Introduction
Atopic dermatitis (AD) is a pruritic and chronic
inflammatory skin disease caused by dysfunction of the skin barrier
and immune response. Initially, skin dysfunction may be caused by
increased protease activity, irritant exposure or genetic mutation
(1). Disturbed skin tissues
exhibit an increased permeability to external antigens or
allergens, an increased release of innate immune cytokines from
keratinocytes and increased infiltration of type 2 helper (Th2) T
cells (2). Infiltrated Th2 cells
secrete various inflammatory cytokines, including interleukin
(IL)-4, IL-5 and IL-13, which are considered to increase
immunoglobulin E (IgE) levels. Th2-promoted pro-inflammatory
mediators further impair epidermal differentiation and integrity,
and subsequently induce the release of pro-inflammatory and
pruritogenic mediators by keratinocytes (2).
AD develops in early childhood up to five years old
and is observed in 60% of adults who have a personal family history
of similar skin diseases or asthma during childhood (3). Methods of treating this disorder
generally include the application of topical steroids or
antibiotics and general skin care. As the number of available
treatments is limited and several side effects are reported,
certain patients opt for alternative treatments such as natural
products (4,5). Various pharmacological compounds from
natural products have been developed that may have the potential to
treat various symptoms in chronic diseases including atopic
dermatitis (AD) (6).
Amomum xanthioides is the seed of Amomum
villosum Lour, which is grown throughout Asia. The seed of
A. xanthioides has traditionally been used to treat
indigestion, diarrhea, flatulence, toothache and sepsis in China
and Vietnam (7). In addition, the
fruit is effective against asthma and also functions as an
antiemetic agent (7). Various
studies have demonstrated that the seed extract of A.
xanthioides (AXE) exerts various pharmacological effects,
including hepatoprotective and anti-gastritis effects, and the
prevention of diabetes mellitus (8,9).
Furthermore, our previous studies demonstrated that seed AXE
inhibited immediate-type hypersensitivity by reducing mast cell
degranulation (10,11). The present study investigated the
effects of AXE on allergic skin inflammation, specifically AD, and
the underlying mechanism of action.
Materials and methods
Animals
Six-week-old BALB/c mice (female, 20 g) were
purchased from Japan SLC, Inc (Hamamatsu, Japan). A total of 30
mice were housed with 5–10 mice per cage in a laminar air flow room
and were maintained at a temperature of 22±2°C with a relative
humidity of 55±5% throughout the study. The food and water were
provided ad libitum, and mice were kept on a 12-h light/12-h dark
cycle. The care and treatment of the mice were in accordance with
the guidelines established by the Public Health Service Policy on
the Humane Care and Use of Laboratory Animals (Kyungpook National
University, Daegu, Republic of Korea) and was approved by the
Institutional Animal Care and Use Committee in Kyungpook National
University, Daegu, Republic of Korea.
Preparation of AXE
The AXE was prepared as previously described
(11). Briefly, A.
xanthioides seeds were purchased from Bohwa Dang (Jeonju,
Korea) and identified by Dr D.K. Kim at the College of Pharmacy,
Woosuk University (Samrye, Korea). A voucher specimen (no.
WSP-16-04) was deposited at the Herbarium of Woosuk University.
Seeds were ground (1,000 rpm for 30 sec) at room temperature using
a Micro Hammer-Cutter Mill (Culatti AG, Zurich, Switzerland). The
particle size after grinding was 0.5–2 mm. The plant sample (60 g)
was extracted twice with purified water (500 ml) at 70°C for 5 h in
a water bath. The extract was filtered through Whatman grade 1
filter paper and the filtrate was lyophilized using 0.45 µm syringe
filters. The dried extract yield from crude materials was ~5.2%.
The dried extract was dissolved in saline or Tyrode's buffer A (10
mM HEPES, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM
MgCl2, 5.6 mM glucose and 0.1% bovine serum albumin
(Affymetrix; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
prior to use.
Drugs and chemicals
All reagents were purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) unless otherwise stated. House dust
mite (Dermatophagoides farinae) extract (DFE; Greer
Laboratories, Inc., Lenoir, NC, USA) was used as an antigen and
2,4-dinitrochlorobenzene (DNCB) was used as a sensitizer to induce
AD-like skin inflammation. Freeze-dried crude DFE powder was
dissolved in PBS containing 0.5% Tween-20. DNCB (1%) was dissolved
in an acetone/olive oil (1:3) solution. Recombinant TNF-α and IFN-γ
were purchased from R&D systems, Inc. (Minneapolis, MN,
USA).
Cell culture and viability
A human keratinocyte cell line, HaCaT (American Type
Culture Collection, Manassas, VA, USA), was maintained in
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc) and antibiotics (100 U/ml penicillin G and 100 µg/ml
streptomycin) at 37°C in 90–95% humidity and 5% CO2.
Cell viability was determined using a MTT assay. HaCaT cells were
treated with various concentration of AXE (0, 0.01, 0.1, 1, 10 and
100 µg/ml) and incubated for 24 h at 37°C in 5% CO2.
Then, MTT (5 mg/ml) was added to each sample well and incubated for
2 h at 37°C. Dimethyl sulfoxide was added to dissolve the formazan
crystals. The absorbance of each sample was at a wavelength of 570
nm compared with the control and expressed as a percentage.
Induction of AD-like skin inflammation
in the mouse ear
The induction of AD-like skin inflammation by DFE
and DNCB was performed using methods based on our previous research
(12). Total 30 mice were divided
into the following six groups (n=5 each group): Vehicle (mice were
treated only with PBS); DFE/DNCB + vehicle; DFE/DNCB + AXE (2, 10
and 50 mg/kg) and tacrolimus (Tac; 1 mg/kg) (Sigma-Aldrich; Merck
KGaA). The surfaces of both ear lobes were stripped very gently
using surgical tape to remove foreign matter or scabbing. After
stripping, 20 µl DNCB (1%) was applied to each ear, which was
followed by 20 µl DFE (10 mg/ml) 4 days later. Treatment with
DFE/DNCB was repeated once a week alternatively for 4 weeks. At 1
week after the first DFE/DNCB treatment, AXE or Tac was orally
administered 5 times weekly between days 7 and 27. Ear thickness
was measured 24 h after DFE or DNCB application with a dial
thickness gauge.
On day 28, blood samples were collected via celiac
artery puncture. Whole blood was incubated at 4°C overnight,
centrifuged at 400 × g for 10 min at 4°C, and serum was collected.
After mice were sacrificed, ears were removed and used for
histopathological analysis. IgG2a levels were measured using an
ELISA kit (cat. no. 552576, BD Biosciences, Franklin Lakes, NJ,
USA). Total IgE and DFE-specific IgE level were assayed with the
same kit (cat. no. 555248). Total IgE levels were measured by
concentration calculation. Mite-specific IgE levels were detected
as optical density values.
Histological observations
The ears were fixed with 10% formaldehyde for one
week at room temperature and embedded in paraffin. Sections of 5 µm
thickness were stained with hematoxylin solution for 5 min and
eosin solution for 1 min at room temperature. Cellular infiltration
of eosinophils and thickening of the epidermis and dermis were
observed via light microscopy. Eosinophils were also counted from
10 different fields at a magnification ×400 in a blinded manner.
Dermal thickness in H&E-stained sections was visualized and
analyzed at a magnification ×200. Thickness was measured from five
randomly selected fields from each sample. For measurement of mast
cell infiltration, skin sections were stained with 0.1% toluidine
blue solution for 2–3 min at room temperature and mast cells from
10 different fields were counted at a magnification ×400 in a
blinded manner.
