Introduction
Parkinson's disease (PD) is a progressive
neurodegenerative disorder characterized by the selective loss of
midbrain dopaminergic neurons in the substantia nigra, and the
development of Lewy bodies. α-synuclein (α-SYN) is a major
component of the Lewy bodies, the misfolding and aggregation of
which contribute to the pathogenesis of both familial and sporadic
PD (1,2). Particularly, phosphorylated Ser129
α-SYN (P-Ser129 α-SYN) serves an important role in the formation of
Lewy bodies and in the neurodegenerative process associated with
PD. Inhibiting the formation and toxicity of the pathogenic
proteins might be an applicable strategy.
Chondroitin sulfate (CS) is a natural
glycosaminoglycan that is present in the extracellular matrix
surrounding cells, which serves an important role in neural
development and repair (3,4), promotes the survival of neuronal
cells (5,6) and protects dopaminergic SH-SY5Y cells
against 6-hydroxydopamine and hydrogen peroxide-induced toxicity
(7,8). It also has been reported that CS
inhibits β-amyloid' fibril formation, shortens the preformed
amyloid fibrils (9) and attenuates
β-amyloid-induced neurotoxicity in vitro and in vivo
(10,11). Both β-amyloid and α-SYN are
pathogenic proteins associated with neurodegenerative disorders.
However, little is known about the effect of CS on the formation
and toxicity of α-SYN.
Previous studies have demonstrated that PD can be
caused by multiplications (duplication and triplication) of or
mutations (A53T, E46K and A30P) in the α-SYN gene (12,13).
Cells and animals overexpressing wild-type (WT) or mutant α-SYN are
often used to study PD pathogenesis and therapeutic interventions
(14,15). The aim of the present study was to
investigate the protective effects of CS on α-SYN-induced damage in
dopaminergic SH-SY5Y cells overexpressing WT or A53T mutant
α-SYN.
Materials and methods
Cell culture
SH-SY5Y human neuroblastoma cells were purchased
from the Typical Culture Preservation Commission Cell Bank, Chinese
Academy of Sciences (Shanghai, China). All cells were maintained in
minimum essential medium and Dulbecco's modified Eagle's medium/F12
(1:1; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a tissue culture
incubator with 5% CO2 and 98% relative humidity.
Stable transfection of SH-SY5Y
cells
For stable transfection of SH-SY5Y cells, the LV5
expression vectors (Shanghai Gene Pharma Co., Ltd., Shanghai,
China) containing a cytomegalovirus promoter were used. WT or A53T
mutant α-SYN green fluorescent protein (GFP) fusion constructs were
polymerase chain reaction-amplified using DNA Polymerase (Takara
Bio, Inc., Otsu, Japan) and expression clones were created in the
LV5 expression vectors. WT α-SYN cDNA insert was generated with
primers AGG GTT CCA AGC TTA AGC GGC CGC G (forward) and GAT CCA TCC
CTA GGT AGA TGC ATT TA (reverse) and the following PCR conditions:
94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, for 30
cycles. The complete A53T mutant α-SYN insert was generated through
three steps. In the first step, A53T mutant α-SYN gene fragment I
was generated with primers AGG GTT CCA AGC TTA AGC GGC CGC G
(forward) and AAG CCA GTG GCT GTT GCA ATG CTC CCT GCT CCC TC
(reverse) and the following PCR conditions: 94°C for 30 sec, 55°C
for 30 sec, and 72°C for 30 sec, for 30 cycles. In the second step,
A53T mutant α-SYN gene fragment II was generated with primers GAG
CAT TGC AAC AGC CAC TGG CTT TGT CAA AAAGG (forward) and GAT CCA TCC
CTA GGT AGA TGC ATT TA (reverse) and the following PCR conditions:
94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec, for 30
cycles. The complete A53T mutant α-SYN gene insert was then
generated with the fragment I and II as templates, primers AGG GTT
CCA AGC TTA AGC GGC CGC G (forward) and GAT CCA TCC CTA GGT AGA TGC
ATT TA (reverse), and the following PCR conditions: 94°C for 30
sec, 55°C for 30 sec, and 72°C for 30 sec, for 30 cycles.
Lentivirus encoding WT or A53T mutant α-SYN-GFP fusion constructs
(Chongqing Western Biological Technology Co., Ltd., Chongqing,
China) were generated by co-transfecting the LV5 expression
construct together with the PG-p1-VSVG, PG-P2-REV and PG-P3-RRE
(Shanghai Gene Pharma Co., Ltd.) into 293T cells. Following this,
WT or A53T mutant α-SYN constructed in lentivirus was transfected
into SH-SY5Y cells. GFP fluorescence intensity was imaged (Fig. 1) and determined in transfected
cells. The transfection efficiency was >70%. The individual
stably transfected colony was subsequently selected in the presence
of puromycin.
Assessment of cell viability
SH-SY5Y cells were seeded at a density of
1.5×104 cells/well in 96-well plates. Cells were treated
with 50, 100, 200, 400 and 800 mg/l CS (CS sodium salt from shark
cartilage; cat. no. C4384; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 24 or 48 h, which is dissolved in sterile water and
added to the medium in a ratio of 1% vehicle, then were incubated
with 10 g/l MTT (Sigma-Aldrich; Merck KGaA) for 4 h. The formazan
dye was eluted by dimethyl sulfoxide. Absorbance was measured at a
wavelength of 490 nm using a microplate reader (BioTek Instruments,
Inc., Winooski, VT, USA).
Apoptosis detection by flow
cytometry
After cells were exposed to 400 mg/l CS for 24 h,
apoptosis was determined by Annexin V (AN)/7-amino-actinomycin D
(7-AAD) staining (559763; BD Biosciences, San Jose, CA, USA)
according to the manufacturer's protocol. Cells were stained with
AN and 7-AAD for 15 min at room temperature. Cells with
AN+/7AAD− (Q3%) and
AN+/7AAD+ (Q2%), which correspond to early
and late apoptotic cells, respectively, were determined by FACS
Vantage SE (BD Influx; BD Biosciences) with BD FACSuite software
version 1.0.6. The apoptotic rate was calculated as Q3%+Q2%.
