Introduction
Alzheimer's disease (AD), also known as senile
dementia, is a degenerative disease of the central nervous system
that is predominantly characterized by progressive degeneration of
cognitive function and memory loss. Previous studies have suggested
that disorders of the Wnt/β-catenin signaling pathway in the brains
of patients with AD may be associated with pathological alterations
(1,2), including formation of neurofibrillary
tangles (NFTs) involving neurites and phosphorylated tau proteins,
and neuronal loss and senile plaques triggered by β-amyloid peptide
(Aβ) accumulation. The Wnt/β-catenin signaling pathway may
therefore be considered the core pathway linking Aβ neurotoxicity
with tau hyperphosphorylation.
Glycogen synthase kinase (GSK)-3β is a
multifunctional serine/threonine kinase and a negative regulator of
Wnt/β-catenin signaling. Its abnormal expression is closely
associated with the pathogenesis, pathological manifestations and
treatment of AD (2). Previous
studies have suggested that GSK-3β is a protein kinase that may
induce abnormal tau phosphorylation in the brains of patients with
AD and phosphorylation of tau at numerous sites (3,4).
Puerarin is the main active ingredient derived from
the Chinese herb root of Pueraria lobata, which is a
selective inhibitor of GSK-3β (5,6).
Previous research has indicated that puerarin downregulates Aβ
expression in the brain, inhibits the abnormal expression of tau
protein induced by Aβ, reduces cellular apoptosis, and improves
learning and memory in animals (7–10).
Puerarin exhibits anti-AD activity by inhibiting the activity of
GSK-3β and activating the Wnt/β-catenin signaling pathway. At
present, it is unknown whether puerarin ameliorates AD via the
Wnt/β-catenin signaling pathway. Therefore, the present study aimed
to investigate the role of oligomeric peptide Aβ1-42 in SH-SY5Y
cell impairment, and the inhibitory effects of puerarin on tau
hyperphosphorylation via the Wnt/β-catenin pathway.
Materials and methods
Drugs and reagents
The SH-SY5Y human neuroblastoma cell line was
obtained from the Chinese Academy of Sciences, Kunming Cell
Biological Research Institute (Kunming, China), and was used in the
present study. Oligomeric peptide Aβ1-42 (cat no. 107761-42-2) was
purchased from GL Biochem (Shanghai), Ltd. (Shanghai, China) and
puerarin (2 ml; 100 mg; 130,803) was obtained from Zhejiang CONBA
Pharmaceutical Co., Ltd. (Zhejiang, China). The reagents used were
as follows: anhydrous lithium chloride (LiCl; cat no. 213233;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), Dulbecco's modified
Eagle's medium (DMEM; high glucose), and fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
following antibodies were used: Rabbit anti-phosphorylated (p)-tau
(Ser396; cat no. ab109390), rabbit anti-p-tau (Ser199; cat no.
ab81268), rabbit anti-p-tau (Tau231; cat no. ab151559), rabbit
anti-β-catenin (cat no. ab32572), rabbit anti-GSK-3β (cat no.
ab32391), rabbit anti-p-GSK-3β (Ser9; cat no. ab75814), rabbit
monoclonal anti-cyclin D1 (cat no. ab134175), mouse anti-β-actin
(cat no. ab179467) (all from Abcam, Cambridge, MA, USA), goat
anti-rabbit IgG (H+L) highly cross-adsorbed Alexa Fluor®
Plus 555-conjugated secondary antibody (cat no. A32732) and goat
anti-mouse IgG (H+L) highly cross-adsorbed Alexa Fluor®
Plus 555-conjugated secondary antibody (cat no. A32727) (both from
Invitrogen; Thermo Fisher Scientific, Inc.).
Cell culture and treatment
SH-SY5Y cells were cultured in DMEM (high glucose)
supplemented with 10% heat-inactivated FBS, 100 µg/ml penicillin
and 100 µg/ml streptomycin at 37°C in a humidified incubator
containing 5% CO2. Cells were passaged once every 5–7
days, and cells in the logarithmic growth period were obtained.
