Introduction
Renal cell carcinoma (RCC) accounts for ~3% of all
adult malignancies and is the most common type of adult kidney
cancer (1). The long-term
prospects for patients with advanced RCC have improved; however,
disease progression-free survival in the absence of debilitating
side effects remains a work-in-progress (2). Various subtypes of RCC have been
reported, with clear cell carcinoma and papillary RCC accounting
for ~75 and ~10% of all RCC cases, respectively (2,3).
Surgical treatment is effective for RCC; however, 20–40% of
patients develop metastatic disease following surgery (4). Furthermore, the five-year survival
rate of patients with distant metastasis remains <10% (5). Thus, it is important to identify
novel diagnostic and prognostic biomarkers of RCC.
MicroRNAs (miRNAs), a type of small non-coding RNA,
may regulate the translation of protein-coding genes by repressing
translation of protein-coding mRNA or enhancing mRNA degradation by
binding to the 3′ untranslated regions of mRNAs (6–8).
miRNA (miR)-24 has been demonstrated to be downregulated in certain
tumors, including breast carcinoma (9), osteosarcoma (10) and gastric cancer (11). Microarray analyses have revealed
that miR-24 was upregulated in RCC (12). However, the expression levels of
miR-24 in RCC remain to be verified by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) in
RCC tissues. In the present study, expressions levels of miR-24-1
and miR-24-2 (two stem-loops of miR-24-3p, the mature sequence of
miR-24) were detected by RT-qPCR in RCC tissues paired with healthy
control tissues and in RCC cell lines. Furthermore, the potential
functions of miRNAs in RCC cell lines were investigated by cell
proliferation, mobility and apoptosis assays.
Materials and methods
Collection of samples
A total of 35 paired tissues were collected from
Peking University Shenzhen Hospital (Shenzhen, China). Written
informed consent was obtained from all patients. The collection and
use of the samples was reviewed and approved by the Ethics
Committee of Peking University Shenzhen Hospital. The tissues were
dissected, immersed in RNAlater (Qiagen GmbH, Hilden, Germany) for
30 min, and stored at −80°C. A pair of tissues included RCC tissue
and adjacent healthy tissue that was 2 cm away from visible RCC
lesions. The tissues collected were reviewed and classified by
hematoxylin and eosin staining. The clinical and pathological
characteristics of the patients are presented in Table I.
 | Table I.Clinicopathological features of renal
cell carcinoma patients. |
Table I.
Clinicopathological features of renal
cell carcinoma patients.
| Characteristic | Number of cases |
|---|
| Mean age (range) | 51 (25–70) |
| Gender |
|
|
Male/female | 21/14 |
| Histological
type |
|
| Clear
cell/papillary | 28/7 |
| pT-stage |
|
|
T1/T2/T3+T4 | 18/13/4 |
| Fuhrman grade |
|
|
I/II/III/IV | 15/13/4/3 |
| AJCC clinical
stages |
|
|
I/II/III+IV | 17/13/5 |
Cell culture
The present study used 293T human embryonic kidney
(293T, Type Culture Collection of the Chinese Academy of Medical
Sciences, Shanghai, China) and 786-O, ACHN and 769P RCC cell lines
(American Type Culture Collection, Manassas, VA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific, Inc.), 1%
antibiotics (100 µl/ml penicillin and 100 mg/ml streptomycin
sulfates) and 1% glutamine, in a humidified incubator in 5%
CO2 at 37°C.
RNA extraction, cDNA synthesis and
RT-qPCR
Total RNA was extracted from tissues and cells using
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and purified with the RNeasy Maxi kit (Qiagen GmbH),
according to the manufacturer's protocol. The concentration of RNA
was measured using a NanoDrop 2000/2000c spectrophotometer (Thermo
Fisher Scientific, Inc.). A total of 1 µg RNA from each sample was
used for reverse transcription using the miScript RT kit (Qiagen
GmbH), following the manufacturer's protocol to acquire cDNA. qPCR
was performed to detect the expression levels of miR-24-1 and
miR-24-2, using the miScript SYBR® Green PCR kit
(Qiagen, Germany) and the Lightcycler 480® Real-Time PCR
system (Roche Applied Science, Penzburg, Germany) according to the
manufacturer's protocol. U6 served as the internal control. Primer
sequences are presented in Table
II. The universal primer, used as reverse primer of miR-24-2,
was provided in the miScript SYBR Green PCR kit. The conditions of
RT-qPCR were: 95°C for 1 min, then 95°C for 10 sec, 55°C for 30
sec, 70°C for 30 sec, for 40 cycles. The expression levels of
miRNAs were analyzed using the 2−ΔΔCq method (13).
 | Table II.Sequences used in the present
study. |
Table II.
Sequences used in the present
study.
| Name | Sequence
(5′-3′) |
|---|
| miR-24–2 | F:
UGCCUACUGAGCUGAAACACAG |
| mimic | R:
GUGUUUCAGCUCAGUAGGCAUU |
| Negative | F:
UUCUCCGAACGUGUCACGUTT |
| control | R:
ACGUGACACGUUCGGAGAATT |
| miR-24-2
inhibitor |
CUGUGUUUCAGCUCAGUAGGCA |
| Inhibitor negative
control |
CAGUACUUUUGUGUAGUACAA |
| miR-24-1 forward
primer |
TGCCTACTGAGCTGATATCAGT |
| miR-24-2 forward
primer |
TGCCTACTGAGCTGAAACACAG |
| U6 forward
primer |
CTCGCTTCGGCAGCACA |
| U6 reverse
primer |
ACGCTTCACGAATTTGCGT |
Cell transfection
To assess the expression levels of miR-24-2, a
synthesized miR-24-2 mimic or inhibitor or their respective
negative controls (NC; Shanghai GenePharma, Co., Ltd., Shanghai,
China), at a concentration of 20 µmol/l, was transfected into cells
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), which was subsequently added to the Opti-Minimum
Essential Medium® I Reduced Serum Medium (Gibco; Thermo
Fisher Scientific, Inc.) and incubated for 24 h. Following this,
qPCR was performed to verify alterations in miR-24-2 expression.
The sequences are presented in Table
II.
Wound scratch assay
A wound scratch assay was performed to assess the
cell migration ability of 786-O and ACHN cells in vitro.
Cells (~6×105 cells/well) were seeded into 6-well
plates, incubated for 24 h and transfected with 200 pmol miR-24-2
mimic, inhibitor, NC or inhibitor NC. A vertical line was scratched
with a sterile 200 µl pipette tip 6 h after transfection. The cells
were rinsed with phosphate-buffered saline (PBS) to remove floating
cells. A digital camera system was used to acquire images of the
scratches at 0 and 24 h. The experiments were performed in
triplicate and repeated at least three times.
