Introduction
Primary nephrotic syndrome (PNS) is a common renal
disease in children. The majority of cases are steroid-sensitive
(SSNS), the prognosis of which is good; however, patients are prone
to relapse (1). The pathogenesis
of PNS remains unclear; however, dysfunction of the
charge-selectively of the glomerular basement membrane (GBM) may
contribute to proteinuria (2). The
anionic filtration of the GBM involves glycosaminoglycans (GAGs),
particularly heparan sulfate proteoglycan (HSPG), a highly anionic
molecule that regulates the charge- and size-selective aspects of
glomerular permselectivity (3).
GAGs are linear polymers of repeating amino sugar uronic acid
disaccharides produced by and associated with the majority of
mammalian cells and certain bacterial cells (4). GAGs may interact with numerous
proteins and participate in matrix organization, cell adhesion,
differentiation, growth, apoptosis and mouse kidney development
(5,6). Certain studies have demonstrated that
reduced GAGs content in the GBM results in increased passage of
proteins across the GBM, particularly negatively charged proteins
(7,8). Urinary GAGs levels have been
suggested as a marker of glomerular damage in PNS (9,10).
GAGs in the GBM may be degraded by certain proteinases, including
heparanase or elastase (Ela) (11). Klebanoff et al (12) revealed that Ela may degrade
subendothelial matrix HSPG in vitro, which may be involved
in proteinuria. By contrast, studies have demonstrated that
proteinuria may be alleviated via the administration of GAGs
(13–15). Low molecular weight heparin (LMWH)
is a negatively charged glycoprotein due to N-sulfate and O-sulfate
on the carbon chain and has a similar structure to HSPG. Previous
studies have revealed that the anti-inflammatory effects of LMWH
were not dependent on its anticoagulant activities, but on
inhibiting Ela activity and adhesion of neutrophils to vascular
endothelial cells, which may have therapeutic potential for the
treatment of chronic inflammatory diseases (16–18).
The influence of LMWH on urinary GAG and serum Ela
levels in children with SSNS remains to be determined. Therefore,
the present study used LMWH as a convenient analogue of HSPG for
experimental purposes to investigate the influence of HSPG on
urinary protein excretion and serum Ela levels. The results of the
present study may facilitate the elucidation of the association
between urinary GAGs, proteinuria and Ela.
Patients and methods
Patients and methods
Children hospitalized as a result of SSNS and
healthy controls from the outpatient department, were recruited for
the present study from the West China Second University Hospital
(Chengdu, China) between March 2010 and July 2014. A total of 40
SSNS patients (male, n=26; female, n=14; age, 7.19±3.02 years) and
20 sex and age-matched healthy controls (male, n=11; female, n=9;
age, 7.32±3.48 years) were included in the present study. All of
the SSNS patients had PNS, as diagnosed according to the criteria
from the International Study of Kidney Disease in Children
(19). Urine protein excretion
>50 mg/kg/24 h was regarded as the nephrotic state, whereas
<4 mg/kg/24 h was regarded as the remission phase. PNS patients
with complications were excluded from the present study. The
coagulation function in all SSNS patients was normal. It was
defined as achieving remission within 4 weeks of treatment with 2
mg/kg/day (with the majority of doses being 60 mg/day) prednisone
(pred). SSNS patients were divided into two groups, which were
similar in sex and age. The LMWH+pred group (n=24) was treated with
pred and LMWH (30–50 IU/kg/day) simultaneously, and the pred group
(n=16) was treated with pred only. The two groups received similar
assistant therapy, including captopril and calcium. Follow-up began
on the date of diagnosis and ended in July 2014. The median
follow-up was 325 days (range, 209–451 days). Regular history,
physical and laboratory examinations were performed on all patients
every one to two months following the disappearance of proteinuria.
The present study was approved by the Institutional Review Board of
West China Second University Hospital, Sichuan University (Sichuan,
China), and blood and urine samples of SSNS patients and healthy
controls were obtained and handled in accordance with its
guidelines. The present study was performed in accordance with the
ethical standards of the Declaration of Helsinki. All parents or
guardians signed informed consent.
Measurement of proteinuria excretion,
urinary GAG excretion and serum parameters
In SSNS patients, two urine samples were collected
during the nephrotic and remission phases. In the healthy controls,
one urine sample was obtained from each individual; 24 h urine
samples were collected and stored at −80°C until analysis. Urinary
proteins were measured by the pyrogallol end-point method (10), while urinary GAGs excretion was
examined using the modified Whiteman process (10,11).