Histamine assay
Histamine content was measured via the
o-phthaldialdehyde spectrofluorometric procedure based on
the method described by a previous report (13). Blood from the mice was centrifuged
at 400 × g for 10 min at 4°C and serum was withdrawn to measure
histamine content. For serum histamine assay, 50 µl serum was used.
Fluorescence was measured on an LS-50B fluorescence spectrometer
(PerkinElmer, Inc., Waltham, MA, USA) using 355 nm excitation and
450 nm emission filters.
Fluorescence-activated cell
sorting
At the end of the experiment, mice were euthanized
with CO2 and both auricular lymph nodes were collected
from each mouse. Auricular lymph nodes were ground using 70 µm
nylon cell strainers to isolate single cells. Cells were
subsequently stained using a Mouse Th1/Th2/Th17 phenotyping kit (BD
Biosciences) according to the manufacturer's protocol, and
fluorescence intensity was detected using a FACSCalibur flow
cytometer (BD Biosciences). Population of cells was analyzed by BD
CellQuest™ Pro software (version 5.1, BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For the quantification of cytokine expression, qPCR
was performed using the TP850 Thermal Cycler Dice Real Time System
(Takara Bio, Inc., Otsu, Japan) according to the manufacturer's
protocol. At the end of the in vivo experimental period, the
ears were excised and total RNA was isolated. Total RNA was
isolated using RNAiso Plus (Takara Bio, Inc.). For the in
vitro analysis, HaCaT cells were pretreated with 0, 0.1, 1 and
10 µg/ml AXE or 10 µg/ml Tac for 1 h at 37°C, and subsequently
stimulated with TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) for 6 h at
37°C. Total cellular RNA was isolated from cells (2×105
cells/24-well plate) according to a method previously described
(14). Complementary (c)DNA was
synthesized with 1 µg of total RNA under condition of 1 h at 45°C
and 5 min at 95°C using Maxime RT PreMix Kit (Intron Biotechnology,
Inc., Seongnam, Korea). A total of 2 µl cDNA (100 ng), 1 µl sense
and antisense primer solution (0.4 µM), 12.5 µl SYBR Premix Ex Taq
(Takara Bio, Inc.) and 9.5 µl dH2O were mixed together
to obtain a 25 µl reaction mixture in each reaction tube. The
relative transcription levels of the mRNAs were calculated
according to the 2−ΔΔCq method (15). β-actin was used as an internal
control. The primers used are shown in Table I. The conditions for amplification
of DNA were 95°C for 30 sec, 45 cycles of 95°C for 5 sec, and 60°C
for 30 sec. A melting curve analysis was done after amplification.
Normalization and quantification of mRNA expression were performed
using the TP850 software supplied by the manufacturer.
 | Table I.Sequences of oligonucleotide
primers. |
Table I.
Sequences of oligonucleotide
primers.
| Mouse | Primer sequence
(5′-3′) | Position | GenBank accession
number |
|---|
| TNF-α |
|
|
|
|
Forward |
GGCAGGTCTACTTTGGAGTCATTGC | 796–1,095 | NM_001278601.1 |
|
Reverse |
ACATTCGAGGCTCCAGTGAATTCG G |
|
|
| IFN-γ |
|
|
|
|
Forward |
TCAAGTGGCATAGATGTGGAAGAA | 224–315 | NM_008337.4 |
|
Reverse |
TGGCTCTGCAGGATTTTCATG |
|
|
| IL-4 |
|
|
|
|
Forward |
ACAGGAGAAGGGACGCCAT | 180–274 | NM_021283.2 |
|
Reverse |
GAAGCCGTACAGACGAGCTCA |
|
|
| IL-13 |
|
|
|
|
Forward |
GCAGCATGGTATGGAGTGTG | 226–451 | NM_008355.3 |
|
Reverse |
TGGCGAAACAGTTGCTTTGT |
|
|
| IL-31 |
|
|
|
|
Forward |
TCGGTCATCATAGCACATCTGGA | 308–637 | NM_029594.1 |
|
Reverse |
GCACAGTCCCTTTGGAGTTAAGTC |
|
|
| IL-17A |
|
|
|
|
Forward |
TCCCTCTGTGATCTGGGAAG | 321–474 | NM_010552.3 |
|
Reverse |
CTCGACCCTGAAAGTGAAGG |
|
|
| β-actin |
|
|
|
|
Forward |
TAGACTTCGAGCAGGAGATG | 771–1,092 | NM_007393.5 |
|
Reverse |
TTGATCTTCATGGTGCTAGG |
|
|
|
| Human | Primer sequence
(5′-3′) | Position | GenBank accession
number |
|
| TNF-α |
|
|
|
|
Forward |
CCTACCAGACCAAGGTCAAC | 660–937 | NM_000594.3 |
|
Reverse |
AGGGGGTAATAAAGGGATTG |
|
|
| IL-1β |
|
|
|
|
Forward |
GCTGATGGCCCTAAACAGATGAA | 163–271 | NM_000576.2 |
|
Reverse |
TGAAGCCCTTGCTGTAGTGGTA |
|
|
| IL-6 |
|
|
|
|
Forward |
AAAGAGGCACTGGCAGAAAA | 365–776 | NM_000600.4 |
|
Reverse |
ATCTGAGGTGCCCATGCTAC |
|
|
| CCL17 |
|
|
|
|
Forward |
ACTGCTCCAGGGATGCCATCGTTT | 296–565 | NM_002987.2 |
|
Reverse |
ACAAGGGGATGGGATCTCCCTCAC |
|
|
| CCL22 |
|
|
|
|
Forward |
AGGACAGAGCATGGCTCGCCTACA | 25–386 | NM_002990.4 |
|
Reverse |
TAATGGCAGGGAGGTAGGGCTCCT |
|
|
| β-actin |
|
|
|
|
Forward |
GGACTTCGAGCAAGAGATGG | 747–980 | NM_001101.3 |
|
Reverse |
AGCACTGTGTTGGCGTACAG |
|
|
Nuclear protein extraction
HaCaT cells were pretreated with AXE for 1 h, and
then stimulated with TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) for 30
min. After stimulation, cells (1×106 cells/6-well plate)
were washed in 1 ml of ice-cold PBS, centrifuged at 1,200 × g for 5
min at 4°C, resuspended in 400 µl of ice-cold hypotonic buffer (10
mM HEPES, 2 mM MgCl2, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT,
0.5 mM PMSF, pH 7.9), left on ice for 10 min, vortexed, and
centrifuged at 5,000 × g for 5 min at 4°C. Pelleted nuclei were
resuspended in 50 µl of ice-cold saline buffer (50 mM HEPES/KOH, 50
mM KCl, 300 mM NaCl, 0.1 mM EDTA, 10% glycerol, 1 mM DTT, 0.5 mM
PMSF, pH 7.9), left on ice for 20 min, vortexed, centrifuged at
15,000 × g for 5 min at 4°C, and supernatant was collected.
Western blot analysis
Samples for western blotting were prepared as
previously described (14). HaCaT
cells (1×106 cells/6-well plate) were stimulated for 20
min with TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) for extracellular
signal-regulated kinase (ERK) and p38 mitogen-activated protein
kinases (MAPKs) and signal transducer and activator of
transcription 1 (STAT1), and 30 min for nuclear factor-κB (NF-κB)
after pretreatment with 10 µg/ml AXE or 10 µg/ml Tac for 1 h.