Transwell assay
A Transwell assay was performed to assess the
migration and invasion ability of 786-O and ACHN cells in
vitro. Transwell chamber inserts (BD Biosciences, Franklin
Lakes, NJ, USA) with or without Matrigel (BD Biosciences) were used
for invasion and migration assays, respectively, according to the
manufacturer's protocol. The transfected cells were seeded into the
upper chamber at a density of 1×104 cells in 200 µl
Dulbecco's Modified Eagle's medium (Gibco, Thermo Fisher
Scientific, Inc.). Medium containing 10% FBS was added to the lower
chamber. The cells were allowed to migrate for 36 h and invade for
48 h. The cells that had migrated or invaded to the bottom of the
inserts were stained with crystal violet and counted using a
microscope. The experiments were performed in triplicate and
repeated at least three times.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
An MTT assay was performed to assess the
proliferation of 786-O and ACHN cells in vitro. Cells
(~3,000 cells/well) were seeded into a 96-well plate, and
subsequently transfected with 5 pmol miR-24-2 mimic, inhibitor, NC
or inhibitor NC. A total of 20 µl MTT (5 mg/ml; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) was added into the wells and
incubated at 37°C for 4 h. Cell proliferation was assessed at 0,
24, 48 or 72 h post-transfection. Following this, the medium was
replaced with 150 µl dimethyl sulfoxide (Sigma-Aldrich; Merck
Millipore) and plates were agitated for 15 min at room temperature.
The optical density (OD) of each well was subsequently measured at
a wavelength of 490 nm using an ELISA microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The experiments were
performed in triplicate and repeated at least three times.
Cell Counting kit-8 (CCK-8) assay
Proliferation of 786-O and ACHN cells was
additionally assessed using the CCK-8 (Beyotime Institute of
Biotechnology, Haimen, China), following the manufacturer's
protocol. Into 96-well plates, ~4,000 cells were seeded per well,
incubated for 24 h and transfected with 5 pmol miR-24-2 mimic,
inhibitor, NC or inhibitor NC. After 0, 24, 48 or 72 h, 15 µl CCK-8
was added into each well and the plate was incubated for 1.5 h.
Following this, the OD of each well was measured at a wavelength of
490 nm using an ELISA microplate reader. The experiments were
performed in triplicate and repeated at least three times.
Flow cytometric analysis of
apoptosis
Apoptosis of 786-O and ACHN cells was measured in
vitro by flow cytometry. Cells were seeded at a density of
~3×105/well into 6-well plates and subsequently
transfected with 200 pmol miR-24-2 mimic, inhibitor, NC or
inhibitor NC. After 48 h, cells were harvested and washed twice
with cold PBS. Cells were subsequently resuspended in 100 µl 1X
binding buffer and 5 µl annexin V-fluorescein isothiocyanate,
following which 3 µl propidium iodide (all from Invitrogen; Thermo
Fisher Scientific, Inc.) was added into each cell suspension. A
total of 15 min later, 400 µl binding buffer was added to each
tube. A COULTER® EPICS® XL™ Flow
Cytometer (Beckman Coulter, Inc., Brea, CA, USA) was subsequently
used to analyze the apoptosis rate. FlowJo (version X) flow
cytometry analysis software (FlowJo LLC, Ashland, OR) was used in
the study.
Statistical analysis
A paired t-test was used to compare the expression
levels of miR-24-2 in matched tissues. An unpaired Student's t-test
was used to analyze assays characterizing the phenotypes of cells.
All statistical analyses were performed using SPSS software version
19.0 (IBM SPSS, Armonk, NY, USA) and data are presented as the mean
± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
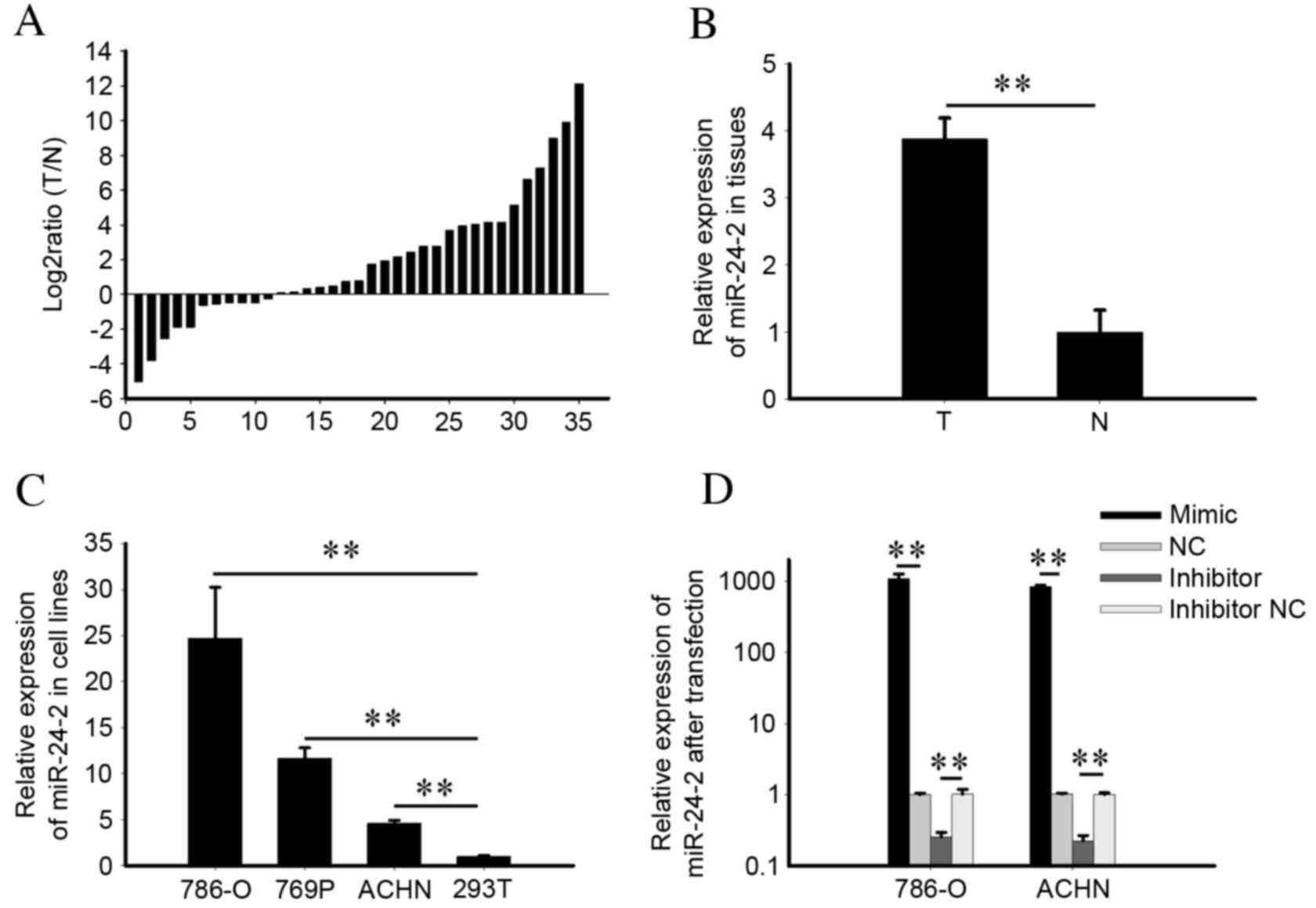
miR-24-2 is upregulated in RCC tissues
and cell lines
The expression levels of miR-24-1 and miR-24-2 were
determined by RT-qPCR in 35 RCC cell lines and paired healthy
tissues. The expression levels of miR-24-1 in tissues and cells
were reduced to the extent that miR-24-1 was detected in less than
half of all tissues, and the majority of quantification cycle
values were >36 (data not shown). The ratios of miR-24-2 in the
35 paired tissues [log2Ratio (T/N)] are presented in
Fig. 1A; miR-24-2 was upregulated
in 24 RCC tissues. The results demonstrated that the expression
levels of miR-24-2 in RCC tissues (mean of relative expression,
3.88) were significantly increased compared with healthy tissues
(P=0.004; Fig. 1B). In addition,
expression levels of miR-24-2 in 786-O, ACHN and 769P RCC cell
lines were significantly increased compared with the 293T human
embryonic kidney cell line (P=0.009, 786-O; P=0.003, 769P;
P=0.0002, ACHN; Fig. 1C). The
results of the present study suggested that miR-24-2 is upregulated
in RCC cell lines and tissues compared with healthy kidney cell
lines or tissues. Therefore, miR-24-2, compared with miR-24-1, may
function as an oncogene in RCC.
