Introduction
Fluid shear stress (FSS), which is generated by
fluid flow inside the lacunar-canalicular networks and trabecular
spaces within bone tissue, is important for the maintenance of
skeletal architectural integrity (1,2).
Previous studies have demonstrated that FSS may promote the
proliferation and differentiation of osteoblasts (3–5). The
cells used in the literature are at various stages of the cell
cycle rather than in a single phase, thus the effect of FSS on
osteoblasts in the G0/G1 phase remains unclear. Proliferation and
differentiation are exclusive physiological processes in cells and
may not be simultaneously induced by FSS in a single cell. It is
therefore necessary to elucidate precisely how the proliferation
and differentiation of osteoblasts are regulated by FSS.
The associated events in the G0/G1 phase for a cell
determine its fate with regards to proliferation or
differentiation. Restriction point passage, which promotes cell
cycle progression and S phase entry, is essential to drive cell
proliferation, whereas cells which do not pass the restriction
point may return to the G0/G1 phase in quiescence or may
differentiate (6). The product of
the retinoblastoma-associated protein gene (Rb) has been reported
to be an important regulatory factor in restriction point passage
(7). The phosphorylation of Rb,
induced by cyclin-dependent kinases 4/6 (CDK4/6) and further
activated by CDK2, promotes cell passage through the restriction
point and S phase entry (8).
Dephosphorylated Rb acts as a transcriptional coactivator required
for runt-related transcription factor 2 (Runx2) activity and
osteogenic differentiation (9,10).
These previous results indicated that the extent of phosphorylation
of phosphorylated (p)Rb may determine the initiation of
proliferation or differentiation in cells. Therefore, the present
study sought to investigate how G0/G1 phase osteoblasts responded
to FSS and whether pRb was involved.
The release of intracellular Ca2+,
activation of extracellular signal regulated kinases 1/2 (ERK1/2),
and Rho signaling have been implicated in the process of osteoblast
proliferation and differentiation induced by FSS (11–18).
Ca2+ is a universal intracellular secondary messenger.
The blockade of Ca2+ signaling was demonstrated to
completely inhibit FSS-induced osteoblast proliferation and
differentiation (4). It has been
demonstrated that the ubiquitously-expressed
calcium/calmodulin-dependent protein kinase type II (CaMK II),
triggered by Ca2+ signaling, is involved in the
regulation of cell proliferation and differentiation (19–20).
ERK1/2 is an important component in the osteogenic action of FSS
(3,4,18).
There is evidence that crosstalk exists between various signaling
pathways, and that the activation of Ca2+ signaling and
ERK1/2 are important downstream events of a number of these
pathways (3,14–15,17,21–25).
Therefore, the present study investigated how FSS affected the
proliferation and differentiation of osteoblasts in the G0/G1
phase, with emphasis on CaMK II and ERK1/2.
Materials and methods
Reagents
The reagents used in the present study were obtained
from the following sources: MC3T3-E1 subclone 14 were from the
American Type Culture Collection (Manassas, VA, USA; cat. no.
CRL-2594); α-minimum essential medium (MEM) was from Hyclone (GE
Healthcare Life Sciences, Logan, UT, USA; cat. no. SH30265.01B);
fetal bovine serum (FBS) was from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA; cat. no. 10082147); mouse anti-Runx2 (cat.
no. ab76956), anti-collagen type I antibody (COLL I; cat. no.
ab34710) and anti-osteocalcin antibody (OCN: cat. no. ab93876) were
from Abcam (Cambridge, UK); rabbit anti-p21Cip/Kip
antibody was from Abcam (cat. no. ab109199); pRb (Ser780) rabbit
monoclonal antibody was from Cell Signaling Technology, Inc.
(Danvers, MA, USA; cat. no. D59B7); rabbit anti-p-ERK1/2 antibody
was from Cell Signaling Technology, Inc. (cat. no. 14474); rabbit
anti-CaMKII-α antibody (cat. no. ab50202), rabbit anti-CaMKII-γ
antibody (cat. no. ab37999) and mouse anti-β-actin antibody (cat.
no. ab6276) were all from Abcam. KN93 was obtained from Calbiochem
(Merck KGaA, Darmstadt, Germany) and was dissolved in dimethyl
sulfoxide. U0126 was obtained from Sigma-Aldrich (Merck KGaA). The
alkaline phosphatase (ALP) activity kit (cat. no. A059-2) and ALP
staining kit (cat. no. D001) were from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Bromodeoxyuridine (BrdU;
cat. no. 11467229001) was from Roche Diagnostics GmbH (Mannheim,
Germany). Trypsin and EDTA were from Irvine Scientific (Santa Ana,
CA, USA).
Cell culture and G0/G1 phase
arrest
The osteoblastic cell line MC3T3-E1 was cultured in
α-MEM with 10% FBS, 100 U/ml penicillin and 100 g/ml streptomycin.
Cells were maintained in a humidified incubator at 37°C with 5%
CO2 and were subcultured every 48 h. For the FSS
experiments, 50,000 cells were seeded onto glass slides. Cell cycle
arrest at the G0/G1 phase was achieved by serum deprivation. Cells
were washed three times with PBS and cultured in serum-free α-MEM
for 24 h. Fluid shear experiments were performed 3 days
subsequently, when cells were 75–80% confluent and G0/G1-arrested.
The flow medium consisted of α-MEM containing 2% FBS, 100 U/ml
penicillin and 100 g/ml streptomycin.
FSS experiment
Fluid flow was applied to cells in a parallel plate
flow chamber using a closed flow loop. The system used a constant
hydrostatic pressure head to drive media through the channel of the
flow chamber, to subject the cell monolayer to steady laminar flow
resulting in a well-defined FSS of 12 dyne/cm2. The
apparatus was maintained at 37°C throughout the duration of
experimentation. The correlation between FSS and flow rate was
calculated using the equation:
τ=6μQbh2
where Q is the flow rate (cm3/s),
µ is the viscosity of the flow media (0.01
dynes/cm2), h is the height of the channel (0.022 cm), b
is the slit width (3.2 cm), and τ is the wall shear stress
(dyne/cm2). A programmable Harvard Syringe Pump (PHD
programmable; Harvard Apparatus, Holliston, MA, USA) was used to
perfuse the flow chamber with fresh media at the aforementioned
shear rate of 12 dyne/cm2.