Nuclear staining
After cells were exposed to 400 mg/l CS for 24 h,
nuclei morphological changes and DNA fragmentation were examined
with 4′6-diamidino-2-phenylindole (DAPI) staining. SH-SY5Y cells
were washed with PBS, stained with 1.0 mg/l DAPI (Sigma-Aldrich;
Merck KGaA) for 5 min at room temperature, then visualized by laser
scanning confocal microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Measurement of the mitochondrial
membrane potential (DΨm)
The mitochondrial membrane potential was measured
with a JC-1 assay kit (551302, BD Biosciences). JC-1 dye exhibits
potential-dependent accumulation in mitochondria, indicated by a
fluorescence emission shift from green (~529 nm) to red (~590 nm).
Consequently, mitochondrial depolarization is indicated by a
decrease in the red/green fluorescence intensity ratio. After
treatment with 400 mg/l CS for 24 h, the cells were incubated with
JC-1 at 37°C for 20 min. The red and green fluorescent intensities
were measured by FACS Vantage SE.
Measurement of reactive oxygen species
(ROS) generation
After cells were exposed to 400 mg/l CS for 24 h,
production of ROS in SH-SY5Y cells was measured by 2,
7-dichlorofuorescin diacetate (DCFH-DA) staining. DCFH-DA passively
enters cells and is converted to DCFH. ROS reacts with DCFH to form
the fluorescent product, DCF. SH-SY5Y cells were incubated with 10
µmol/l DCFH-DA (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min, and
then analyzed by FACS Vantage SE.
Western blot analysis of α-SYN, B-cell
lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax) and cytochrome
c (Cyt-c)
After cells were exposed to 400 mg/l CS for 24 h,
the mitochondria and total protein were prepared using a protein
extraction kit (Boster Biological Technology, Pleasanton, CA, USA).
Protein concentration was quantified using a Bradford protein assay
reagent. Equal amounts (30 µg) of proteins were separated by 10%
SDS-PAGE and blotted onto nitrocellulose membranes. After blocking
with 5% non-fat dry milk in TBS with Tween-20 buffer, blots were
incubated with primary monoclonal antibodies at 4°C overnight:
Rabbit anti-α-SYN (ab138501; 1:500; Abcam, Cambridge, MA, USA),
rabbit anti-P-Ser129 α-SYN (ab51253; 1:500; Abcam), rabbit
anti-Bcl-2 (ab32124; 1:1,000; Abcam), rabbit anti-Bax (ab32503;
1:1,000; Abcam), rabbit anti-Cyt-c (ab133504; 1:1,000;
Abcam), anti-GAPDH (A01622-40; 1:3,000; GenScript, Nanjing, China)
and anti-cytochrome c oxidase (COX IV; 1:1,000, sc-376731;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), then incubated
with goat horseradish peroxidase-conjugated corresponding secondary
antibodies (ab6721 and ab6789; 1:3,000; Abcam) at room temperature
for 1 h. Proteins were detected using an enhanced chemiluminescence
plus kit (Pierce; Thermo Fisher Scientific, Inc.) and determined by
Labworks™ Analysis software version 4.6 (UVP, Inc., Upland, CA,
USA). GAPDH and COX IV served as loading controls.
Caspase-9 and caspase-3 assay
Following exposure to 400 mg/l CS for 24 h,
caspase-3 activity in SH-SY5Y cells was analyzed with a
phycoerythrin-conjugated anti-active caspase-3 antibody (550914; BD
Biosciences) by FACS Vantage SE according to the kit instructions.
The cells were incubated with the phycoerythrin-conjugated
anti-active caspase-3 antibody for 30 min at room temperature.
Caspase-9 activity was analyzed with active caspase-9 FITC staining
kit (ab65615; Abcam) by FACS Vantage SE. The cells were incubated
with FITC-LEHD-FMK for 30 min at 37°C.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance followed by a Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. All analyses were performed by using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Effect of CS on proliferation in
SH-SY5Y cells
Compared with vector cells, the WT α-SYN and A53T
α-SYN transgenic SH-SY5Y cells showed decreased cell viability. In
WT α-SYN group, treatment with 50, 100, 200, 400 and 800 mg/l CS
for 24 h and 100, 200, 400 and 800 mg/l CS for 48 h prevented cell
loss (P<0.05). In the A53T α-SYN group, treatment with 100, 200,
400 and 800 mg/l CS for 24 h, and 200, 400 and 800 mg/l CS for 48
h, prevented cell loss (P<0.05). The most significant protective
effect of CS was achieved at the concentration of 400 mg/l
(P<0.05; Fig. 2).
 | Figure 2.CS prevents SH-SY5Y cells loss. The
transfected SH-SY5Y cells were treated with 50, 100, 200, 400 and
800 mg/l CS for 24 or 48 h. Cell viability assay was performed with
MTT. Data are presented as the mean ± standard deviation (n=3).
**P<0.01 vs. Vector group, #P<0.05 vs. 0 mg/l WT,
##P<0.01 vs. 0 mg/l WT, &P<0.05 vs.
0 mg/l A53T, &&P<0.01 vs. 0 mg/l A53T. WT,
wild-type; α-SYN, α-synuclein; CS, chondroitin sulfate; OD, optical
density; Con, control; Vec, vector. |
The effect of CS on control SH-SY5Y cells was
investigated by MTT test. Incubation of control SH-SY5Y cells with
400 mg/l CS for 24 h did not significantly affect the OD value
(0.45±0.01 vs. 0.46±0.02, n=3, P>0.05) (data not shown). This
result is consistent with the finding of Cañas et al
(7).
