Introduction
Wound healing is clinically defined as the
completion of the closure of the wounded skin area, which is an
interactive and dynamic process. It includes phases of hemostasis,
cellular proliferation, inflammation, angiogenesis, matrix
synthesis and remodeling, and formation of granulation tissue
(1). The evolution of the wound
healing process involves a series of phenomena which represent
attempts in reestablishing the anatomical structure and function of
the normal tissue. One aspect of wound repair that has always been
considered to be essential for adequate healing is the creation of
new vasculature via angiogenesis (2).
Understanding the correct sequence of molecular and
cellular processes that occur during wound healing is critical for
the development of novel treatments. Previous studies have
demonstrated that pulsed electromagnetic fields (PEMFs) are capable
of altering the structure of the cell membranes, and diversifying
the permeability of different ion channels and the potential of the
cellular membranes. At the molecular and cellular level, PEMFs have
been advocated to exert a direct effect on the production of
proteins and promote the synthesis of extracellular matrix proteins
that regulate gene transcription (3). Additionally, previous studies have
suggested that the process of angiogenesis, both in vivo and
in vitro, may be influenced by various forms of electrical
stimulation, including direct current, combined electromagnetic
fields and PEMFs (4). In another
study, Athanasiou et al (3)
demonstrated that short duration PEMFs appear to facilitate and
improve the quality of skin wound healing in a rat model.
Nevertheless, further studies are required to define the optimal
characteristics of the PEMFs, in order to ensure a faster and more
effective wound healing process. Furthermore, PEMFs may also affect
several membrane receptors and stimulate endothelial cells to
secrete several growth factors, including vascular endothelial
growth factor, connective tissue growth factor and endothelial
nitric oxide synthase in vivo and in vitro (5,6).
In vitro, PEMFs have been demonstrated to inhibit the
process of hypoxia-induced apoptosis and augmented tube formation,
migration and proliferative capacities of human umbilical vein
endothelial cells (HUVECs) (7). To
date, PEMFs have been applied experimentally and clinically to
promote wound healing for many years (8,9).
Hydroxytyrosol (HTY), or 3,4-dihydroxyphenylethanol,
is one of the hydroxyaromatic components of secoiridoids, which is
a very bioactive alcoholic ortho-diphenol. Accumulating
experimental, clinical and epidemiological data have indicated that
HTY is antioxidant and antimicrobial and that it has beneficial
effects on the cardiovascular system and in several human diseases
(10,11). Zrelli et al (12) revealed that HTY upregulates heme
oxygenase-1 expression by stimulating the nuclear accumulation and
stabilization of nuclear factor erythroid 2-related factor 2, which
leads to the wound repair of vascular endothelial cells crucial in
the prevention of atherosclerosis. In another study, Scoditti et
al (13) revealed that HTY
could reduce inflammatory angiogenesis in cultured endothelial
cells, through matrix metalloproteinase-9 and cyclooxygenase-2
inhibition, supporting a potential protective role for dietary
polyphenols in atherosclerotic vascular disease and cancer.
However, the exact function of HTY on endothelial cells has not
been fully uncovered until now, especially in association with
co-treatment with PEMFs.
When developing a wounding and healing model for
PEMFs studies, endothelial cells appear to be an ideal system to
investigate (14). The present
study investigated the impact of the co-treatment of HYT and PEMFs
on HUVEC function, and evaluated their influence on proliferation
and migration in primary cultures of HUVECs. The present study also
investigated the expression of functional parameters such as
migration, viability and apoptosis, and the gene expression of
protein kinase B (Akt), mechanistic target of rapamycin (mTOR),
transforming growth factor (TGF)-β1 and p53 under the influence of
different doses of HYT and PEMFs on HUVECs. The results
demonstrated that PEMFs may provide novel research options in the
field of wounding healing by promoting endothelial cell growth and
by enhancing the healing response of the endothelium.
Materials and methods
Cell culture
HUVECs were obtained from the American Type Culture
Collection (Manassas, VA, USA). The HUVECs were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences,
Logan, UT, USA). The cells were cultured in 95% air and 5% carbon
dioxide at 37°C.
PEMF exposure
The PEMFs used in the current study was one that was
clinically available for the treatment of wound healing. The
devices were supplied by Electro-Biology, Inc. (Parsippany, NJ,
USA). The model required a voltage of 230 V with a frequency of
50/60 Hz to generate PEMF with a maximum intensity of 0.015 T (150
Gauss) and an impulse repetition frequency of up to 100 Hz. At the
beginning of the experiment, the intensity of the magnetic fields
generated inside the solenoid was measured with a Gaussimeter
PCE-G28 (PCE Group, Albacete, Spain) with a triaxial probe. The
intensity of the electromagnetic field used in this protocol was
2.25 mT, the frequency of the bursts was 50 Hz and the application
time was 15 min. PEMF irradiation was performed on days 1, 2, 3 and
4 of culture, with the solenoid inside the incubator, under the
same conditions of temperature.
Cell viability assay
The cell viability was calculated using the Cell
Counting kit-8 (CCK-8; Beyotime Institute of Biotechnology,
Jiangsu, China) according to the manufacturer's protocol. Briefly,
HUVECs (1×104/well) were seeded into 96-well plates in DMEM
supplemented with 10% FBS for different time points. Following
this, the cells were exposed to PEMFs at days 0, 1, 2, 3 or 4, or
treated with HTY (0, 10, 30, 50, 100, 150 µM) at day 2, or treated
with a combination on days 0, 1, 2 or 4. Finally, the viable cells
were detected using by CCK-8 assay, where the absorbance for each
sample was assessed at 450 nm using a microplate reader (Tecan,
Männedorf, Switzerland).