GAGs levels were calculated using a calibration curve with HS as a
standard, and corrected with urinary creatinine (Cr).
At the same time, blood samples (4 ml) were
collected from SSNS patients and healthy controls and stored in
tubes containing EDTA to prevent coagulation, and subsequently
centrifuged at 400 × g for 5 min at 4°C. Enzymatic activity of Ela
was quantified by ELISA according to the manufacturer's protocols
(ab119553; Abcam, London, Cambridge, UK).
Urine levels of Creatinine (Cr) were measured using
an Hitachi 7600 Chemistry Analyzer (Hitachi, Ltd., Tokyo,
Japan).
Statistical analysis
All statistical analyses were performed in SPSS
software version 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous
data were presented as the mean ± standard deviation. Comparison of
continuous data was performed with an independent t-test between
the two groups, whereas the correlation among categorical variables
was analyzed by a two-sided Chi-square test. Multiple groups were
compared using one way Analysis of variance. Spearman's test was
used to analyze correlations. P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient clinical profile
A total of 40 children hospitalized with SSNS and 20
healthy controls were enrolled in the present study. The clinical
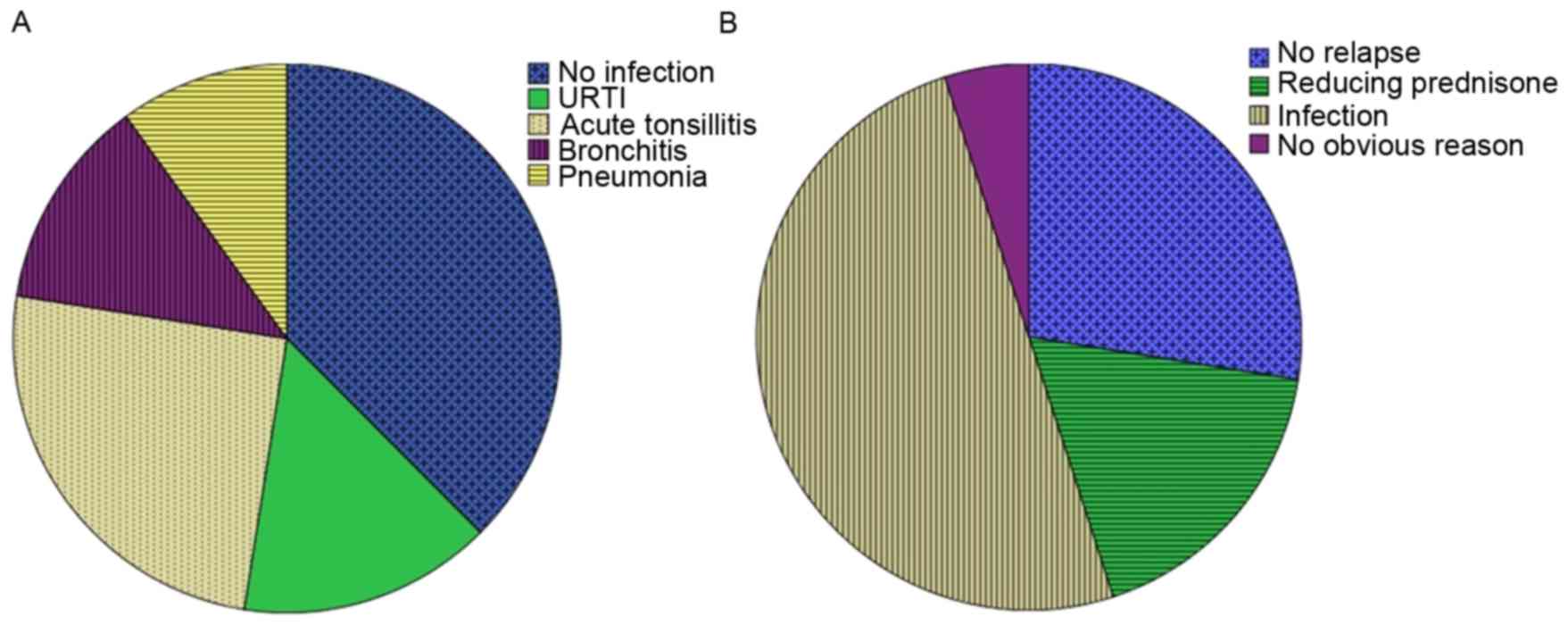
characteristics of the participants are presented in Table I. Of the 40 SSNS patients, 25
patients had an infection history 7 to 14 days prior to the
diagnosis of nephrotic syndrome (upper respiratory tract infection,
n=6; acute tonsillitis, n=10; bronchitis, n=5; pneumonia, n=4;
Fig. 1A). In the pred group, 10
patients had an infection (upper respiratory tract infection, n=2;
acute tonsillitis, n=5; bronchitis, n=2; pneumonia, n=1). In the
LMWH+pred group, 15 patients had an infection (upper respiratory
tract infection, n=3; acute tonsillitis, n=7; bronchitis, n=3;
pneumonia, n=2).
 | Table I.Clinical profiles of 40 SSNS patients
and 20 healthy controls. |
Table I.
