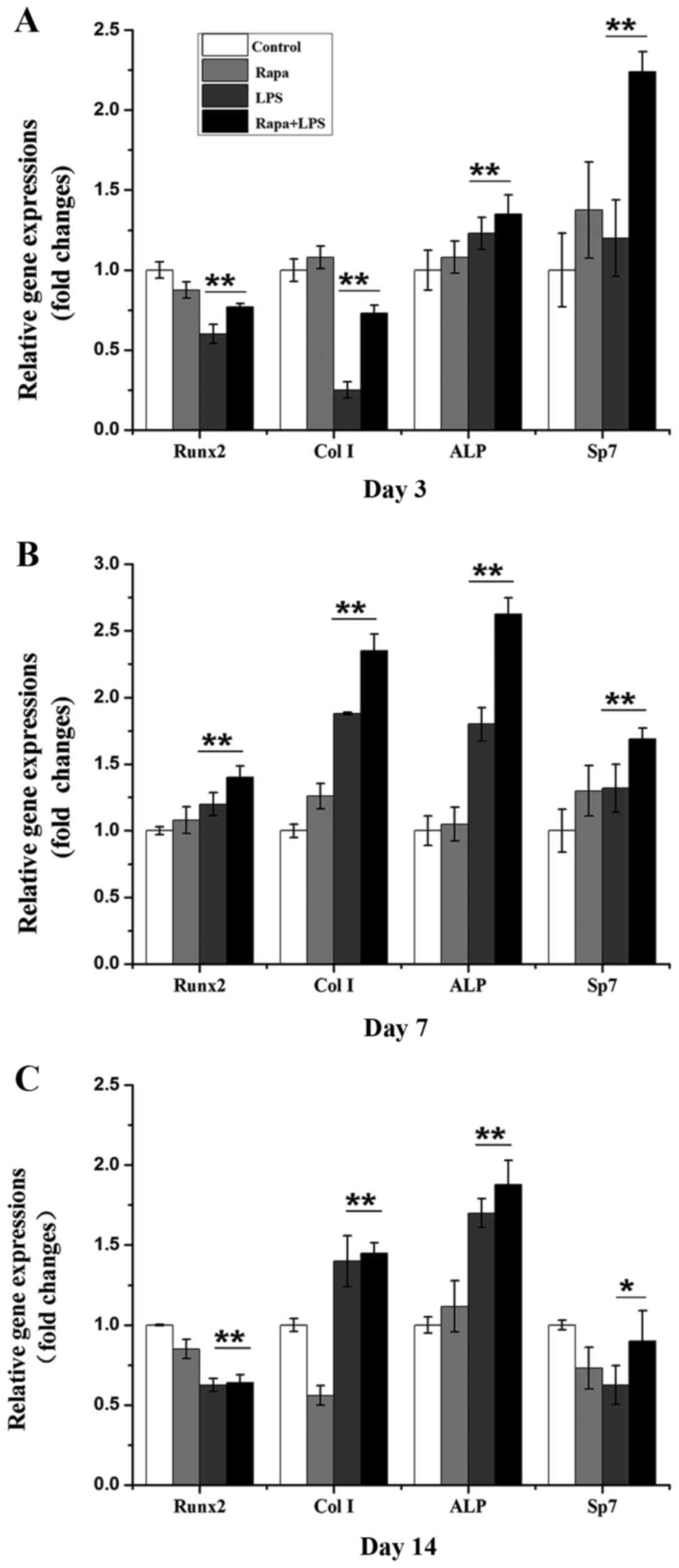 | Figure 2.Gene expressions of Runx2, Col I,
ALP, and Sp7 after 3, 7, and 14 days post-treatment. (A) Gene
expressions on day 3. (B) Gene expressions on day 7. (C) Gene
expressions on day 14. Data are represented as means ± SD from
three experiments. *P<0.05, **P<0.01. Runx2, runt related
transcription factor 2; Col I, collagen I; ALP, alkaline
phosphatase; Sp7, Sp7 transcription factor; SD, standard deviation;
Rapa, rapamycin; LPS, lipopolysaccharide. |
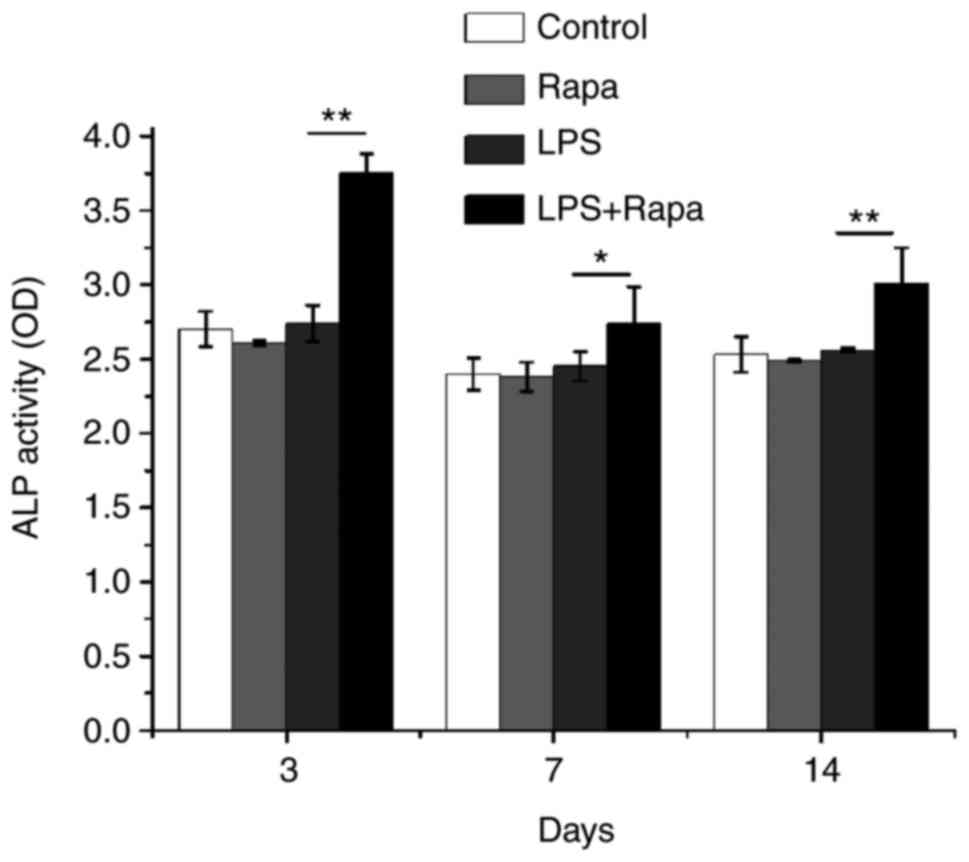
Rapamycin plus LPS increased ALP
activity
Next, we directly measured ALP activity in BMSC
cultures to assess osteoblast differentiation. Interestingly, only
rapamycin plus LPS enhanced ALP activity compared to the control,
rapamycin and LPS groups on days 3, 7, and 14 (Fig. 3). These data demonstrate that the
rapamycin alone is not enough to affect osteoblast differentiation
while the rapamycin plus LPS can induce osteoblast differentiation
in vitro.
