Introduction
The glomerular podocyte functions as a charge
barrier to prevent proteinuria. Proteinuira is the most critical
clinical signature of podocyte injury predominantly by anatomical
and molecular abnormalities of podocyte foot processes (FP) and the
interposed slit diaphragm (SD) (1). The consistent proteinuria and
podocyte apoptosis damages glomerular structure and results in
progressive glomerular consecutive tubular destruction (2,3),
resulting in chronic kidney disease (CKD), which is a worldwide
health problem, of which the prevalence is increasing (4). Due to the fact that CKD is a
worldwide chronic disease and annually millions die of
cardiovascular or renal complications directly due to CKD,
exploring factors intervening in the pathogenesis of podocyte
injury is necessary in the development of novel strategies for CKD
clinical therapy.
Previous genetic studies have highlighted the
molecular makeup of SD and podocyte FP (5–6). SD
is comprised by molecular proteins functioning as ‘protein
gatekeepers’ in the junctional domain of the podocyte. The first
observed SD protein, Nephrin, was identified by Ruotsalainen et
al (7) in 1999, and more
recently additional and more complex SD proteins have been
identified, including podocin, CD2 associated protein (CD2AP),
nephrin-like protein 1, zona occludens 1 and dendrin (8–11).
Certain SD proteins have been identified to be critical in renal
injury; genetic deletion of CD2AP, a scaffolding protein at the SD,
leads to progressive renal failure in mice (12,13),
and ectopic expression of CD2AP in podocyte prevents the
development of proteinuria (14,15).
Dendrin, another newly identified SD complex, interacts with CD2AP,
and loss of CD2AP could drive dendrin protein from the SD to the
nucleus (16). Nuclear relocation
of dendrin has been identified in various podocyte injury models
in vitro and in human renal diseases (17,18).
In addition, nuclear dendrin could further promote apoptosis of
podocytes (19). Despite of these
results, the precise molecular mechanism by which dendrin regulates
function of podocyte remains to be fully understood.
In yeast two hybrid screens with the human isoform
of dendrin, Kremerskothen et al (20) isolated a cDNA coding for a novel
protein, WWC1 (also termed KIBRA) (20). WWC1, a kidney- and brain-associated
protein, was observed to be predominantly expressed in the brain
and kidney, and in the kidney, WWC1 was identified to be expressed
in glomerular podocytes, tubular structures and collecting ducts
(21). WWC1, interaction directly
with protein associated to tight junctions (PATJ), impacted the
proteins associated with Lin seven-PATJ-Crumbs 3 cell polarity
complex and modulated the migration of podocytes (21). However, the bio-function of WWC1 in
the podocytes remains to be fully elucidated, and the functional
association between WWC1 and dendrin in the podocytes was
investigated in the current study.
In the present study, it was indicated that
expression of WWC1 significantly was decreased in injured
podocytes. Further investigation indicated that loss of WWC1 in
vitro directly induced podocyte apoptosis and promoted SD
protein dendrin relocation from SD into the nuclei. The results
present novel insight into the molecular events in podocyte
injury.
Materials and methods
Cell cultures
The conditionally immortalized mouse podocyte cell
line (MPC-5) was donated by Dr Jochen Reiser (Rush University
Medical Center, Chicago, IL, USA), and cultured as described
previously (22–23). Cells were treated with adriamycin
(ADR; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in
RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
to a final concentration of 0.25 µg/ml or of lipopolysaccharides
(LPS; Sigma Aldrich; Merck KGaA, Darmstadt, Germany) to 1 µg/ml for
24 h. Equal amounts of phosphate-buffered saline (PBS) were treated
as the negative control.