Cell migration analysis
For the migration assay, cells were treated with
PEMFs/HTY or a combination for 48 h, and then re-suspended cells
were removed on the top layer of a Transwell chamber with 8 µm
pores (EMD Millipore, Billerica, MA, USA). The lower layer of the
chamber contained 10% FBS as a chemoattractant. The chambers were
placed at 37°C in 5% CO2 for 48 h. Non-migrating cells
on the top of the membrane were removed with cotton swabs. Cells
that had migrated to the bottom were fixed with 95% ethanol,
stained with 0.2% crystal violet staining (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), and imaged by bright-field microscopy
(IX71; Olympus Corporation, Tokyo, Japan) and counted using
Image-Pro Plus v6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). Each experiment was performed in triplicate.
TUNEL assay
DNA fragmentation in HUVECs was detected by TUNEL
assay using a Cell Death Detection kit (Roche Applied Science,
Mannheim, Germany). Briefly, air-dried slides were fixed with 4%
paraformaldehyde for 30 min at room temperature and cleaned three
times with PBS for 10 min, and then permeabilized with 1% Triton
X-100 for 4 min at 4°C. Then, the TdT-labelled nucleotide mix was
added to each slide and incubated at 37°C for 60 min in dark.
Slides were washed twice with PBS and then counterstained with 10
mg/ml 4,6-diamidino-2-phenylindole for 5 min at 37°C.
Calcein-acetoxymethyl (AM)/propidium
iodide (PI) dual-staining assay
A calcein-AM/PI dual-staining assay (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was performed to
verify the effect of PEMFs and HTY on cell apoptosis. The
calcein-AM/PI/CoCl2 assay relies on the intracellular esterase
activity within living cells. Living cells are stained with green
fluorescence, and dead cells are stained red by PI. HUVECs were
treated with PEMFs and HTY for 48 h in DMEM with 10% FBS.
Fluorescence was analyzed by microscopy (Olympus Corporation).
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Total RNA samples from cultured HUVECs were isolated
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. Total RNA (1 µg) was
then reverse transcribed using the High-Capacity cDNA Reverse
Transcription kit (Toyobo Co., Ltd., Osaka, Japan) to obtain cDNA,
with the following temperature protocol: 37°C for 15 min, then 98°C
for 5 min. The SYBR Green PCR Master Mix kit (Toyobo Co., Ltd.) was
used for qPCR to quantify RNA levels of silent information
regulator 1 (SIRT1). GAPDH served as an internal control. qPCR was
performed on the 7300 FAST Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) for 40 cycles. The
thermocycling conditions were as follows: 95°C for 10 min followed
by 95°C for 15 sec, then 40 cycles of 60°C for 30 sec and 72°C for
30 sec. The results were quantified using the 2−ΔΔCq
method (15). Primer sequences
were as follows: Akt, forward, 5′-GGTGATCCTGGTGAAGGAGA-3′ and
reverse, 5′-CTTAATGTGCCCGTCCTTGT-3′; mTOR, forward,
5′-TTCTGGTGCGACACCGAATC-3′ and reverse 5′-CATCGGGTTGTAGGCCTGTG-3′;
TGF-β1, forward, 5′-CCCCGAGGGCGGCATG-3′ and reverse,
5′-CATGCCGCCCTCGGGG-3′; p53, forward, 5′-ACGACGGTGACACGCTTCCCTG-3′
and reverse, 5′-CGCTAGGATCTGACTGCGGCTC-3′; and GAPDH, forward,
5′-GCTCTCTGCTCCTCCTGTTC-3′ and reverse,
5′-ACGACCAAATCCGTTGACTC-3′.
Scratch-wound assay
The confluent HUVECs cultured in 6-well plates were
scratched with pipette tips, which led to a 1-mm-wide lane per
well, and the ablate cells were cleaned with PBS. Subsequently, the
cells were treated with or without hydroxytyrosol (Shanghai Pureone
Biotechnology Co., Ltd, Shanghai, China) with DMEM supplemented
with 10% FBS at 37°C for 1–4 days. Wounded areas were imaged by
bright-field microscopy (magnification, ×200; IX71 Olympus) and
analyzed using Image-Pro Plus v6.0 software (Media Cybernetics,
Inc.) at time point zero and after a 48 h treatment.
Western blot analysis
HUVECs were treated with PEMFs or HTY in DMEM with
10% FBS for 48 h. Proteins were solubilized and extracted with 200
µl lysis buffer (Beyotime Institute of Biotechnology) and kept for
20 min on ice. Following this, the lysates were sonicated and
centrifuged at 12,000 × g for 15 min at 4°C, and the insoluble
fractions were discarded. Equal amounts of protein (~60 µg) from
each sample were separated by 10% SDS-PAGE, and transferred onto a
nitrocellulose membrane (EMD Millipore). After a 1 h incubation
with 5% non-fat dry milk powder, the membranes were probed with the
following primary antibodies: anti-GADPH (1:5,000 dilution; cat.
no. 97166), anti-Akt (1:2,000; cat. no. 9272), anti-mTOR (1:1,000;
cat. no. 2983), anti-human TGF-β1 (1:2,000; cat. no. 3711) and
anti-p53 (1:2,000; cat. no. 2524; all Cell Signaling Technology,
Inc., Danvers, MA, USA) overnight at 4°C. Membranes were washed
three times for 10 min in PBS containing 0.5% Tween-20, then
incubated for 1 h at room temperature with the following Alexa
Fluor™-conjugated secondary antibodies: IRDye® 800CW
goat anti-rabbit IgG conjugated to IRDye® 800CW (cat.
no. 926-32211; 1:10,000 dilution; LI-COR Biosciences, Lincoln, NE,
USA) and IRDye® 800CW goat anti-mouse IgG conjugated to
IRDye® 800CW (cat. no. 926-32210; 1:10,000 dilution;
LI-COR Biosciences). The bands were visualized using the Odyssey
Imaging system (LI-COR Biosciences) and semi-quantified using the
Odyssey software version 3.0. Relative protein expression was
normalized to that of GAPDH, which was used as an internal control,
and experiments were repeated three times, unless otherwise
stated.