Whole-cell extracts were prepared by washing cells twice with
ice-cold PBS and incubating them in lysis buffer [20 mM Tris (pH
7.4), 137 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 100 µM
DTT] with the addition of phosphatase inhibitor cocktail (Roche
Diagnostics, Indianapolis, IN, USA) for 30 min at 4°C. The lysates
were collected by centrifugation at 13,000 × g for 15 min at 4°C,
and protein quantification was performed with a Bradford protein
assay kit (Bio-Rad, Hercules, CA, USA). For western blot analysis,
30 µg of total protein was separated using 8–12% sodium dodecyl
sulfate polyacrylamide gels and transferred to nitrocellulose
membranes (Pall Life Sciences, Port Washington, NY, USA). The
membranes were stained with reversible Ponceau S stain for 10–20
sec at room temperature to ascertain equal loading of samples in
the gel. All blots were blocked in 3% blocking buffer [3% w/v skim
milk, 0.1% Tween-20, Tris-buffered saline (pH 7.4)] at room
temperature for 1 h and incubated in primary antibody (1:1,000
dilution) at 4°C for overnight. Then, secondary antibody (1:2,000
dilution) were incubated at room temperature for 1 h. Detection was
performed using an enhanced chemiluminescence detection kit (GE
Healthcare, Chicago, IL, USA). β-actin and lamin B were used as
internal loading controls for cytosolic and nuclear extraction,
respectively. Phospho-ERK (Thr202/Tyr204; cat. no. 9101),
phospho-p38 MAPK (Tyr180/Tyr182; cat. no. 9211), phospho-STAT1
(Tyr701; cat. no. 9171), ERK (ERK1/2; cat. no. 9102), p38 MAPK
(cat. no. 9212) and STAT1 (cat. no. 9172) were from Cell Signaling
Technology Inc. (Danvers, MA, USA). NF-κB p65 (cat. no. sc-109),
IκB-α (cat. no. sc-371), lamin B (cat. no. sc-6217), β-actin (cat.
no. sc-8432), and horseradish peroxidase-linked anti-rabbit IgG
(cat. no. sc-2004) antibodies were from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 7 (La Jolla, CA, USA). Treatment effects were analyzed using
one-way analysis of variance followed by Dunnett's test. Data are
presented as the mean ± standard error. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of AXE on ear thickness and
histopathological changes in AD-like skin inflammation
To investigate the effects of AXE on AD-like skin
inflammation, AXE was orally administered to BALB/c mice 5 times
weekly from days 7 to 27. Repeated application of DFE/DNCB on the
ear lobes of mice increased ear thickness, swelling, reddening and
scaling of the skin. Ear thickening was unaffected until 1 week
after initial oral administration of AXE (2, 10 and 50 mg/kg).
Thereafter, thickening was suppressed in a dose- and time-dependent
manner by AXE (Fig. 1A). Ear
photographs at the end of the experiment demonstrated the
amelioration of the skin lesion, including swelling and reddening,
by AXE treatment (Fig. 1B). Tac,
also termed FK-506 or fujimycin, is an immunosuppressive drug used
for the treatment of inflammatory skin diseases such as AD and
psoriasis. It was used as a positive control.
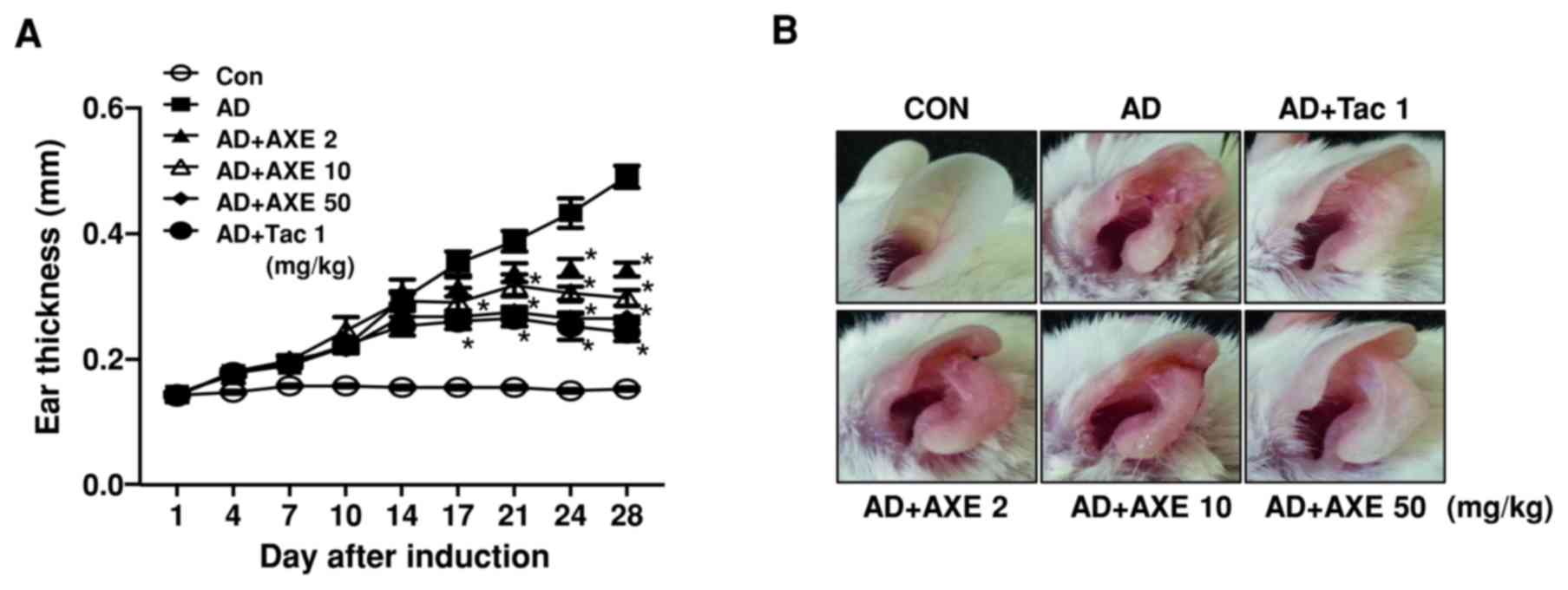 | Figure 1.Effects of AXE on DFE/DNCB-induced AD
mice. (A) To measure the effects of AXE (2, 10 and 50 mg/kg) or Tac
(1 mg/kg) on DFE/DNCB-induced AD, ear thickness was measured 24 h
after DFE or DNCB application with a dial thickness gauge. (B)
Gross observation of morphological recovery at day 28. (C) Ear
tissues were stained with H&E or toluidine blue for
histological analysis and the determination of dermal and epidermal
thickness, and eosinophil and mast cell infiltration. Original
magnification, ×200. (D) Dermal and (E) epidermal thickness. Number
of (F) eosinophils and (G) mast cells. Data are presented as the
mean ± standard error of the mean of five determinants. *P<0.05
vs. AD group. AXE, Amomum xanthioides extract; DFE,
Dermatophagoides farinae extract; DNCB,
dinitrochlorobenzene; AD, atopic dermatitis; Tac, tacrolimus; CON,
control. |
To analyze the effects of AXE on skin hypertrophy
and infiltration of eosinophils and mast cells, which are important
effector cells in AD, tissue sections of ears were stained with
H&E or toluidine blue (Fig.