siRNA transfection
Oligonucleotide siRNA was synthesized by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). WWC1 or scramble siRNA (Scr)
was transfected into podocytes using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. WWC1 siRNA sequences used were as follows:
5′-GCACAGAGACCAGGUACUUdTdT-3′, 5′-GCACAAGAGUGAGUUGCAAdTdT-3′ and
5′-GGUGGACAGAGAGACCAAUdTdT-3′.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA samples were prepared using TRIzol RNA isolation
system (Invitrogen; Thermo Fisher Scientific, Inc.) and were
reverse transcribed into cDNA (50 ng) using superscript reverse
transcriptase. The cDNA was used for RT-qPCR analysis using a
Platinum SYBR-Green SuperMix-UDG kit (Takara Bio, Inc., Otsu,
Japan) as described previously (21). The primer sequences used are listed
as follows: WWC1, forward 5′-TGCTGAGGGAAACCAAAGCC-3′ and reverse
5′-CTGGACCATAGGTCGGAGTG-3′; Bcl-2-associated X (Bax), forward,
5′-CTGGACCATAGGTCGGAGTG-3′ and reverse 5′-AATTCGCCGGAGACACTCG-3′;
Bcl-2, forward 5′-TGTGGCCTGATGTTGGATAAC-3′ and reverse
5′-GGTGACGAATGTGCAAATCTACT-3′; GAPDH, forward
5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse
5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Immunofluorescence of cultured
podocytes
Subsequent to being subjected to various treatments,
podocytes were fixed with 4% paraformaldehyde at −20°C for 20 min,
then were blocked using blocking solution of 5% fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc.) in PBS. The primary
antibodies were incubated overnight at 4°C, followed by 1 h of
incubation with goat anti-rabbit Alexa Fluor 488 (4412S; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) or Texas
red-donkey anti-goat IgG (ab6883; 1:250; Abcam, Cambridge, MA, USA)
at room temperature. DAPI (1:1,000; Sigma-Aldrich; Merck KGaA) was
stained for counterstaining. The primary antibodies used in the
present study were as follows: Rabbit anti-WWC1 (sc-133374; 1:100;
Santa Cruz Biotechnology, Inc.), rabbit anti-dendrin (ABT59; 1:400;
EMD Millipore, Billerica, MA, USA) and goat anti-dendrin
(sc-167616; 1:100; Santa Cruz Biotechnology, Inc.). All images were
analyzed by two investigators blinded to the identity of the
samples.
Western blotting
Protein lysates were resolved by SDS
(7.5–10%)-polyacrylamide gel electrophoresis, transferred onto a