Clinical profiles of 40 SSNS patients
and 20 healthy controls.
|
| SSNS patients
(n=40) | Healthy controls
(n=20) |
|---|
|
|
|
|
|---|
| Parameter | Pred (n=16) | LMWH+pred (n=24) | P-value | All SSNS (n=40) | All control
(n=20) | P-value |
|---|
| Age (years) | 7.43±3.52 | 7.02±2.69 | 0.092a | 7.19±3.02 | 7.32±3.48 | 0.214a |
| Gender |
|
| 0.685b |
|
| 0.453b |
| Male | 11 (68.8%) | 15 (62.5%) |
| 26 (65.0%) | 11 (55%) |
|
|
Female | 5
(31.2%) | 9
(37.5%) |
| 14 (35.0%) | 9
(45%) |
|
| Respiratory
infection |
|
| 1b |
|
| / |
| No | 6
(37.5%) | 9
(37.5%) |
| 15 (37.5%) | 0 |
|
| Yes | 10 (62.5%) | 15 (62.5%) |
| 25 (62.5%) | 0 |
|
| LMWH |
|
| / |
|
| / |
| No | 16 (100%) | 0 (0%) |
| 16 (40%) | 0 |
|
| Yes | 0 (0%) | 24 (100%) |
| 24 (60%) | 0 |
|
| Relapse |
|
| 0.643b |
|
| / |
| No | 3
(18.8%) | 6
(25.0%) |
| 9
(22.5%) | 0 |
|
|
Yes | 13 (81.2%) | 18 (75.0%) |
| 31 (77.5%) | 0 |
|
| Nephrotic period
(days) | 18.63±6.49 | 14.13±4.56 | 0.014a | 15.93±5.78 | 0 |
|
The nephrotic period of SSNS was 15.93±5.78 days
(range, 6–27 days). The nephrotic period of SSNS in the LMWH+pred
group was 14.13±4.56 days, which was significantly reduced compared
with the pred group (18.63±6.49 days, Table I).
All patients were followed up for an average of 325
days (range from 209 to 451 days). During the follow-up period,
there were 31 relapse cases of SSNS. Of these cases, 3 were of
frequent relapse; one in the LMWH+pred group, whose renal pathology
indicated minimal change disease, and two in the pred group, whose
renal pathology indicated minimal change disease and focal
segmental glomerulosclerosis. As presented in Fig. 1B, the 31 relapse cases of SSNS were
suspected to be the result of infection (n=21, 67.7%), reducing
pred dose (n=7, 22.6%) or no clear reason (n=3, 9.7%). In the 18
relapse patients in the LMWH+pred group, the suspected factors were
infection (n=12, 66.7%), reducing pred dose (n=4, 22.2%) or no
clear reason (n=2, 11.1%). In the 13 relapse patients in the pred
group, the suspected factors were infection (n=9, 69.2%), reducing
pred dose (n=3, 23.1%) or no clear reason (n=1, 7.7%). There was no
statistically significant difference in number of relapses between
the LMWH+pred and pred groups (P>0.05, Table I).