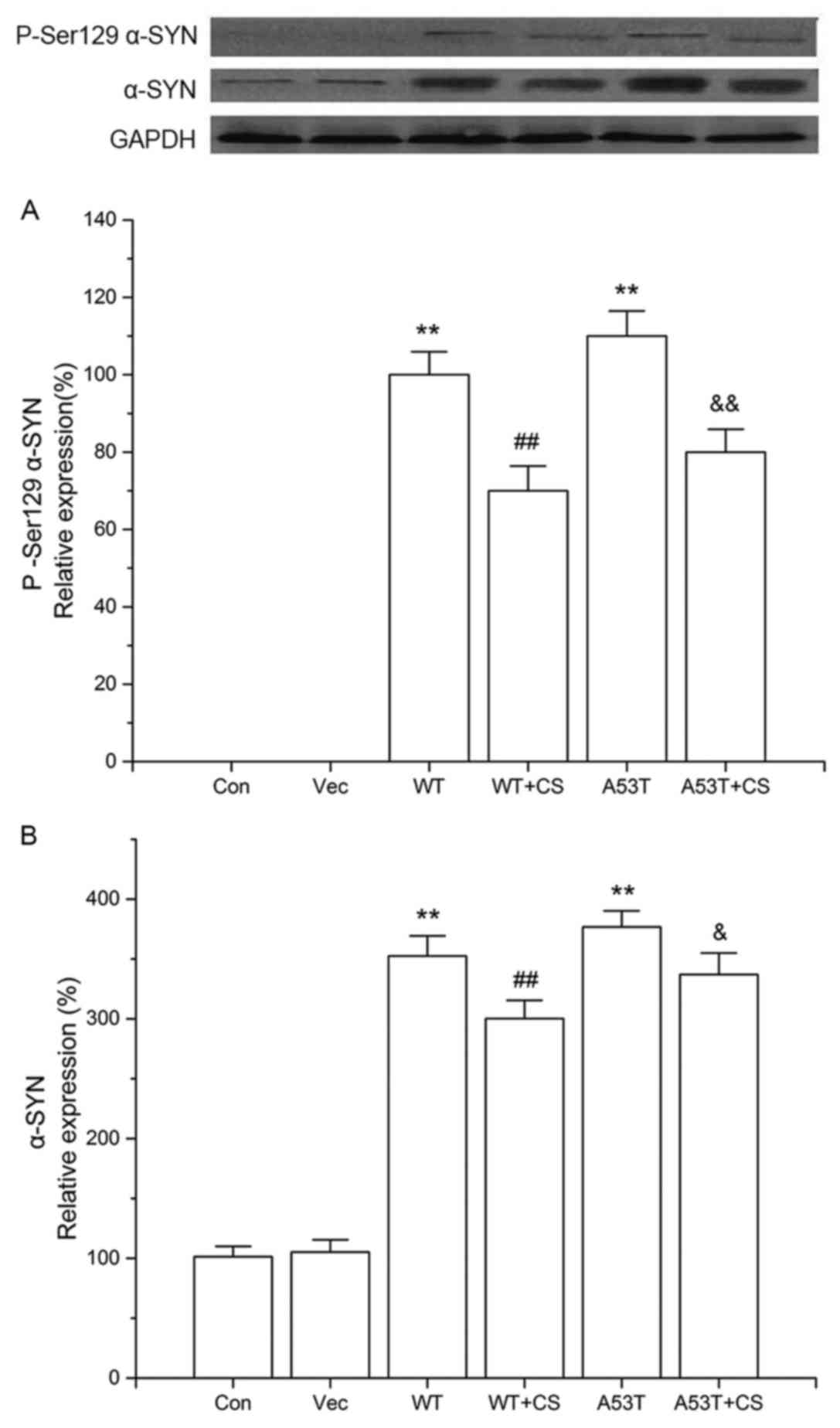
Effect of CS on P-Ser 129 α-SYN and
total α-SYN protein expression
After transfection, cells overexpressing α-SYN (both
WT and A53T) had significant levels of P-Ser129 α-SYN, while the
levels of P-Ser 129 α-SYN in the vector and control groups were too
low to detect. The increased P-Ser129 α-SYN observed in the WT and
A53T α-SYN overexpressing cells was reduced by CS treatment
(P<0.01; Fig. 3A).
Additionally, cells overexpressing WT and A53T α-SYN had
significant increases in the levels of total α-SYN compared with
vector cells, while 400 mg/l CS inhibited total α-SYN protein
expression (P<0.05; Fig. 3B).
No significant differences were observed between cells transfected
with WT vs. A53T α-SYN.
Effect of CS on apoptosis in
transfected SH-SY5Y cells
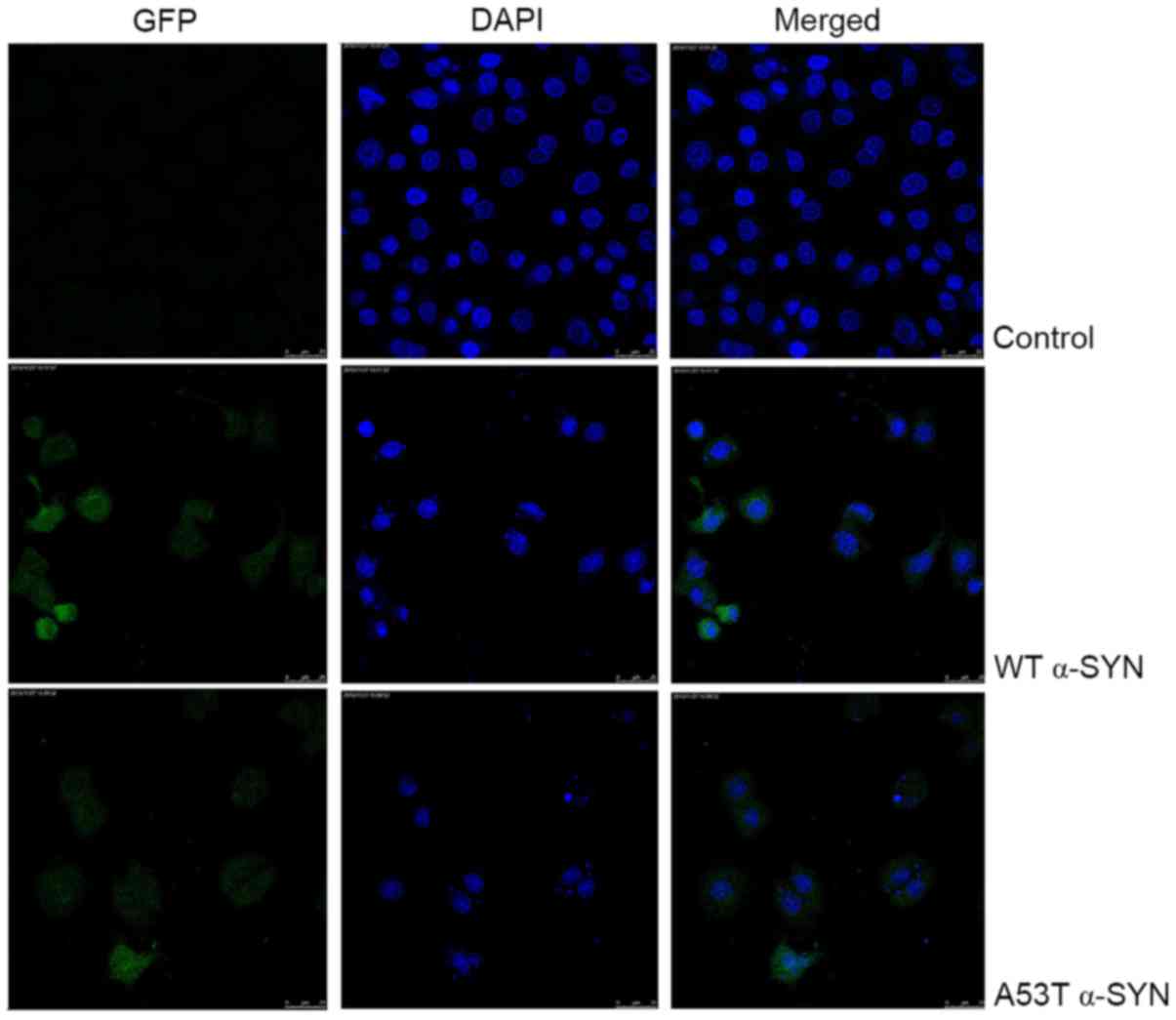
As presented in Fig.