BrdU assay
The BrdU ELISA (Amersham Cell Proliferation Biotrak
ELISA system, version 2; cat. no. 11647229001; GE Healthcare Life
Sciences, Little Chalfont, UK) is based on the incorporation of
BrdU during DNA synthesis in proliferating cells. Prior to
labeling, cells were seeded at a density of 50,000/ml in 96-well
plates. In order to quantify the cell proliferation, 10 µM BrdU
labeling reagent was added to each well (100 µl/well) and the cells
were incubated for 2–12 h in a humidified incubator at 37°C with
95% air and 5% CO2. (Following stimulation, the DNA of
MC3T3 cells will be duplicated during the first 12 h. Thus, the
times points of 2–12 h were selected to identify the cell
proliferation rate.) The BrdU labeling reagent was removed from the
wells and 200 µl FixDenat solution (for cell fixation and DNA
denaturation) was added, and the cells were incubated for 30 min at
15–25°C. The FixDenat solution was removed, 100 µl/well
anti-BrdU-POD working solution was added and the cells were
incubated for 90 min at 15–25°C. The antibody conjugate was removed
and the wells were rinsed three times with 200–300 µl/well washing
solution. The washing solution was subsequently removed and 100
µl/well substrate solution was added, followed by incubation at
15–25°C until color development was sufficient for photometric
detection (5–30 min). The reaction was stopped by adding 25 µl 2 M
H2SO4 solution to each well. The optical
density (absorbance) of 150 µl of the resultant yellow-colored
solution was read at 450 nm in a 96-well microplate
spectrophotometer. The absorbance values correlated directly with
the amount of DNA synthesis and thereby to the number of
proliferating cells in culture.
ALP activity and staining
Cells were washed with PBS and frozen (−70°C) in 300
ml Tris-Triton (0.1 M Tris-base; 0.2% TritonX-100). Following
thawing, the cells were centrifuged (13,800 × g for 5 min at 4°C)
and the supernatant was used for analysis. ALP substrate was added
to supernatant at a ratio of 1:1, and then the mixture was
incubated at 37°C for 40 min. Then 5 g/l NaOH was added to stop the
reaction and the OD value detected at a wavelength of 410 nm. ALP
activity was normalized to the total protein content. ALP staining
was performed using the ALP staining kit, according to the
manufacturer's protocol. The staining of ALP was observed by an
inverted microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Flow cytometry
MC3T3 cells were pelleted and fixed with 70% ethanol
at 4°C for 2 min. After the cells were digested with RNase, the DNA
was stained with propidium iodide at 4°C for 30 mins in the dark,
and then analyzed with a flow cytometer (FACSCalibur, BD).