MTT assay
The MTT assay was used to evaluate cell survival as
a function of mitochondrial viability; the following procedure was
performed at 37°C. SH-SY5Y cells were subcultured in 96-well plates
at a density of 5×105 cells/ml/well. After 24 h
attachment, cells were treated with 10, 50, 100 and 150 µmol/l
puerarin. Following a 24 h pretreatment with puerarin, 30 µmol/l
oligomeric peptide Aβ1-42 was added to the wells and the plate was
cultured for a further 24 h. Subsequently, 20 µl MTT (5 mg/ml PBS
stock solution) was added to each well and the cells were incubated
for 4 h at 37°C, after which, the medium was removed and the cells
were treated with 150 µl DMSO. The culture plate was gently
agitated until the crystals were completely dissolved, and optical
density values were measured at 540 nm using a microplate reader
and the inhibitory ratio of cell proliferation was calculated.
Cell morphological analysis
SH-SY5Y cells in the logarithmic growth phase were
seeded at a density of 5×105 cells/ml/well in a 6-well
plate. Cells were divided into the following groups: The control
group, the AD model group (30 µmol/l Aβ1-42), the puerarin
treatment groups (10, 50 or 100 µmol/l), and the positive control
group (10 mmol/l LiCl). After cell attachment on day 2, the medium
in the 6-well plate was removed. Serum-free DMEM was added to the
control and AD model groups; DMEM with puerarin, at a final
concentration of 10, 50 and 100 µmol/l, was added to the drug
treatment groups; and DMEM combined with LiCl at a final
concentration of 10 mmol/l was added to the positive control group;
50 µl was added per well for all groups. After 24 h, with the
exception of the control group, 30 µmol/l Aβ1-42 was added to the
remaining groups. After culturing for a further 24 h, cell
morphology was observed under an inverted fluorescence microscope.
All procedures described were performed at 37°C.
Protein expression detection by
western blotting
Cells were treated as aforementioned. Subsequently,
the medium was removed and cells were washed twice with pre-cooled
PBS. Radioimmunoprecipitation assay lysis buffer [50 mM Tris (pH
7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
SDS, 2 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM
EDTA, 1 mM Na3VO4 and 0.5 mg/ml leupeptin;
200 µl) was added to each well and the cells were lysed for 30 min
over ice. The cell lysates were then centrifuged at 12,000 × g for
15 min at 4°C, the supernatants were collected, and protein content
was quantified using the bicinchoninic acid method. Equal protein
samples (20 µg) from each group were separated by 10% SDS-PAGE and
were transferred to polyvinylidene fluoride membranes by semi-dry
transfer. The membranes were then blocked with 5% non-fat milk for
2 h at room temperature, after which the membranes were incubated
with the following primary antibodies at 4°C overnight: Rabbit
anti-p-tau (Ser396; 1:10,000), rabbit anti-p-tau (Ser199; 1:5,000),
rabbit anti-p-tau (Thr231; 1:1,000), rabbit anti-β-catenin
(1:5,000), rabbit anti-GSK-3β (1:10,000), rabbit anti-p-GSK-3β
(Ser9; 1:10,000) and rabbit anti-cyclin D1 (1:10,000); β-actin
(1:10,000) served as an internal control. After washing the
membranes with TBS containing 20% Tween-20 (TBST), Alexa
Fluor® Plus 555-conjugated secondary antibodies
(1:1,000) were added to the membranes and incubated for 1.5 h in
the dark at 37°C. Blots were visualized using chemiluminescence
(Invitrogen; Thermo Fisher Scientific, Inc.) and were exposed to
radiographic films. The same experiment was repeated three times.
Density of the bands was measured and analyzed using a gel
electrophoresis image analyzer (GS-900™ Calibrated Densitometer
with integrated Image Lab™ v6.0 software; cat no. 1707991; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), and are expressed as the
absorbance ratio of the target protein to the internal control.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA) were used to conduct
statistical analyses. Data were statistically analyzed using
one-way analysis of variance, with either the Kruskal-Wallis H, the
least significant difference or the Games-Howell post hoc tests
depending on the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of puerarin on the viability
of Aβ1-42-treated SH-SY5Y cells
As presented in Fig.
1, 30 µmol/l Aβ1-42 significantly reduced the viability of
SH-SY5Y cells compared with the control group (P<0.01). However,
treatment with puerarin (10, 50, 100 and 150 µmol/l) significantly
enhanced the viability of SH-SY5Y cells in a dose-dependent manner,
compared with the Aβ group (P<0.05). These results suggested
that puerarin may inhibit Aβ1-42-induced decreases in SH-SY5Y cell
viability and exert a cytoprotective effect.