Validation of cell transfection
efficiency
To quantify the expression levels of miR-24-2
following transfection of an miR-24-2 mimic, inhibitor, NC or
inhibitor NC, RT-qPCR was performed. The expression levels of
miR-24-2 in the miR-24-2 mimic group were 1,075.55- and 840.69-fold
greater in the 786-O and ACHN groups, respectively, compared with
the NC group (P=0.009, 786-O; P=0.004, ACHN). miR-24-2 expression
levels in the inhibitor group were 0.25- and 0.22-fold greater in
the 786-O and ACHN groups, respectively, compared with the
inhibitor NC group (P=0.008, 786-O; P=0.005, ACHN; Fig.1D).
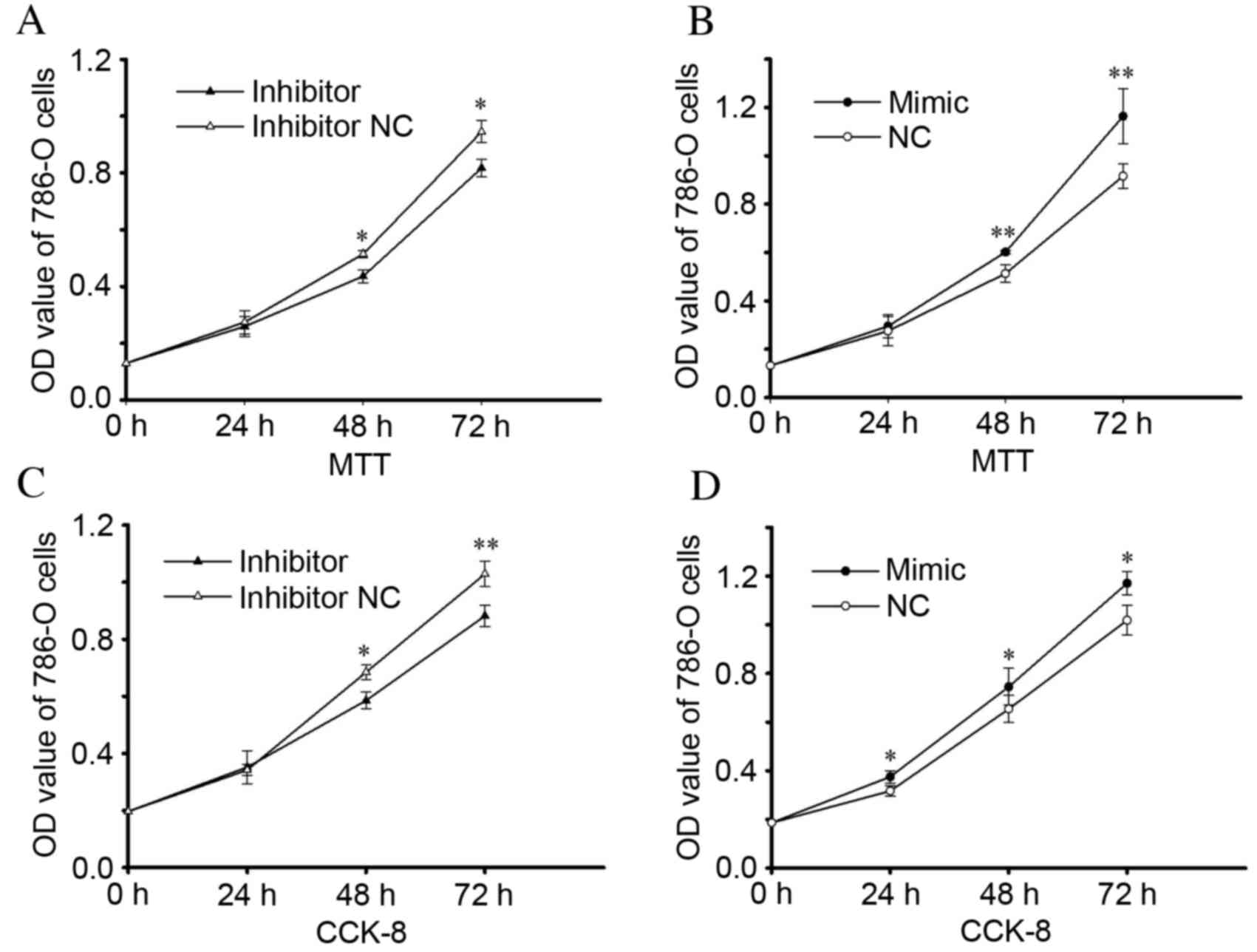
miR-24-2 promotes cell
proliferation
MTT and CCK-8 assays were performed in vitro
to detect cell proliferation. These results suggested that
upregulation of miR-24-2 may mediate RCC cell proliferation,
whereas downregulation of miR-24-2 may attenuate cell
proliferation. An MTT assay revealed that proliferation of 786-O
cells transfected with an miR-24-2 inhibitor was reduced by 5.62
(P=0.503), 7.45 (P=0.032) and 8.29% (P=0.012) at 24, 48 and 72 h,
respectively, compared with the inhibitor NC group (Fig. 2A). Cell proliferation was increased
in the mimic group by 7.27 (P=0.630), 14.42 (P=0.0091) and 27.07%
(P=0.009) at 24, 48 and 72 h following transfection, respectively,
compared with the NC group (Fig.