CytExpert version 1.2.11.0 (Beckman Coulter, Inc., Brea, CA, USA)
was used for date analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from cells using the RNA Easy
kit (Takara Biotechnology Co., Ltd., Dalian, China). RT was
performed using a Prime Script™ RT reagent kit (Takara
Biotechnology Co., Ltd.). qPCR assays were performed using SYBR
Premix Ex Taq™ (Takara Biotechnology Co., Ltd.) and the
ABI Prism 7900 sequence detection system (Shanghai Ying Huai Jie
Trading Co. Ltd., Shanghai, China) for quantitation. The RT
reactions were performed with the following cycling conditions:
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 56°C
for 30 sec. The gene expression was normalized to β-actin gene
expression. The 2−ΔΔCq method was used for normalization
(26). Each sample was analyzed in
triplicate, in a total volume of 20 µl amplification mixture
containing 10 µl SYBR Premix Ex Taq™ Mix (2X), 2 µl
cDNA, 0.8 of each primer, 0.4 µl Rox I and 6 µl of H2O.
Thermal cycling was performed by denaturation at 95°C for 5 min,
followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec
(annealing). All primer sequences are presented in Table I.
 | Table I.Primers used for the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for the reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer |
|---|
| COLL I |
5′-CGAGTCACACCGGAACTTGG-3′ |
5′-GCAGGCAGGGCCAATGTCTA-3′ |
| OCN |
5′-CTCTGTCTCTCTGACCTCACAG-3′ |
5′-CAGGTCCTAAATAGTGATACCG-3′ |
| Runx2 |
5′-GACTGTGGTTACCGTCATGGC-3′ |
5′-ACTTGGTTTTTCATAACAGCGGA-3′ |
| β-actin |
5′-CGTGGACATCCGCAAAGAC-3′ |
5′-GCATTTGCGGTGGACGAT-3′ |
Western blot analysis
ERK, p-ERK, CaMK II α and γ, OCN, COLL I, Runx2, pRb
(S780) and the cell cycle marker p21Cip/Kip in MC3T3
cells were analyzed by western blotting. For inhibition of ERK and
CaMK II by U0126 and KN93, 10 µM KN93 or U0126 were added into
a-MEM with 10% FBS for 12 h following FSS stimulation. Following
treatment, cells were washed by PBS (4°C) 3 times, then lysed by
200 µl RIPA (Beyotime Biotechnology Research Institute, P0013B) for
5 mins on ice. The cells were centrifuged (16,200 × g for 25 min at
4°C) and the supernatant was used for analysis. The concentration
of the protein was determined by a BCA kit (NCI3227CH; Thermo
Fisher Scientific, Inc.). Equal amounts of total cellular protein
(30 µg per lane) were separated via 10% of the SDS-PAGE gel, and
then electrotransferred to polyvinylidene difluoride membranes
(Immobilon-P; EMD Millipore, Billerica, MA, USA) at 340 mA for 65
mins. Blots were incubated with a 1:1,000 dilution of each primary
antibody overnight at 4°C. Primary antibody binding was detected