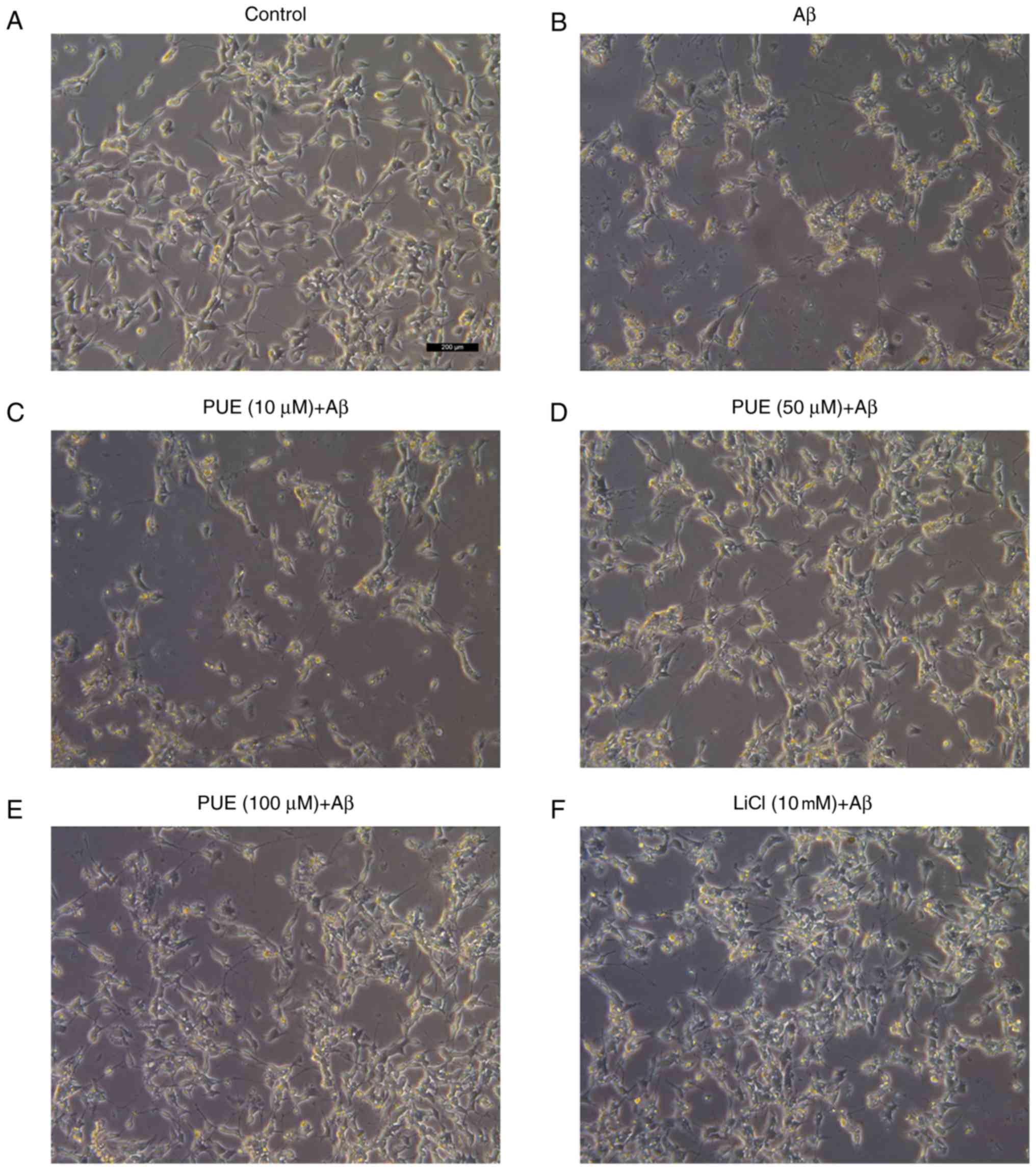
Effects of puerarin on the growth of
Aβ1-42-treated SH-SY5Y cells
As presented in Fig.