2B). A CCK-8 assay demonstrated that proliferation of 786-O
cells in the inhibitor group was reduced by 7.15 (P=0.016) and
11.51% (P=0.002), compared with cells transfected with an inhibitor
NC for 48 and 72 h, respectively (Fig.
2C). Cell proliferation was increased by 18.38 (P=0.019), 14.00
(P=0.046) and 14.96% (P=0.011) following transfection for 24, 48
and 72 h, respectively, compared with cells transfected with NC
(Fig. 2D).
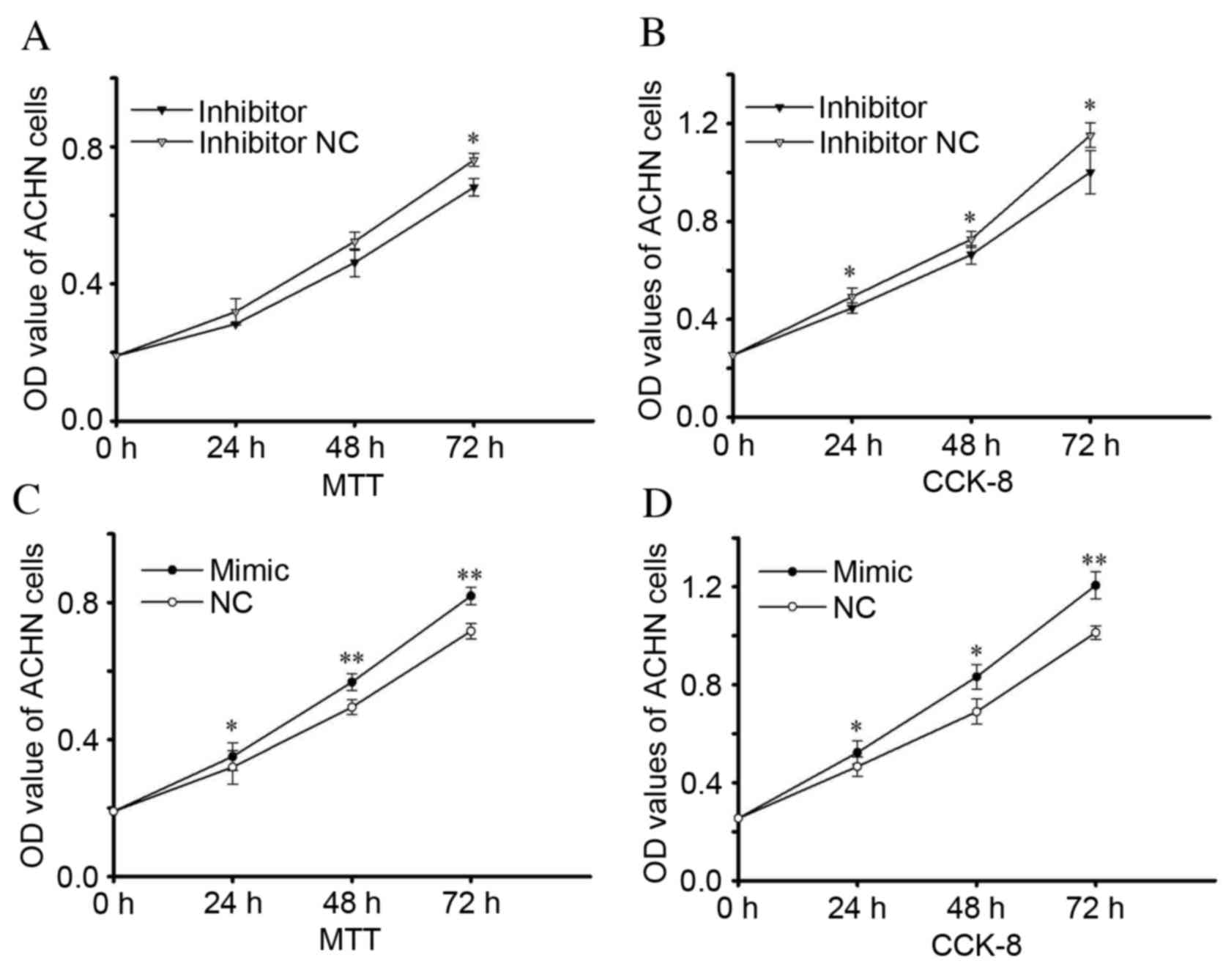
Similar results were observed in ACHN
cells
As assessed by MTT assay, proliferation of cells
transfected with an miR-24-2 inhibitor was reduced by 7.75%
(P=0.011) 72 h after transfection (Fig. 3A). After 24, 48 and 72-h
transfection, proliferation was reduced by 9.40 (P=0.035), 8.62
(P=0.034) and 13.06% (P=0.034) respectively, compared with the
inhibitor NC group, as determined by a CCK-8 assay (Fig. 3B). Proliferation of ACHN cells in
the miR-24-2 mimic group increased by 9.65 (P=0.040), 14.77
(P=0.003) and 10.17% (P=0.001; MTT assay; Fig. 3C), and 12.25 (P=0.015), 20.70
(P=0.020) and 19.03% (P=0.001; CCK-8 assay; Fig. 3D) 24, 48 and 72 h after
transfection, respectively, compared with cells transfected with
NC. Therefore, the upregulation of miR-24-2 expression levels may
mediate RCC cell proliferation, whereas downregulation of miR-24-2
may inhibit cell proliferation.