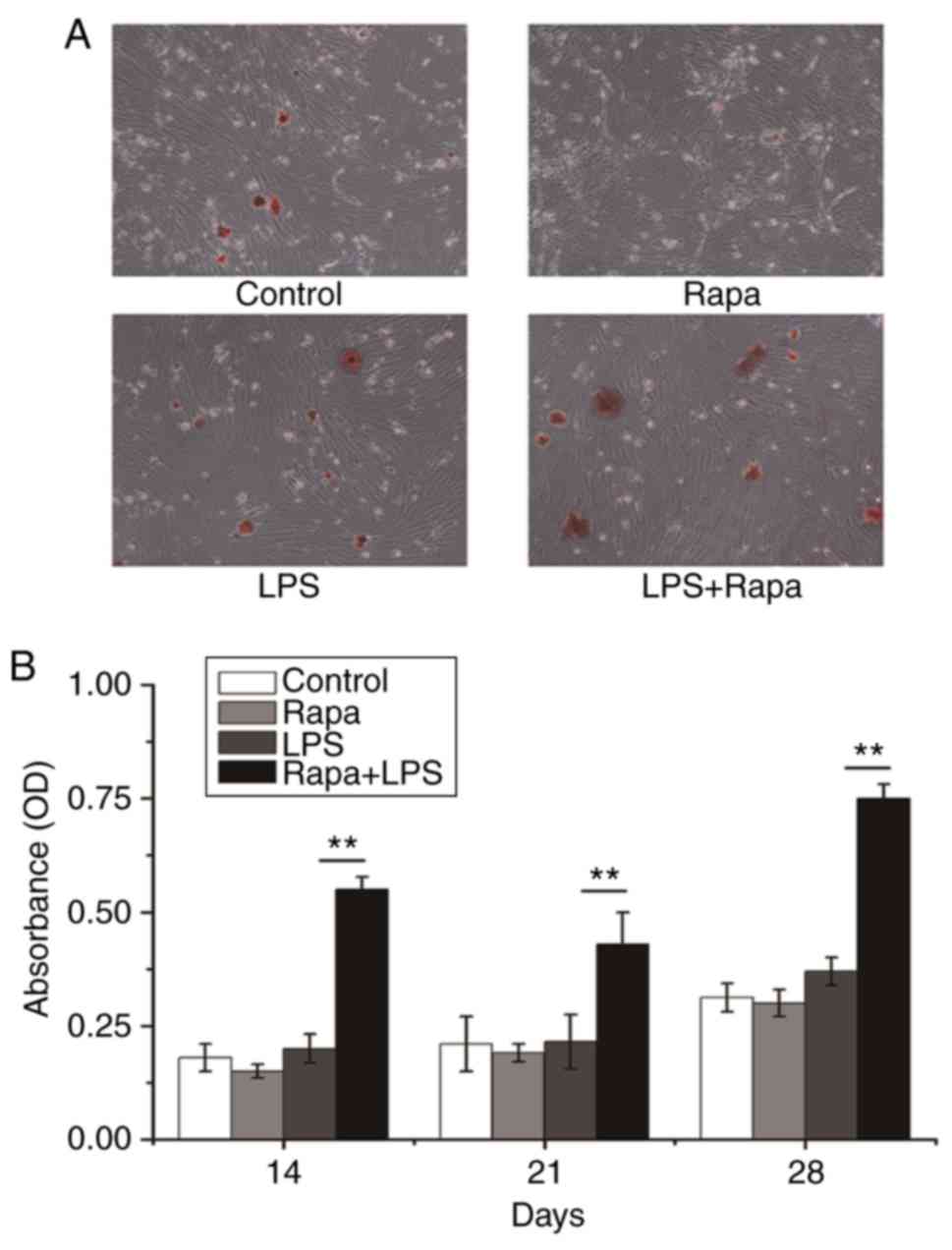
Effects of rapamycin on formation of
calcium nodule
Alizarin red staining is used to identify calcium in
tissue section or cell culture which can indicate osteogenesis
differentiation. The data clearly showed that only LPS plus
rapamycin had significantly more calcium nodule compared to any
other groups on days 14, 21, and 28 (Fig. 4). These data further suggest that
the rapamycin plus LPS can promote osteoblast differentiation in
vitro.
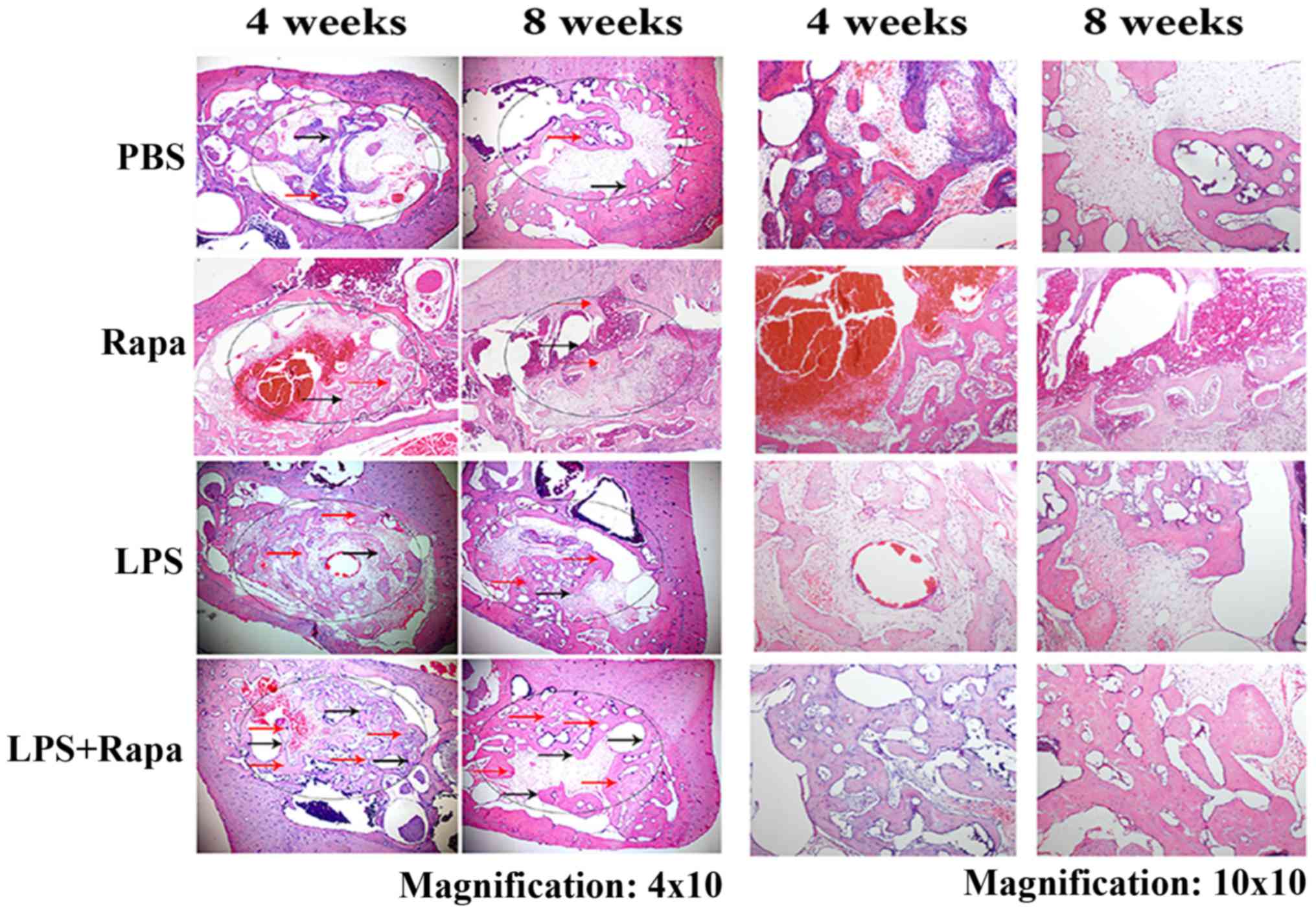
Effect of rapamycin on bone formation
in vivo
To further evaluate the effect of rapamycin on bone
formation, we performed in vivo experiments using the
alveolar bone regeneration model. Data from BV/TV, BMD, TMD, Tb.N,
and Tb.Th showed that rapamycin plus LPS significantly increased
bone formation compared to PBS control and rapamycin control (data
not shown here) on weeks 4 and 8 while LPS alone only significantly
increased Tb.Th, and both LPS and rapamycin plus LPS decreased