polyvinylidene difluoride membrane, and hybridized with the
corresponding antibodies. The membranes were washed 3 times for 5
min with PBS following antibody incubation. The antibodies used in
the present study were as follows: Rabbit anti-WWC1 (sc-133374;
1:500; Santa Cruz Biotechnology, Inc.), rabbit anti-dendrin (ABT59;
1:1,000; EMD Millipore), rabbit anti-Bax (sc-493; 1:500; Santa Cruz
Biotechnology, Inc.), rabbit anti-Bcl-2 (3498; 1:1,000; Cell
Signaling Technology, Inc.), rabbit anti-GAPDH (G9545; 1:5,000;
Sigma-Aldrich; Merck KGaA) and rabbit anti-histone (4499; 1:2,000;
Cell Signaling Technology, Inc.). The membrane was then incubated
with anti-rabbit or anti-mouse IgG (211002217 and 515005071;
1:10,000; Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) at room temperature for 1 h. Enhanced chemiluminescence
(Pierce Biotechnology, Inc., Rockford, IL, USA) reagents were used
for detection.
Annexin V and propidium iodide (PI)
staining
Apoptotic cells were determined using an Annexin
V/PI apoptosis detection kit (Nanjing KeyGEN Biotech., Co., Ltd.,
Nanjing, China) as described previously (20).
Statistical analysis
The statistical analysis was performed using SPSS
15.0 (SPSS, Inc., Chicago, IL, USA). All experiments in
vitro were performed at least in triplicate. Continuous values
were expressed as mean ± standard error using one-way analysis of
variance and Bonferroni non-parametric test for statistical
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of WWC1 was decreased in
injured podocytes in vitro
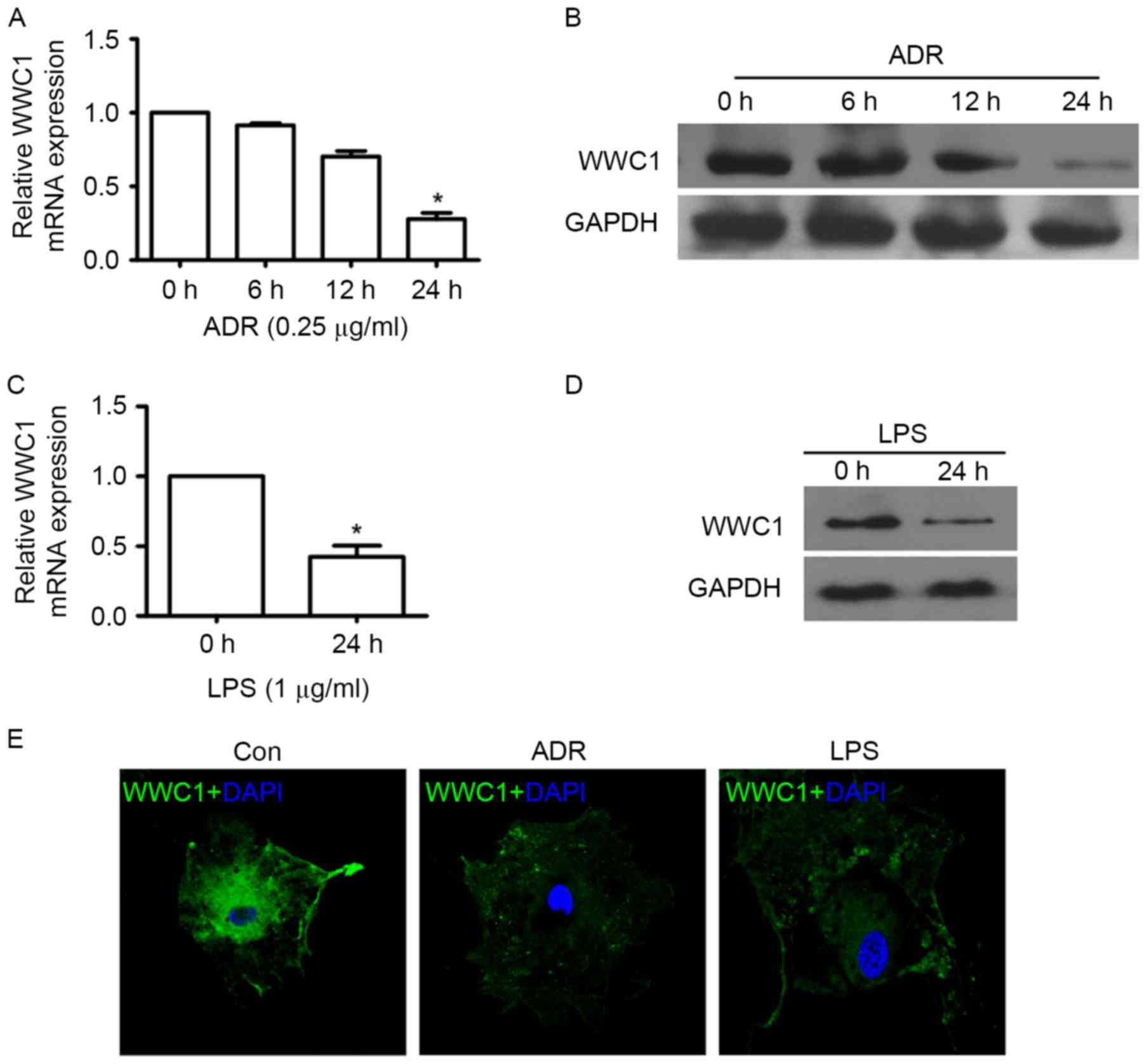
In order to evaluate whether loss of WWC1 could be
induced by ADR or LPS stimulation particularly in podocytes, WWC1
expression was assessed under different exposures by RT-qPCR,
immunoblotting and immunofluorescence staining. The time-response
experiment indicated that ADR induced loss of WWC1 expression in a
time-dependent manner. A significant decrease of WWC1 gene and
protein expression was observed following 24 h exposure to 0.25
µg/ml of ADR (Fig. 1A and B).
Following stimulation by LPS (1 µg/ml) for 24 h, WWC1 expression
significant decreased in cultured podocyte (Fig. 1C and D). Similar results also
indicated that ADR and LPS stimulation induced WWC1 expression
reduction by immunofluorescence staining (Fig. 1E) (P<0.05).