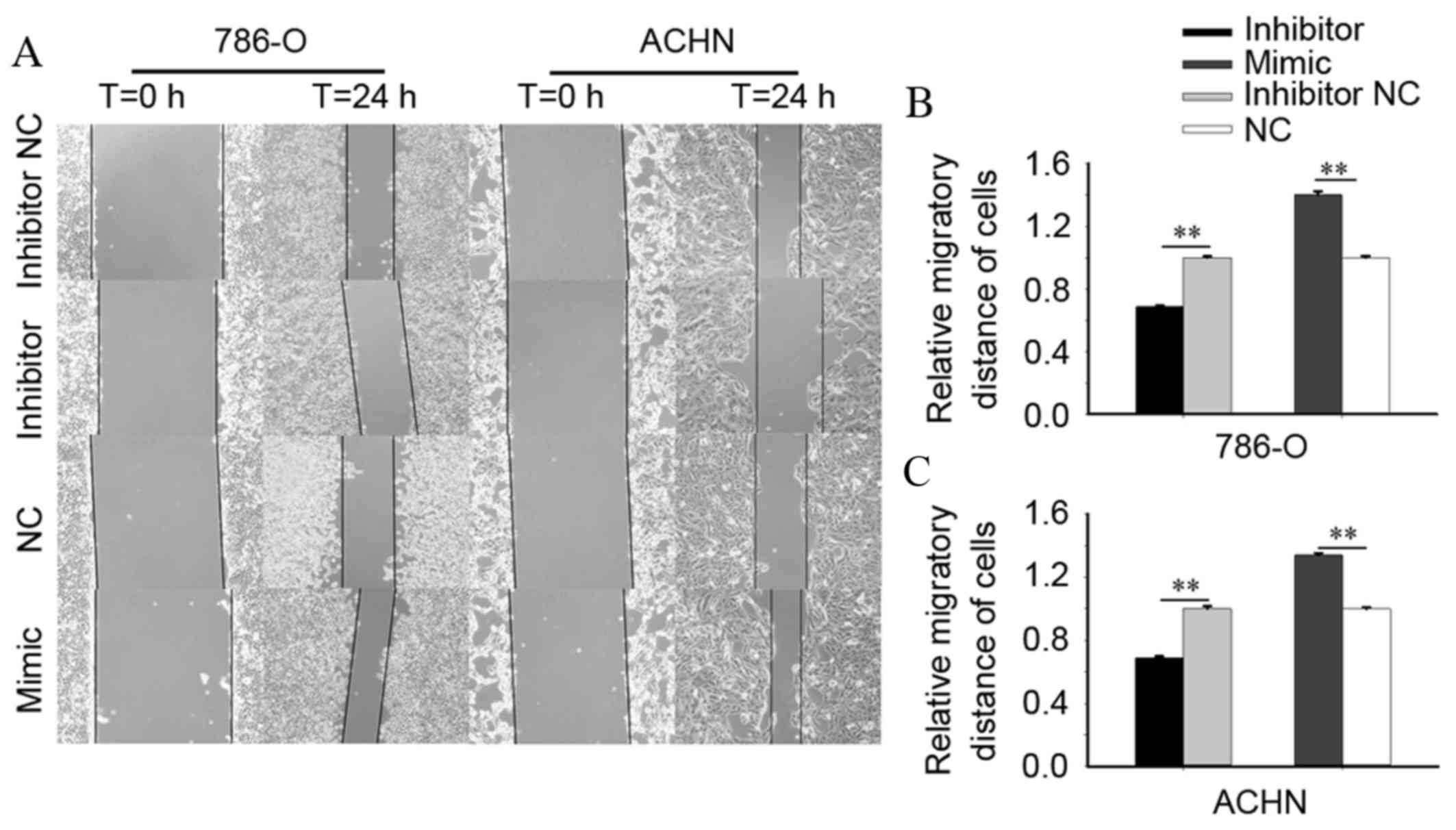
miR-24-2 promotes RCC cell
mobility
To investigate the effect of miR-24-2 on RCC cell
mobility, Transwell and wound scratch assays were performed. As
presented in Fig. 4A and B, the
wound scratch assay of 786-O cells demonstrated that 24 h
post-transfection, the migratory distance of cells transfected with
an miR-24-2 inhibitor was reduced significantly by 31.43%
(P=0.004), compared with cells transfected with an inhibitor NC.
The upregulation of miR-24-2 increased cell migration by 40.05%
(P=0.003) compared with the NC group. In ACHN cells, 24 h
post-transfection, downregulation of miR-24-2 reduced the migratory
distance by 31.23% (P=0.009), whereas upregulation of miR-24-2
using an miR-24-2 mimic increased migratory distances by 33.92%
(P=0.002), compared with cells transfected with an inhibitor NC and
NC, respectively (Fig. 4A and
C).
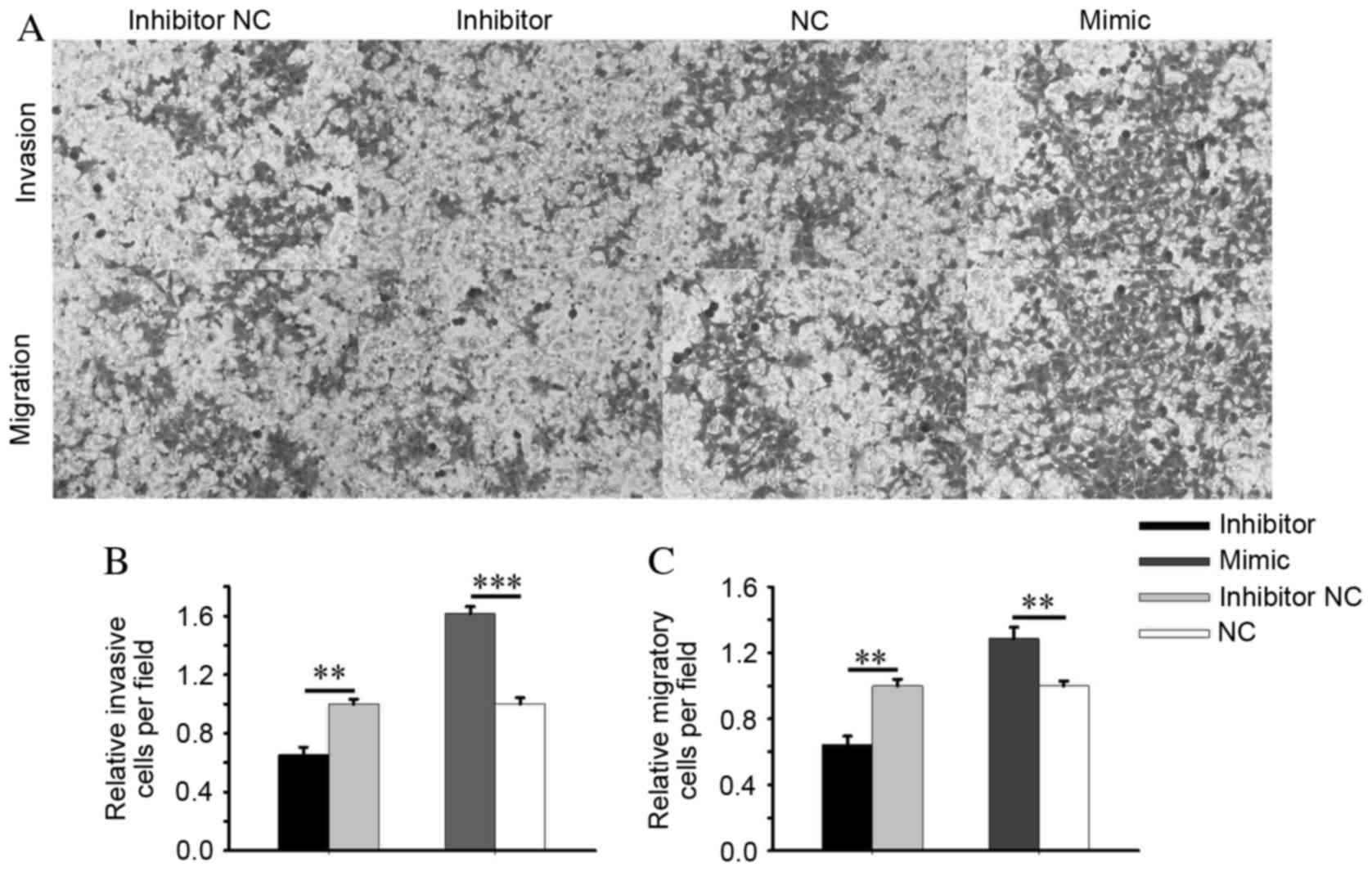
The Transwell assay revealed that the invasiveness
of 786-O cells transfected with an miR-24-2 inhibitor was reduced