using a secondary antibody conjugated to horseradish peroxidase
with a 1:5,000 dilution for 1 h at room temperature, and enhanced
using an enhanced chemiluminescence assay kit (Amersham; GE
Healthcare Life Sciences), according to the manufacturer's
protocol. Chemiluminescence was imaged on a FUJIFILM LAS-3000
system (Fujifilm Holdings Corporation, Tokyo, Japan). Image Reader
LAS 3000 (Fujifilm Holdings Corporation, Tokyo, Japan) was used for
densitometric analysis. The basal levels of proteins were
normalized to the level of β-actin protein.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed using SPSS version
13.0 (SPSS Inc., Chicago, IL, USA). A one-way analysis of variance
with Duncan's multiple range test was used to examine the
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Fluid shear stress in the G0/G1 phase
promotes osteoblast differentiation
In order to determine the response of osteoblasts
induced by FSS, MC3T3-E1 cells in the G0/G1 phase were subjected to
12 dyne/cm2 FSS for 1 h, and subsequently cultured in
α-MEM containing 10% FBS for a further 24 h. Cell differentiation
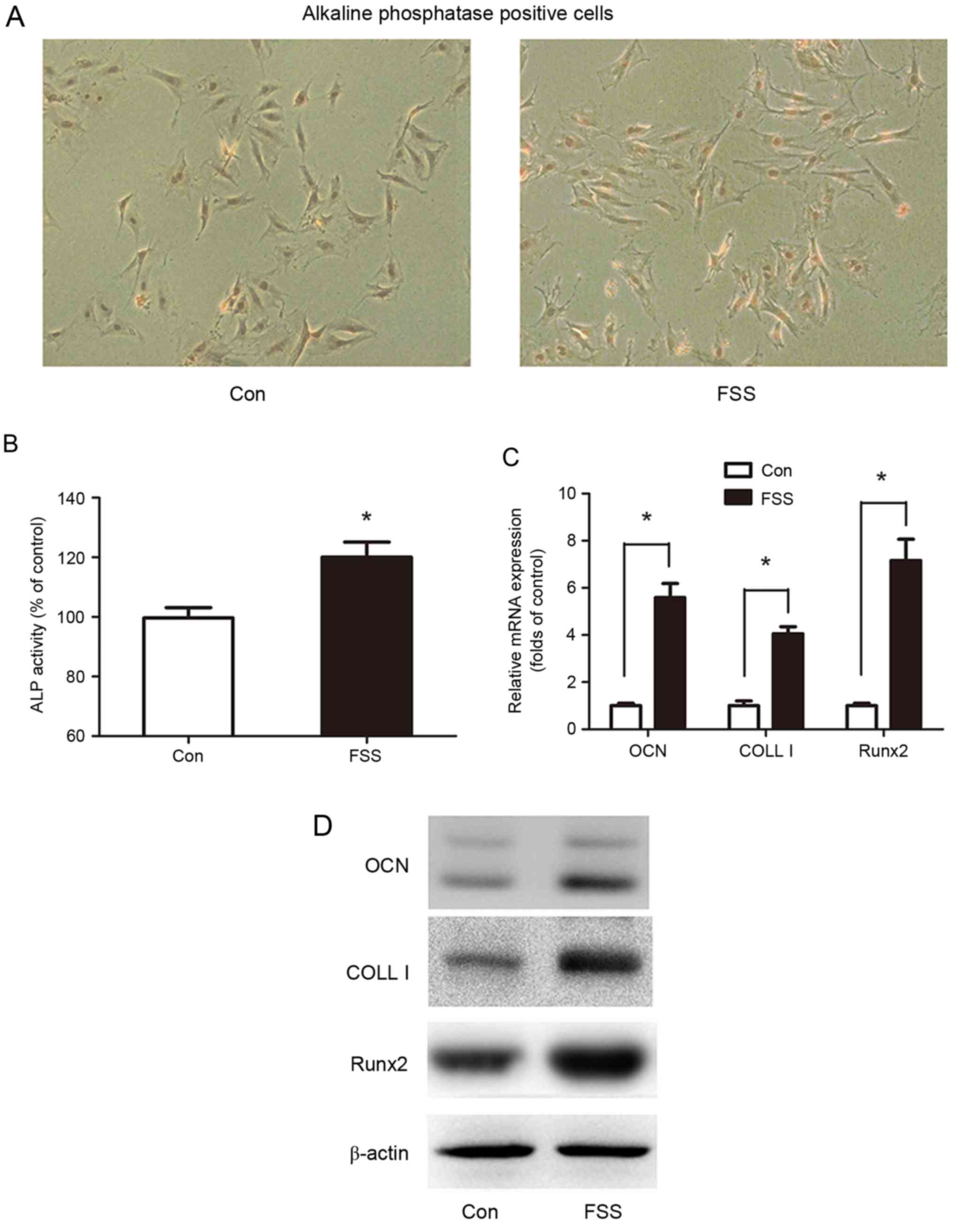
was determined by analysis of ALP activity and by ALP staining.
Compared with the control, FSS significantly increased ALP activity
by 28% (Fig. 1A and B).
Runx2, which promotes the transcription of
differentiated genes, is an important indicator of cell
differentiation induced by FSS (27). OCN and COLL I, which are
transcriptionally-regulated by Runx2, are two early markers of
osteoblast differentiation. The results of the present study
demonstrated that OCN, COLL I and Runx2 mRNA expression was
elevated by 5.59, 4.05 and 7.16-fold, respectively (Fig. 1C). In addition, OCN, COLL I and
Runx2 protein levels were upregulated by FSS (Fig. 1D).
The results of the present study indicated that FSS
promoted osteoblast differentiation in the G0/G1 phase.
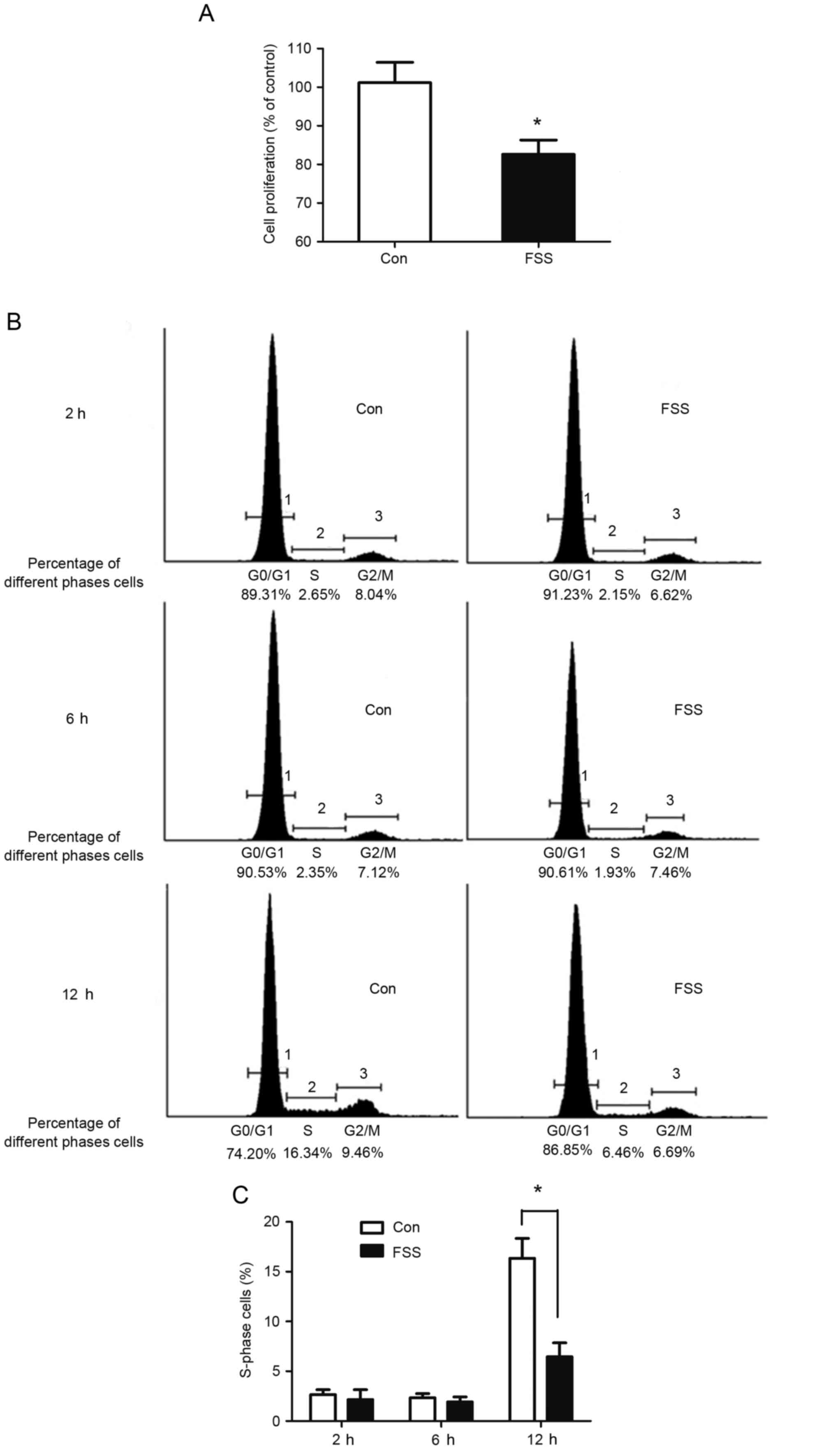
FSS arrests the cell cycle at the
G0/G1 phase
Following exposure to FSS, the DNA content of cells
was decreased by 11.9% compared with the control (Fig. 2A). The percentage of cells in the S
phase decreased to 6.46% compared with the control value of 16.34%
(Fig. 2B and C). The results
indicated that FSS inhibited proliferation through inhibition of
the transition between the G0/G1 and S phases.
Promotion of osteoblast
differentiation induced by FSS in the G0/G1 phase is associated
with CaMK II and the ERK1/2 signaling pathway
CaMK II and ERK1/2 have been reported to regulate
osteoblast differentiation induced by FSS (28). The present study further
investigated the potential involvement of ERK1/2 and CaMK II during