2, cells in the control group were normal with high refraction,
clearly visible axons and adherence, when observed under an
inverted microscope. A day after the addition of Aβ1-42 to the
model group, cell diopter was degraded, cell processes were
significantly shortened or broken, and the cell number was
decreased. Cell morphology in the puerarin (10, 50 and 100 µmol/l)
+ Aβ group was markedly improved compared with that of the model
group. Puerarin inhibited the loss of cellular synapse, and
partially enhanced cell diopter and number in a dose-dependent
manner. The effects of puerarin (50 µmol/l) were comparable to
those of LiCl, thus indicating that various doses of puerarin
protected SH-SY5Y cells from Aβ1-42-induced damage.
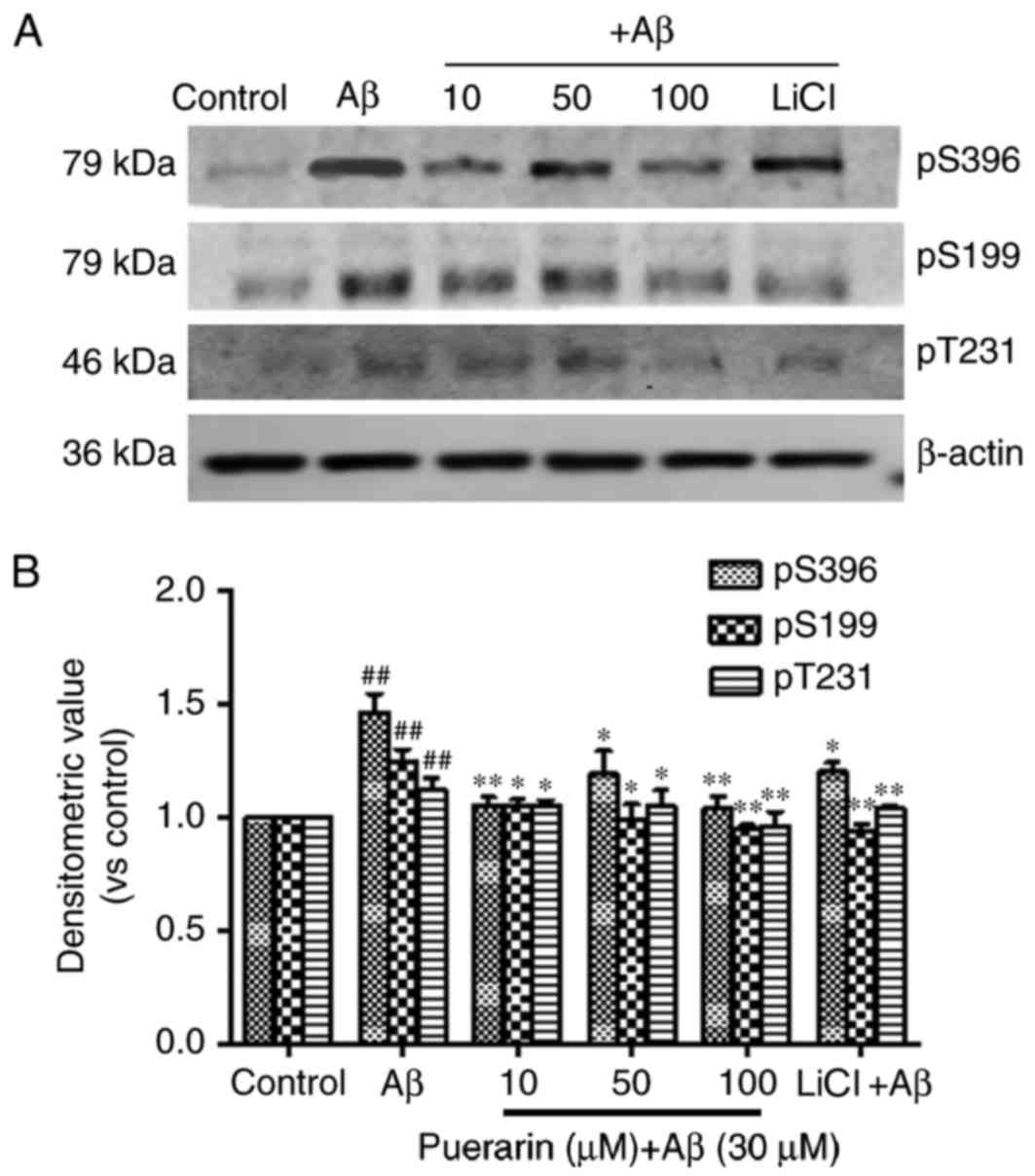
Effects of puerarin on tau protein
phosphorylation in Aβ1-42-treated SH-SY5Y cells
As presented in Fig.