significantly by 34.58% compared with cells transfected with an
inhibitor NC (P=0.001), and was markedly increased by 61.63% in the
miR-24-2 mimic group, compared with the NC group (P=0.0004;
Fig. 5A and B). The migration
ability of 786-O cells transfected with miR-24-2 inhibitors was
reduced by 35.53% (P=0.001) and increased by 28.57% (P=0.007) in
cells transfected with an miR-24-2 mimic, compared with the
inhibitor NC and NC groups, respectively (Fig. 5A and C). In ACHN cells, the
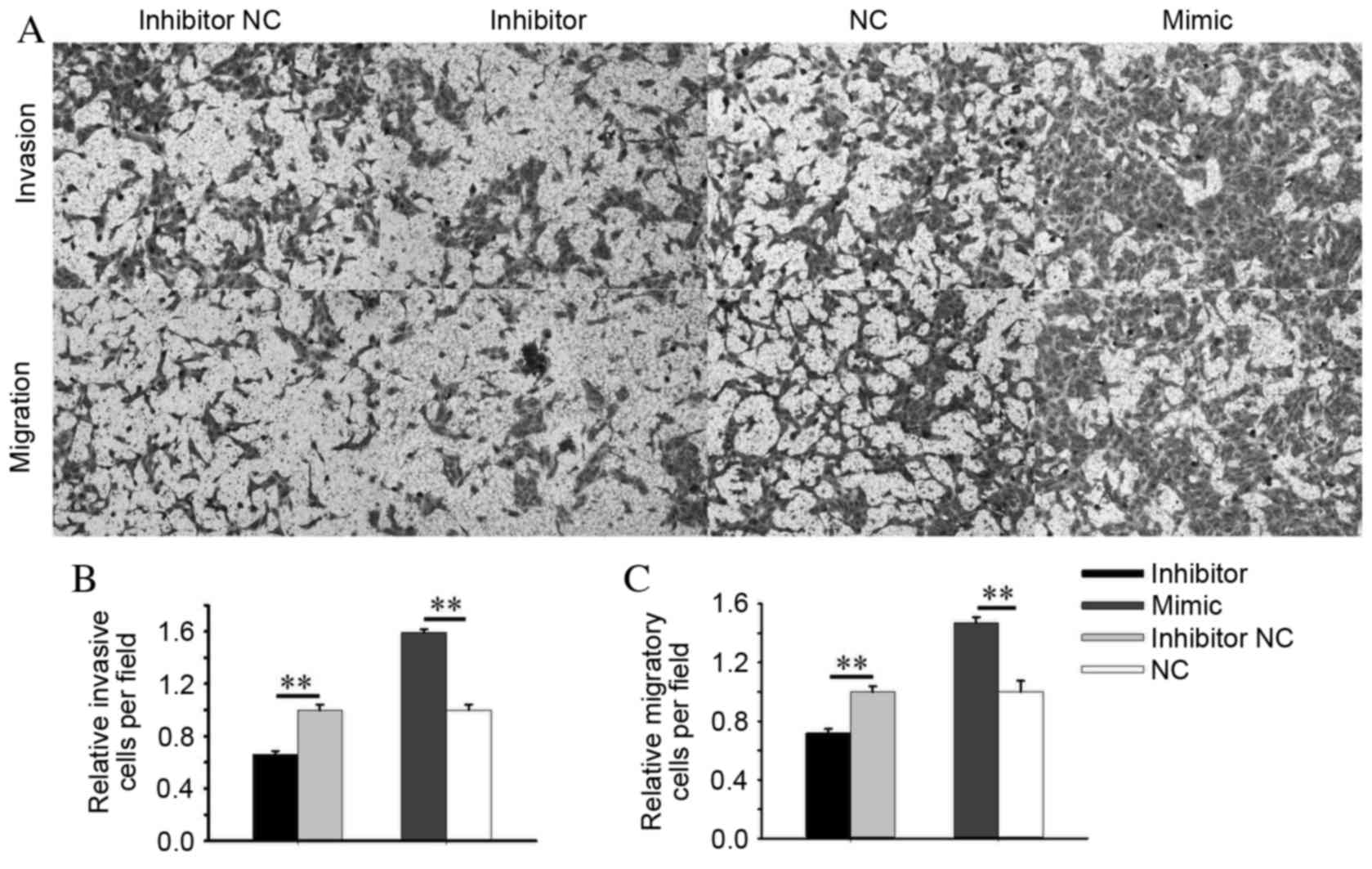
invasiveness of cells transfected with an miR-24-2 inhibitor was
reduced by 34.07% (P=0.009) and increased by 59.36% (P=0.003) in
cells transfected with an miR-24-2 mimic compared with cells
transfected with an inhibitor NC and NC, respectively (Fig. 6A and B). The migratory ability of
cells was reduced by 28.05% (P=0.003) in the inhibitor group and
increased by 47.26% (P=0.001) in the mimic group, compared with the
inhibitor NC and NC groups, respectively (Fig. 6A and C). Therefore, miR-24-2 may
mediate RCC cell mobility.