Proteinuria in 24 h
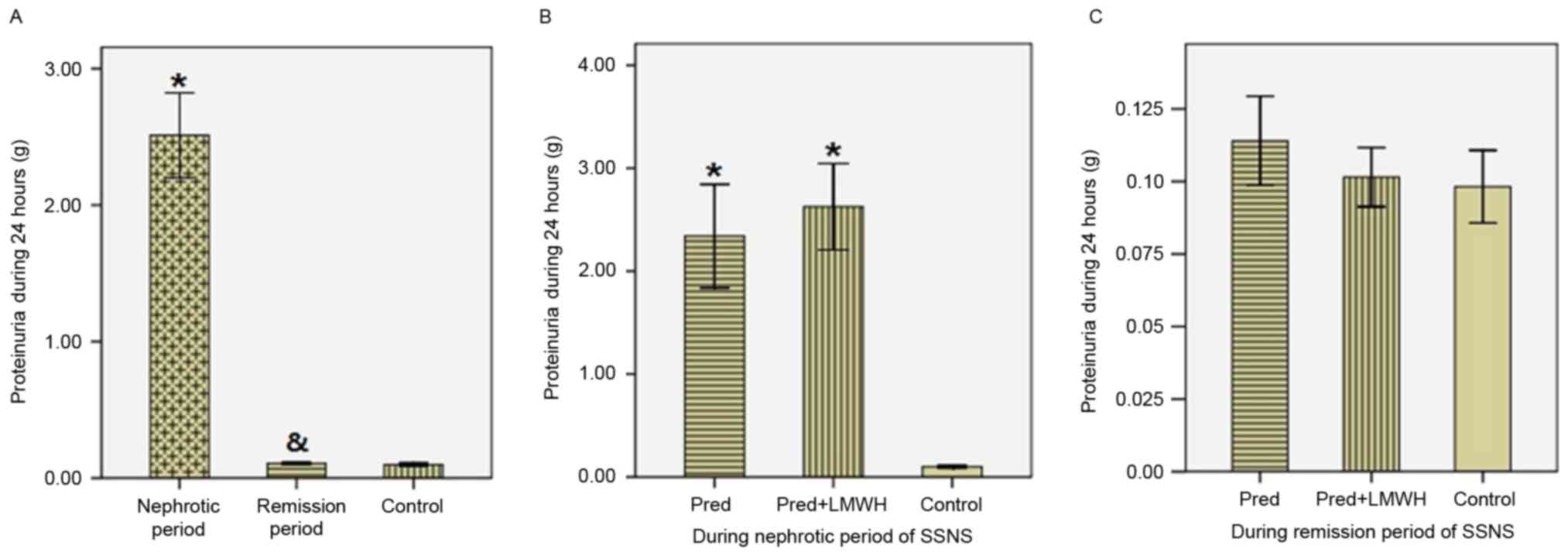
As presented in Fig.
2, proteinuria in the nephrotic period of SSNS (2.51±0.97 g/24
h) was significantly greater compared with the remission period of
SSNS (0.107±0.026 g/24 h) and the healthy control group
(0.098±0.027 g/24 h, P<0.05), whereas proteinuria was not
significantly different between the remission period of SSNS and
the healthy control group (P>0.05, Fig. 2A). In the nephrotic period of SSNS,
proteinuria in the LMWH+pred group was similar to the pred group
(2.62±0.99 g/24 h vs. 2.34±0.94 g/24 h, P>0.05, Fig. 2B). In the remission period of SSNS,
there were no significant differences in proteinuria between the
LMWH+pred (0.102±0.024 g/24 h), pred (0.114±0.029 g/24 h) and
healthy control groups (0.098±0.027 g/24 h, P>0.05, Fig. 2C).
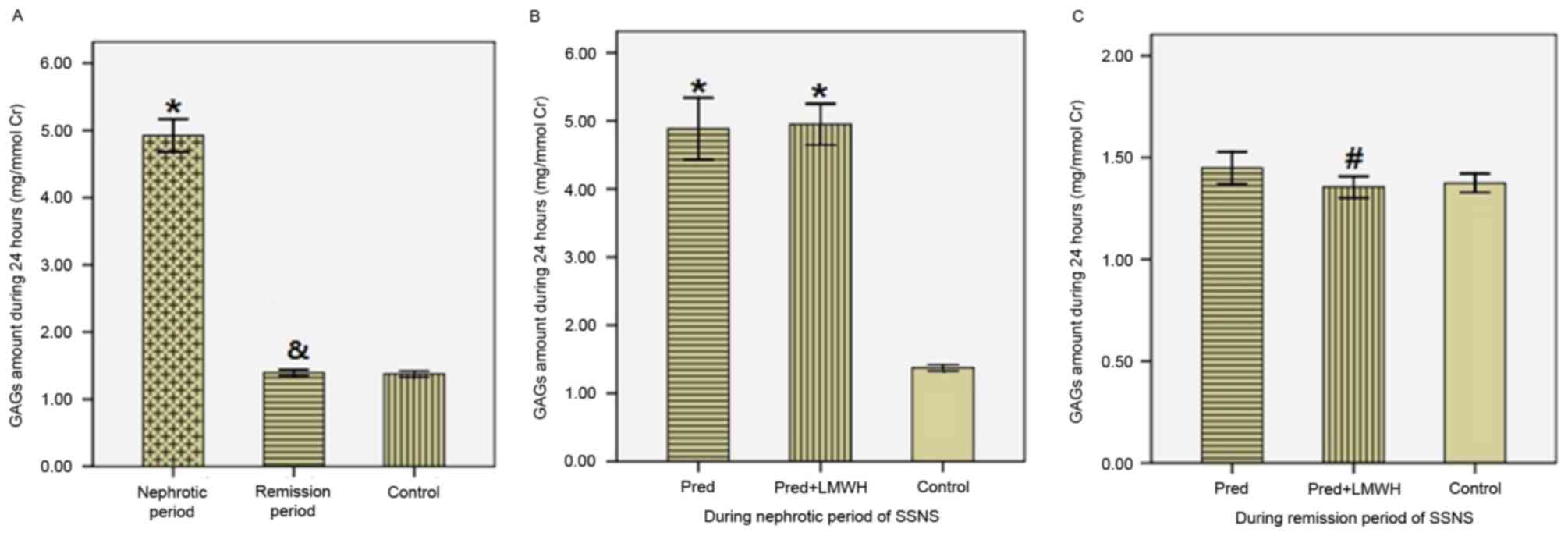
Urinary GAG excretion in 24 h
The results of urinary GAG excretion were corrected
by the ratio of urinary GAGs (UGAGs) to urinary Cr (UCr). As
presented in Fig. 3, in SSNS
patients the ratio of UGAGs/UCr in the nephrotic period (4.92±0.76