FSS-induced differentiation in the G0/G1 phase (Fig. 3). Western blotting demonstrated
that both ERK1/2 and p-ERK1/2 in MC3T3-E1 cells increased following
stimulation with FSS (Fig. 3A).
Among the four isoforms of CaMKII, only CaMK II-α and -γ have been
observed to be expressed in osteoblasts, and CaMK II-α is involved
in differentiation (20). The
results of the present study demonstrated that the expression of
CaMK II-α was increased following stimulation with FSS, while CaMK
II-γ was not significantly different between the control and FSS
groups (Fig. 3A). In addition, FSS
increased Runx2 activity, and the induction of Runx2 was
accompanied by an increase in ALP activity and the expression of
OCN and COLL I (Fig. 1).
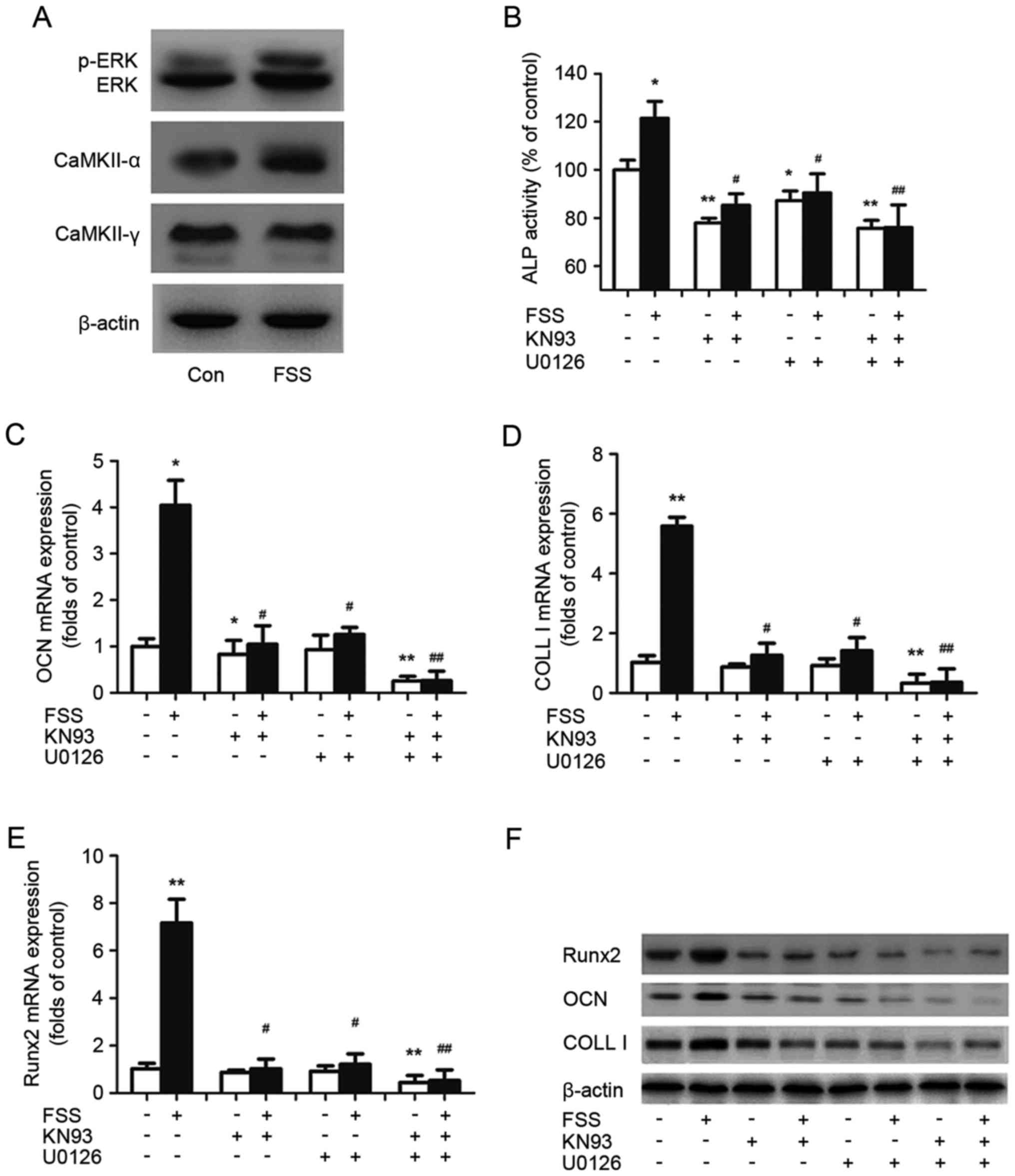 | Figure 3.ERK and CaMK II pathways regulate
FSS-induced osteoblast differentiation. (A) p-ERK, ERK, CaMK II α
and CaMK II γ levels were determined by western blotting and
compared with β-actin. (B) Alkaline phosphatase activity was
assessed by the conversion of p-nitrophenyl phosphate to
p-nitrophenol. The mRNA expression of (C) OCN, (D) COLL I and (E)
Runx2 was determined using reverse transcription-quantitative
polymerase chain reaction analysis and compared with β-actin. (F)
COLL I, OCN and Runx2 activity was determined by western blot
analysis and compared with β-actin. The data are presented as the
mean ± standard deviation from at least three independent
experiments. *P<0.05, **P<0.01 vs. control group,
#P<0.05, ##P<0.01 vs. FSS group. ERK,
extracellular signal-regulated kinase; p, phosphorylated; CaMK II,
calcium/calmodulin-dependent protein kinase type II; OCN,
osteocalcin; COLL I, collagen type I; Runx2, runt-related
transcription factor 2; FSS, fluid shear stress; Con, control. |
To ascertain whether the CaMK II or ERK1/2 pathways
mediated osteoblast differentiation in response to FSS, KN93, an
inhibitor of CaMK II, and U0126, an inhibitor of ERK1/2, were used
to treat the cells. The results of the present study demonstrated
that the induction of Runx2 by FSS was partially inhibited when
CaMK II or ERK1/2 was inhibited (Fig.
3E and F). It was additionally observed that treatment with
KN93 and U0126 in the presence of FSS abolished ALP activity, and
the transcription and expression of OCN and COLL I (Fig. 3B-D and F). The present results
indicated that the CaMK II and ERK1/2 signaling pathways were
necessary for osteoblast differentiation induced by FSS.
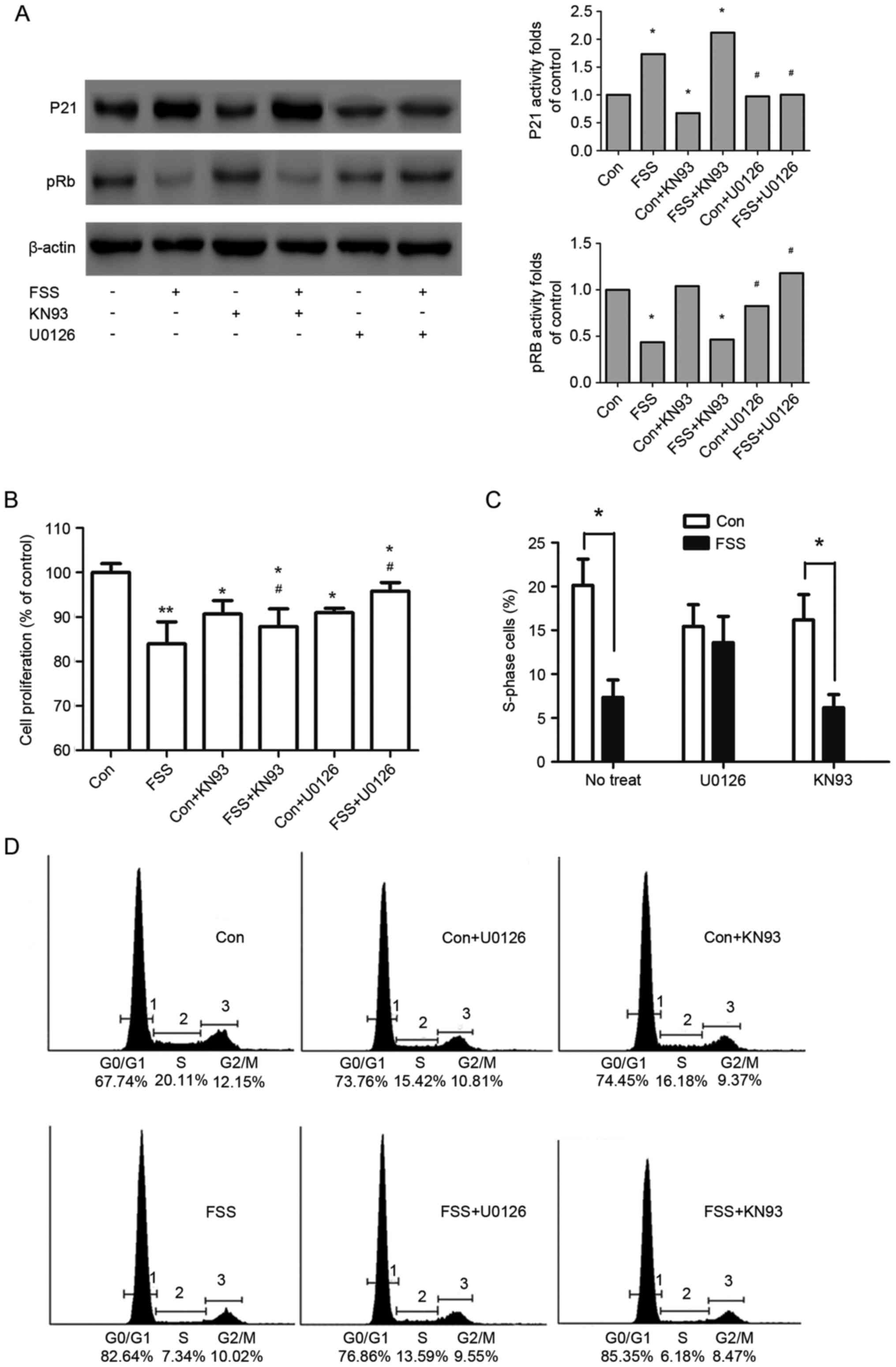
ERK1/2, not CaMK II, mediates
FSS-induced p21Cip/Kip upregulation and pRb
dephosphorylation
Induction of the cell cycle regulator
p21Cip/Kip is an important indicator of ERK1/2-induced
inhibition of proliferation (29–32).