Statistical analysis
All quantitative data are expressed as the mean ±
standard error. Statistical analysis was performed using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA). A Student's t-test or
one-way analysis of variance followed by Dunnett's test was
performed where appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
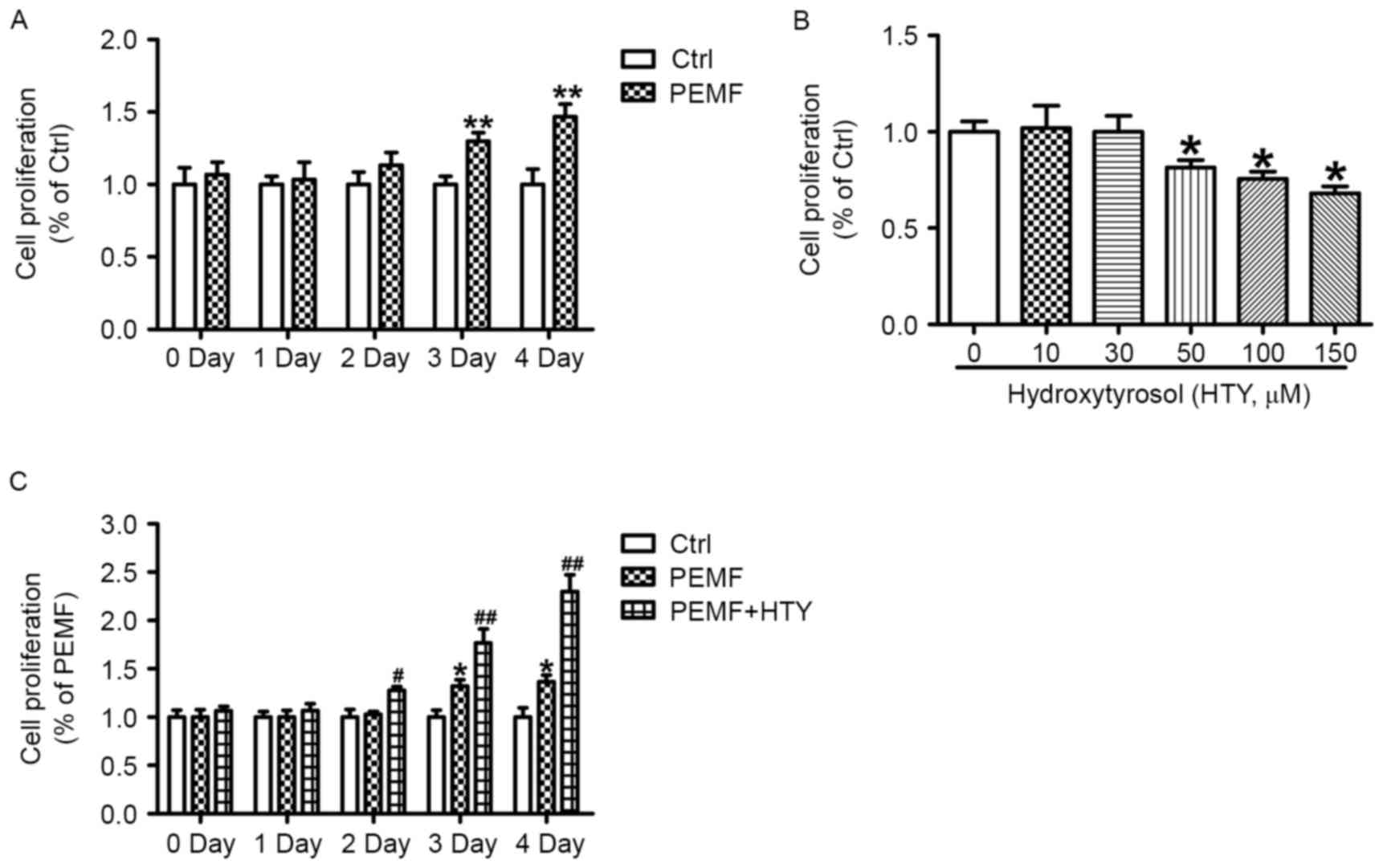
HTY promotes HUVEC proliferation
mediated by PEMFs in a time-dependent manner
As endothelial cell proliferation represents one of
the critical processes for wound healing, the present study
investigated the effect of PEMFs for <24 h on the cell
proliferation of HUVECs. PEMFs increased the proliferation rate of
HUVECs in a time-dependent manner, significantly so after 72 and 96
h of exposure (Fig. 1A). In order
to investigate whether HTY could promote proliferation induced by
treatment with PEMFs, different dosages of HTY was used. The
results demonstrated that 50, 100 and 150 µM HTY could markedly
reduce cell proliferation at the 24 h time point (Fig. 1B). Therefore, 30 µM HTY was
selected for following experiments. As presented in Fig. 1C, HTY could substantially increase
the cell proliferation in PEMF-treated cells at the 24 h time
point, while PEMF treatment alone produced no response on cell
proliferation.