4, the nucleus of SH-SY5Y cells overexpressing WT and A53T
α-SYN was condensed, and the nuclear apoptotic bodies were formed
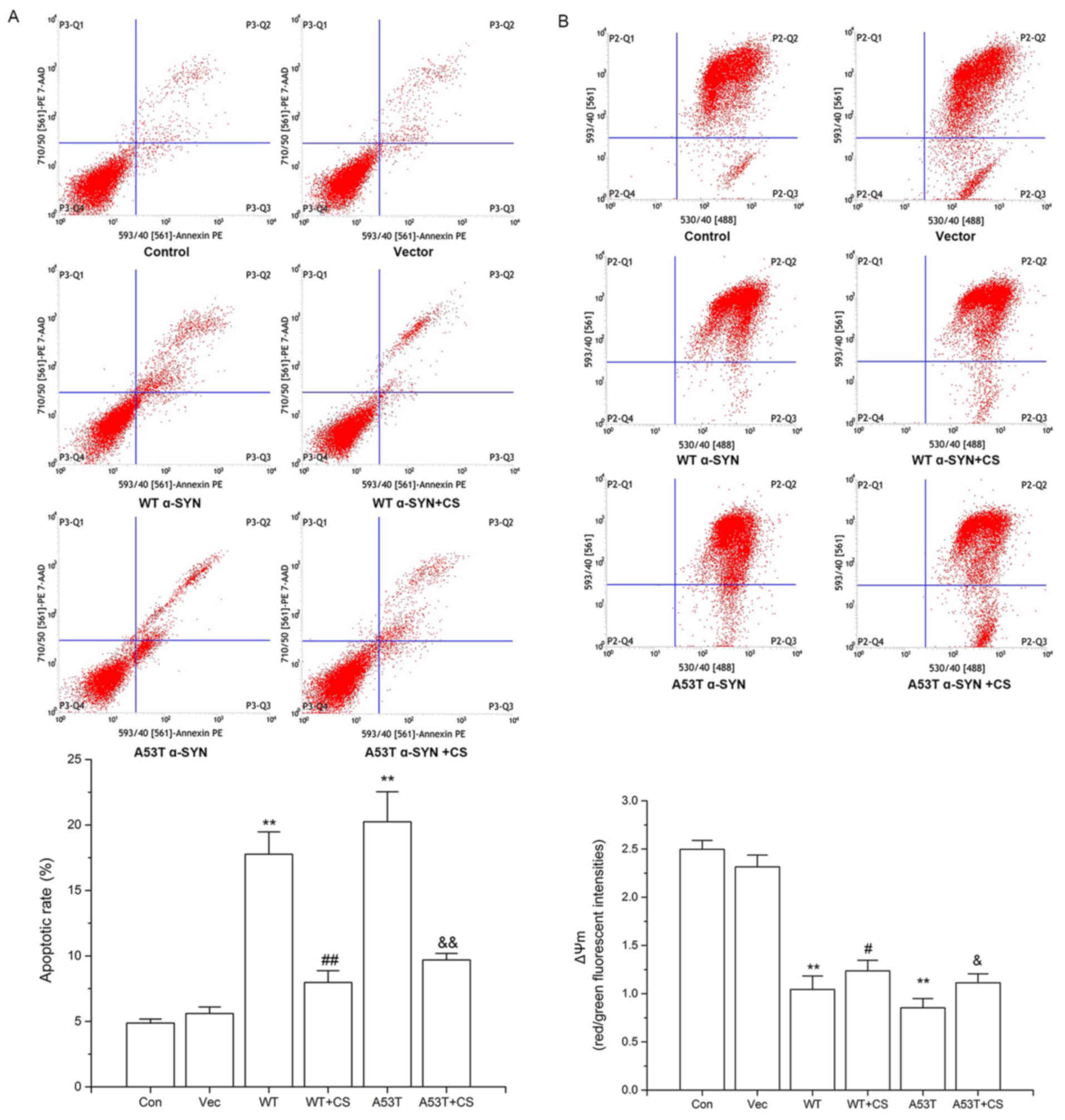
and brighter. The apoptotic rates of WT α-SYN and A53T α-SYN
transgenic SH-SY5Y cells were higher than that of vector cells
(17.77±1.7%, 20.24±2.3% vs. 4.88±0.3%, respectively; P<0.01;
Fig. 5A). When treated with 400
mg/l CS for 24 h, apoptotic rates were decreased to 7.98±0.9 and
9.69±0.5%, respectively (P<0.01; Fig. 5A). No significant differences were
observed between cells transfected with WT vs. A53T α-SYN.
 | Figure 5.Effect of CS on apoptotic rates and
ΔΨm in transfected SH-SY5Y cells. The transfected SH-SY5Y cells
were treated with 400 mg/l CS for 24 h. (A) Apoptosis was
determined by AN/7-AAD staining. (B) ΔΨm was determined by JC-1
staining. Data are presented as the mean ± standard deviation
(n=4). **P<0.01 vs. Vector group, #P<0.05 vs. WT,
##P<0.01 vs. WT, &P<0.05 vs. A53T,
&&P<0.01 vs. A53T. WT, wild-type; α-SYN,
α-synuclein; CS, chondroitin sulfate; Con, control; Vec, vector;
AN/7-AAD, Annexin V/-amino-actinomycin D; ΔΨm, mitochondrial
membrane potential. |
Effect of CS on ∆Ψm in transfected
SH-SY5Y Cells
Decreased ∆Ψm is an early event occurring in
mitochondrial dysfunction and apoptosis. In WT α-SYN and A53T α-SYN
groups, the red/green fluorescence ratios of JC-1 were decreased
compared with the control and vector groups (P<0.01; Fig. 5B). Treatment with 400 mg/l CS
resulted in significant increases of both groups (P<0.05;
Fig. 5B).