mg/mmol Cr) was significantly greater compared with the remission
period (1.39±0.14 mg/mmol Cr) and the healthy control group
(1.37±0.10 mg/mmol Cr; P<0.05), whereas the ratio of UGAGs/UCr
in the remission period was similar to the control group
(P>0.05; Fig. 3A). Comparison
of the LMWH+pred and pred groups revealed no significant difference
in the ratio of UGAGs/UCr in the nephrotic period (4.95±0.71 vs.
4.89±0.85 mg/mmol Cr; P>0.05; Fig.
3B). In the remission period, the ratio of UGAGs/UCr in the
LMWH+pred group was significantly reduced compared with the pred
group (1.36±0.12 vs. 1.45±0.15 mg/mmol Cr; P<0.05), whereas
these two groups were not significantly different compared with the
healthy control group (P>0.05; Fig.
3C). Therefore, following steroid therapy in SSNS patients,
there was a significant reduction in GAG excretion, and pred
accompanied by LMWH treatment was more efficient in reducing
urinary GAGs excretion compared with pred monotherapy.
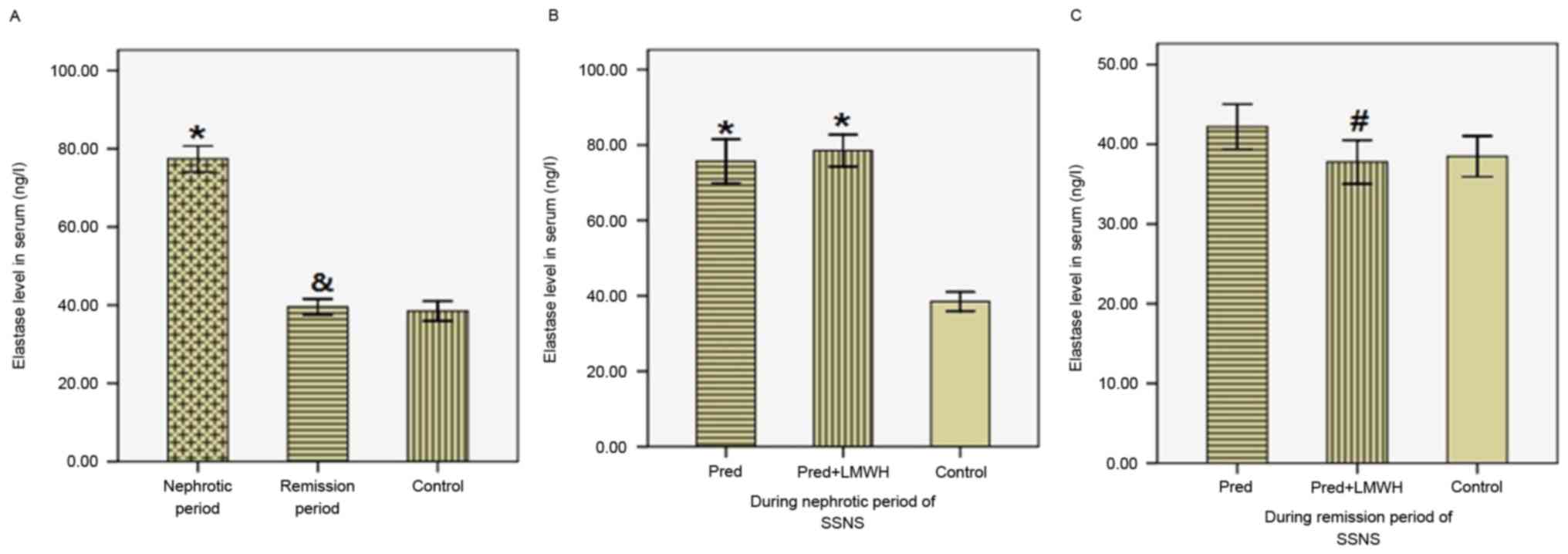
Serum Ela levels
As presented in Fig.