Tb.Sp (Fig. 5). H&E staining
from Fig. 6 clearly demonstrated
that the rapamycin plus LPS-treated group had much more new bone
formation with scattered osteoblasts compared to control, Rapa and
LPS groups on weeks 4 and 8. At 8 weeks, bone deposition continued
to increase, and the new bone showed bigger and higher density of
bone trabecula in rapamycin plus LPS-treated group (Fig. 6). H&E staining data were
consistent with the micro-CT data. These data suggest that
rapamycin affects bone homeostasis by stimulating osteoblast
activity under LPS-induced inflammatory condition.
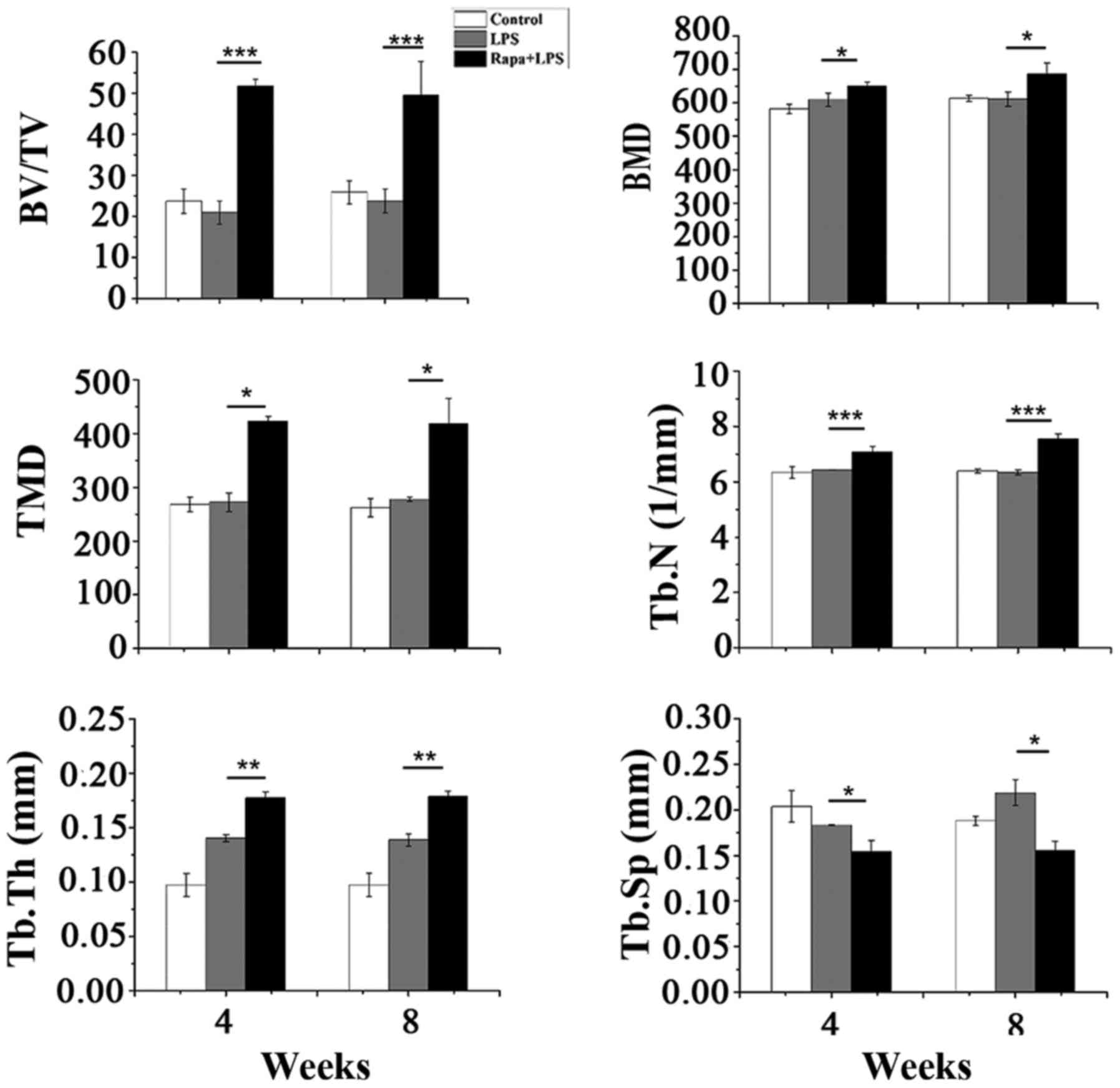 | Figure 5.Measurements of BV/TV, BMD, TMD,
Tb.N, Tb.Th, and Tb.Sp of mandible trabecular bone after 4 and 8
weeks post-treatment. Data are represented as means ± SD from 20
rats. *P<0.05, **P<0.01, ***P<0.001. BV/TV, trabecular
bone volume fraction; BMD, bone mineral density; TMD, tissue
mineral density; Tb.N, trabecular number; Tb.Th, trabecular