3, compared with the control group, the levels of tau
phosphorylation at Ser396, Ser199 and Thr231 sites were
significantly increased in the AD model group (P<0.01).
Conversely, the levels of tau phosphorylation in the puerarin and
LiCl groups were much lower than in the model group (P<0.05).
The levels of tau phosphorylation were most significantly decreased
following pretreatment with 100 µmol/l puerarin. Therefore, a
specific dose of puerarin, similar to that of the GSK-3β inhibitor
LiCl, inhibited tau hyperphosphorylation in Aβ-treated SH-SY5Y
cells.
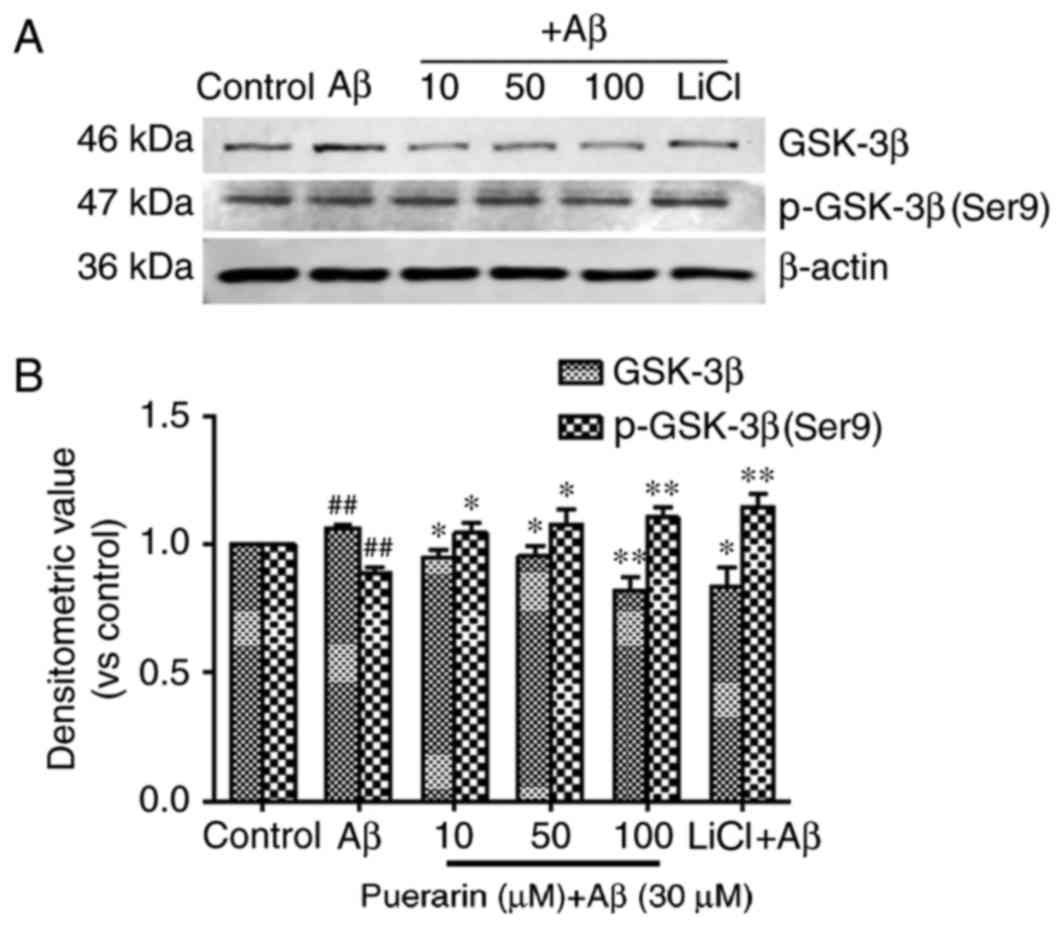
Effects of puerarin on the expression
of GSK-3β and p-GSK-3β (Ser9) in Aβ1-42-treated SH-SY5Y cells
As shown in Fig. 4,
treatment of SH-SY5Y cells with Aβ1-42 for 24 h significantly
reduced the levels of GSK-3β phosphorylation at Ser9 (the
inhibitory phosphorylated site) (P<0.01) and significantly
increased the expression levels of GSK-3β compared with the control
group (P<0.01). The expression levels of p-GSK-3β (Ser9) in the
SH-SY5Y cells following treatment with puerarin and LiCl were
significantly increased compared with in the model group
(P<0.05); however, GSK-3β expression was significantly decreased
compared with the model group. A reduction in GSK-3β expression and
an increase in p-GSK-3β (Ser9) expression were most prominent
following pretreatment with 100 µmol/l puerarin, thus suggesting
that puerarin increased p-GSK-3β (Ser9)-mediated inhibition of
GSK-3β, and GSK-3β mediated Aβ1-42-induced tau phosphorylation.