4, serum Ela levels in the nephrotic period (77.39±10.38 ng/l)
were significantly greater compared with the remission period
(39.55±6.35 ng/l, P<0.05) and the healthy control group
(38.48±5.44 ng/l, P<0.05), whereas Ela levels in the remission
period were similar to those in the control group (Fig. 4A). Ela levels were not
significantly different between the LMWH+pred and pred group during
the nephrotic period (78.53±10.05 vs. 75.69±10.96 ng/l, P>0.05,
Fig. 4B). In the remission period,
Ela levels in the LMWH+pred group were significantly reduced
compared with the pred group (37.78±6.49 vs. 42.20±5.28 ng/l,
P<0.05), whereas these two groups were not significantly
different compared with the healthy control group (P>0.05,
Fig. 4C). Therefore, following
steroid therapy in SSNS patients, there was a significant reduction
in serum Ela levels, and pred accompanied by LMWH treatment was
more efficient in reducing serum Ela levels compared with pred
monotherapy.
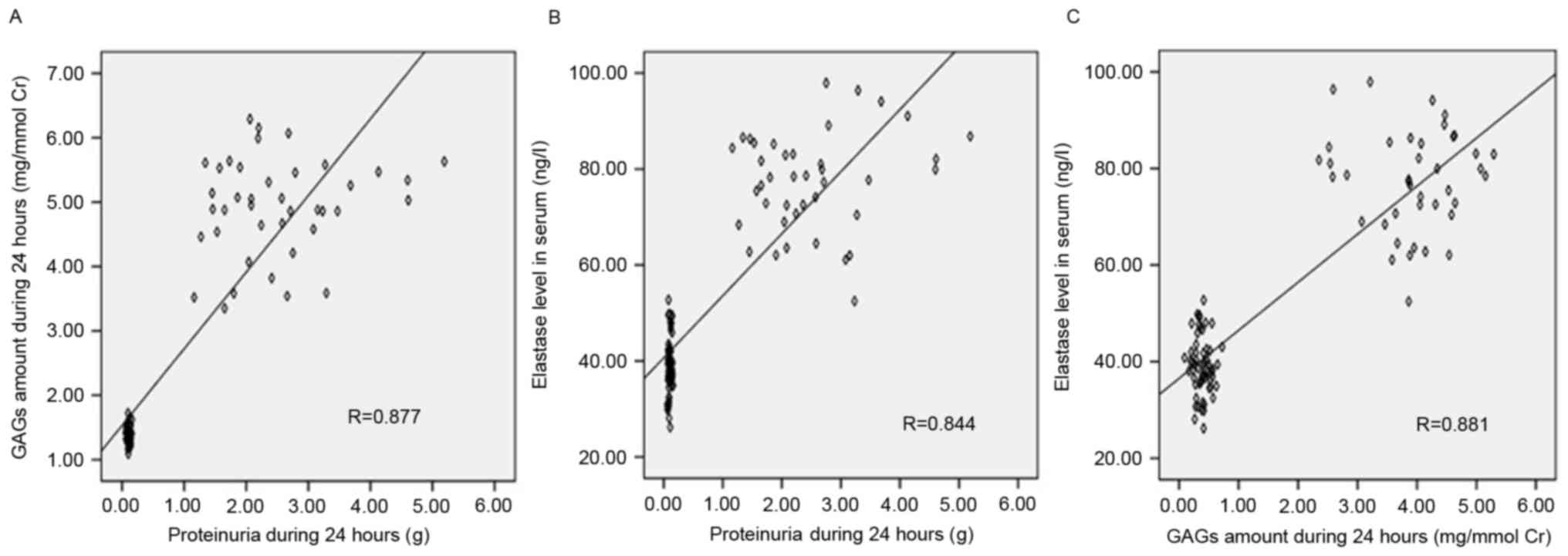
Association between urinary GAG
excretion, proteinuria and serum Ela levels
Correlation analysis of all participants revealed
significant positive correlations between urinary GAG levels and
proteinuria (r=0.877, P<0.05, Fig.