1C). Quantitative values based on microscopic observations were
analyzed (Fig. 1D-G). Compared
with the control, repeated DFE/DNCB exposure caused a marked
increase in the thickening of the dermis and epidermis, in addition
to the infiltration of eosinophils and mast cells. However, oral
administration of AXE reduced these histopathological changes in a
dose-dependent manner (Fig.
1D-G).
Effect of AXE on serum histamine and
Ig levels
To evaluate the serum levels of histamine and Igs in
AD, serum was isolated following blood collection. Excessive
production of histamine, a representative symptom of AD, was
detected in DFE/DNCB-sensitized mice (Fig. 2A). In addition, elevation of total
IgE, DFE-specific IgE and IgG2a (Fig.
2B-D) was also observed in the DFE/DNCB-sensitized mice. These
increased levels of histamine and Igs were significantly decreased
following AXE application.
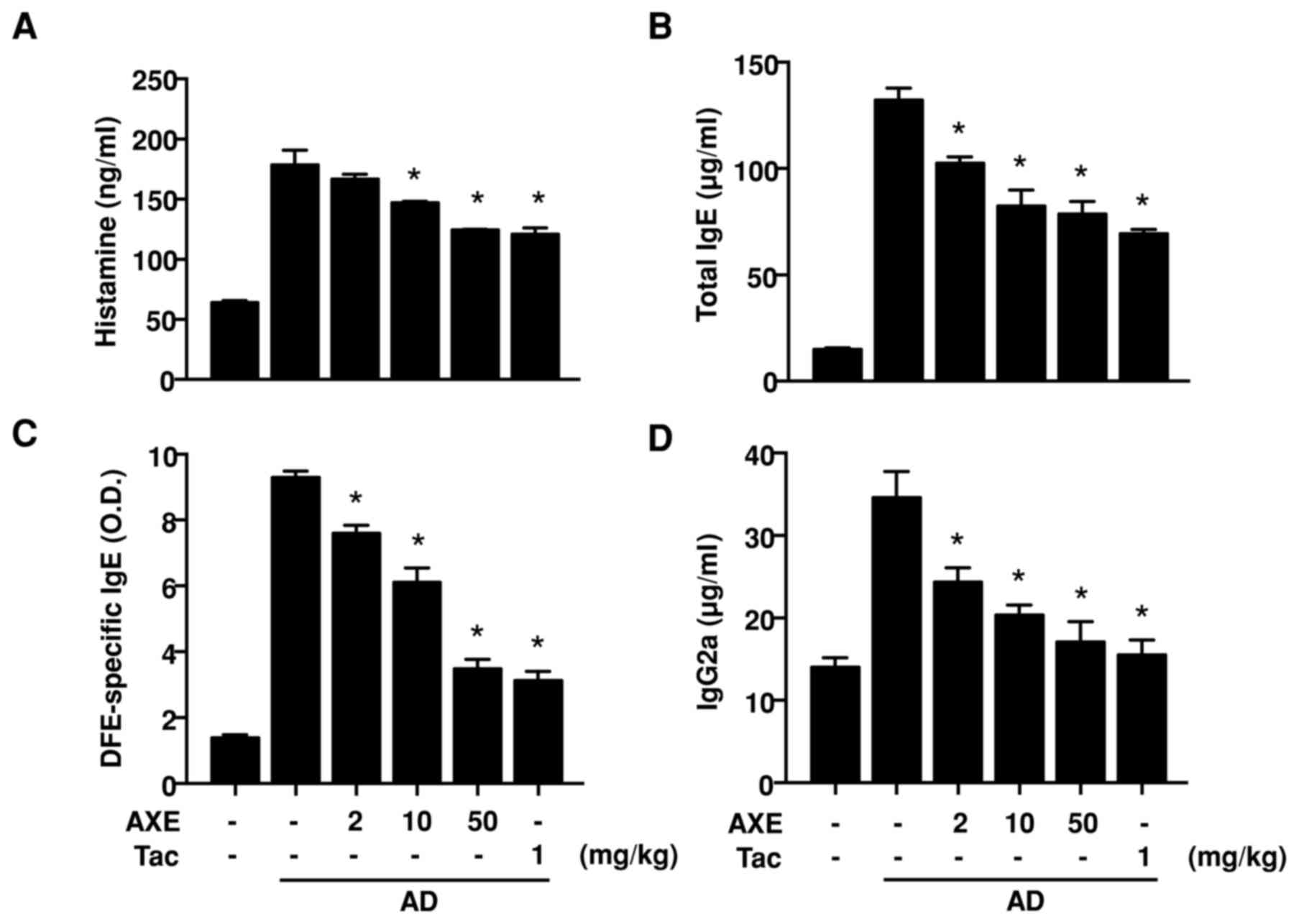 | Figure 2.Levels of (A) serum histamine and
(B-D) Igs in DFE/DNCB-induced AD mice. Blood samples from vehicle,
DFE/DNCB + vehicle, and DFE/DNCB + AXE (2, 10 and 50 mg/kg) or Tac
(1 mg/kg) groups were obtained from the celiac artery at day 28,
and serum was isolated. Serum histamine and Igs were quantified by
ELISA. Data are presented as the mean ± standard error of the mean
of five determinants. *P<0.05 vs. AD group. Ig, immunoglobulin;
AD, atopic dermatitis; DFE, Dermatophagoides farinae
extract; DNCB, dinitrochlorobenzene; AXE, Amomum xanthioides
extract; Tac, tacrolimus; O.D., optical density. |
Effects of AXE on the polarization of
T lymphocytes and expression of cytokines in the ear tissue of AD
mice
The progression of AD is characterized by an
alteration in the polarization of the T cell population,