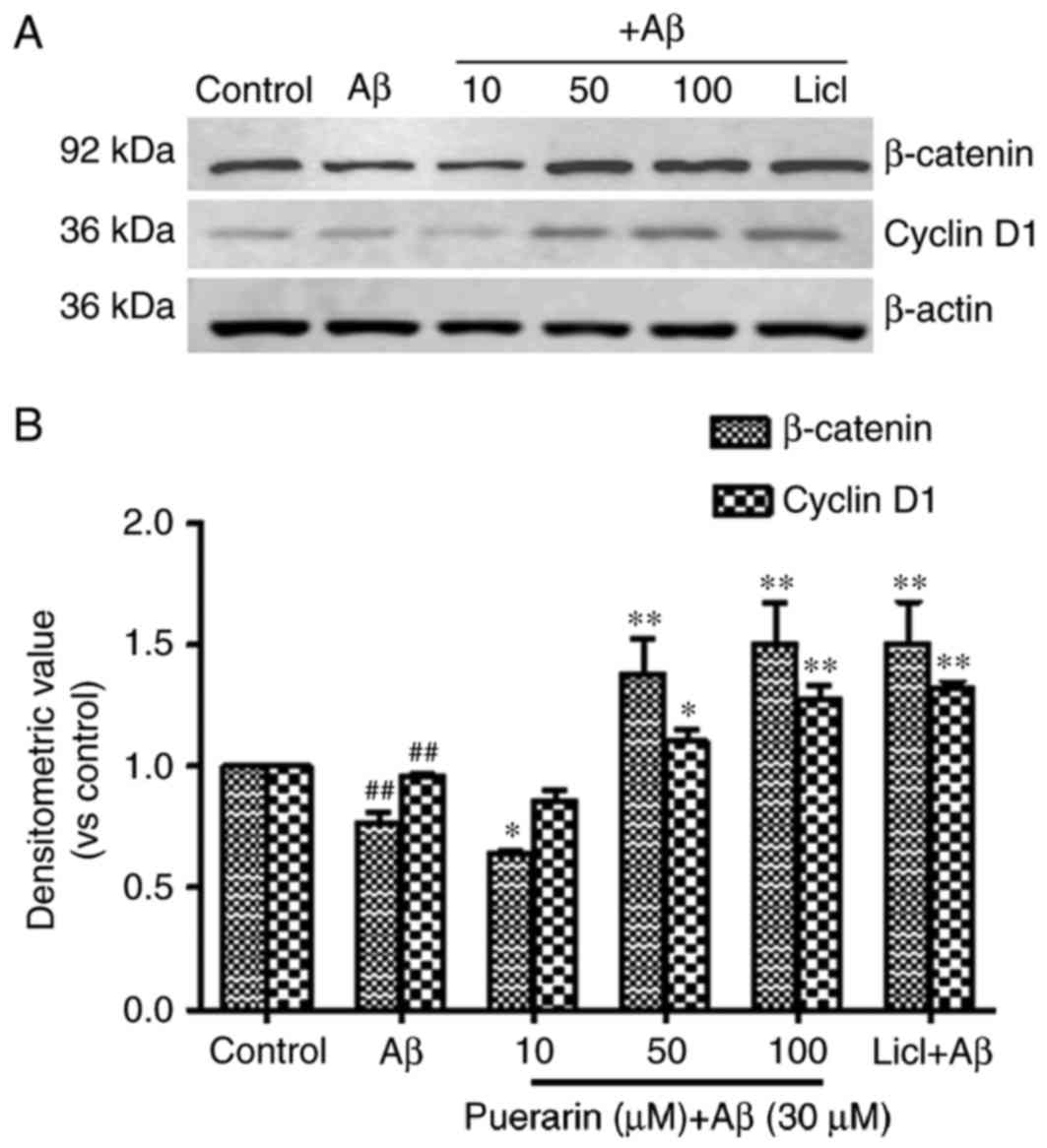
Effects of puerarin on the expression
of β-catenin and cyclin D1 in Aβ1-42-treated SH-SY5Y cells
As presented in Fig.