In the present study, FSS increased p21Cip/Kip (Fig. 4A), ERK1/2 and p-ERK1/2 activity
(Fig. 3A). The induction of
p21Cip/Kip was accompanied by pRb dephosphorylation and
an 11.82% decrease in the number of proliferating cells (Fig. 4B).
To ascertain whether ERK1/2 mediated
p21Cip/Kip induction and MC3T3 cell proliferation in
response to FSS, the effect of inhibiting ERK1/2 on osteoblasts
stimulated with FSS was investigated. It was observed that the
induction of p21Cip/Kip by FSS was partly decreased when
ERK1/2 was inhibited. It was additionally demonstrated that
treatment with U0126 in the presence of FSS increased cell numbers
(Fig. 4B) and S phase re-entry
(Fig. 4C and D), compared with the
FSS group. The present results indicated that FSS inhibited MC3T3
cell proliferation, in part through the
ERK1/2-p21Cip/Kip signaling pathway.
Although CaMK II mediated the differentiation of
MC3T3 cells exposed to FSS, the induction of p21Cip/Kip
by FSS was not decreased when cells were pretreated with KN93.
Additionally, the inhibition of CaMK II during FSS stimulation did
not increase cell numbers (Fig.
4B) compared with the FSS group. The results indicated that
CaMK II was not implicated in the proliferation inhibited by
FSS.
Discussion
Previous studies reported that FSS promoted the
proliferation and differentiation of osteoblasts. However, the
conditions of osteoblast proliferation and differentiation induced
by FSS have rarely been reported. Proliferation and differentiation
are exclusive cellular events. Cells enter into the cell cycle to
commence proliferation, whereas cells exit the cell cycle prior to
differentiation. Therefore, the present study investigated the
effects of FSS on osteoblast-like MC3T3 cells in the G0/G1 phase,
the period during which cell proliferation or differentiation is
determined. It was observed that FSS in the G0/G1 phase promoted
the differentiation of MC3T3 cells through the ERK1/2 and CaMK II
signaling pathways, and partly arrested the cell cycle at the G0/G1
phase via the ERK1/2 pathway. The results of the present study
provided evidence for the fact that proliferation and
differentiation may not be promoted by FSS simultaneously, and
demonstrated the necessity of further research to elucidate the
mechanobiology of the effect of FSS on osteoblasts.
Loading of bone causes movement of interstitial
fluid within bone. Bone exhibits the unique ability to modify its
structure via bone-associated cells in response to fluid flow.
Previous studies have focused on how the cells, including
osteoblasts, respond to FSS (3–5).
Although certain studies noted that FSS promoted osteoblast
proliferation or differentiation (33,34),
further details of the process were not reported. Proliferation and
differentiation are distinct cellular events that occur at
different cell stages. Therefore, further studies using different
cell populations induced by FSS are required. In the present study,
in order to demonstrate when FSS altered the differentiation or
proliferation of osteoblasts, osteoblasts at the G0/G1 phase, the
period during which cell differentiation or entry into the cell
cycle is determined, were obtained and the roles of FSS were
investigated. The results of the present study demonstrated that
FSS decreased the cell numbers, although it increased the
expression of Runx2, COLL I and OCN, which are markers of
osteoblast differentiation. The results of the present study
demonstrated that FSS during the G0/G1 phase induced the
differentiation of osteoblasts while arresting the cell cycle at
the G0/G1 phase.
It has been reported that osteoblasts express Runx2
in the G0/G1 phase. Runx2 is important for the differentiation of
osteoblasts (35–38), and is able to directly stimulate
the transcription of osteoblast-associated genes, including ALP,
COLL I and OCN, by binding to specific enhancer regions (19,39,40).
The results of the present study demonstrated that FSS induced an
increase in Runx2 expression, which indicated that FSS promoted
Runx2 activity in MC3T3 cells. It was hypothesized that Runx2 may
be an important factor enabling FSS to promote the differentiation
of osteoblasts in the G0/G1 phase. The results of the present study
demonstrated that the increase in Runx2 activity was accompanied by
activation of CaMK II α and ERK1/2. Additionally, the inhibition of
CaMK II or ERK1/2 decreased the protein level of Runx2, and reduced
ALP activity and the expression of OCN and COLL I. The combination
of KN93 and U0126 inhibited the expression of FSS-induced
differentiation markers. Previous studies have reported that CaMK
II and ERK1/2 may be involved in regulating proliferation and
differentiation (29–31,41,42).
Inhibition of ERK1/2 by U0126 and CaMK II by KN93 decreased
FSS-induced Runx2 activity, indicating that Runx2 was the
downstream regulator of ERK1/2 and CaMK II in osteoblast
differentiation induced by FSS in the G0/G1 phase. The results of
the present study demonstrated that FSS in the G0/G1 phase promoted
the differentiation of osteoblasts by enhancing the protein level
of Runx2 via the CaMK II and ERK1/2 signaling pathways.
The notable markers of proliferative inhibition are
pRb dephosphorylation and G0/G1 phase arrest.
p21Cip/Kip, which inhibits pRb phosphorylation (43–45),
has been reported to inhibit proliferation in different cell types
(35,46,47).
Runx2 interacts with pRb (9,10)
and the overexpression of Runx2 suppresses proliferation and causes
a delay in the G1 phase of MC3T3 preosteoblasts (27). It was considered that increased
expression of Runx2 induced by FSS may be associated with pRb
regulation, which was necessary for S phase entry. Notably, the
results of the present study demonstrated that the high expression
of Runx2 induced by FSS was accompanied by pRb dephosphorylation
and an upregulation of p21Cip/Kip. In addition, the
present study investigated whether CaMK II and ERK1/2 were involved
in cell cycle regulation. It was observed that inhibition of ERK1/2
by U0126 increased cell numbers and S phase re-entry. However,
inhibition of ERK1/2 in static control only partially inhibited
cell entry into S phase. The ERK1/2 pathway has been demonstrated
to be involved in the positive and negative regulation of cell
growth (29–31). The results of the present study
demonstrated that ERK1/2 induced by FSS primarily exerted negative
regulation of the cell cycle. An additional signaling pathway, CaMK
II, was observed to be irrelevant to cell cycle induction by FSS.