thickness; Tb.Sp, trabecular separation; SD, standard deviation;
Rapa, rapamycin; LPS, lipopolysaccharide. |
Discussion
Osteogenesis is one of two major processes, a
process of new bone formation by osteoblast in bone remodeling.
Bone resorption is another process by osteoclast in bone
remodeling. It is important to maintain the balance between bone
formation and bone resorption. Complex molecules and signaling
pathways are involved in these processes. Rapamycin is a common
immunosuppressant widely used to retain a renal allograft.
Recently, it was recognized that mammalian target of rapamycin
complex 2 (mTORC2) signaling pathway is involved in osteogenesis
processes, and that rapamycin can also affect bone homeostasis
under certain conditions, but this is still unclear and remains
controversial (29). For example,
it showed that osteoblast-targeted inhibition of PPARc activated
mTOR signaling pathway resulting in osteoblast differentiation
(30) while Wang et al
demonstrated that the rapamycin inhibited mTOR signaling pathway
leading to BMSCs differentiation/proliferation to osteoblasts
(31). The study herein indicates
that rapamycin can induce osteoblast differentiation and lead to
new bone formation under LPS-induced inflammatory conditions.
Bacterial endotoxin LPS is a potent stimuli for
monocytes, which produce pro-inflammatory cytokines such as TNFα,
IL-1, and IL-6 to play an important role in pathogenesis bacterial
sepsis (32–34). LPS can also activate NF-κB
(35). Interestingly, cytokines of
TNFα, IL-1, and IL-6 can play critical roles in osteoclast
formation resulting in osteoclastic bone loss (36,37).
These cytokines can induce production of RANKL (38,39),
decrease production of osteoprotegerin (OPG) (40), upregulate RANK on osteoclast
precursors, and increase RANKL to affect RANK/RANKL/OPG signaling
pathway (41). LPS can also
interact with toll-like receptor (TLR)-2 and/or TLR-4, resulting in
RANKL expression or release increase and manipulation of
RANK/RANKL/OPG signal pathway (41).