5, the expression levels of β-catenin and cyclin D1 were
significantly reduced in SH-SY5Y cells following treatment with
Aβ1-42 compared with the control group (P<0.01). Compared with
the model group, the expression levels of β-catenin and cyclin D1
were significantly enhanced in cells exposed to various doses of
puerarin with increasing drug concentration, and in cells exposed
to LiCl. The expression levels were significantly increased in
cells treated with 50 and 100 µmol/l puerarin groups compared with
in the model group. These results suggested that puerarin induced
neuroprotection via activation of β-catenin and cyclin D1 in the
Wnt/β-catenin signaling pathway. It may be hypothesized that
puerarin activates β-catenin and cyclin D1 expression by inhibiting
the activity of GSK-3β.
Discussion
Tau hyperphosphorylation is a key event in the
pathogenesis of AD. Abnormal signal transduction in early AD
triggers an imbalance in protein kinase and phosphatase levels, and
the accumulation of hyperphosphorylated microtubule-associated
protein tau in neurons leads to the formation of NFTs (11) resulting in synaptic damage and
neural degeneration. Tau contains 21 tau-Ser and
tau-Thr-phosphorylated sites; however, only a few sites serve a
role in the microtubule-binding activity of tau. Nevertheless,
abnormal phosphorylation at Ser396, Ser199 and Thr231 sites occurs
in AD (12,13). In AD pathogenesis, the accumulation
of Aβ is correlated with hyperphosphorylation of tau, and in
vitro studies have demonstrated that Aβ induces tau
hyperphosphorylation (14,15). In the present study, SH-SY5Y cells
were treated with 30 µmol/l oligomeric peptide Aβ1-42. After 24 h,
a cell model of tau hyperphosphorylation resembling AD was
successfully established. Conversely, the levels of tau
phosphorylation at the aforementioned sites were reduced, following
pretreatment of the cells with various concentrations of puerarin.
A reduction in tau protein phosphorylation was most prominent
following treatment with 100 µmol/l puerarin. Therefore, puerarin
inhibited Aβ1-42-induced hyperphosphorylation of tau.
GSK-3β is the key protein kinase underlying tau
phosphorylation. GSK-3β is a kinase rich in Ser and Thr, and its
activity is inhibited by phosphorylation at the Ser9 residue
(16). The present study
demonstrated that GSK-3β expression was increased in Aβ1-42-treated
SH-SY5Y cells, whereas p-GSK-3β (Ser9) expression was reduced.
Treatment with puerarin and LiCl, which is a specific inhibitor of
GSK-3β, increased p-GSK-3β (Ser9) expression and reduced GSK-3β
expression. It may be hypothesized that Aβ1-42 triggers tau
hyperphosphorylation predominantly via activation of GSK-3β,
whereas puerarin inhibits GSK-3β activity by increasing p-GSK-3β
(Ser9) expression, which further inhibits tau hyperphosphorylation
in Aβ-treated SH-SY5Y cells.
A previous study reported that Wnt/β-catenin
signaling is closely associated with the incidence and development
of AD (2). GSK-3β is a key
negative regulator of the Wnt signaling pathway, which reduces the
transcription and expression of β-catenin and the target gene
cyclin D1 via phosphorylation and ubiquitination (4). Loss of regulation of normal cell
growth and differentiation leads to neuron death (17). The present study suggested that
puerarin may activate β-catenin and cyclin D1 in the Wnt/β-catenin
signaling pathway to inhibit GSK-3β activity, and exert
neuroprotective effects.
In conclusion, the present study demonstrated that
puerarin is able to inhibit Aβ1-42-induced tau
hyperphosphorylation. The underlying mechanism is potentially
mediated via inhibition of GSK-3β, which may induce activation of
β-catenin and the downstream factor cyclin D1, and reduce
hyperphosphorylation of microtubule-associated tau in nerve cells,
consequently resulting in neuroprotection. These results provide
novel ideas and strategies regarding the clinical treatment of AD
with puerarin.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of Zhejiang Province (grant no.