Effect of CS on ROS generation in
transfected SH-SY5Y cells
As presented in Fig.
6, the levels of intracellular ROS in the WT α-SYN and A53T
α-SYN groups were increased significantly (P<0.01), and the
increases were attenuated by 400 mg/l CS (P<0.01). No
significant differences were observed between cells transfected
with WT vs. A53T α-SYN.
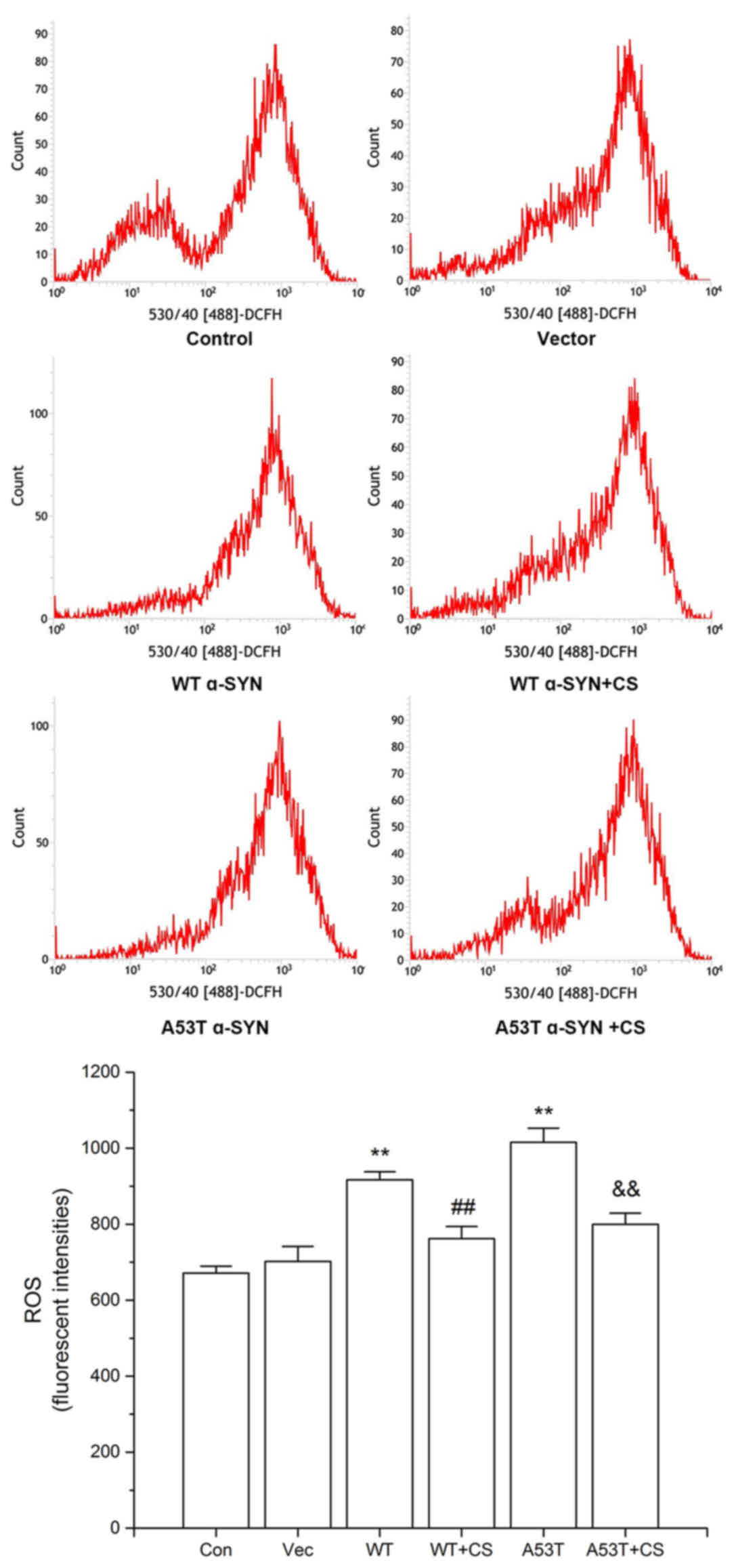 | Figure 6.Effect of CS on ROS generation. The
transfected SH-SY5Y cells were treated with 400 mg/l CS for 24 h.
ROS was determined by DCFH-DA staining. Data are presented as the
mean ± standard deviation (n=4). **P<0.01 vs. Vector group,
##P<0.01 vs. WT, &&P<0.01 vs.
A53T. ROS, reactive oxygen species; DCFH-DA, 2, 7-dichlorofuorescin
diacetate; WT, wild-type; α-SYN, α-synuclein; CS, chondroitin
sulfate; Con, control; Vec, vector. |
Effect of CS on Bcl-2, Bax and Cyt-c
protein expression levels
Compared with the vector group, the protein
expression of anti-apoptotic Bcl-2 and mitochondrial Cyt-c
in the WT α-SYN and A53T α-SYN groups were downregulaed, and
expression of pro-apoptotic Bax was upregulaed, while 400 mg/l CS
reversed these effects (P<0.01; Fig. 7).