particularly that of Th1, Th2 and Th17 cells (16). To assess alterations in T
lymphocyte polarization, single cells from auricular lymph nodes
were isolated and analyzed using specific antibodies for signature
cytokines expressed by polarized lymphocytes, including IFN-γ for
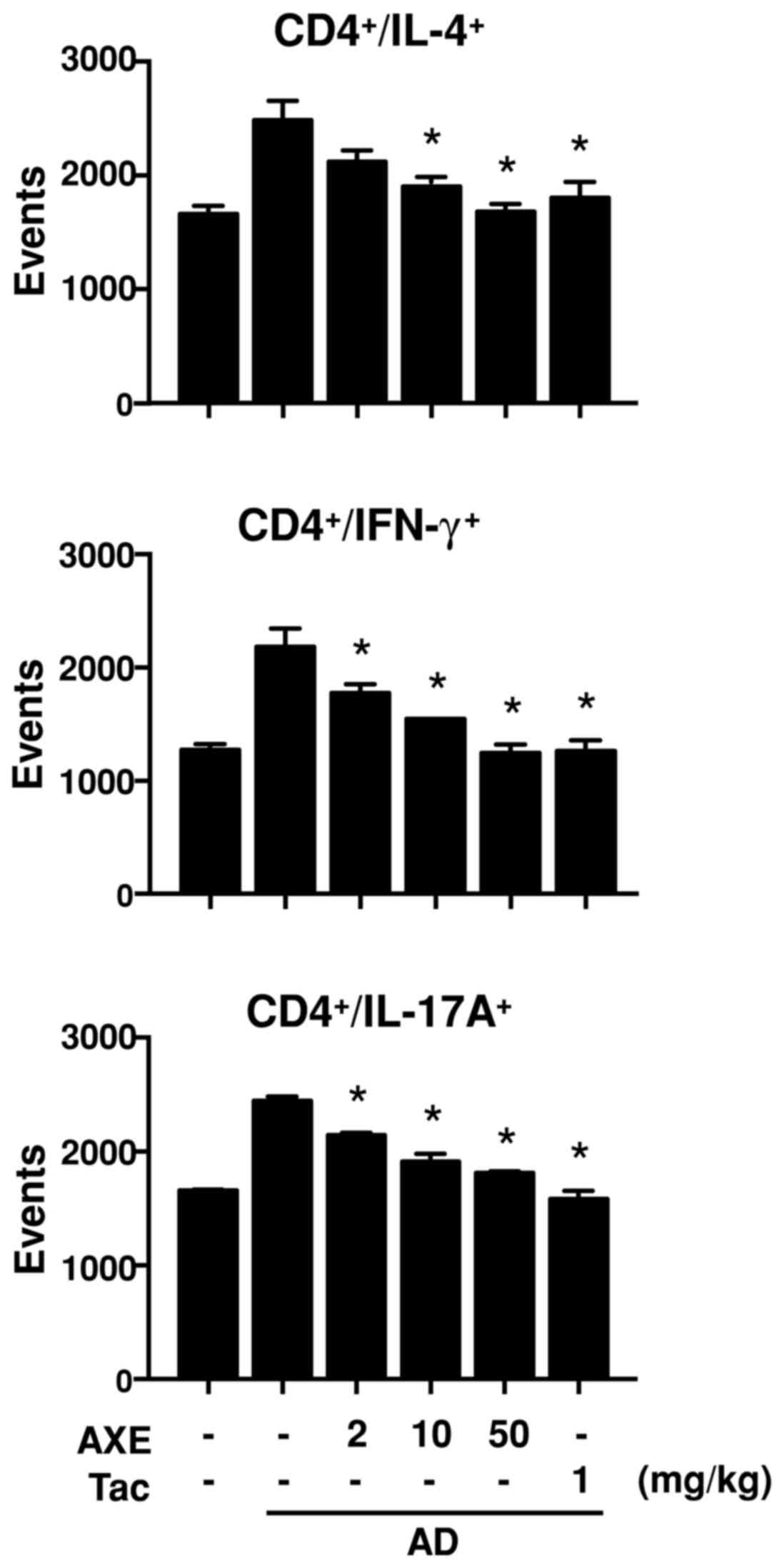
Th1, IL-4 for Th2 and IL-17A for Th17 (Fig. 3). The results demonstrated that
populations of CD4+/IFN-γ+,
CD4+/IL-4+ and
CD4+/IL-17A+ were increased in the
DFE/DNCB-sensitized mice, and were dose-dependently decreased
following AXE treatment.
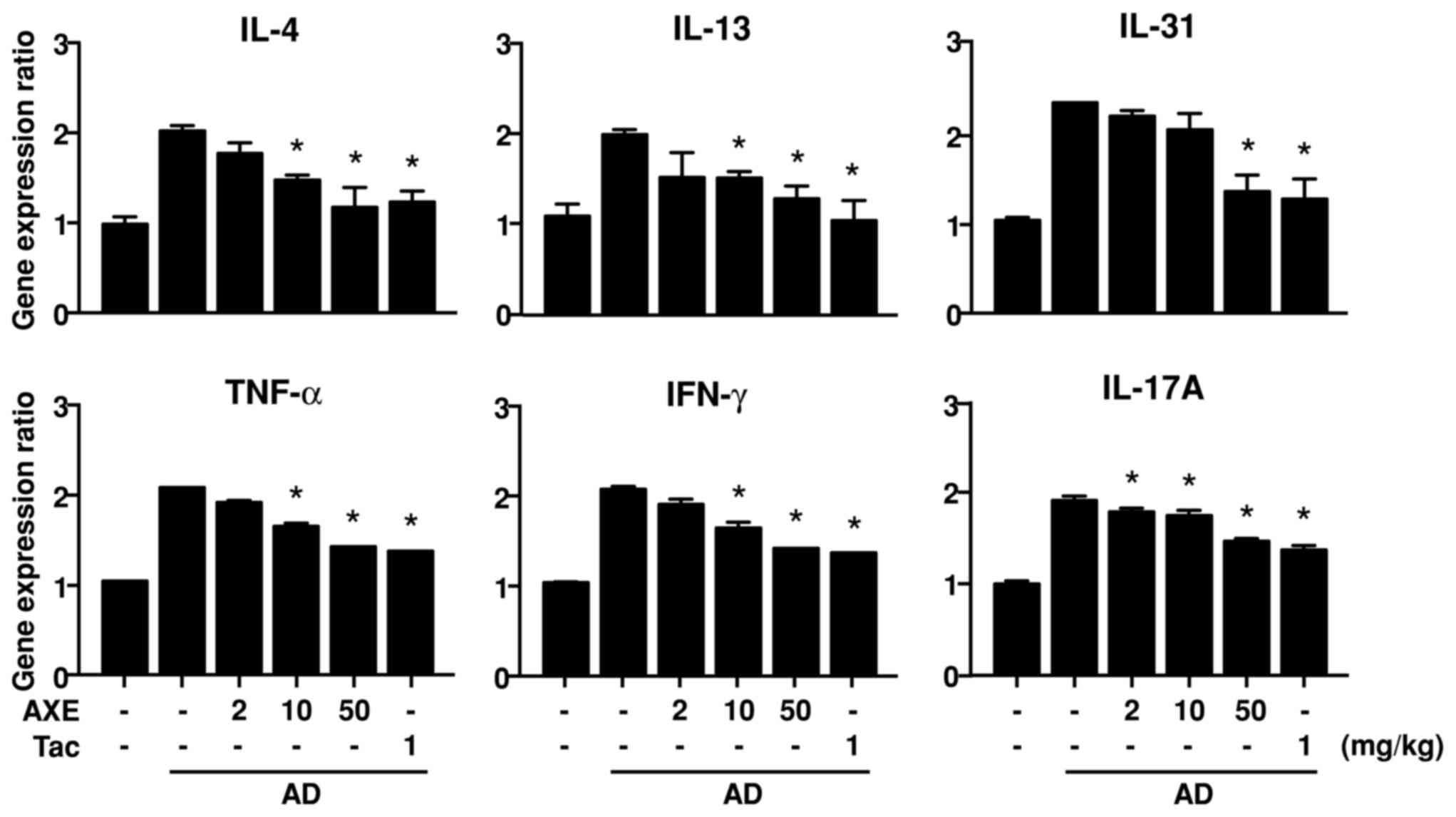
In AD, infiltrated immune cells, including
lymphocytes and resident keratinocytes, continuously interact via
the production of various cytokines, and thus exacerbate the
disease (2). To assess the gene
expression of cytokines in AD skin, RNA was isolated from ear
tissue and qPCR was performed. Repeated application of DFE/DNCB
increased the expression levels of IL-4, IL-13, IL-31, TNF-α, IFN-γ
and IL-17A. However, treatment with AXE significantly reduced the
expression of these cytokines at certain doses (Fig. 4).
Effects of AXE on the activation of
keratinocytes
In AD, keratinocytes are an important source of
cytokines that accelerate chronic, self-amplifying loops of immune
activation. In skin inflammation, the increased production of TNF-α
and IFN-γ by keratinocytes promotes the amplification of the
inflammatory response. TNF-α and IFN-γ stimulate the synthesis and
secretion of various inflammatory mediators by keratinocytes
(14,17). To determine the effect of AXE on
the production of inflammatory cytokines and its signaling
mechanism, HaCaT cells were stimulated with TNF-α/IFN-γ with or
without pretreatment with AXE (Fig.