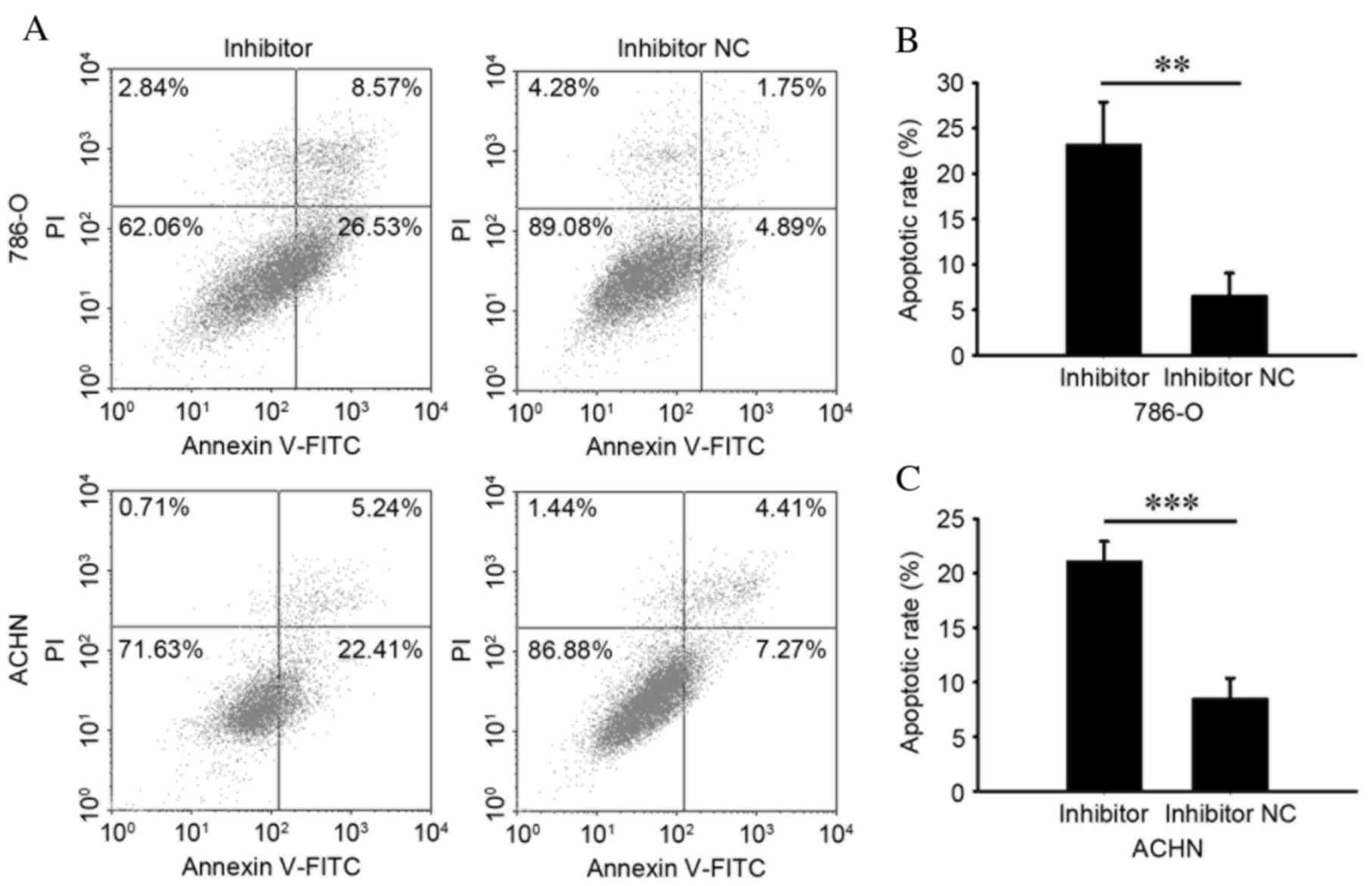
Knockdown of miR-24-2 induces
apoptosis
To assess cell apoptosis following transfection,
flow cytometry was performed. As presented in Fig. 7, the early apoptosis rate of cells
transfected with an miR-24-2 inhibitor or inhibitor NC was 23.29
vs. 6.62% in 786O cells (P=0.003; Fig.
7A and B) and 21.18 vs. 8.56% in ACHN cells (P<0.001;
Fig. 7A and C). However, no
significant differences were observed between levels of apoptosis
in cells transfected with an miR-24-2 mimic or NC (results not
shown). These results demonstrated that knockdown of miR-24-2 may
induce cell apoptosis.
Discussion
RCC, the most common type of kidney cancer, is a
highly vascularized tumor originating from the renal cortex. The
incidence of RCC is increasing at a rate of 2.6% annually (5,14).
Traditional chemotherapy and radiotherapy have limited success in
the treatment of advanced RCC (15), whereas surgical treatment has been
demonstrated to be more effective. However, 20–40% patients develop
metastatic disease following surgery (4). miRNAs may provide novel strategies
for the diagnosis, prognosis and development of therapeutic
applications for RCC.
miR-24-1 and miR-24-2 are two members of the miR-24
family, which possess different stem-loops. The present study
detected the expression levels of miR-24-1 and miR-24-2 by RT-qPCR.
The results indicated that miR-24-1 was almost absent in the
majority of RCC and paired healthy tissues, whereas miR-24-2 was
upregulated in RCC tissues and cell lines, compared with paired
healthy tissues and the HEK-293T cell line, respectively. A
synthesized miR-24-2 mimic, inhibitor, NC or inhibitor NC was
transfected into 786-O and ACHN RCC cells to investigate the role
of miR-24-2 in RCC tumorigenesis. The results demonstrated that
upregulation of miR-24-2 may promote RCC cell proliferation,
migration and invasion, whereas downregulation may inhibit these
processes. Furthermore, downregulation may induce RCC cell
apoptosis. Therefore, miR-24-2 may be involved in RCC tumorigenesis
and development. Additionally, the present study identified
miR-24-2 as an oncogene in RCC, which is in contrast to previous
studies (10,11).
miR-24-2 is a member of miR-23a/-27a/-24-2 cluster,
which exists intergenically in the vertebrate genome (6). Srivastava et al (16) demonstrated that miR-24-2 may
regulate H2A histone family member X gene expression and induce
MCF-7 breast cancer cell apoptosis by targeting B-cell lymphoma 2.
A subsequent study by the same group revealed that these effects
may be enhanced following treatment with 2 nM docetaxel (17). A previous study identified that
overexpression of miR-24-2 may suppress MCF-7 cell survival by
targeting protein kinase C α (18). Therefore, miR-24-2 may serve a role
as a tumor suppressor in breast cancer and is a potential target
for the treatment of breast cancer.
The miR-23a/-27a/-24-2 cluster has been demonstrated
to be associated with apoptosis of human embryonic kidney cells.
Chhabra et al (19)
demonstrated that the miR-23a/-27a/-24-2 cluster induces apoptosis
in HEK-293T cells via c-Jun N-terminal kinases. Apoptosis in
HEK-293T cells induced by the cluster was revealed to be associated
with the endoplasmic reticulum stress and unfolded protein response
signaling pathways (20). The
miR-23a/-27a/-24-2 cluster was additionally demonstrated to be
regulated by macrophage M1 and M2 cytokines, and may in turn
regulate M1 and M2 polarization via a negative feedback loop.
Furthermore, macrophages overexpressing the miR-23a/-27a/-24-2
cluster exhibited antitumor effects (21). Notably, in the present study,
knockdown of miR-24-2, a member of the cluster, was revealed to
induce RCC cell apoptosis. Overexpression of miR-27a, another
member of the cluster, has previously been demonstrated to mediate
RCC cell migration, invasion and proliferation (22,23).