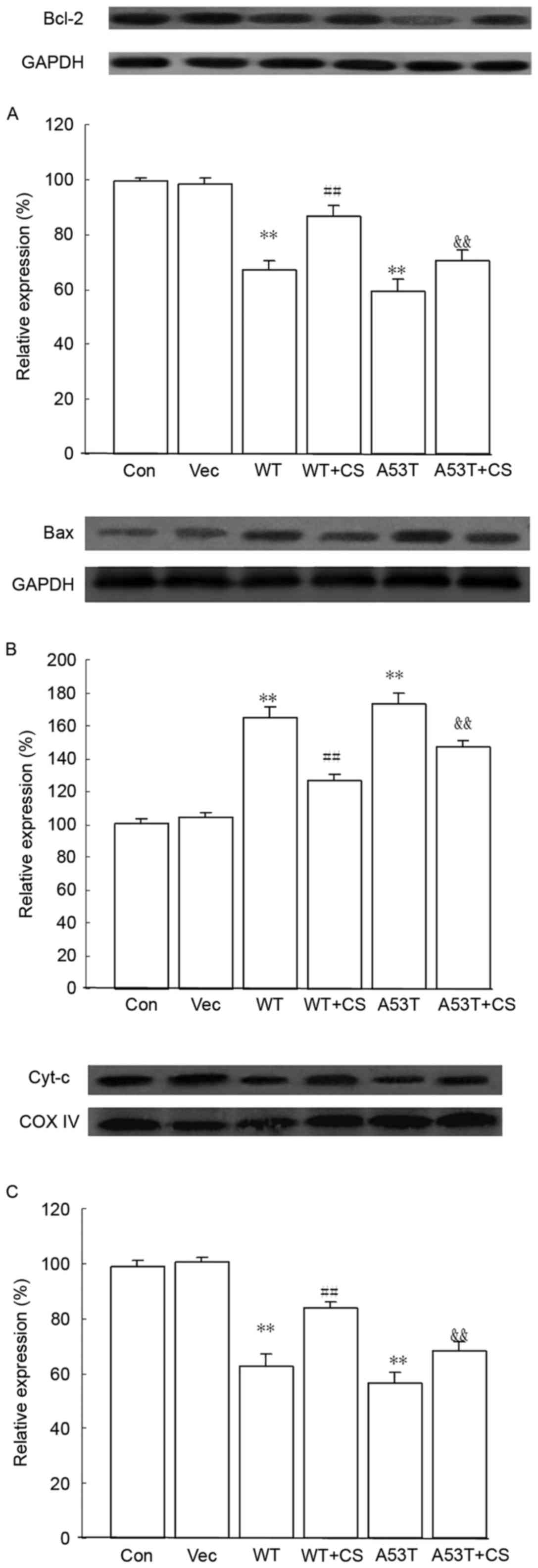 | Figure 7.Effects of CS on Bcl-2, Bax and
Cyt-c protein expression levels. The transfected SH-SY5Y
cells were treated with 400 mg/l CS for 24 h. (A) Bcl-2, (B) Bax
and (C) mitochondrial Cyt-c protein expression were assessed
by western blotting. GAPDH and COX IV served as internal loading
controls. Data are presented as the mean ± standard deviation
(n=4). **P<0.01 vs. Vector group, ##P<0.01 vs. WT,
&&P<0.01 vs. A53T. WT, wild-type; α-SYN,
α-synuclein; CS, chondroitin sulfate; Con, control; Vec, vector.
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
Cyt-c, cytochrome-c; COX IV, cytochrome c
oxidase. |
Effect of CS on activated caspase-9
and caspase-3
Compared with vector group, there was increased
caspase-3 and caspase-9 in activity in the WT α-SYN and A53T α-SYN
groups, while 400 mg/l CS inhibited the activation (P<0.05;
Table I).
 | Table I.Effects of CS on caspase-3 and
caspase-9 activities in transfected SH-SY5Y cells (n=4). |
Table I.
Effects of CS on caspase-3 and
caspase-9 activities in transfected SH-SY5Y cells (n=4).
| Group | Activated caspase-3
(%) | Activated caspase-9
(%) |
|---|
| Control |
13.99±1.03 |
14.41±1.35 |
| Vector |
13.75±1.67 |
15.66±1.85 |
| WT α-SYN |
26.97±1.30a |
31.34±2.76a |
| WT α-SYN + 400 mg/l
CS |
23.16±1.25c |
27.19±1.77b |
| A53T α-SYN |
28.33±1.53a |
32.96±1.61a |
| A53T α-SYN + 400
mg/l CS |
23.49±2.43d |
28.44±2.12d |
Discussion
Elevated α-SYN levels are deleterious to
dopaminergic neurons (16,17). Previous studies have demonstrated
that α-SYN protein aggregates, causing oxidative stress, and
increase cell vulnerability in cells and animals overexpressing
α-SYN (18–21). In the present study, the
overexpression of WT and A53T α-SYN in SH-SY5Y cells was
successfully utilized in a PD model of cytotoxicity, with cells
overexpressing a 3.5 and 3.7X increase their normal levels of the
protein. These increases of WT and A53T α-SYN in SH-SY5Y cells were
associated with decreased proliferation and increased apoptosis. It
was confirmed that overexpression of either WT or A53T α-SYN is
toxic to dopaminergic SH-SY5Y cells.
Immunohistochemical and biochemical studies have
demonstrated that ~90% α-SYN deposits in Lewy bodies are
phosphorylated at Ser129 (22,23).
Although the precise contribution of P-S129 α-SYN to the
pathogenesis of PD remains to be elucidated, recent studies have
revealed that P-Ser129 α-SYN induces intracellular aggregate
formation and endoplasmic reticulum stress (24), and accelerates A53T mutant α-SYN
neurotoxicity in a rat model of familial PD (25). This is in line with the observation
that P-Ser129 α-SYN expression increases progressively and
concomitantly with the neurodegenerative degree in mice
overexpressing α-SYN (26).
Consistently, in the present study, the cell model expressing WT
and A53T α-SYN exhibited higher levels of Ser129 phosphorylation.
Due to the general increase in total α-SYN, the increased
expression of P-Ser129 α-SYN was possibly due to accumulation of
substrate available for phosphorylation. It was observed that CS
attenuated α-SYN-induced cytotoxicity, increased cell viability,
inhibited apoptosis and decreased total α-SYN and P-Ser129 α-SYN
levels, suggesting that these processes are linked.
Small amounts of ROS are necessary to undergo normal
physiological processes. When ROS concentration greatly outnumbers
antioxidant concentration, oxidative stress arises subsequently.
Excessive accumulation of ROS contributes to neuronal losses and
dysfunction. Oxidative stress serves an important role in the
degeneration of dopaminergic neurons (27). Overexpression of WT α-SYN or its
A53T mutant forms increases intracellular ROS levels and
susceptibility to dopamine (28,29).