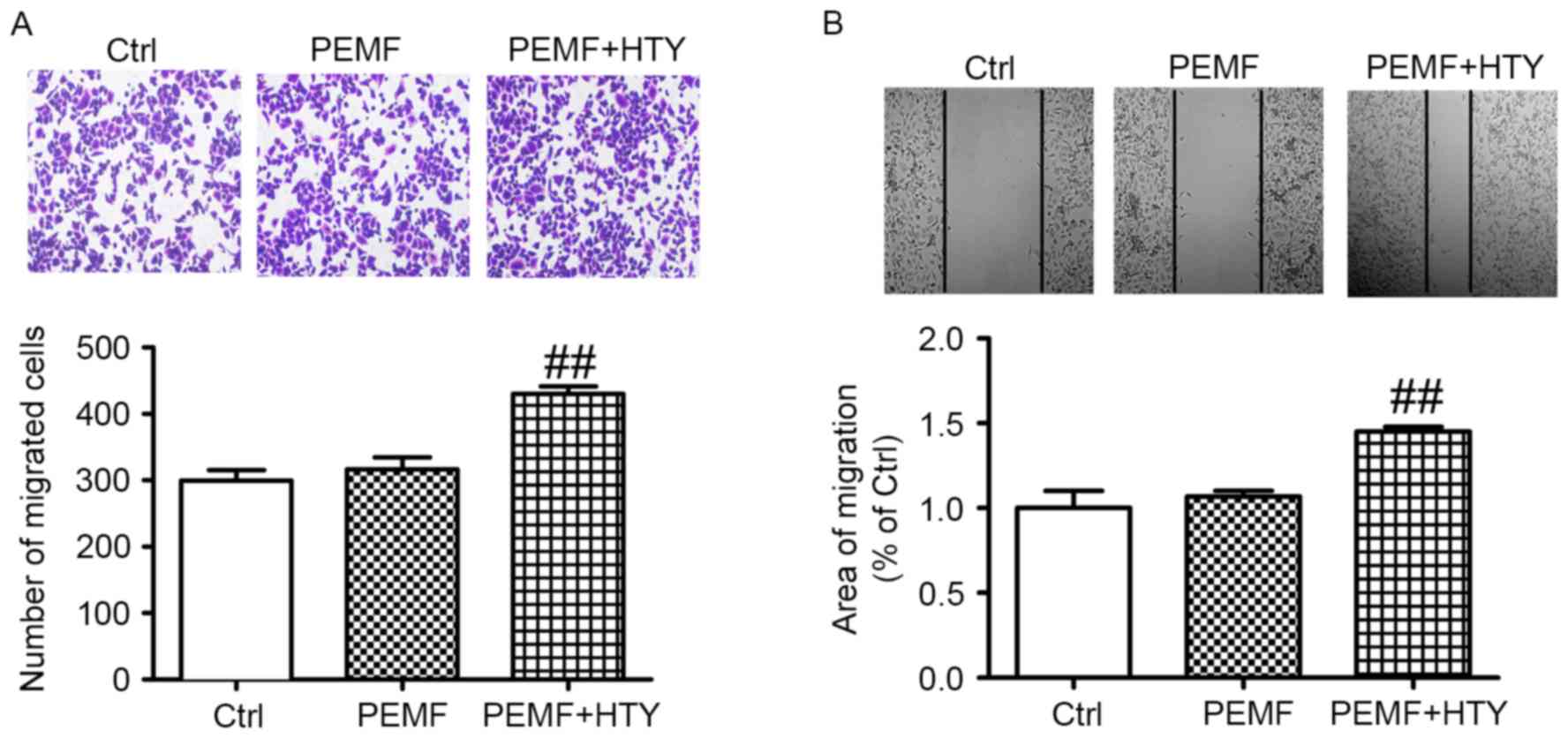
HTY enhances HUVEC migration induced
by PEMF
To further determine the effect of HTY on cell
migration, Transwell and scratch wound assays were performed. The
results demonstrated that the number of migratory cells was
significantly enhanced following combined HTY and PEMF treatment,
compared with the control and PEMF treatment only (Fig. 2).
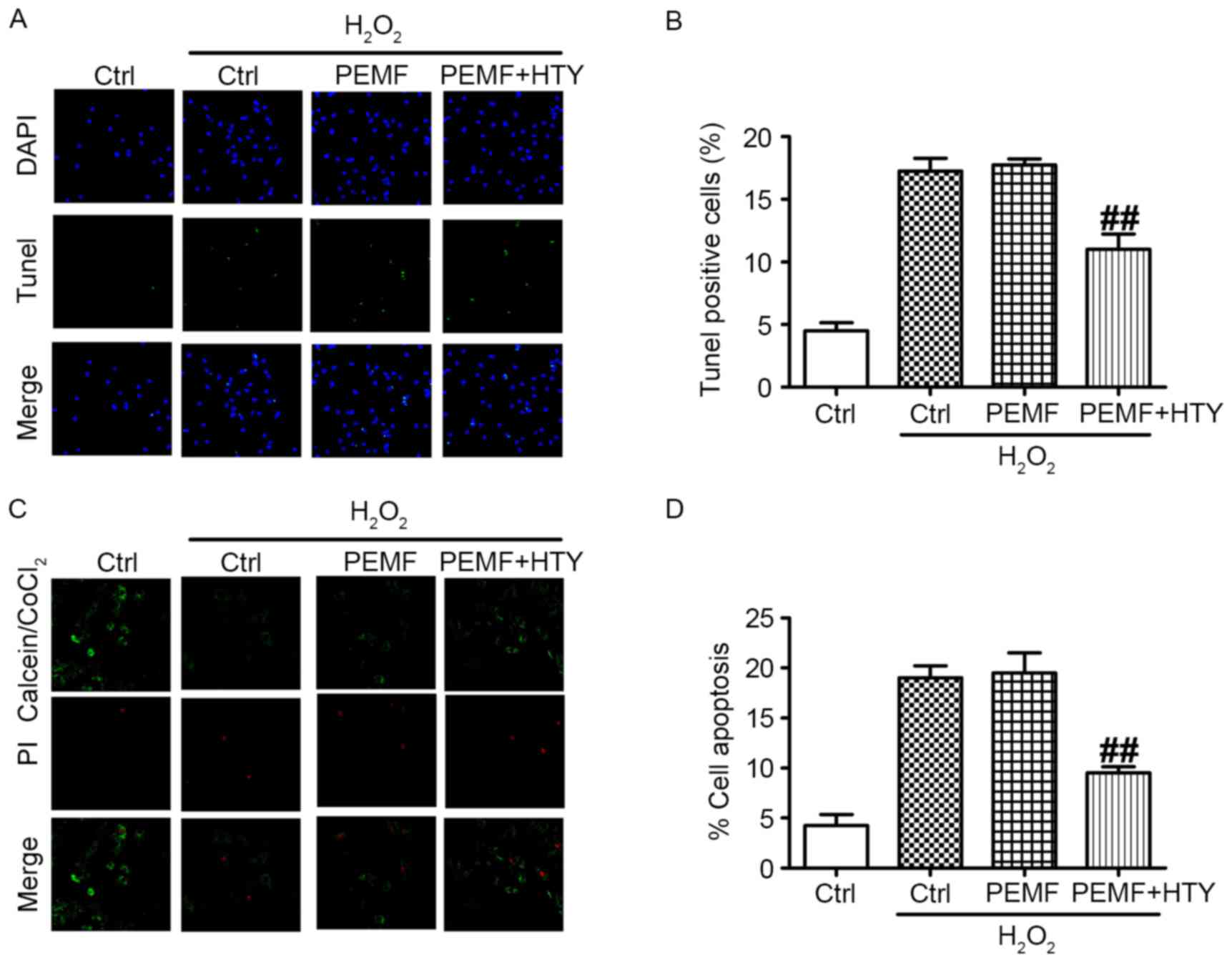
Co-treatment of HTY and PEMFs has
anti-apoptosis effects, and ameliorates mitochondrial dysfunction
induced by H2O2
An increase in apoptotic cells is associated with
wound healing. Therefore, a TUNEL assay was performed. Briefly,
HUVECs were treated with HTY/PEMFs or combined with H2O2,
(representative images in Fig.
3A). The results demonstrated that combined PEMF and HTY could
significantly reduce the number of apoptotic HUVECs, while PEMF
treatment alone produced no effects (Fig. 3B). In addition,
calcein/PI/CoCl2 staining was used to verify cell apoptosis
associated with mitochondrial dysfunction (representative images
are presented in Fig. 3C). In
Fig. 3D, apoptotic cells were