It is known that rapamycin possesses potent
anti-proliferative and anti-angiogenic properties (42). Previous studies show that rapamycin
downregulates expressions of RANKL, M-CSF, and TNFα to suppress
proliferation and differentiation of osteoclast, and also block
cytokine release to further inhibit differentiation of osteoclast
(15,16). These data indicate that rapamycin
potentially reduces bone resorption and contributes to a
bone-protective effect or new bone formation through
anti-inflammatory influence (20,21).
Rapamycin could partly activate osteocyte autophagy and reduce
age-related bone loss in trabecular bone of old male rats (43). Indeed, it has been reported that
rapamycin could also inhibit osteoblast proliferation and
differentiation in MC3T3-E1 cells and primary mouse bone marrow
stromal cell (17). Therefore,
there is uncertain effect of rapamycin on bone formation.
Our data demonstrate that rapamycin alone did not
influence gene expression of Runx2, Col I, ALP, and Sp7 compared to
the control group on days 3, 7, and 14, except that it slightly
decreased Runx2 on days 3 and 14 (Fig.
2). Rapamycin alone also could not increase ALP enzyme activity
on days 3, 7, and 14 (Fig. 3). LPS
decreased gene expression of Runx2 and Col I compared to the
control group on days 3 and 14, and Sp7 on day 14 (Fig. 1), and also had no effect on ALP
enzyme activity on days 3, 7 and 14 (Fig. 3). Interestingly, rapamycin from the
rapamycin plus LPS group could either enhance or bring back gene
expression of Runx2, Col I, ALP, and Sp7 compared to LPS alone on
all three days in which LPS induced an increase or decrease
(Fig. 2), and also increased ALP
enzyme activity (Fig. 3). Data
from calcium nodule assay showed that only rapamycin plus
LPS-treated BMSCs significantly increased the number of calcium
nodules (Fig. 4). These data
clearly indicate that rapamycin alone cannot fully induce BMSC
differentiation to become functional osteoblasts in vitro;
it only occurred when rapamycin plus LPS was used in vitro.
Recently published study, however, demonstrates that rapamycin can
induce their BMSCs differentiation to the osteoblast in
vitro (31). Compared both
culture systems, we recognized that the difference could cause by
differences of culture contents and species of rat.
In our in vivo animal model (although we knew
that this model was not perfect model), we delivered rapamycin
locally to the extraction defect area. Micro-CT data show that bone
tissue volume markedly increased in the defect area and that
density of alveolar bone significantly increased in the LPS plus
rapamycin-treated group (Fig. 5).
H&E staining revealed that rapamycin from the LPS plus
rapamycin-treated group markedly increased new bone formation with
scattered osteoblasts in the defect area (Fig. 6). Therefore, our data suggest that
LPS is a precondition for rapamycin induction of osteoblast
differentiation and new bone formation.
Results from our cell culture model and animal model
clearly indicate that rapamycin alone is not enough to fully
promote osteoblast differentiation in vitro and in
vivo. However, when LPS creates an inflammatory condition
resulting in cytokine production and leads RANK/RANKL/OPG signaling
pathway to activate osteoclasts, then rapamycin may block the
inflammation and turn around the RANK/RANKL/OPG signaling pathway
to the osteoblast differentiation and new bone formation in
vitro and in vivo.
Periodontitis is a chronic inflammatory disease with
dense inflammatory cell infiltrates. Porphyromonas gingivalis is
the major bacterial cause of periodontitis. The LPS produced by
Porphyromonas gingivalis can induce cytokine production and
increase soluble RANKL release to affect RANK/RANKL/OPG signaling
pathway resulting in bone loss (41). Therefore, our results suggest that
rapamycin can be used to treat periodontitis in order to block bone
resorption and promote bone regeneration.
Acknowledgements
We would like to thank Cindy Clark, NIH Library
Editing Service, for reviewing and editing the manuscript. We thank
Guibin Zhu, Medical Laboratory, for her excellent technical
assistance. This study was supported by grants from the National
Key Research and Development Program of China (2016YFC1102800), the
National Natural Science Foundation of China (81320108011,
81600879, 30830108, 81500820 and 81400488) and the JLU Science and
Technology Innovative Research Team (2017TD-11).