5A), serum Ela levels and proteinuria (r=0.844, P<0.05,
Fig. 5B), and serum Ela and
urinary GAG levels (r=0.881, P<0.05, Fig. 5C).
Discussion
The results of the present study suggested that pred
and LMWH treatment reduced proteinuria and urinary GAGs levels and
decreased the nephrotic period in SSNS patients, but had no effect
on SSNS relapse.
It had previously been reported that the GAGs
content of GBM was reduced in nephrotic syndrome and that this
decrease in GAGs induced the loss of the GBM negative charge, which
might contribute to an increase in urinary proteins (20,21).
Girardin et al suggested that various lymphocytic factors
may decrease the anionic charge of the GBM, and so increase
permeability to albumin (22).
Mitsuhashi et al (23)
investigated adult patients with minimal change disease and
revealed that they excreted significantly greater levels of HSPG
compared with controls. Consistent with these results, in child
SSNS patients, the present study detected elevated levels of
urinary GAGs excretion during the nephrotic period and normal
levels during the remission period. Heeringa et al (24) reported that in rats, renal
perfusion with Ela might induce proteinuria by GBM HSPG
degradation. Our previous studies of rats infected with respiratory
syncytial virus (RSV) indicated that RSV might induce renal minimal
change similar to human minimal change disease, and degradation of
GAGs on GBM potentially via elevated heparanase and Ela (25–27).
The present study demonstrated that LMWH administration may reduce
excretion of urinary proteins and that pred accompanied by LMWH
treatment was more efficient compared with pred monotherapy in
reducing nephrotic period and urinary GAGs excretion, and
decreasing the level of serum Ela. Previous studies have reported
that LMWH may inhibit the release of Ela from human neutrophils
(28,29). Therefore, LMWH may be beneficial in
SSNS via inhibiting Ela and reducing degradation of GBM GAGs. The
present study also revealed that LMWH had no preventive and
predictive effect on the relapse of SSNS.
In conclusion, the results of the present study
indicated that urinary excretion of GAGs was associated with the
degree of proteinuria and serum Ela levels in children with SSNS.
Treatment with LMWH may benefit proteinuria remission.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation of China (grant no. 81370807).
The authors thank Dr Nan Li (College of Preclinical and Forensic
Medicine, Sichuan University) for language checking and
proofreading the manuscript.
References
|
1
|
Working Group For National Survey On
Status Of Diagnosis And Treatment Of Childhood Renal Diseases, .
Multicenter survey of diagnostic and therapeutic status in Chinese
childhood patients with steroid-sensitive,
relaping/steroid-dependent nephrotic syndrome. Zhonghua Er Ke Za
Zhi. 52:194–200. 2014.(In Chinese). PubMed/NCBI
|
|
2
|
Brenner BM, Hostetter TH and Humes HD:
Glomerular permselectivity: Barrier function based on
discrimination of molecular size and charge. Am J Physiol.
234:F455–F460. 1978.PubMed/NCBI
|
|
3
|
Bridges CR, Myers BD, Brenner BM and Deen
WM: Glomerular charge alterations in human minimal change
nephropathy. Kidney Int. 22:677–684. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miner JH: Renal basement membrane
components. Kidney Int. 56:2016–2024. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamada T and Kawasaki T: Microbial
synthesis of hyaluronan and chitin: New approaches. J Biosci
Bioeng. 99:521–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davies JA, Fisher CE and Barnett MW:
Glycosaminoglycans in the study of mammalian organ development.