5). Treatment with TNF-α/IFN-γ promoted the gene expression of
the pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and
IL-8, CCL17 and CCL22. These increased levels were reduced by
pretreatment with AXE (Fig. 5A).
Cell viability of HaCaT cells was unaffected by AXE concentrations
of up to 100 µg/ml (Fig. 5B).
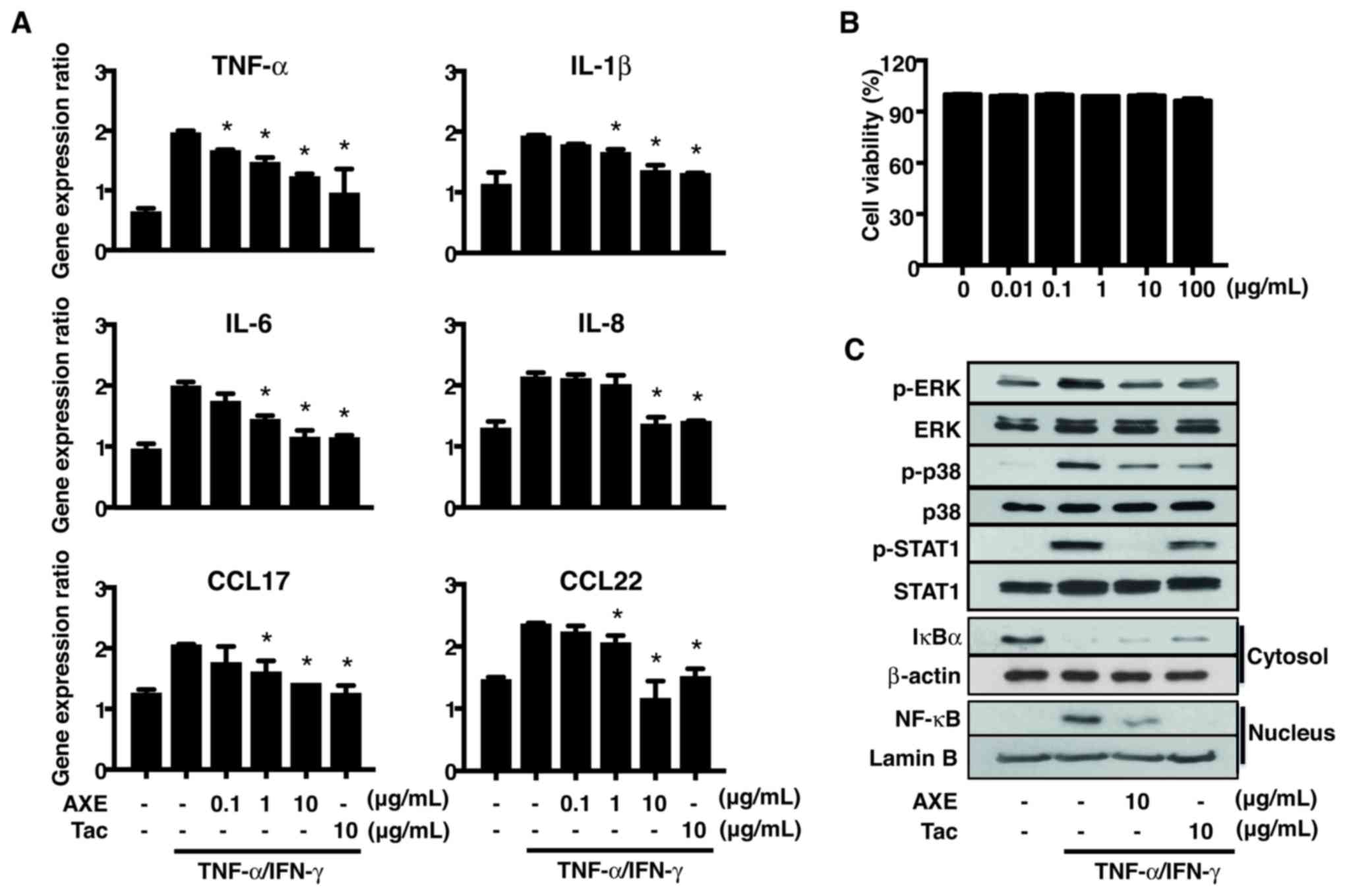 | Figure 5.Effects of AXE on
TNF-α/IFN-γ-stimulated HaCaT cells. Cells were pretreated with AXE
or Tac for 1 h prior to stimulation with TNF-α (10 ng/ml) and IFN-γ
(10 ng/ml). (A) Gene expression of TNF-α, IL-1β, IL-6, IL-8, CCL17
and CCL22 in HaCaT cells were detected by reverse
transcription-quantitative polymerase chain reaction. (B) Cell
viability in HaCaT cells following AXE treatment was determined by
an MTT assay after 24 h. (C) Representative western blot of three
independent experiments for the protein expression of p-ERK, p-p38,
p-STAT1, NF-κB and IκBα. Data are presented as the mean ± standard
error of the mean of five determinants. *P<0.05 vs. TNF-α/IFN-γ
stimulation. AXE, Amomum xanthioides extract; TNF, tumor
necrosis factor; IFN, interferon; Tac, tacrolimus; IL, interleukin;
CCL, C-C motif chemokine ligand; p-, phosphorylated-; ERK,
extracellular signal-regulated kinase; STAT1, signal transducer and
activator of transcription 1; NF-κB, nuclear factor-κB; IκBα, NF-κB
inhibitor α. |
To investigate the mechanism responsible for the
inhibitory effects of AXE on the expression of cytokines and
chemokines in HaCaT cells, the activation of MAPKs, STAT1, IkBα and
NF-κB, which regulate the expression of cytokines and chemokines,
was analyzed by Western blot (Fig.
5C). TNF-α/IFN-γ-induced phosphorylation of ERK, p38 and STAT1,
as well as degradation of cytosolic IκBα and nuclear translocation
of NF-κB, were inhibited by AXE.
Discussion
A. xanthioides has been used in Asia to treat
various disorders, including stomach and digestive disorders, for a
long time (7). The seed of A.
xanthioides is listed in the Japanese Pharmacopoeia as ‘Amomum
seed’ (18). Although their total
composition remains unclear, the seeds commonly contain five
primary classes of components, including volatile oils, saponins,
flavonoid glycosides, organic acids and inorganic components
(19). Specifically, they contain
1–1.5% essential oil that is rich in monoterpenoids (borneol,
linalool, camphene and nerolidol), which are reported to exhibit
numerous pharmacological properties, including antimicrobial,
anti-inflammatory, anti-oxidant, anti-pruritic, hypotensive and
analgesic activities (20).
The present study investigated the pharmacological
effects of a water-soluble component of A. xanthioides in a
mouse AD-like skin inflammation model. AXE alleviated clinical and
histopathological alterations in AD-like skin inflammation,
including severe ear thickening, ulcers, epidermal thickening and
infiltration of immune cells. Gross observation prior to sacrifice
demonstrated severe clinical phenotypes, including as hemorrhage,
edema, excoriation and scaling in DFE/DNCB-induced skin
inflammation. However, the oral administration of AXE ameliorated
those symptoms.
The activation of mast cells and eosinophils, and
increased IgE levels, are hallmarks of Th2-mediated immune
responses (21,22). Mast cells typically accumulate in
DFE and/or DNCB-stimulated skin lesions (23,24).