markedly decreased in the PEMF- and HTY-treated group, while PEMFs
administered alone had no effect on cell apoptosis. Therefore, the
anti-apoptotic effect of HTY may be associated with ameliorating
mitochondrial dysfunction.
HTY increases the transcription
activities of Akt, mTOR and TGF-β, but not p53, in HUVECs
To clarify the anti-apoptosis and pro-proliferation
effects of HTY following PEMF treatment, the mRNA expression levels
of Akt, mTOR, TGF-β and p53 were detected. Combined PEMF and HTY
treatment significantly increased the mRNA expression levels of Akt
(Fig. 4A), mTOR (Fig. 4B) and TGF-β (Fig. 4C) compared with the control. In
addition, the mRNA expression of mTOR was substantially elevated
following both HTY and PEMF treatment (Fig. 4B). Notably, no significant
differences were observed in the mRNA expression levels of p53
(Fig. 4D).
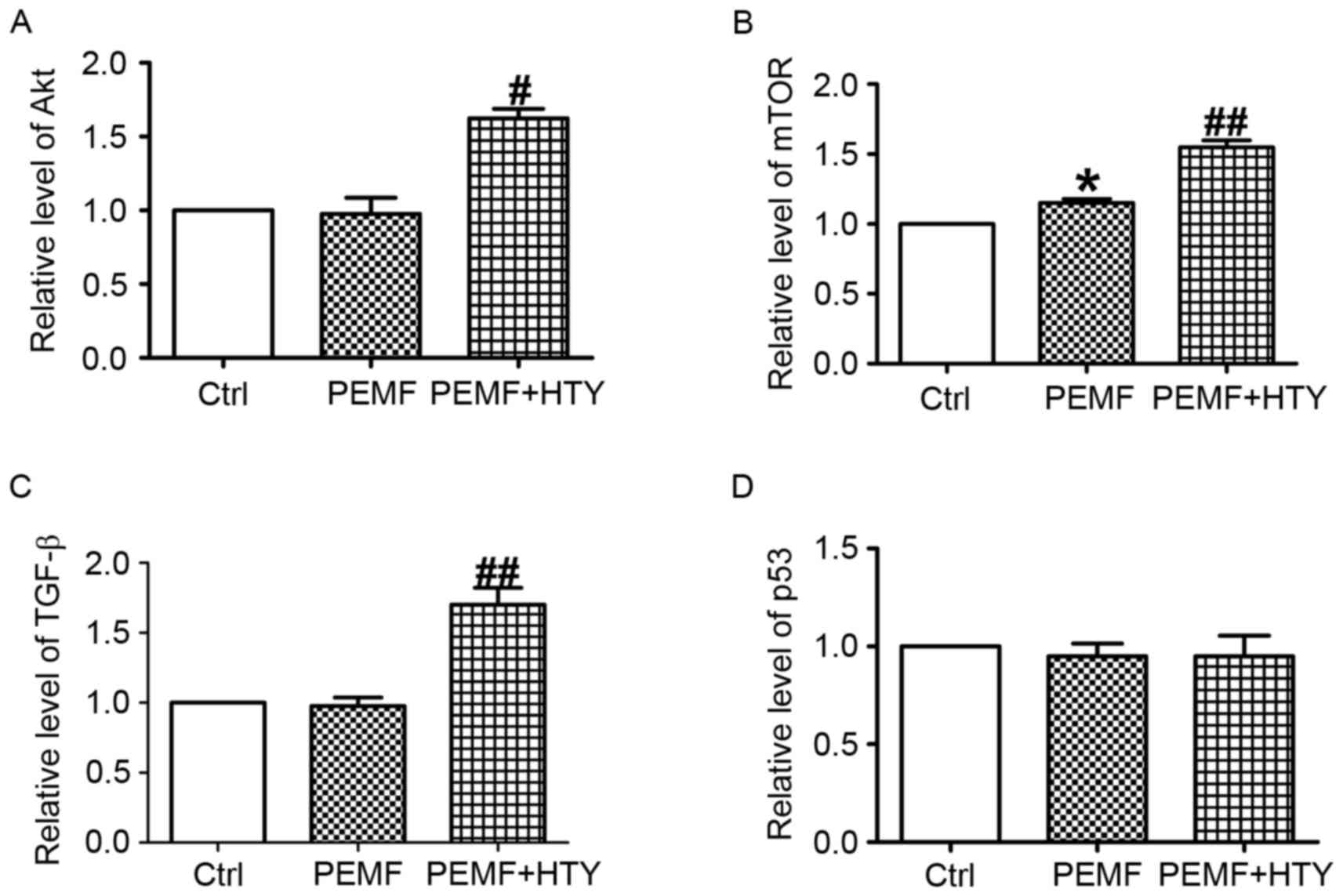 | Figure 4.Effect of HTY and PEMF on mRNA
expression levels of Akt, mTOR, TGF-β and p53 at 24 h. (A) Akt, (B)
mTOR, (C) TGF-β and (D) p53 mRNA expression levels in human
umbilical vein endothelial cells. Data are expressed as the mean ±