Loss of WWC1 directly induced podocyte
apoptosis in vitro
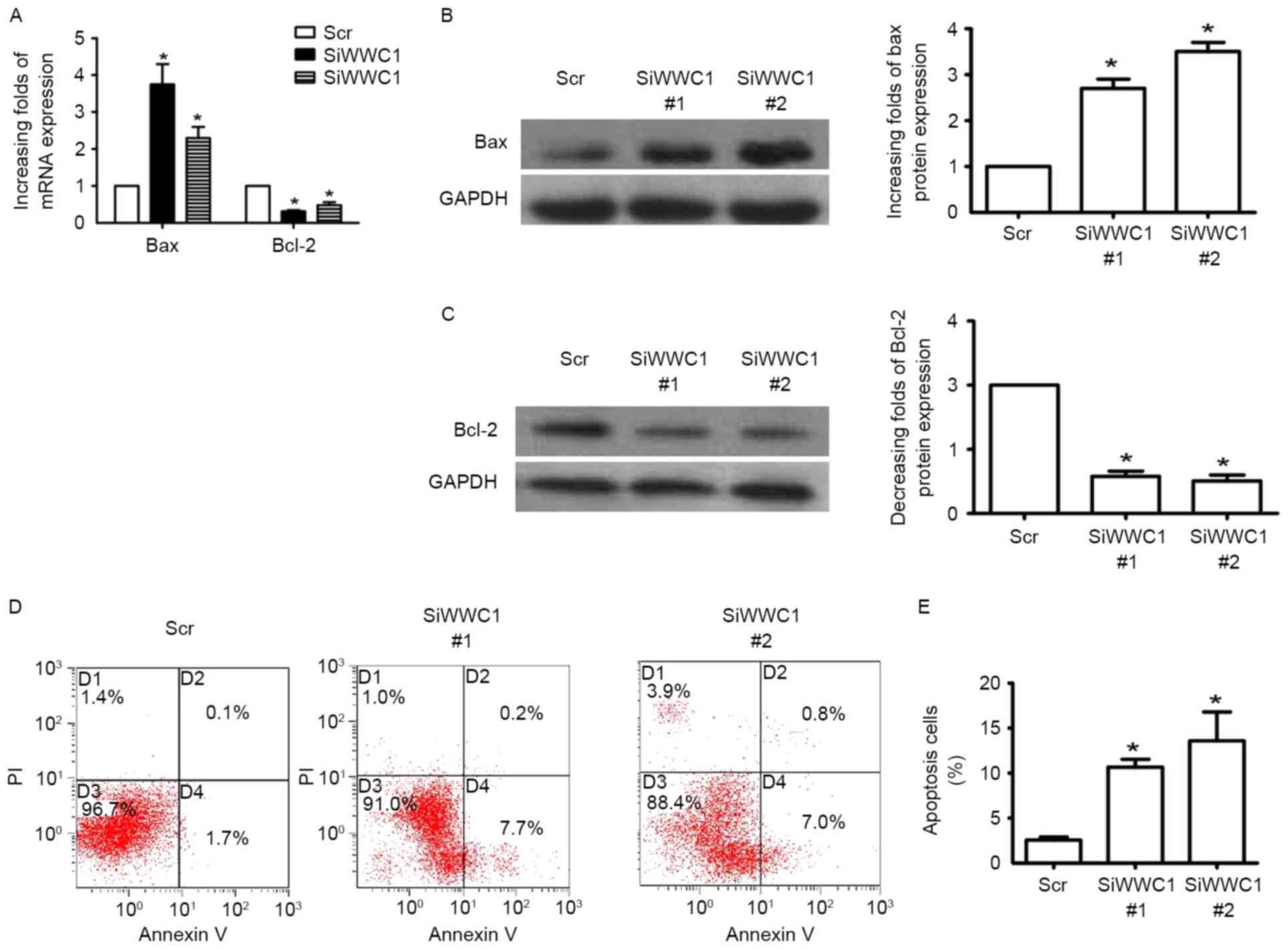
In order to investigate the direct affection of WWC1
in podocyte, WWC1 gene expression was altered in podocytes in
vitro by WWC1 siRNA. Three pairs of WWC1 siRNA were transfected
into podocytes and RT-qPCR was performed to detect WWC1 gene
expression 48 h subsequent to transfection. Gene and protein
analysis of the apoptotic factor Bax exhibited increased expression
in WWC1-silenced podocytes (Fig. 2A
and B), while the anti-apoptotic factor Bcl-2 was reduced
(Fig. 2A and C). Annexin V and PI
staining assay indicated that loss of WWC1 directly induced
apoptosis process in podocytes (2.56±0.34 vs. 10.67±0.88 and
13.60±3.19, respectively; n=3) (Fig.
2D and E) (P<0.05).