The CaMK II pathway has been reported to be a stimulus for cell
proliferation (34,42). The present findings indicated that
inhibition of CaMK II reduced cells numbers in static control, in
accordance with these previous studies. The results of the present
study demonstrated that CaMK II was not involved in the FSS-induced
inhibition of S phase entry. Another notable feature of MC3T3 cells
in the G0/G1 phase is the increased expression of
p21Cip/Kip activity (27). The present study investigated the
role of the ERK1/2 and CaMK II signaling pathways in
p21Cip/Kip regulation. It was observed that FSS promoted
p21Cip/Kip activity in MC3T3 cells. Inhibition of ERK1/2
by U0126 suppressed p21Cip/Kip activity induced by FSS,
although inhibition of CaMK II had little effect on
p21Cip/Kip. Previous studies have reported that the
inhibition of CaMK II induced p21Cip/Kip activity, which
was mediated by cell proliferation in other cell types (45,48,49).
However, the results of the present study demonstrated that CaMK II
was not involved in p21Cip/Kip expression induced by FSS
in MC3T3 cells.
In conclusion, in the present study, we found that
in the G0/G1 phase, FSS promoted osteoblast differentiation via the
CaMK II and ERK1/2 signaling pathways although it inhibited
proliferation via the ERK1/2-p21 pathway only. The present findings
provided a deeper understanding of the mechanism underlying
osteoblastic mechanobiology.
Acknowledgements
The present study was supported by National Natural
Scientific Foundation of China (grant nos. 11372244 and
31170893).
Glossary
Abbreviations
Abbreviations:
|
FSS
|
fluid shear stress
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
ALP
|
alkaline phosphatase
|
|
pRb
|
phosphorylated
retinoblastoma-associated protein
|
|
CaMK II
|
calcium/calmodulin-dependent protein
kinase type II
|
|
Runx2
|
runt-related transcription factor
2
|
|
COLL I
|
collagen type I
|
|
OCN
|
osteocalcin
|
|
CDK
|
cyclin-dependent kinase
|
|
FBS
|
fetal bovine serum
|
|
BrdU
|
bromodeoxyuridine
|
|
MEM
|
minimum essential medium
|
References
|
1
|
Tate ML Knothe, Knothe U and Niederer P:
Experimental elucidation of mechanical load-induced fluid flow and
its potential role in bone metabolism and functional adaptation. Am
J Med Sci. 316:189–195. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kufahl RH and Saha S: A theoretical model
for stress-generated fluid flow in the canaliculi-lacunae network
in bone tissue. J Biomech. 23:171–180. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapur S, Baylink DJ and Lau KH: Fluid flow
shear stress stimulates human osteoblast proliferation and
differentiation through multiple interacting and competing signal
transduction pathways. Bone. 32:241–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Zhang X and Lee I: A quantitative
study on morphological responses of osteoblastic cells to fluid
shear stress. Acta Biochim Biophys Sin (Shanghai). 42:195–201.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu W, Qu H, Hu G, Zhang Q, Song K, Guan H,
Liu T and Qin J: A microfluidic-based multi-shear device for
investigating the effects of low fluid-induced stresses on
osteoblasts. PLoS One. 9:e899662014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prescott DM: Regulation of cell
reproduction. Cancer Res. 28:1815–1820. 1968.PubMed/NCBI
|
|
7
|
Dyson N: The regulation of E2F by
pRB-family proteins. Genes Dev. 12:2245–2262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spencer SL, Cappell SD, Tsai FC, Overton
KW, Wang CL and Meyer T: The proliferation-quiescence decision is
controlled by a bifurcation in CDK2 activity at mitotic exit. Cell.
155:369–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luan Y, Yu XP, Xu K, Ding B, Yu J, Huang
Y, Yang N, Lengyel P, Di Cesare PE and Liu CJ: The retinoblastoma
protein is an essential mediator of osteogenesis that links the
p204 protein to the Cbfa1 transcription factor thereby increasing
its activity. J Biol Chem. 282:16860–16870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas DM, Carty SA, Piscopo DM, Lee JS,
Wang WF, Forrester WC and Hinds PW: The retinoblastoma protein acts
as a transcriptional coactivator required for osteogenic
differentiation. Mol Cell. 8:303–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akhter MP, Wells DJ, Short SJ, Cullen DM,
Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F and
Recker RR: Bone biomechanical properties in LRP5 mutant mice. Bone.
35:162–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen NX, Geist DJ, Genetos DC, Pavalko FM
and Duncan RL: Fluid shear-induced NFkappaB translocation in
osteoblasts is mediated by intracellular calcium release. Bone.
33:399–410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen NX, Ryder KD, Pavalko FM, Turner CH,
Burr DB, Qiu J and Duncan RL: Ca(2+) regulates fluid shear-induced
cytoskeletal reorganization and gene expression in osteoblasts. Am
J Physiol Cell Physiol. 278:C989–C997. 2000.PubMed/NCBI
|
|
14
|
Jing D, Baik AD, Lu XL, Zhou B, Lai X,
Wang L, Luo E and Guo XE: In situ intracellular calcium
oscillations in osteocytes in intact mouse long bones under dynamic
mechanical loading. FASEB J. 28:1582–1592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu XL, Huo B, Park M and Guo XE: Calcium
response in osteocytic networks under steady and oscillatory fluid
flow. Bone. 51:466–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pilbeam CC, Raisz LG, Voznesensky O,
Alander CB, Delman BN and Kawaguchi H: Autoregulation of inducible
prostaglandin G/H synthase in osteoblastic cells by prostaglandins.
J Bone Miner Res. 10:406–414. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wadhwa S, Choudhary S, Voznesensky M,
Epstein M, Raisz L and Pilbeam C: Fluid flow induces COX-2
expression in MC3T3-E1 osteoblasts via a PKA signaling pathway.
Biochem Biophys Res Commun. 297:46–51. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang B, Du T, Wang Y, Yang C, Zhang S and
Cao X: Focal adhesion kinase signaling pathway is involved in
mechanotransduction in MG-63 cells. Biochem Biophys Res Commun.
410:671–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: A transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zayzafoon M, Fulzele K and McDonald JM:
Calmodulin and calmodulin-dependent kinase IIalpha regulate
osteoblast differentiation by controlling c-fos expression. J Biol
Chem. 280:7049–7059. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gebken J, Lüders B, Notbohm H, Klein HH,
Brinckmann J, Müller PK and Bätge B: Hypergravity stimulates
collagen synthesis in human osteoblast-like cells: Evidence for the
involvement of p44/42 MAP-kinases (ERK 1/2). J Biochem.