References
|
1
|
Hattner R, Epker BN and Frost HM:
Suggested sequential mode of control of changes in cell behaviour
in adult bone remodelling. Nature. 206:489–490. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Shi C, Kim J, Chen Y, Ni S, Jiang L,
Zheng C, Li D, Hou J, Taichman RS and Sun H: Erythropoietin
promotes bone formation through EphrinB2/EphB4 signaling. J Dent
Res. 94:455–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takayanagi H: Osteoimmunology: Shared
mechanisms and crosstalk between the immune and bone systems. Nat
Rev Immunol. 7:292–304. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Taubman MA and Kawai T: Involvement of
T-lymphocytes in periodontal disease and in direct and indirect
induction of bone resorption. Crit Rev Oral Biol Med. 12:125–135.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dequeker J, Maenaut K, Verwilghen J and
Westhovens R: Osteoporosis in rheumatoid arthritis. Clin Exp
Rheumatol. 12(13 Suppl): S21–S26. 1995.
|
|
6
|
Kotake S, Udagawa N, Hakoda M, Mogi M,
Yano K, Tsuda E, Takahashi K, Furuya T, Ishiyama S, Kim KJ, et al:
Activated human T cells directly induce osteoclastogenesis from
human monocytes: Possible role of T cells in bone destruction in
rheumatoid arthritis patients. Arthritis Rheum. 44:1003–1012. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harrison DE, Strong R, Sharp ZD, Nelson
JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter
CS, et al: Rapamycin fed late in life extends lifespan in
genetically heterogeneous mice. Nature. 460:392–395.
2009.PubMed/NCBI
|
|
8
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, et al: Rapamycin inhibits primary and metastatic tumor
growth by antiangiogenesis: Involvement of vascular endothelial
growth factor. Nat Med. 8:128–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng N, Ding X and Jahan R: Low
concentration of rapamycin inhibits hemangioma endothelial cell
proliferation, migration, and vascular tumor formation in mice.
Curr Ther Res Clin Exp. 76:99–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho HJ, Park J, Lee HW, Lee YS and Kim JB:
Regulation of adipocyte differentiation and insulin action with
rapamycin. Biochem Biophys Res Commun. 321:942–948. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antonarakis ES, Carducci MA and
Eisenberger MA: Novel targeted therapeutics for metastatic
castration-resistant prostate cancer. Cancer Lett. 291:1–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarbassov DD, Ali SM, Sengupta S, Sheen
JH, Hsu PP, Bagley AF, Markhard AL and Sabatini DM: Prolonged
rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell.
22:159–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Q and Guan KL: Expanding mTOR
signaling. Cell Res. 17:666–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lam J, Takeshita S, Barker JE, Kanagawa O,
Ross FP and Teitelbaum SL: TNF-alpha induces osteoclastogenesis by
direct stimulation of macrophages exposed to permissive levels of
RANK ligand. J Clin Invest. 106:1481–1488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fielhaber JA, Carroll SF, Dydensborg AB,
Shourian M, Triantafillopoulos A, Harel S, Hussain SN, Bouchard M,
Qureshi ST and Kristof AS: Inhibition of mammalian target of
rapamycin augments lipopolysaccharide-induced lung injury and
apoptosis. J Immunol. 188:4535–4542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tulek B, Kiyan E, Toy H, Kiyici A, Narin C
and Suerdem M: Anti-inflammatory and anti-fibrotic effects of
sirolimus on bleomycin-induced pulmonary fibrosis in rats. Clin
Invest Med. 34:E3412011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singha UK, Jiang Y, Yu S, Luo M, Lu Y,
Zhang J and Xiao G: Rapamycin inhibits osteoblast proliferation and
differentiation in MC3T3-E1 cells and primary mouse bone marrow
stromal cells. J Cell Biochem. 103:434–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shui C, Riggs BL and Khosla S: The
immunosuppressant rapamycin, alone or with transforming growth
factor-beta, enhances osteoclast differentiation of RAW264.7
monocyte-macrophage cells in the presence of RANK-ligand. Calcif
Tissue Int. 71:437–446. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phornphutkul C, Lee M, Voigt C, Wu KY,
Ehrlich MG, Gruppuso PA and Chen Q: The effect of rapamycin on bone
growth in rabbits. J Orthop Res. 27:1157–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Viñals F, López-Rovira T, Rosa JL and
Ventura V: Inhibition of PI3K/p70 S6K and p38 MAPK cascades
increases osteoblastic differentiation induced by BMP-2. FEBS Lett.
510:99–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee KW, Yook JY, Son MY, Kim MJ, Koo DB,
Han YM and Cho YS: Rapamycin promotes the osteoblastic
differentiation of human embryonic stem cells by blocking the mTOR
pathway and stimulating the BMP/Smad pathway. Stem Cells Dev.
19:557–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lian JB, Javed A, Zaidi SK, Lengner C,
Montecino M, van Wijnen AJ, Stein JL and Stein GS: Regulatory
controls for osteoblast growth and differentiation: Role of
Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 14:1–41. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: Reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stricker S, Fundele R, Vortkamp A and
Mundlos S: Role of Runx genes in chondrocyte differentiation. Dev
Biol. 245:95–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohamadnia AR, Shahbazkia HR, Sharifi S
and Shafael I: Bone-specific alkaline phosphatase as a good
indicator of bone formation in sheepdogs. Comp Clin Path.