They produce histamine, a major contributor to pruritus, which is
key symptom of AD (25). Tissue
eosinophilia in AD skin is associated with epidermal hyperplasia,
spongiosis, skin hypertrophy and disease severity (26). Mast cells and eosinophils express
an IgE receptor (FcεRI) on their surface and are activated by IgE
(21,27). In general, the IgE level is an
appropriate marker for Th2 response, whereas IgG2a level is a
marker for Th1 response (28).
Excessive Th2 responses are primarily observed in the acute stages
of AD, whereas a mixed Th1/Th2 pattern of inflammation is usually
observed during the chronic stage. Stimulation with DFE/DNCB for 4
weeks induced a chronic AD-like response in the mouse model of our
previous study (17). We
hypothesize that the suppressive effect of AXE on DFE/DNCB-induced
allergic skin inflammation may be due to a decrease in Th2-mediated
immune responses leading to a decrease in Th1 responses.
T cells, as one of the major components of adaptive
immunity, have important roles in the pathogenesis of AD. In AD
progression, T cell polarization is biphasic, primarily involving
Th2 cells in the acute phase and Th1 cells in the chronic phase
(16). Each subset is
characterized by the expression of specific cytokines.
Th2-polarized cells express Th2 cytokines, including IL-4 and IL-13
(29), while Th1 cells express
IFN-γ (30). In addition, Th17
cells, as a specific subset of memory CD4+ T cells, have
been reported to contribute to the pathogenesis of AD. Analysis of
the IL-17+/CD4+ population in peripheral
blood mononuclear cells isolated from patients with AD previously
revealed that a higher percentage was present in the severely
affected group compared with the healthy control group (31). Furthermore, our previous report
demonstrated that all three T cell subsets increased in draining
lymph nodes following DFE/DNCB exposure (14). In the present study, the results
indicated that AXE pretreatment in AD rats reduced Th1, Th2 and
Th17 subsets in draining lymph nodes. These findings indicate that
AXE may control the differentiation of naive T lymphocytes to
effector T cells.
Various immune biomarkers characterize the
inflammatory phenotype of lesional skin in AD. IL-4, IL-13 and
IL-31 represent the Th2 response, TNF-α and IFN-γ for the Th1
response and IL-17A for Th17 response (32). In the present study, repeated ear
skin exposure to DFE/DNCB markedly increased the expression of
these biomarkers. IL-4 and IL-13 are the key upstream drivers of
the Th2 response in allergic inflammation. IL-31 originating from
inflammatory infiltrates is considered to be critical in the
regulation of keratinocyte differentiation and induction of
pruritus in AD (33,34). As mentioned above, the chronic
stage of AD reveals a mixed Th1/Th2 response pattern. IFN-γ and
TNF-α are secreted by activated Th1 cells and keratinocytes,
respectively, and affect the fate of keratinocytes and the skin
microenvironment. Overexpression of IFN-γ is implicated in skin
hypertrophy (35), and TNF-α was
reported to induce spongiosis and flatten suprabasal keratinocytes
(36). IFN-γ produced by Th1 cells
promotes the expression of IgG2a in B cells (37). IL-17 produced by Th17 cells induces
the production of certain cytokines, chemokines and antimicrobial
peptides by keratinocytes (38).
Therefore, the inhibitory effects of AXE on the expression of each
biomarker in DFE/DNCB-treated ear skin may occur due to a decrease
in the number and activity of infiltrated immune cells.
Keratinocyte activation in inflamed skin has an
important role in the pathogenesis of prolonged inflammatory skin
disorders through the regulation of innate immunity (39). Stimulation of epidermal
keratinocytes with TNF-α and IFN-γ has been reported to induce the
expression of various pro-inflammatory cytokines and chemokines,
including TNF-α itself, IL-1 and IL-8, and the expression of
Th2-attracting chemokines, such as CCL17 and CCL22, was also
induced (40,41). These cytokines and chemokines are
considered to be important mediators for the development of AD.
Cotreatment of keratinocytes with TNF-α and IFN-γ was reported to
activate several transcription factors, including NF-κB and STAT1,
through regulation of the MAPK signaling cascade (42). Notably, TNF-α/IFN-γ-induced
expression of the Th2 chemokines CCL17 and CCL22 was directly
altered by specific inhibitors for NF-κB and STAT (43). Therefore, the inhibitory effects of
AXE on TNF-α/IFN-γ-stimulated keratinocytes may occur via the
suppression of cytokine and chemokine production through the
coordination of MAPK, NF-κB and STAT1. We hypothesize that AXE may
inhibit chronic and self-amplifying loops of immune activation by
blocking the activation of keratinocytes and the subsequent
production of inflammatory cytokines and chemokines.
In conclusion, the results of the present study
demonstrate that oral administration of AXE suppressed
DFE/DNCB-induced AD-like skin inflammation, which may occur due to
decreased infiltration of immune cells and subsequent Th1/Th2/Th17
responses in AD skin, and reduced TNF-α/IFN-γ-induced immune
activation through the regulation of inflammatory cytokines and
chemokines in keratinocytes. The present study employed whole
aqueous extract of A. xanthioides rather than a purified
single compound. Therefore, the biological effects of the
individual active components are not clear at present. Efforts to
identify the active component from AXE on AD symptoms are ongoing
in our laboratory. However, the results presented in this report
give an insight into the mechanism that is potentially responsible
for the anti-AD activity of AXE. Therefore, these results indicate
that AXE may be applied to effectively control AD, as a useful
natural pharmacological agent.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean Government
(grant nos. 2014R1A5A2009242, 2012M3A9B6055416, 2016R1A2B4008513,
2017R1D1A1B03031032 and 2015R1D1A3A01016229), the Korea Research
Institute of Bioscience and Biotechnology Research Initiative
Program (grant no. KGM4251723) and the High Value-added Food
Technology Development Program, Ministry of Agriculture, Food and
Rural Affairs.
References
|
1
|
Cork MJ, Danby SG, Vasilopoulos Y,
Hadgraft J, Lane ME, Moustafa M, Guy RH, Macgowan AL, Tazi-Ahnini R
and Ward SJ: Epidermal barrier dysfunction in atopic dermatitis. J
Invest Dermatol. 129:1892–1908. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weidinger S and Novak N: Atopic
dermatitis. Lancet. 387:1109–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sidbury R and Hanifin JM: Old, new, and
emerging therapies for atopic dermatitis. Dermatol Clin. 18:1–11.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vender RB: Alternative treatments for
atopic dermatitis: A selected review. Skin Therapy Lett. 7:1–5.