In this study, the levels of ROS in WT α-SYN and A53T α-SYN groups
were increased significantly; 400 mg/l CS was capable of blocking
α-SYN-induced ROS generation, which demonstrated that the
neuroprotective effect of CS may be mediated through inhibiting ROS
overproduction.
Mitochondrial dysfunction due to the accumulation of
α-SYN has been implicated as one of the mechanisms leading to PD
(30,31). α-SYN overexpression in cell culture
models and animals has demonstrated that α-SYN can cause
mitochondrial dysfunction, including mitochondrial depolarization,
Ca2+ dyshomeostasis, and Cyt-c release (18–21).
In the present study, overexpression of WT α-SYN or A53T α-SYN
impaired the mitochondrial membrane, resulting in the collapse of
ΔΨm, Cyt-c release and caspase activation, thus inducing
apoptosis. CS (400 mg/l) reduced mitochondrial transmembrane
potential loss, inhibited the release of Cyt-c from the
mitochondria and the activation of caspase-9 and caspase-3, and
inhibited apoptosis.
The anti-apoptotic Bcl-2 and pro-apoptotic Bax
proteins are key regulators of mitochondria by initiating
mitochondrial remodeling, mitochondrial outer membrane
permeabilization and the release of apoptotic factors such as
Cyt-c from the mitochondria to cytosol. It has been reported
that α-SYN regulates neuronal survival via Bcl-2 family expression
(32). High levels of α-SYN
downregulae Bcl-2 expression and upregulae Bax expression (33). The present study confirmed this in
cells overexpressing WT and A53T α-SYN. Furthermore, CS upregulaed
the anti-apoptotic Bcl-2 expression, and downregulaed the
pro-apoptotic Bax expression, then inhibited mitochondrial
dysfunction.
Our previous study demonstrated that CS protects
SH-SY5Y cells against 6-hydroxydopamine-induced injury through the
upregulaion of nuclear NF-E2-related factor-2 (Nrf2) and inhibition
of the mitochondria-mediated pathway (8). Cañas et al (7) have reported that CS protects SH-SY5Y
cells under oxidative stress conditions by activating protein
kinase C (PKC), which phosphorylates protein kinase B (Akt) via the
phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, and induces
the synthesis of the antioxidant protein heme oxygenase-1 (HO-1)
and Nrf2 nuclear translocation, which is subsequently activated by
PKC and PI3 K/Akt upstream of HO-1 expression (34,35).
Therefore, it may be hypothesized that the PI3K/Akt/Nrf2/HO-1
signaling pathway might also be responsible for the protective
effect of CS in SH-SY5Y cells overexpressing WT or A53T mutant
α-SYN.
In conclusion, to the best of our knowledge, this is
the first report to study the neuroprotective effects of CS using
α-SYN-based cell models. The data demonstrated that CS attenuates
α-SYN-induced cytotoxicity. The neuroprotective effect may be
associated with downregulation of P-Ser129 α-SYN and total α-SYN
expression, inhibiting ROS overproduction and changes of
mitochondrion mediated apoptotic pathways. Therefore, CS might be
useful agent for the treatment of α-SYN-associated
neurodegeneration.
Acknowledgements
The present study was supported by the National
Natural Science Fund (grant no. 81441094), the Natural Science
Foundation of Shandong Province (grant nos. ZR2013HQ010 and
ZR2016HM46), the China Postdoctoral Science Foundation (grant no.
2015M571998), Medical Scientific Foundation of Shandong Province
(grant no. 2013WS0256), Qingdao Municipal Science and Technology
Foundation (grant no. 13-1-3-48-nsh) and the Young Foundation of
Qingdao University.
References
|
1
|
Chiba-Falek O, Lopez GJ and Nussbaum RL:
Levels of alpha-synuclein mRNA in sporadic Parkinson disease
patients. Mov Disord. 21:1703–1708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singleton AB, Farrer M, Johnson J,
Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra
A, Nussbaum R, et al: alpha-Synuclein locus triplication causes
Parkinson's disease. Science. 302:8412002. View Article : Google Scholar
|
|
3
|
Galtrey CM and Fawcett JW: The role of
chondroitin sulfate proteoglycans in regeneration and plasticity in
the central nervous system. Brain Res Rev. 54:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Purushothaman A, Fukuda J, Mizumoto S, ten
Dam GB, van Kuppevelt TH, Kitagawa H, Mikami T and Sugahara K:
Functions of chondroitin sulfate/dermatan sulfate chains in brain
development. Critical roles of E and iE disaccharide units
recognized by a single chain antibody GD3G7. J Biol Chem.
282:19442–19452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okamoto M, Mori S, Ichimura M and Endo H:
Chondroitin sulfate proteoglycans protect cultured rat's cortical
and hippocampal neurons from delayed cell death induced by
excitatory amino acids. Neurosci Lett. 172:51–54. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato Y, Nakanishi K, Tokita Y, Kakizawa H,
Ida M, Maeda H, Matsui F, Aono S, Saito A, Kuroda Y, et al: A
highly sulfated chondroitin sulfate preparation, CS-E, prevents
excitatory amino acid-induced neuronal cell death. J Neurochem.
104:1565–1576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cañas N, Valero T, Villarroya M, Montell
E, Vergés J, García AG and López MG: Chondroitin sulfate protects
SH-SY5Y cells from oxidative stress by inducing heme oxygenase-1
via phosphatidylinositol 3-kinase/Akt. J Pharmacol Exp Ther.
323:946–953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju C, Hou L, Sun F, Zhang L, Zhang Z, Gao
H, Wang L, Wang D, Lv Y and Zhao X: Anti-oxidation and
antiapoptotic effects of chondroitin sulfate on
6-hydroxydopamine-induced injury through the up-regulation of nrf2
and inhibition of mitochondria-mediated pathway. Neurochem Res.
40:1509–1519. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McLaughlin RW, De Stigter JK, Sikkink LA,
Baden EM and Ramirez-Alvarado M: The effects of sodium sulfate,
glycosaminoglycans, and Congo red on the structure, stability, and
amyloid formation of an immunoglobulin light-chain protein. Protein
Sci. 15:1710–1722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Q, Li J, Liu C, Song C, Li P, Yin F,
Xiao Y, Li J, Jiang W, Zong A, et al: Protective effects of low
molecular weight chondroitin sulfate on amyloid beta (Aβ)-induced
damage in vitro and in vivo. Neuroscience. 305:169–182. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woods AG, Cribbs DH, Whittemore ER and
Cotman CW: Heparan sulfate and chondroitin sulfate
glycosaminoglycan attenuate beta-amyloid(25–35) induced
neurodegeneration in cultured hippocampal neurons. Brain Res.