LQ14H280001), the Medical and Health Technology Project of Zhejiang
Province (grant no. 2015KYA062) and the National Natural Science
Foundation (grant no. 81403128).
References
|
1
|
Inestrosa NC, Montecinos-Oliva C and
Fuenzalida M: Wnt signaling: Role in Alzheimer disease and
schizophrenia. J Neuroimmune Pharmacol. 7:788–807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silva-Alvarez C, Arrázola MS, Godoy JA,
Ordenes D and Inestrosa NC: Canonical Wnt signaling protects
hippocampal neurons from Ab oligomers: Role of non-canonical
Wnt-5a/Ca(2+) in mitochondrial dynamics. Front Cell Neurosci.
7:972013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng Y and Wang XL: Tau protein and
Alzheimers disease. Chin Pharmacol Bull. 20:601–605. 2004.
|
|
4
|
Engel T, Hernández F, Avila J and Lucas
JJ: Full reversal of Alzheimer's disease-like phenotype in a mouse
model with conditional overexpression of glycogen synthase
kinase-3. J Neurosci. 26:5083–5090. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zou Y, Hong B, Fan L, Zhou L, Liu Y, Wu Q,
Zhang X and Dong M: Protective effect of puerarin against
beta-amyloid-induced oxidative stress in neuronal cultures from rat
hippocampus: Involvement of the GSK-3b/Nrf2 signaling pathway. Free
Radic Res. 47:55–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin F, Xie B, Cai F and Wu G: Protective
effect of Puerarin on b-amyloid-induced neurotoxicity in rat
hippocampal neurons. Arzneimittelforschung. 62:187–193. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang HY, Hu HT, Liu YH, Wang HQ, Feng GF
and Chen GM: Effect of puerarin on PC12 cells apoptosis induced by
Abeta25-35 in vitro. Zhong Yao Cai. 31:543–546. 2008.(In Chinese).
PubMed/NCBI
|
|
8
|
Nie JW, Li Y and Wang RT: Protective
effect of puerarin on PC-12 cell impairment induced by Aβ25-35.
Chin J Gerontol. 27:2283–2285. 2007.(In Chinese).
|
|
9
|
Yan FL, Guo L, Wang YQ and Hong Z: Effects
of puerarin on the expression of Aβ1-40 and Bax in brain of AD
rats. Chin J Neuromed. 5:158–161. 2006.
|
|
10
|
Yan FL, Wang YQ and Guo L: Effects of
Puerarin on protein expression of hyperphosphorylated tau and ChAT
in hippocampus of Alzheimer's disease rats. J Clin Neurol.
19:191–193. 2006.
|
|
11
|
Steinhilb ML, Dias-Santagata D, Fulga TA,
Felch DL and Feany MB: Tau phosphorylation sites work in concert to
promote neurotoxicity in vivo. Mol Biol Cell. 18:5060–5068. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Léger J, Kempf M, Lee G and Brandt R:
Conversion of serine to aspartate imitates phosphorylation-induced
changes in the structure and function of microtubule-associated
protein tau. J Biol Chem. 272:8441–8446. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie H, Litersky JM, Hartigan JA, Jope RS
and Johnson GV: The interrelationship between selective tau
phosphorylation and microtubule association. Brain Res.
798:173–183. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olivieri G, Baysang G, Meier F,
Müller-Spahn F, Stähelin HB, Brockhaus M and Brack C:
N-acetyl-L-cysteine protects SHSY5Y neuroblastoma cells from
oxidative stress and cell cytotoxicity: Effects on beta-amyloid
secretion and tau phosphorylation. J Neurochem. 76:224–233. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rapoport M and Ferreira A: PD98059
prevents neurite degeneration induced by fibrillar beta-amyloid in
mature hippocampal neurons. J Neurochem. 74:125–133. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frame S, Cohen P and Biondi RM: A common
phosphate binding site explains the unique substrate specificity of
GSK3 and its inactivation by phosphorylation. Mol Cell.
7:1321–1327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang QG, Wang R, Khan M, Mahesh V and
Brann DW: Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin
signaling pathway, in estrogen-induced neuroprotection and
attenuation of tau phosphorylation. J Neurosci. 28:8430–8441. 2008.
View Article : Google Scholar : PubMed/NCBI
|