standard error of six independent experiments. *P<0.05 vs. Ctrl;
#P<0.05, ##P<0.01 vs. PEMF-treated group. HTY,
hydroxytyrosol; PEMFs, pulse electromagnetic fields; Ctrl, control;
Akt, protein kinase B; mTOR, mechanistic target of rapamycin;
TGF-β, transforming growth factor-β. |
HTY increases the protein expression
levels of Akt, mTOR and TGF-β, but not p53, in PEMF-treated
HUVECs
Western blotting was used to assess the protein
expression levels of Akt, mTOR and TGF-β in PEMF- and HTY-treated
HUVECs. PEMF treatment alone had no effect on the protein
expression levels of Akt; however, combined treatment with HTY
significantly upregulated these levels (Fig. 5A). PEMF treatment alone upregulated
the protein expression levels of mTOR, and this effect was further
enhanced by combined treatment with HTY (Fig. 5B). TGF-β protein expression levels
demonstrated the same pattern of effect as Akt (Fig. 5C). Both HTY and PEMF had no effect
on the protein expression level of p53.
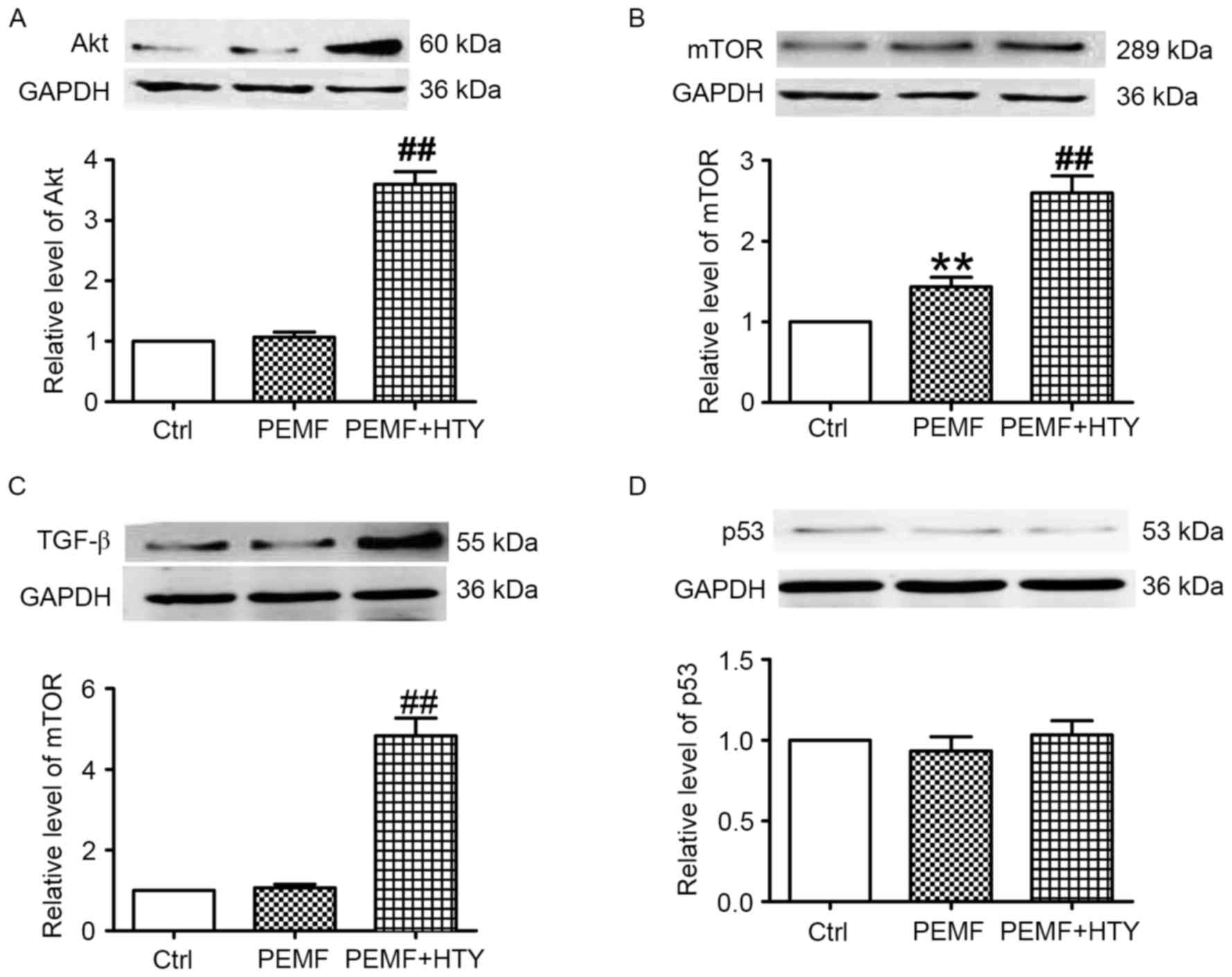 | Figure 5.Effect of HTY and PEMF on protein
expression levels of Akt, mTOR, TGF-β and p53 at 24 h.