697:53–62. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chartier-Harlin MC, Kachergus J, Roumier
C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J,
Hulihan M, et al: Alpha-synuclein locus duplication as a cause of
familial Parkinson's disease. Lancet. 364:1167–1169. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paumier KL, Rizzo SJ Sukoff, Berger Z,
Chen Y, Gonzales C, Kaftan E, Li L, Lotarski S, Monaghan M, Shen W,
et al: Behavioral characterization of A53T mice reveals early and
late stage deficits related to Parkinson's disease. PLoS One.
8:e702742013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chesselet MF: In vivo alpha-synuclein
overexpression in rodents: A useful model of Parkinson's disease?
Exp Neurol. 209:22–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lashuel HA and Hirling H: Rescuing
defective vesicular trafficking protects against alpha-synuclein
toxicity in cellular and animal models of Parkinson's disease. ACS
Chem Biol. 1:420–424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Periquet M, Fulga T, Myllykangas L,
Schlossmacher MG and Feany MB: Aggregated alpha-synuclein mediates
dopaminergic neurotoxicity in vivo. J Neurosci. 27:3338–3346. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saha AR, Ninkina NN, Hanger DP, Anderton
BH, Davies AM and Buchman VL: Induction of neuronal death by
alpha-synuclein. Eur J Neurosci. 12:3073–3077. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu LJ, Sagara Y, Arroyo A, Rockenstein E,
Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M and Masliah E:
alpha-synuclein promotes mitochondrial deficit and oxidative
stress. Am J Pathol. 157:401–410. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parihar MS, Parihar A, Fujita M, Hashimoto
M and Ghafourifar P: Mitochondrial association of alpha-synuclein
causes oxidative stress. Cell Mol Life Sci. 65:1272–1284. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parihar MS, Parihar A, Fujita M, Hashimoto
M and Ghafourifar P: Alpha-synuclein overexpression and aggregation
exacerbates impairment of mitochondrial functions by augmenting
oxidative stress in human neuroblastoma cells. Int J Biochem Cell
Biol. 41:2015–2024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin LJ, Semenkow S, Hanaford A and Wong
M: Mitochondrial permeability transition pore regulates Parkinson's
disease development in mutant α-synuclein transgenic mice.
Neurobiol Aging. 35:1132–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson JP, Walker DE, Goldstein JM, de
Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee
M, et al: Phosphorylation of Ser-129 is the dominant pathological
modification of alpha-synuclein in familial and sporadic Lewy body
disease. J Biol Chem. 281:29739–29752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujiwara H, Hasegawa M, Dohmae N,
Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K and Iwatsubo
T: alpha-Synuclein is phosphorylated in synucleinopathy lesions.
Nat Cell Biol. 4:160–164. 2002.PubMed/NCBI
|
|
24
|
Sugeno N, Takeda A, Hasegawa T, Kobayashi
M, Kikuchi A, Mori F, Wakabayashi K and Itoyama Y: Serine 129
phosphorylation of alpha-synuclein induces unfolded protein
response-mediated cell death. Biol Chem. 283:23179–23188. 2008.
View Article : Google Scholar
|
|
25
|
Sato H, Arawaka S, Hara S, Fukushima S,
Koga K, Koyama S and Kato T: Authentically phosphorylated
α-synuclein at Ser129 accelerates neurodegeneration in a rat model
of familial Parkinson's disease. J Neurosci. 31:16884–16894. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliveras-Salvá M, Van der Perren A,
Casadei N, Stroobants S, Nuber S, D'Hooge R, Van den Haute C and
Baekelandt V: rAAV2/7 vector-mediated overexpression of
alpha-synuclein in mouse substantia nigra induces protein
aggregation and progressive dose-dependent neurodegeneration. Mol
Neurodegener. 8:442013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dias V, Junn E and Mouradian MM: The role
of oxidative stress in Parkinson's disease. J Parkinsons Dis.
3:461–491. 2013.PubMed/NCBI
|
|
28
|
Junn E and Mouradian MM: Human
alpha-synuclein over-expression increases intracellular reactive
oxygen species levels and susceptibility to dopamine. Neurosci
Lett. 320:146–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Devi L, Raghavendran V, Prabhu BM,
Avadhani NG and Anandatheerthavarada HK: Mitochondrial import and
accumulation of alpha-synuclein impair complex I in human
dopaminergic neuronal cultures and Parkinson disease brain. J Biol
Chem. 283:9089–9100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chinta SJ, Mallajosyula JK, Rane A and
Andersen JK: Mitochondrial α-synuclein accumulation impairs complex
I function in dopaminergic neurons and results in increased
mitophagy in vivo. Neurosci Lett. 486:235–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Y, Duan C, Lü L, Gao H, Zhao C, Yu S,
Uéda K, Chan P and Yang H: α-Synuclein overexpression impairs
mitochondrial function by associating with adenylate translocator.
Int J Biochem Cell Biol. 43:732–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seo JH, Rah JC, Choi SH, Shin JK, Min K,
Kim HS, Park CH, Kim S, Kim EM, Lee SH, et al: α-synuclein
regulates neuronal survival via Bcl-2 family expression and PI3/Akt
kinase pathway. FASEB J. 16:1826–1828. 2002.PubMed/NCBI
|
|
33
|
Yuan Y, Jin J, Yang B, Zhang W, Hu J,
Zhang Y and Chen NH: Overexpressed alpha-synuclein regulated the
nuclear factor-kappaB signal pathway. Cell Mol Neurobiol. 28:21–33.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niture SK, Kaspar JW, Shen J and Jaiswal
AK: Nrf2 signaling and cell survival. Toxicol Appl Pharmacol.
244:37–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hwang YP and Jeong HG: Mechanism of
phytoestrogen puerarin-mediated cytoprotection following oxidative
injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and
HO-1. Toxicol Appl Pharmacol. 233:371–381. 2008. View Article : Google Scholar : PubMed/NCBI
|