2002.PubMed/NCBI
|
|
5
|
Gardiner P and Kemper KJ: Herbs in
pediatric and adolescent medicine. Pediatr Rev. 21:44–57. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun Y, Kim K, Choi I and Ko SG: Topical
herbal application in the management of atopic dermatitis: A review
of animal studies. Mediators Inflamm. 2014:7521032014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quattrocchi U: CRC world dictionary of
medicinal and poisonous plants: Common names, scientific names,
eponyms, synonyms and etymology. CRC Press; Boca Raton: 2012,
View Article : Google Scholar
|
|
8
|
Lee YS, Kang MH, Cho SY and Jeong CS:
Effects of constituents of Amomum xanthioides on gastritis in rats
and on growth of gastric cancer cells. Arch Pharm Res. 30:436–443.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HG, Han JM, Lee JS and Son CG: Ethyl
acetate fraction of Amomum xanthioides improves bile duct
ligation-induced liver fibrosis of rat model via modulation of
pro-fibrogenic cytokines. Sci Rep. 5:145312015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SH and Shin TY: Amomum xanthiodes
inhibits mast cell-mediated allergic reactions through the
inhibition of histamine release and inflammatory cytokine
production. Exp Biol Med (Maywood). 230:681–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SH, Lee S, Kim IK, Kwon TK, Moon JY,
Park WH and Shin TY: Suppression of mast cell-mediated allergic
reaction by Amomum xanthiodes. Food Chem Toxicol. 45:2138–2144.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi EJ, Lee S, Kim HH, Singh TS, Choi JK,
Choi HG, Suh WM, Lee SH and Kim SH: Suppression of dust mite
extract and 2,4-dinitrochlorobenzene-induced atopic dermatitis by
the water extract of Lindera obtusiloba. J Ethnopharmacol.
137:802–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh TS, Lee S, Kim HH, Choi JK and Kim
SH: Perfluorooctanoic acid induces mast cell-mediated allergic
inflammation by the release of histamine and inflammatory
mediators. Toxicol Lett. 210:64–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi JK and Kim SH: Inhibitory effect of
galangin on atopic dermatitis-like skin lesions. Food Chem Toxicol.
68:135–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Auriemma M, Vianale G, Amerio P and Reale
M: Cytokines and T cells in atopic dermatitis. Eur Cytokine Netw.
24:37–44. 2013.PubMed/NCBI
|
|
17
|
Choi JK, Oh HM, Lee S, Kwon TK, Shin TY,
Rho MC and Kim SH: Salvia plebeia suppresses atopic dermatitis-like
skin lesions. Am J Chin Med. 42:967–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Japan. Kōsei Rōdōshō. Nihon
yakkyokuhō=The Japanese pharmacopoeia. Kōsei Rōdōshō; Tokyo:
2001
|
|
19
|
Wang JH, Wang J, Choi MK, Gao F, Lee DS,
Han JM and Son CG: Hepatoprotective effect of Amomum xanthoides
against dimethylnitrosamine-induced sub-chronic liver injury in a
rat model. Pharm Biol. 51:930–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guimarães AG, Quintans JS and Quintans LJ
Jr: Monoterpenes with analgesic activity-a systematic review.
Phytother Res. 27:1–15. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawakami T, Ando T, Kimura M, Wilson BS
and Kawakami Y: Mast cells in atopic dermatitis. Curr Opin Immunol.
21:666–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stone KD, Prussin C and Metcalfe DD: IgE,
mast cells, basophils, and eosinophils. J Allergy Clin Immunol.
125(2 Suppl 2): S73–S80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitchell EB, Crow J, Williams G and
Platts-Mills TA: Increase in skin mast cells following chronic
house dust mite exposure. Br J Dermatol. 114:65–73. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujii Y, Takeuchi H, Sakuma S, Sengoku T
and Takakura S: Characterization of a
2,4-dinitrochlorobenzene-induced chronic dermatitis model in rats.
Skin Pharmacol Physiol. 22:240–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yarbrough KB, Neuhaus KJ and Simpson EL:
The effects of treatment on itch in atopic dermatitis. Dermatol
Ther. 26:110–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Simon D, Braathen LR and Simon HU:
Eosinophils and atopic dermatitis. Allergy. 59:561–570. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu FT, Goodarzi H and Chen HY: IgE, mast
cells, and eosinophils in atopic dermatitis. Clin Rev Allergy
Immunol. 41:298–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adel-Patient K, Créminon C, Bernard H,
Clément G, Négroni L, Frobert Y, Grassi J, Wal JM and Chatel JM:
Evaluation of a high IgE-responder mouse model of allergy to bovine
beta-lactoglobulin (BLG): Development of sandwich immunoassays for
total and allergen-specific IgE, IgG1, and IgG2a in BLG-sensitized
mice. J Immunol Methods. 235:21–32. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandt EB and Sivaprasad U: Th2 cytokines
and atopic dermatitis. Clin Dev Immunol. 2:pii: 1102011.
|
|
30
|
Zhu J, Yamane H and Paul WE:
Differentiation of effector CD4 T cell populations. Annu Rev
Immunol. 28:445–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koga C, Kabashima K, Shiraishi N,
Kobayashi M and Tokura Y: Possible pathogenic role of Th17 cells
for atopic dermatitis. J Invest Dermatol. 128:2625–2630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mansouri Y and Guttman-Yassky E: Immune
pathways in atopic dermatitis and definition of biomarkers through
broad and targeted therapeutics. J Clin Med. 4:858–873. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cornelissen C, Marquardt Y, Czaja K,
Wenzel J, Frank J, Lüscher-Firzlaff J, Lüscher B and Baron JM:
IL-31 regulates differentiation and filaggrin expression in human
organotypic skin models. J Allergy Clin Immunol. 129:426–433,
433.e1-8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raap U, Weißmantel S, Gehring M, Eisenberg
AM, Kapp A and Fölster-Holst R: IL-31 significantly correlates with
disease activity and Th2 cytokine levels in children with atopic
dermatitis. Pediatr Allergy Immunol. 23:285–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin H, He R, Oyoshi M and Geha RS: Animal
models of atopic dermatitis. J Invest Dermatol. 129:31–40. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Danso MO, van Drongelen V, Mulder A, van
Esch J, Scott H, van Smeden J and El Ghalbzouri A: TNF-α and Th2
cytokines induce atopic dermatitis-like features on epidermal
differentiation proteins and stratum corneum lipids in human skin
equivalents. J Invest Dermatol. 134:1941–1950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bossie A and Vitetta ES: IFN-gamma
enhances secretion of IgG2a from IgG2a-committed LPS-stimulated
murine B cells: Implications for the role of IFN-gamma in class
switching. Cell Immunol. 135:95–104. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Albanesi C, Cavani A and Girolomoni G:
IL-17 is produced by nickel-specific T lymphocytes and regulates
ICAM-1 expression and chemokine production in human keratinocytes:
Synergistic or antagonist effects with IFN-gamma and TNF-alpha. J
Immunol. 162:494–502. 1999.PubMed/NCBI
|
|
39
|
McGirt LY and Beck LA: Innate immune
defects in atopic dermatitis. J Allergy Clin Immunol. 118:202–208.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vestergaard C, Bang K, Gesser B, Yoneyama
H, Matsushima K and Larsen CG: A Th2 chemokine, TARC, produced by
keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional
atopic dermatitis skin. J Invest Dermatol. 115:640–646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mizuno K, Morizane S, Takiguchi T and
Iwatsuki K: Dexamethasone but not tacrolimus suppresses
TNF-α-induced thymic stromal lymphopoietin expression in lesional
keratinocytes of atopic dermatitis model. J Dermatol Sci. 80:45–53.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang JH, Yoo JM, Cho WK and Ma JY:
Anti-inflammatory effects of Sanguisorbae Radix water extract on
the suppression of mast cell degranulation and STAT-1/Jak-2
activation in BMMCs and HaCaT keratinocytes. BMC Complement Altern
Med. 16:3472016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jung M, Lee TH, Oh HJ, Kim H, Son Y, Lee
EH and Kim J: Inhibitory effect of 5,6-dihydroergosteol-glucoside
on atopic dermatitis-like skin lesions via suppression of NF-κB and
STAT activation. J Dermatol Sci. 79:252–261. 2015. View Article : Google Scholar : PubMed/NCBI
|