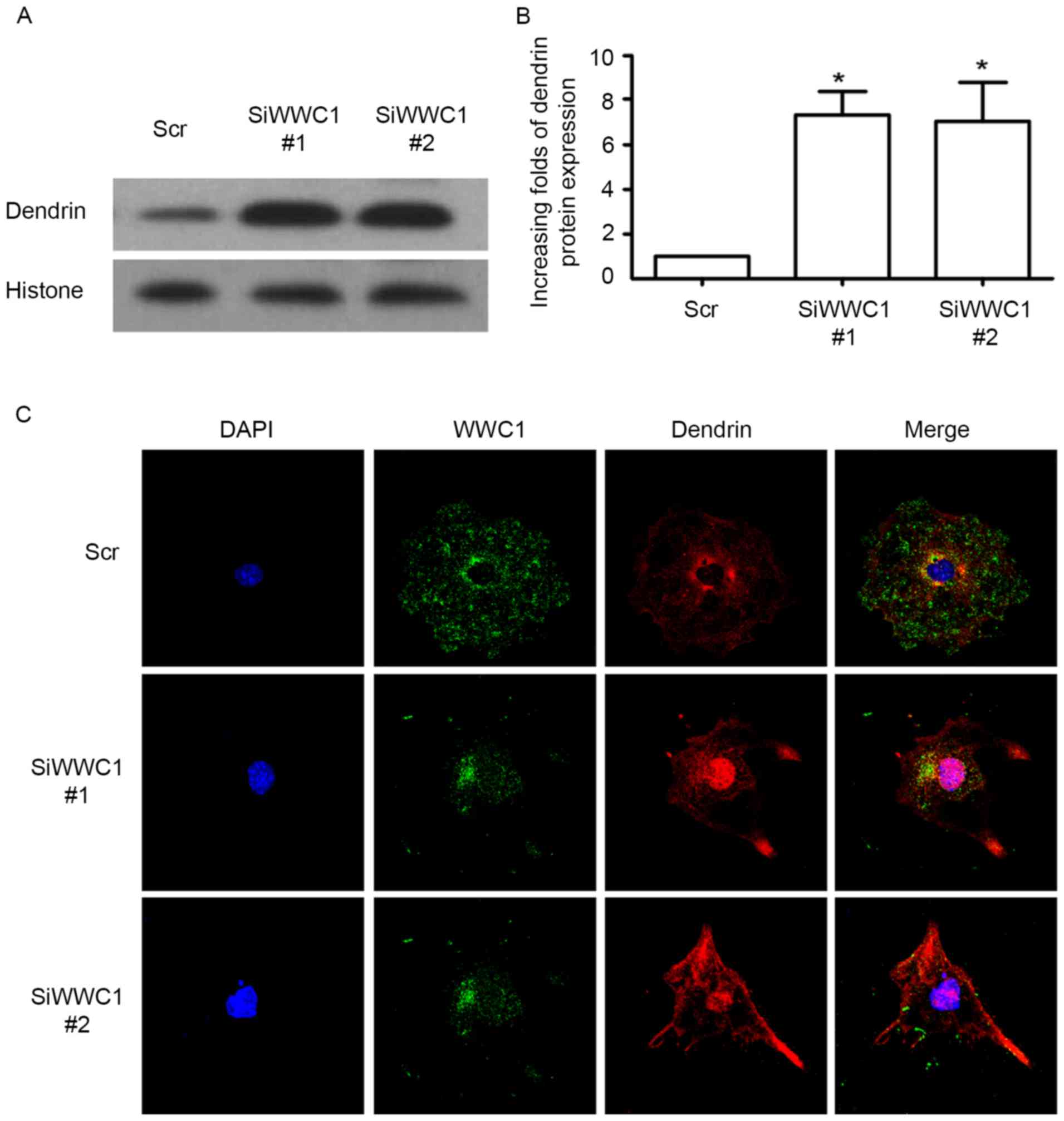
Loss of WWC1 induced dendrin nucleus
relocation in podocytes
As we know, nucleus relocation of SD protein dendrin
is one of the newly confirmed apoptotic mechanisms in podocytes
(19). Considering the
interactions between WWC1 and dendrin in normal podocytes,
subcellular localization of dendrin in podocytes was assessed with
silenced WWC1 gene. Notably, following silencing of the WWC1 gene
in podocytes, dendrin nuclear accumulation was significantly
increased (Fig. 3A and B). By
immunofluorescence staining, it was identified that WWC1 and
dendrin were co-located in the cytoplasm, with low levels in the
nuclei of the physiological podocyte, whereas an accumulation of
dendrin was detected in the podocyte nuclei in WWC1 silenced
podocytes (Fig. 3C). These results
indicate that loss of WWC1 could directly drive dendrin relocating
into the nuclei of podocyte.
Discussion
The terminally differentiated podocyte functions as
a critical barrier to prevent proteinuria, and proteinuria is the
clinical signature for podocyte injury, with or without loss of
renal functions. Emerging experimental and clinical studies have
highlighted that loss of podocyte directly causes proteinuria and
glomerulosclerosis, owing to podocyte apoptosis or detachment
(24–27). The present study demonstrated, to
the best of our knowledge for the first time, that kidney and brain
associated protein WWC1, is a critical molecular in podocyte
injury. Reduced WWC1 expression was identified in injured
podocytes, and loss of WWC1 directly induced podocyte apoptosis. In
addition further evidence was obtained that WWC1 protected
podocytes from apoptosis by preventing SD protein dendrin from
relocating into nuclei.