16:265–270. 2007. View Article : Google Scholar
|
|
26
|
Matsubara T, Kida K, Yamaguchi A, Hata K,
Ichida F, Meguro H, Aburatani H, Nishimura R and Yoneda T: BMP2
regulates Osterix through Msx2 and Runx2 during osteoblast
differentiation. J Biol Chem. 283:29119–29125. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishio Y, Dong Y, Paris M, O'Keefe RJ,
Schwarz EM and Drissi H: Runx2-mediated regulation of the zinc
finger Osterix/Sp7 gene. Gene. 372:62–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elsubeihi ES and Heersche JN: Quantitative
assessment of post-extraction healing and alveolar ridge
remodelling of the mandible in female rats. Arch Oral Biol.
49:401–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanchez CP and He YZ: Bone growth during
rapamycin therapy in young rats. BMC Pediatr. 9:32009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun H, Kim JK, Mortensen R, Mutyaba LP,
Hankenson KD and Krebsbach PH: Osteoblast-targeted suppression of
PPARγ increases osteogenesis through activation of mTOR signaling.
Stem Cells. 31:2183–2192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Yi XD and Li CD: Suppression of
mTOR signaling pathway promotes bone marrow mesenchymal stem cells
differentiation into osteoblast in degenerative scoliosis: In vivo
and in vitro. Mol Biol Rep. 44:129–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van der Bruggen T, Nijenhuis S, van Raaij
E, Verhoef J and van Asbeck BS: Lipopolysaccharide-induced tumor
necrosis factor alpha production by human monocytes involves the
raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun. 67:3824–3829.
1999.PubMed/NCBI
|
|
33
|
Fisher CJ Jr, Agosti JM, Opal SM, Lowry
SF, Balk RA, Sadoff JC, Abraham E, Schein RM and Benjamin E:
Treatment of septic shock with the tumor necrosis factor receptor:
Fc fusion protein. The soluble TNF receptor sepsis study group. N
Engl J Med. 334:1697–1702. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Glauser MP, Zanetti G, Baumgartner JD and
Cohen J: Septic shock: Pathogenesis. Lancet. 338:732–736. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sweet MJ and Hume DA: Endotoxin signal
transduction in macrophages. J Leukoc Biol. 60:8–26.
1996.PubMed/NCBI
|
|
36
|
Pacifici R, Brown C, Puscheck E, Friedrich
E, Slatopolsky E, Maggio D, McCracken R and Avioli LV: Effect of
surgical menopause and estrogen replacement on cytokine release
from human blood mononuclear cells. Proc Natl Acad Sci USA. 88:pp.
5134–5138. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manolagas SC, Bellido T and Jilka RL: New
insights into the cellular, biochemical, and molecular basis of
postmenopausal and senile osteoporosis: Roles of IL-6 and gp130.
Int J Immunopharmacol. 17:109–116. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hofbauer LC, Lacey DL, Dunstan CR,
Spelsberg TC, Riggs BL and Khosla S: Interleukin-1beta and tumor
necrosis factor-alpha, but not interleukin-6, stimulate
osteoprotegerin ligand gene expression in human osteoblastic cells.
Bone. 25:255–259. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei S, Kitaura H, Zhou P, Ross FP and
Teitelbaum SL: IL-1 mediates TNF-induced osteoclastogenesis. J Clin
Invest. 115:282–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weitzmann MN, Roggia C, Toraldo G,
Weitzmann L and Pacifici R: Increased production of IL-7 uncouples
bone formation from bone resorption during estrogen deficiency. J
Clin Invest. 110:1643–1650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krajewski AC, Biessei J, Kunze M, Maersch
S, Perabo L and Noack MJ: Influence of lipopolysaccharide and
interleukin-6 on RANKL and OPG expression and release in human
periodontal ligament cells. APMIS. 117:746–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Powell N, Till S, Bungre J and Corrigan C:
The immunomodulatory drugs cyclosporin A, mycophenolate mofetil,
and sirolimus (rapamycin) inhibit allergen-induced proliferation
and IL-5 production by PBMCs from atopic asthmatic patients. J
Allergy Clin Immunol. 108:915–917. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luo D, Ren H, Li T, Lian K and Lin D:
Rapamycin reduces severity of senile osteoporosis by activating
osteocyte autophagy. Osteoporos Int. 27:1093–1101. 2016. View Article : Google Scholar : PubMed/NCBI
|