Representative western blot images and quantification of (A) Akt,
(B) mTOR, (C) TGF-β and (D) p53 protein expression levels in human
umbilical vein endothelial cells. Data are expressed as the mean ±
standard error of three independent experiments. **P<0.01 vs.
Ctrl; ##P<0.01 vs. PEMF-treated group. HTY,
hydroxytyrosol; PEMFs, pulse electromagnetic fields; Ctrl, control;
Akt, protein kinase B; mTOR, mechanistic target of rapamycin;
TGF-β, transforming growth factor-β. |
Discussion
In the present study, several novel findings were
demonstrated. To the best of our knowledge, we demonstrated for the
first time that HTY promotes HUVEC proliferation mediated by PEMFs
in a time-dependent manner. Secondly, the data from the study
revealed that HTY and PEMFs serve synergistic roles in
anti-apoptosis and ameliorating mitochondrial dysfunction induced
by H2O2 incubation. Thirdly, to clarify the anti-apoptosis and
pro-proliferation effects of HTY following PEMF treatment, the mRNA
and protein expression levels of Akt, mTOR, TGF-β and p53 were
detected. It was demonstrated that HTY increases the transcription
activities of Akt, mTOR and TGF-β, but not p53, in HUVECs in
vitro. To the best of our knowledge, the present study was the
first to reveal the molecular mechanism of co-treatment with PEMFs
and HTY on the biological characteristics in HUVECs. These results
not only facilitate understanding of the mechanisms underlying the
effects of HTY on HUVECs, but also improve the view of co-treatment
of HTY and PEMFs that may serve as potential therapeutic strategy
in wound healing in the future.
Previous studies have demonstrated that PEMFs are a
non-invasive and non-pharmacological intervention, and have been
reported to repair damaged tissue (16–18).
Several clinical studies have demonstrated that PEMFs can
effectively alleviate tissue swell, traumatic pain and promote
wound healing (19,20). Guerriero et al (21) described the first reported case of
an innovative PEMF therapy, emysimmetric bilateral stimulation
(EBS), used to successfully treat refractory skin ulcers in two
elderly and fragile patients. They demonstrated a supportive role
of PEMFs in the treatment of skin ulceration in diabetes, which was
suggestive of a potential benefit of EBS for this clinical
condition. HTY is a major phenolic compound in virgin olive oil in
both free and complex forms. Catalán et al (22) revealed the protective effect of HTY
and its predominant plasmatic human metabolites, synthetized for
the first time by using an intestinal cell model, which may be
responsible in part for the protection against endothelial
dysfunction. However, no previous findings have suggested that the
combined treatment of PEMFs with HTY is a potentially promising
therapeutic strategy for treating wound healing. To further
investigate the mechanism of PEMF- and HTY-mediated
neovascularization, the present study investigated the effects of
co-treatment of PEMF and HTY on endothelial biological modification
in vitro. The synergistic effect of PEMFs and HTY were
identified, which may improve multiple endothelial functions
including migration, proliferation, anti-apoptosis and
mitochondrial dysfunction, suggesting the therapeutic potential of
PEMF- and HTY-combined treatment on pre-existing endothelial
vasculature. However, other mechanisms involved in PEMF- and
HTY-mediated neovascularization may exist; therefore, several
limitations of the current research should be highlighted. For
example, the present study used cell models, and the findings may
not be extrapolated directly to animal models and humans. The gene
activation and protein production of the HTY-stimulated HUVECs will
require further study to confirm the beneficial effects of PEMFs on
wound healing in vivo. Precaution should be taken when
applying the results of this study to patients. Nevertheless, these
results provide a basis for future studies to investigate whether
the effects of co-treatment of PEMFs and HTY are also applicable in
a clinical setting.
In conclusion, the results of the present study
revealed that combined exposure of PEMFs and HTY to HUVECs
demonstrated protective effects on proliferation, migration and
apoptosis in vitro, implicating the potential benefits of
this treatment for wound healing in the future.
References
|
1
|
Janis JE, Attinger CE and Lavery L:
Introduction to ‘current concepts in wound healing: Update 2016’.