The expression of WWC1 (KIBRA), the mammalian
ortholog of Kibra, has been observed to be enriched in kidney and
brain (18). In Drosophila, Kibra
predominantly acts in the Merlin branch upstream of the core kinase
cascade to regulate Hippo signaling, a well-recognized pathway by
restricting proliferation and promoting apoptosis (28–30).
In mammals, WWC1 additionally exhibits MST-independent functional
regulation of Hippo signaling pathway in breast cancer (31). In a breast cancer cell line, loss
of WWC1 expression displays epithelial-to-mesenchymal transition
features as well as promoted migration. Directional migration
regulation is additionally observed in podocytes (21). The current study focused on the
observation of the response of WWC1 to podocyte apoptosis injury. A
reduction of WWC1 expression was observed in ADR- and LPS-mediated
podocyte injury. Due to the fact that ADR and LPS induce
proteinuria in mice, these results indicated a probable endogenic
protective role of WWC1 in podocytes.
Apoptosis has been reported to cause podocyte loss
and glomerulosclerosis, and according to the biogenetic pleiotropy
of WWC1 (31–34), it was hypothesized that reduction
of WWC1 may be associated with podocyte apoptosis as a response to
injury. Subsequently, it was investigated whether loss of WWC1
directly induces podocyte apoptosis. Increased apoptotic cells were
observed following knockdown of endogenic WWC1 gene in podocyte by
Annexin V and PI staining. In addition, the apoptosis-associated
gene Bax and Bcl-2 were observed to be activated and inhibited
respectively with WWC1 gene silencing.
These results suggested that endogenic WWC1 could
serve a critical role in protecting podocytes from apoptotic
injury. Total podocyte number is a balance between proliferation
and loss, owing to several critical processes including DNA
synthesis, DNA damage, hypertrophy, detachment and apoptosis. The
proteins or molecules those are stably present in podocyte are
essential for normal function, such as survival. For example,
several SD proteins, such as CD2AP and nephrin, had been
demonstrated to serve a critical role in proteinuria protection
(35,36). The results provide new evidence
supporting the role of WWC1 in podocyte protection.
The mechanisms of WWC1 in the protection of podocyte
from apoptosis require further investigation. WWC1 was originally
isolated by dendrin in a yeast two hybrid screen (20), and accumulating evidence has
indicated that dendrin is additionally one of the SD complex
proteins present in podocytes (37,38).
Nuclear relocation of dendrin has been identified both in human
glomerular diseases and various podocyte injury models, and
notably, it was observed that nuclear dendrin induced podocyte
apoptosis (17,18). It was investigated whether WWC1
could contribute to normal location of dendrin in the SD domain; it
was identified that loss of WWC1 in podocytes directly promoted
movement of dendrin proteins from SD into the nuclei, indicating
that WWC1 is a crucial molecular component in regulating the
stabilization of dendrin in podocytes.
The present study systematically evaluated the
functions of WWC1 in the podocyte. The results demonstrated that
expression of WWC1 was significantly decreased in injured
podocytes. In addition, loss of WWC1 in podocytes directly induced
apoptosis of podocytes. Nuclear relocation of the SD complex
protein dendrin was promoted following WWC1 gene silencing in
podocytes. These results indicate that endogenic WWC1 could serve a
critical role in podocyte apoptosis. Taken together, the results
demonstrate that WWC1 may serve as a promising molecular target to
tackle proteinuric kidney diseases.
Acknowledgements
The present study was supported by the National
Nature and Science Grants (grant nos. 81270784, 181470930 and
81500560); Nature and Science Grant of Guangdong Province (grant
nos. 2014A030310106 and 2014A030310107) and National Clinical Key
Specialty Construction Projects.
References
|
1
|
Somlo S and Mundel P: Getting a foothold
in nephrotic syndrome. Nat Genet. 24:333–335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartz MM: The role of podocyte injury
in the pathogenesis of focal segmental glomerulosclerosis. Ren
Fail. 22:663–684. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kriz W and Lemley KV: The role of the
podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens.
8:489–497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schieppati A and Remuzzi G: Chronic renal
diseases as a public health problem: Epidemiology, social, and
economic implications. Kidney Int Suppl (Suppl). S7–S10. 2005.