126:676–682. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jessop HL, Rawlinson SC, Pitsillides AA
and Lanyon LE: Mechanical strain and fluid movement both activate
extracellular regulated kinase (ERK) in osteoblast-like cells but
via different signaling pathways. Bone. 31:186–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuda N, Morita N, Matsuda K and
Watanabe M: Proliferation and differentiation of human osteoblastic
cells associated with differential activation of MAP kinases in
response to epidermal growth factor, hypoxia, and mechanical stress
in vitro. Biochem Biophys Res Commun. 249:350–354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wadhwa S, Godwin SL, Peterson DR, Epstein
MA, Raisz LG and Pilbeam CC: Fluid flow induction of
cyclo-oxygenase 2 gene expression in osteoblasts is dependent on an
extracellular signal-regulated kinase signaling pathway. J Bone
Miner Res. 17:266–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
You J, Reilly GC, Zhen X, Yellowley CE,
Chen Q, Donahue HJ and Jacobs CR: Osteopontin gene regulation by
oscillatory fluid flow via intracellular calcium mobilization and
activation of mitogen-activated protein kinase in MC3T3-E1
osteoblasts. J Biol Chem. 276:13365–13371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galindo M, Pratap J, Young DW,
Hovhannisyan H, Im HJ, Choi JY, Lian JB, Stein JL, Stein GS and van
Wijnen AJ: The bone-specific expression of Runx2 oscillates during
the cell cycle to support a G1-related antiproliferative function
in osteoblasts. J Biol Chem. 280:20274–20285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Franceschi RT, Xiao G, Jiang D,
Gopalakrishnan R, Yang S and Reith E: Multiple signaling pathways
converge on the Cbfa1/Runx2 transcription factor to regulate
osteoblast differentiation. Connective Tissue Res. 44 Suppl
1:S109–S116. 2003. View Article : Google Scholar
|
|
29
|
Enarsson M, Erlandsson A, Larsson H and
Forsberg-Nilsson K: Extracellular signal-regulated protein kinase
signaling is uncoupled from initial differentiation of central
nervous system stem cells to neurons. Mol Cancer Res. 1:147–154.
2002.PubMed/NCBI
|
|
30
|
Howe AK and Juliano RL: Distinct
mechanisms mediate the initial and sustained phases of
integrin-mediated activation of the Raf/MEK/mitogen-activated
protein kinase cascade. J Biol Chem. 273:27268–27274. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park KS, Lee NG, Lee KH, Seo JT and Choi
KY: The ERK pathway involves positive and negative regulations of
HT-29 colorectal cancer cell growth by extracellular zinc. Am J
Physiol Gastrointest Liver Physiol. 285:G1181–G1188. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Woods D, Parry D, Cherwinski H, Bosch E,
Lees E and McMahon M: Raf-induced proliferation or cell cycle
arrest is determined by the level of Raf activity with arrest
mediated by p21Cip1. Mol Cell Biol. 17:5598–5611. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vezeridis PS, Semeins CM, Chen Q and
Klein-Nulend J: Osteocytes subjected to pulsating fluid flow
regulate osteoblast proliferation and differentiation. Biochem
Biophys Res Commun. 348:1082–1088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YJ, Batra NN, You L, Meier SC, Coe IA,
Yellowley CE and Jacobs CR: Oscillatory fluid flow affects human
marrow stromal cell proliferation and differentiation. J Orthop
Res. 22:1283–1289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chau JF, Leong WF and Li B: Signaling
pathways governing osteoblast proliferation, differentiation and
function. Histol Histopathol. 24:1593–1606. 2009.PubMed/NCBI
|
|
36
|
Komori T: Cbfa1/Runx2, an essential
transcription factor for the regulation of osteoblast
differentiation. Nihon Rinsho. 60(Suppl 3): S91–S97. 2002.(In
Japanese).
|
|
37
|
Komori T: Regulation of osteoblast
differentiation by Runx2. Adv Exp Med Biol. 658:43–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao LG, Chen SL, Teng YJ, An LP, Wang J,
Ma JL and Xia YY: The MEK5/ERK5 pathway mediates fluid shear stress
promoted osteoblast differentiation. Connect Tissue Res. 55:96–102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kern B, Shen J, Starbuck M and Karsenty G:
Cbfa1 contributes to the osteoblast-specific expression of type I
collagen genes. J Biol Chem. 276:7101–7107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Selvamurugan N, Chou WY, Pearman AT,
Pulumati MR and Partridge NC: Parathyroid hormone regulates the rat
collagenase-3 promoter in osteoblastic cells through the
cooperative interaction of the activator protein-1 site and the
runt domain binding sequence. J Biol Chem. 273:10647–10657. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Braun AP and Schulman H: The
multifunctional calcium/calmodulin-dependent protein kinase: From
form to function. Annu Rev Physiol. 57:417–445. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Colomer JM and Means AR: Chronic elevation
of calmodulin in the ventricles of transgenic mice increases the
autonomous activity of calmodulin-dependent protein kinase II,
which regulates atrial natriuretic factor gene expression. Mol
Endocrinol. 14:1125–1136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Akimoto S, Mitsumata M, Sasaguri T and
Yoshida Y: Laminar shear stress inhibits vascular endothelial cell
proliferation by inducing cyclin-dependent kinase inhibitor
p21(Sdi1/Cip1/Waf1). Circ Res. 86:185–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jung GA, Yoon JY, Moon BS, Yang DH, Kim
HY, Lee SH, Bryja V, Arenas E and Choi KY: Valproic acid induces
differentiation and inhibition of proliferation in neural
progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway.
BMC Cell Biol. 9:662008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yuan K, Chung LW, Siegal GP and Zayzafoon
M: Alpha-CaMKII controls the growth of human osteosarcoma by
regulating cell cycle progression. Lab Invest. 87:938–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Plasilova M, Schonmeyr B, Fernandez J,
Clavin N, Soares M and Mehrara BJ: Accelerating stem cell
proliferation by down-regulation of cell cycle regulator p21.
Plastic Reconstr Surg. 123:149S–157S. 2009. View Article : Google Scholar
|
|
47
|
James AW: Review of Signaling Pathways
Governing MSC Osteogenic and Adipogenic Differentiation.
Scientifica. 2013:6847362013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
An P, Tian Y, Chen M and Luo H:
Ca(2+)/calmodulin-dependent protein kinase II mediates transforming
growth factor-β-induced hepatic stellate cells proliferation but
not in collagen α1(I) production. Hepatol Res. 42:806–818. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Choi J and Husain M: Calmodulin-mediated
cell cycle regulation: New mechanisms for old observations. Cell
Cycle. 5:2183–2186. 2006. View Article : Google Scholar : PubMed/NCBI
|