Based on previous studies, two members of the miR-23a/-27a/-24-2
cluster, miR-27a and −24-2, may act as oncogenes in RCC.
In conclusion, the results of the present study
identified miR-24-2 as an oncogene associated with cell
proliferation, migration, invasion and apoptosis in RCC, which
indicated that miR-24-2 may be involved in RCC tumorigenesis and
development. Further studies are required to investigate the
signaling pathways of miR-24-2, and the potential of miR-24-2 as a
therapeutic target or biomarker for the early detection of RCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101922), the
Science and Technology Development Fund Project of Shenzhen (grant
nos. JCYJ20150403091443329 and JCYJ20170307111334308), the fund of
‘San-ming’ Project of Medicine in Shenzhen (2017) and the fund of
Guangdong Key Medical Subject (2015).
References
|
1
|
Chen D, Li Y, Su Z, Yu Z, Yu W, Li Y, Gui
Y, Yang S and Lai Y: Identification of miR-125a-5p as a tumor
suppressor of renal cell carcinoma, regulating cellular
proliferation, migration and apoptosis. Mol Med Rep. 11:1278–1283.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sellitti DF and Doi SQ: MicroRNAs in renal
cell carcinoma. Microrna. 4:26–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang HM, Yang FQ, Chen SJ, Che J and
Zheng JH: Upregulation of long non-coding RNA MALAT1 correlates
with tumor progression and poor prognosis in clear cell renal cell
carcinoma. Tumour Biol. 36:2947–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu M, Gu M, Zhang K, Zhou J, Wang Z and Da
J: miR-203 inhibition of renal cancer cell proliferation, migration
and invasion by targeting of FGF2. Diagn Pathol. 10:242015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan Y, Yang FQ, Zhang HM, Che J and Zheng
JH: Up-regulation of flotillin-2 is associated with renal cell
carcinoma progression. Tumour Biol. 35:10479–10486. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li X, Liu X, Xu W, Zhou P, Gao P, Jiang S,
Lobie PE and Zhu T: c-MYC-regulated miR-23a/24-2/27a cluster
promotes mammary carcinoma cell invasion and hepatic metastasis by
targeting Sprouty2. J Biol Chem. 288:18121–18133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Tang W, Zhang LR and Zhang CY: FMRP
regulates miR196a-mediated repression of HOXB8 via interaction with
the AGO2 MID domain. Mol Biosyst. 10:1757–1764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang BS, Liu Z, Xu WX and Sun SL:
Functional polymorphisms in microRNAs and susceptibility to liver
cancer: A meta-analysis and meta-regression. Genet Mol Res.
13:5426–5440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du WW, Fang L, Li M, Yang X, Liang Y, Peng
C, Qian W, O'Malley YQ, Askeland RW, Sugg SL, et al: MicroRNA
miR-24 enhances tumor invasion and metastasis by targeting PTPN9
and PTPRF to promote EGF signaling. J Cell Sci. 126:1440–1453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song L, Yang J, Duan P, Xu J, Luo X, Luo
F, Zhang Z, Hou T, Liu B and Zhou Q: MicroRNA-24 inhibits
osteosarcoma cell proliferation both in vitro and in vivo by
targeting LPAATβ. Arch Biochem Biophys. 535:128–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan Y, Hu L, Liu B, Yu B, Li J, Yan M, Yu
Y, Li C, Su L, Zhu Z, et al: Tumor suppressor miR-24 restrains
gastric cancer progression by downregulating RegIV. Mol Cancer.
13:1272014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Dai Y, Yang J, Chen T, Yin Y,
Tang M, Hu C and Zhang L: Microarray analysis of microRNA
expression in renal clear cell carcinoma. Eur J Surg Oncol.
35:1119–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Z, Ni L, Chen D, Su Z, Yu W, Zhang Q,
Wang Y, Li C, Gui Y and Lai Y: Expression and clinical significance
of RCDG1 in renal cell carcinoma: A novel renal cancerassociated
gene. Mol Med Rep. 10:1583–1589. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhai Q, Zhou L, Zhao C, Wan J, Yu Z, Guo
X, Qin J, Chen J and Lu R: Identification of miR-508-3p and
miR-509-3p that are associated with cell invasion and migration and
involved in the apoptosis of renal cell carcinoma. Biochem Biophys
Res Commun. 419:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Srivastava N, Manvati S, Srivastava A, Pal
R, Kalaiarasan P, Chattopadhyay S, Gochhait S, Dua R and Bamezai
RN: miR-24-2 controls H2AFX expression regardless of gene copy
number alteration and induces apoptosis by targeting antiapoptotic
gene BCL-2: A potential for therapeutic intervention. Breast Cancer
Res. 13:R392011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manvati S, Mangalhara KC, Kalaiarasan P,
Srivastava N and Bamezai RN: miR-24-2 regulates genes in survival
pathway and demonstrates potential in reducing cellular viability
in combination with docetaxel. Gene. 567:217–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin EC, Elliott S, Rhodes LV, Antoon
JW, Fewell C, Zhu Y, Driver JL, Jodari-Karimi M, Taylor CW,
Flemington EK, et al: Preferential star strand biogenesis of
pre-miR-24-2 targets PKC-alpha and suppresses cell survival in
MCF-7 breast cancer cells. Mol Carcinog. 53:38–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chhabra R, Dubey R and Saini N: Gene
expression profiling indicate role of ER stress in miR-23a~27a~24-2
cluster induced apoptosis in HEK293T cells. RNA Biol. 8:648–664.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chhabra R, Adlakha YK, Hariharan M, Scaria
V and Saini N: Upregulation of miR-23a-27a-24-2 cluster induces
caspase-dependent and -independent apoptosis in human embryonic
kidney cells. PLoS One. 4:e58482009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma S, Liu M, Xu Z, Li Y, Guo H, Ge Y, Liu
Y, Zheng D and Shi J: A double feedback loop mediated by
microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage
polarization and thus regulates cancer progression. Oncotarget.
22:13502–13519. 2016. View Article : Google Scholar
|
|
22
|
Peng H, Wang X, Zhang P, Sun T, Ren X and
Xia Z: miR-27a promotes cell proliferation and metastasis in renal
cell carcinoma. Int J Clin Exp Pathol. 8:2259–2266. 2015.PubMed/NCBI
|
|
23
|
Nakata W, Uemura M, Sato M, Fujita K,
Jingushi K, Ueda Y, Kitae K, Tsujikawa K and Nonomura N: Expression
of miR-27a-3p is an independent predictive factor for recurrence in
clear cell renal cell carcinoma. Oncotarget. 6:21645–21654. 2015.
View Article : Google Scholar : PubMed/NCBI
|