View Article : Google Scholar
|
|
5
|
Kawachi H, Han GD, Miyauchi N, Hashimoto
T, Suzuki K and Shimizu F: Therapeutic targets in the podocyte:
Findings in anti-slit diaphragm antibody-induced nephropathy. J
Nephrol. 22:450–456. 2009.PubMed/NCBI
|
|
6
|
Kriz W and Lemley KV: Potential relevance
of shear stress for slit diaphragm and podocyte function. Kidney
Int. 91:1283–1286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruotsalainen V, Ljungberg P, Wartiovaara
J, Lenkkeri U, Kestilä M, Jalanko H, Holmberg C and Tryggvason K:
Nephrin is specifically located at the slit diaphragm of glomerular
podocytes. Proc Natl Acad Sci USA. 96:pp. 7962–7967. 1999;
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roselli S, Gribouval O, Boute N, Sich M,
Benessy F, Attié T, Gubler MC and Antignac C: Podocin localizes in
the kidney to the slit diaphragm area. Am J Pathol. 160:131–139.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schwarz K, Simons M, Reiser J, Saleem MA,
Faul C, Kriz W, Shaw AS, Holzman LB and Mundel P: Podocin, a
raft-associated component of the glomerular slit diaphragm,
interacts with CD2AP and nephrin. J Clin Invest. 108:1621–1629.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shih NY, Li J, Cotran R, Mundel P, Miner
JH and Shaw AS: CD2AP localizes to the slit diaphragm and binds to
nephrin via a novel C-terminal domain. Am J Pathol. 159:2303–2308.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahola H, Heikkilä E, Aström E, Inagaki M,
Izawa I, Pavenstädt H, Kerjaschki D and Holthöfer H: A novel
protein, densin, expressed by glomerular podocytes. J Am Soc
Nephrol. 14:1731–1737. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JM, Wu H, Green G, Winkler CA, Kopp
JB, Miner JH, Unanue ER and Shaw AS: CD2-associated protein
haploinsufficiency is linked to glomerular disease susceptibility.
Science. 300:1298–1300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shih NY, Li J, Karpitskii V, Nguyen A,
Dustin ML, Kanagawa O, Miner JH and Shaw AS: Congenital nephrotic
syndrome in mice lacking CD2-associated protein. Science.
286:312–315. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huber TB, Kwoh C, Wu H, Asanuma K, Gödel
M, Hartleben B, Blumer KJ, Miner JH, Mundel P and Shaw AS: Bigenic
mouse models of focal segmental glomerulosclerosis involving
pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin
Invest. 116:1337–1345. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grunkemeyer JA, Kwoh C, Huber TB and Shaw
AS: CD2-associated protein (CD2AP) expression in podocytes rescues
lethality of CD2AP deficiency. J Biol Chem. 280:29677–29681. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yaddanapudi S, Altintas MM, Kistler AD,
Fernandez I, Möller CC, Wei C, Peev V, Flesche JB, Forst AL, Li J,
et al: CD2AP in mouse and human podocytes controls a proteolytic
program that regulates cytoskeletal structure and cellular
survival. J Clin Invest. 121:3965–3980. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asanuma K, Akiba-Takagi M, Kodama F, Asao
R, Nagai Y, Lydia A, Fukuda H, Tanaka E, Shibata T, Takahara H, et
al: Dendrin location in podocytes is associated with disease
progression in animal and human glomerulopathy. Am J Nephrol.
33:537–549. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kodama F, Asanuma K, Takagi M, Hidaka T,
Asanuma E, Fukuda H, Seki T, Takeda Y, Hosoe-Nagai Y, Asao R, et
al: Translocation of dendrin to the podocyte nucleus in acute
glomerular injury in patients with IgA nephropathy. Nephrol Dial
Transplant. 28:1762–1772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asanuma K, Campbell KN, Kim K, Faul C and
Mundel P: Nuclear relocation of the nephrin and CD2AP-binding
protein dendrin promotes apoptosis of podocytes. Proc Natl Acad Sci
USA. 104:pp. 10134–10139. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kremerskothen J, Plaas C, Büther K, Finger
I, Veltel S, Matanis T, Liedtke T and Barnekow A: Characterization
of KIBRA, a novel WW domain-containing protein. Biochem Biophys Res
Commun. 300:862–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duning K, Schurek EM, Schluter M, Bayer M,
Reinhardt HC, Schwab A, Schaefer L, Benzing T, Schermer B, Saleem
MA, et al: KIBRA modulates directional migration of podocytes. J Am
Soc Nephrol. 19:1891–1903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li R, Zhang L, Shi W, Zhang B, Liang X,
Liu S and Wang W: NFAT2 mediates high glucose-induced glomerular
podocyte apoptosis through increased Bax expression. Exp Cell Res.