Plast Reconstr Surg. 138(3 Suppl): 7S–8S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshida S, Yoshimoto H, Hirano A and Akita
S: Wound healing and angiogenesis through combined use of a
vascularized tissue flap and adipose-derived stem cells in a rat
hindlimb irradiated ischemia model. Plast Reconstr Surg.
137:1486–1497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Athanasiou A, Karkambounas S, Batistatou
A, Lykoudis E, Katsaraki A, Kartsiouni T, Papalois A and Evangelou
A: The effect of pulsed electromagnetic fields on secondary skin
wound healing: Aexperimental study. Bioelectromagnetics.
28:362–368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monache S Delle, Alessandro R, Iorio R,
Gualtieri G and Colonna R: Extremely low frequency electromagnetic
fields (ELF-EMFs) induce in vitro angiogenesis process in human
endothelial cells. Bioelectromagnetics. 29:640–648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li F, Lei T, Xie K, Wu X, Tang C, Jiang M,
Liu J, Luo E and Shen G: Effects of extremely low frequency pulsed
magnetic fields on diabetic nephropathy in streptozotocin-treated
rats. Biomed Eng Online. 15:82016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martino CF, Perea H, Hopfner U, Ferguson
VL and Wintermantel E: Effects of weak static magnetic fields on
endothelial cells. Bioelectromagnetics. 31:296–301. 2010.PubMed/NCBI
|
|
7
|
Li RL, Huang JJ, Shi YQ, Hu A, Lu ZY, Weng
L, Wang SQ, Han YP, Zhang L, Hao CN and Duan JL: Pulsed
electromagnetic field improves postnatal neovascularization in
response to hindlimb ischemia. Am J Transl Res. 7:430–444.
2015.PubMed/NCBI
|
|
8
|
Cheing GL, Li X, Huang L, Kwan RL and
Cheung KK: Pulsed electromagnetic fields (PEMF) promote early wound
healing and myofibroblast proliferation in diabetic rats.
Bioelectromagnetics. 35:161–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goudarzi I, Hajizadeh S, Salmani ME and
Abrari K: Pulsed electromagnetic fields accelerate wound healing in
the skin of diabetic rats. Bioelectromagnetics. 31:318–323.
2010.PubMed/NCBI
|
|
10
|
Lamy S, Ben Saad A, Zgheib A and Annabi B:
Olive oil compounds inhibit the paracrine regulation of
TNF-alpha-induced endothelial cell migration through reduced
glioblastoma cell cyclooxygenase-2 expression. J Nutr Biochem.
27:136–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morbidelli L: Polyphenol-based
nutraceuticals for the control of angiogenesis: Analysis of the
critical issues for human use. Pharmacol Res. 111:384–393. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zrelli H, Kusunoki M and Miyazaki H: Role
of hydroxytyrosol-dependent regulation of HO-1 expression in
promoting wound healing of vascular endothelial cells via Nrf2 De
Novo synthesis and stabilization. Phytother Res. 29:1011–1018.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scoditti E, Calabriso N, Massaro M,
Pellegrino M, Storelli C, Martines G, De Caterina R and Carluccio
MA: Mediterranean diet polyphenols reduce inflammatory angiogenesis
through MMP-9 and COX-2 inhibition in human vascular endothelial
cells: A potentially protective mechanism in atherosclerotic
vascular disease and cancer. Arch Biochem Biophys. 527:81–89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferroni L, Bellin G, Emer V, Rizzuto R,
Isola M, Gardin C and Zavan B: Treatment by Therapeutic Magnetic
Resonance (TMR) increases fibroblastic activity and keratinocyte
differentiation in an in vitro model of 3D artificial skin. J
Tissue Eng Regenerat med. 11:1332–1342. 2015. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goto T, Fujioka M, Ishida M, Kuribayashi
M, Ueshima K and Kubo T: Noninvasive up-regulation of
angiopoietin-2 and fibroblast growth factor-2 in bone marrow by
pulsed electromagnetic field therapy. J Orthop Sci. 15:661–665.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osti L, Buono AD and Maffulli N: Pulsed
electromagnetic fields after rotator cuff repair: A randomized,
controlled study. Orthopedics. 38:e223–e228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YT, Hei WH, Kim S, Seo YK, Kim SM,
Jahng JW and Lee JH: Co-treatment effect of pulsed electromagnetic
field (PEMF) with human dental pulp stromal cells and FK506 on the
regeneration of crush injured rat sciatic nerve. Int J Neurosci.
125:774–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bagnato GL, Miceli G, Marino N, Sciortino
D and Bagnato GF: Pulsed electromagnetic fields in knee
osteoarthritis: A double blind, placebo-controlled, randomized
clinical trial. Rheumatology (Oxford). 55:755–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Menini M, Bevilacqua M, Setti P, Tealdo T,
Pesce P and Pera P: Effects of pulsed electromagnetic fields on
swelling and pain after implant surgery: A double-blind, randomized
study. Int J Oral Maxillofac Surg. 45:346–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guerriero F, Botarelli E, Mele G, Polo L,
Zoncu D, Renati P, Sgarlata C, Rollone M, Ricevuti G, Maurizi N, et
al: Effectiveness of an innovative pulsed electromagnetic fields
stimulation in healing of untreatable skin ulcers in the frail
elderly: Two case reports. Case Rep Dermatol Med.
2015:5765802015.PubMed/NCBI
|
|
22
|
Catalán Ú, López de Las Hazas MC, Rubió L,
Fernández-Castillejo S, Pedret A, de la Torre R, Motilva MJ and
Solà R: Protective effect of hydroxytyrosol and its predominant
plasmatic human metabolites against endothelial dysfunction in
human aortic endothelial cells. Mol Nutr Food Res. 59:2523–2536.
2015. View Article : Google Scholar : PubMed/NCBI
|