Biochem Soc Trans. 29:166–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cengiz N, Bayazit AK, Noyan A, Anarat R
and Anarat A: Glycosaminoglycan excretion in children with
nephrotic syndrome. Pediatr Nephrol. 20:486–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carrie BJ, Salyer WR and Myers BD: Minimal
change nephropathy: An electrochemical disorder of the glomerular
membrane. Am J Med. 70:262–268. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baggio B, Gambaro G, Briani G, Favaro S
and Borsatti A: Urinary excretion of glycosaminoglycans and brush
border and lysosomal enzymes as a marker of glomerular and tubular
involvement in kidney disease. Contrib Nephrol. 42:107–110. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotz P, Pilliod J, Berode M, Rey F and
Boillat MA: Glycosaminoglycans, albuminuria and hydrocarbon
exposure. Nephron. 58:184–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu XM, Wang Z and Guo Y: Respiratory
syncytial virus nephropathy in rats. Kidney Int. 71:388–396. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klebanoff SJ, Kinsella MG and Wight TN:
Degradation of endothelial cell matrix heparan sulfate proteoglycan
by elastase and the
myeloperoxidase-H2O2-chloride system. Am J
Pathol. 143:907–917. 1993.PubMed/NCBI
|
|
13
|
Baggio B and Gambaro G: Antiproteinuric
effect of glycosaminoglycans? Nephron. 64:643–644. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vehaskari VM, Root ER, Germuth FG Jr and
Robson AM: Glomerular charge and urinary protein excretion: Effect
of systemic and intrarenal polycation infusion in the rat. Kidney
Int. 22:127–135. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S-J, Zhang S-W, Wei M, Xiong Y, Gao
Y, Hu Y, et al: Glycosaminoglycans on CRP and UAER of early
diabetic nephropathy. Zhong Guo Lao Nian Xue Za Zhi. 27:1779–1781.
2007.(In Chinese).
|
|
16
|
Lever R and Page CP: Novel drug
development opportunities for heparin. Nat Rev Drug Discov.
1:140–148. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
PAGE CP: One explanation of the asthma
paradox: Inhibition of natural anti-inflammatory mechanism by beta
2-agonists. Lancet. 337:717–720. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown RA, Leung E, Kankaanranta H,
Moilanen E and Page CP: Effects of heparin and related drugs on
neutrophil function. Pulm Pharmacol Ther. 25:185–192. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Birmele B, Thibault G, Nivet H, de
Agostini A and Girardin EP: In vitro decrease of glomerular heparan
sulfate by lymphocytes from idiopathic nephrotic syndrome patients.
Kidney Int. 59:913–922. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salant DJ: The structural biology of
glomerular epithelialcells in proteinuric diseases. Curr Opin
Nephrol Hypertens. 3:569–574. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vernier RL, Klein DJ, Sisson SP, Mahan JD,
Oegema TR and Brown DM: Heparan sulphate-rich anionic sites in the
human glomerular basement membrane. Decreased concentration in
congenital nephrotic syndrome. N Engl J Med. 309:1001–1009. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Girardin EP, Birmele B, Benador N, Neuhaus
T, Hosseini G, Van Den Heuvel LP and De Agostini A: Effect of
plasma from patients with idiopathic nephrotic syndrome on
proteoglycan synthesis by human and rat glomerular cells. Pediatr
Res. 43:489–495. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitsuhashi H, Tsukada Y, Ono K, Yano S and
Naruse T: Urine glycosaminoglycans and heparan sulfate excretions
in adult patients with glomerular diseases. Clin Nephrol.
39:231–238. 1993.PubMed/NCBI
|
|
24
|
Heeringa P, van den Born J, Brouwer E,
Dolman KM, Klok PA, Huitema MG, Limburg PC, Bakker MA, Berden JH,
Daha MR and Kallenberg CG: Elastase, but not proteinase 3 (PR3),
induces proteinuria associated with loss of glomerular basement
membrane heparan sulphate after in vivo renal perfusion in rats.
Clin Exp Immunol. 105:321–329. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo Y, Wang Z, Dong L, Wu J, Zhai S and
Liu D: Ability of low-molecular-weight heparin to alleviate
proteinuria by inhibiting respiratory syncytial virus infection.
Nephrology (Carlton). 13:545–553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tao YH, Wang Z and Zhou YR: Expression of
heparanase in kidney of rats with respiratory syncytial virus
nephropathy and its relationship with proteinurina. Sichuan Da Xue
Xue Bao Yi Xue Ban. 45:212–224. 2014.(In Chinese). PubMed/NCBI
|
|
27
|
Dong LQ, Wang XQ, Guo YN, Wu J, Li S, Yu P
and Wang Z: HS N-sulfation and iduronic acids play an important
role in the infection of respiratory syncytial virus in vitro. Eur
Rev Med Pharmacol Sci. 17:1864–1868. 2013.PubMed/NCBI
|
|
28
|
Brown RA, Lever R, Jones NA and Page CP:
Effects of heparin and related molecules upon neutrophil
aggregation and elastase release in vitro. Br J Pharmacol.
139:845–853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cadène M, Boudier C, de Marcillac GD and
Bieth JG: Influence of low molecular mass heparin on the kinetics
of neutrophil elastase inhibition by mucus proteinase inhibitor. J
Biol Chem. 270:13204–13209. 1995. View Article : Google Scholar : PubMed/NCBI
|