319:992–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma J, Zhang B, Liu S, Xie S, Yang Y, Ma J,
Deng Y, Wang W, Xu L, Li R, et al: 1,25-dihydroxyvitamin D(3)
inhibits podocyte uPAR expression and reduces proteinuria. PLoS
One. 8:e649122013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verzola D, Gandolfo MT, Ferrario F,
Rastaldi MP, Villaggio B, Gianiorio F, Giannoni M, Rimoldi L,
Lauria F, Miji M, et al: Apoptosis in the kidneys of patients with
type II diabetic nephropathy. Kidney Int. 72:1262–1272. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eid AA, Gorin Y, Fagg BM, Maalouf R,
Barnes JL and Benya RV: Mechanisms of podocyte injury in diabetes:
Role of cytochrome P450 and NADPH oxidases. Diabetes. 58:1201–1211.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schiffer M, Bitzer M, Roberts IS, Kopp JB,
ten Dijke P, Mundel P and Böttinger EP: Apoptosis in podocytes
induced by TGF-beta and Smad7. J Clin Invest. 108:807–816. 2011.
View Article : Google Scholar
|
|
27
|
Kim YH, Goyal M, Kurnit D, Wharram B,
Wiggins J, Holzman L, Kershaw D and Wiggins R: Podocyte depletion
and glomerulosclerosis have a direct relationship in the
PAN-treated rat. Kidney Int. 60:957–968. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baumgartner R, Poernbacher I, Buser N,
Hafen E and Stocker H: The WW domain protein Kibra acts upstream of
Hippo in Drosophila. Dev Cell. 18:309–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Genevet A, Wehr MC, Brain R, Thompson BJ
and Tapon N: Kibra is a regulator of the Salvador/Warts/Hippo
signaling network. Dev Cell. 18:300–308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao B, Tumaneng K and Guan KL: The Hippo
pathway in organ size control, tissue regeneration and stem cell
self-renewal. Nat Cell Biol. 13:877–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moleirinho S, Chang N, Sims AH,
Tilston-Lünel AM, Angus L, Steele A, Boswell V, Barnett SC, Ormandy
C, Faratian D, et al: KIBRA exhibits MST-independent functional
regulation of the Hippo signaling pathway in mammals. Oncogene.
32:1821–1830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Basu-Roy U, Bayin NS, Rattanakorn K, Han
E, Placantonakis DG, Mansukhani A and Basilico C: Sox2 antagonizes
the Hippo pathway to maintain stemness in cancer cells. Nat Commun.
6:64112015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Papenberg G, Salami A, Persson J,
Lindenberger U and Bäckman L: Genetics and functional imaging:
Effects of APOE BDNF, COMT, and KIBRA in aging. Neuropsychol Rev.
25:47–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang S, Ji M, Zhang L, Chen Y, Wennmann
DO, Kremerskothen J and Dong J: Phosphorylation of KIBRA by the
extracellular signal-regulated kinase (ERK)-ribosomal S6 kinase
(RSK) cascade modulates cell proliferation and migration. Cell
Signal. 26:343–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schiffer M, Mundel P, Shaw AS and
Böttinger EP: A novel role for the adaptor molecule CD2-associated
protein in transforming growth factor-beta-induced apoptosis. J
Biol Chem. 279:37004–37012. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huber TB, Hartleben B, Kim J, Schmidts M,
Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, et
al: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase
and stimulate AKT-dependent signaling. Mol Cell Biol. 23:4917–4928.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kremerskothen J, Kindler S, Finger I,
Veltel S and Barnekow A: Postsynaptic recruitment of Dendrin
depends on both dendritic mRNA transport and synaptic anchoring. J
Neurochem. 96:1659–1666. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patrakka J, Xiao Z, Nukui M, Takemoto M,
He L, Oddsson A, Perisic L, Kaukinen A, Szigyarto CA, Uhlén M, et
al: Expression and subcellular distribution of novel
glomerulus-associated proteins dendrin, ehd3, sh2d4a, plekhh2, and
2310066E14Rik. J Am Soc Nephrol. 18:689–697. 2007. View Article : Google Scholar : PubMed/NCBI
|