Introduction
Osteonecrosis of the femoral head (ONFH) is a common
and progressive disease that predominantly impairs the femoral head
(1). With the development of the
pathological process, it often progresses and leads to the collapse
of the femoral head and osteoarthritis. Late stages of ONFH cause
severe pain and require surgical arthrodesis, which severely
affects the quality of life and the ability to work, and
simultaneously causes economic burden for patients and society.
Although multiple factors have been demonstrated to contribute to
ONFN, long-term administration of glucocorticoid hormones (GCs) is
considered a predominant cause. Currently, surgery is the primary
treatment option, which can markedly improve the symptoms among
young and middle-age patients. However, multiple revision surgeries
are frequently required during the lifetime of the patient.
Therefore, there is a need to develop novel medical therapies for
the treatment of ONFH.
Erythropoietin (EPO), a pleiotropic cytokine mainly
secreted by kidneys, stimulates the production of red blood cells
(2). Previous studies reported
beneficial effects of EPO on the non-hematopoietic system and on
the bone fracture repair process, including stimulation of
angiogenesis and bone formation (3,4).
Moreover, Bakhshi et al (5)
hypothesized that local administration of recombinant human
erythropoietin (rhEPO) combined with core decompression surgery can
enhance angiogenesis and bone regeneration in the early stages of
ONFH. However, the mechanism underlying the role of EPO on ONFH
remain to be elucidated.
The pathomechanism underlying avascular necrosis of
the femoral head induced by steroids can be ultimately ascribed to
the damage of the local vasculature and restriction of oxygen
supply to the femoral head (6).
Vascularization in bone tissue supplies nutrients for the normal
metabolic process, and angiogenesis is essential for bone tissue
regeneration in the necrotic area of the femoral head. Vascular
endothelial growth factor (VEGF) regulates the coupling of
angiogenesis and bone formation, and regeneration (7). It has been demonstrated that EPO
serves an important role in capillary vessel formation and
up-regulation of VEGF mRNA expression during tendon repair
(8). In addition, studies have
demonstrated that GCs can induce apoptosis of osteoblasts or
osteocytes in the steroid-induced ONFH (9,10).
Therefore, the authors of the present study
hypothesized that the administration of EPO prevents bone loss in a
mouse model of ONFH by increasing osteocalcin- and vascular
endothelial growth factor (VEGF)-mediated angiogenesis,
up-regulating runt-related transcription factor 2 (Runx2)-mediated
osteogenesis and inhibiting cell apoptosis.
Materials and methods
Animals
In the present study, male C57BL/6J mice (weight,
23.5–29.4 g; age, 3 months; n=80) were provided by the Center of
Experimental Animals, Zhejiang Chinese Medical University
(Hangzhou, China). Mice were kept in a temperature- and
humidity-controlled environment (22±1°C, 40–60% humidity,
atmospheric CO2) with a free access to standard pellet
food and tap water and a 12-h light/dark cycle. The present study
was approved by the local Governmental Animal Care Committee
(Hangzhou, China) and all the experimental procedures adhered to
the guidelines set by the Center of Experimental Animals, Zhejiang
Chinese Medical University (Hangzhou, China).
Model of ONFH
The model of ONFH was established to study the
effect of EPO treatment on ONFH. Mice were randomly and equally
divided into 2 groups: The EPO group and the control group. The
mouse model of ONFH was established by the administration of
prednisolone (PDS; Reyon Pharmaceutical Co., Ltd., Seoul, Korea)
and co-treatment with lipopolysaccharide (LPS; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), as previously described (11). Intraperitoneal (ip) administration
of LPS (1 mg/kg) was immediately followed by an intramuscular
injection of PDS (100 mg/kg). A second double-injection was
performed 1 week following the initial co-injection of PDS/LPS and
injections were continued every 3 days until the mice were
sacrificed. The mice in the control group were treated with the
same dosing regimen, using saline as a substitute for LPS and
PDS.
Intervention
Mice in the EPO group were intramuscularly
administered recombinant mouse EPO (500 U/kg/day; cat. no.
959-ME-010; R&D Systems, Inc., Minneapolis, MN, USA) daily
starting 1 week following the initial co-injection of PDS/LPS. The
mice in the control group were administered an equivalent amount of
saline. At 2, 4, 6 and 8 weeks following intervention, 6 mice from
each group were sacrificed by carbon dioxide (CO2)
narcosis. Subsequently, the mice were dissected and right femurs
were harvested. Following removal of the skin and muscle from the
legs, the entire femur with the hip joint was removed from the
mouse's body by cutting above the hip joint and in the knee joint.
Then the hip joint was dissected to expose the femoral head and
remove the acetabular tissue. This tissue was used for micro
computed tomography (micro-CT) and histological analyses.
Micro-CT analysis
Micro-CT was performed using a Skyscan 1172X-ray
microtomograph (resolution, 2.47 µm; Bruker Corporation, Billerica,
MA, USA). Femurs were placed vertically in the microtomograph
sample holder and images were captured at 65 kV (153 µA) using a
0.5-mm aluminum filter. A series of 483 projection images was
captured with a rotation step of 0.40° between each image for each
specimen. For each sample, 3-dimensional projection images were
reconstructed from a stack of 2-dimensional images, using the
NRecon software (version 1.6.10; Skyscan; Bruker Corporation). In
the trabecular region of the femoral head, a 0.8×0.8 mm3
region of interest (ROI) was selected in the center of the femoral
head using a semiautomatic contouring method, and the contouring of
images was performed every 50 axial slices (proximal to distal).
Bone morphometric parameters of ROI, including tissue volume (TV),
bone volume (BV) and bone mineral density (BMD), were calculated
using CTAn analysis software (version 1.15; Skyscan; Bruker
Corporation), as previously described (12).
Histological analysis
Following harvesting of right femoral heads, samples
were fixed with 10% buffered formalin for 3 days and then
decalcified in 14% ethylenediamine tetraacetic acid for 2 weeks.
Decalcified samples were embedded in paraffin and 3 µm sections
were prepared for histological analysis through haematoxylin and
eosin (H&E) staining. The sections were stained in CAT
hematoxylin (no. CATHE-MM; Biocare Medical, LLC, Pacheco, CA, USA)
for 1 min and counterstained in alcoholic-eosin (no. 17372-87-1;
Sigma-Aldrich; Merck KGaA) for 1 min. All the procedures were
performed at room temperature. Successful induction of ONFH was
defined as diffuse presence of empty lacunae or pyknotic nuclei of
bone cells in the trabecular bone, accompanied by surrounding bone
marrow cell necrosis (13,14). Histopathological changes of ONFH
were examined under a light microscope (Axio Scope A1; Carl Zeiss
AG, Oberkochen, Germany) in a blinded manner by three independent
investigators. At least 50 lacunae in each field were counted and 5
fields in each slide were selected randomly. The fraction of empty
lacunae to total lacunae was defined as the ratio of empty lacuna
number to the total lacuna number. The trabecular bone volume in
the area of femoral heads was measured by Image-Pro Plus software
(version, 6.0; Media Cybernetics, Inc., Rockville, MD, USA) and the
percentage of trabecular BV/TV of femoral heads was calculated.
Immunohistochemical analysis
To investigate the expression levels of Runx2,
osteocalcin, VEGF and platelet endothelial cell adhesion molecule
(CD31) in femoral heads, bone sections were prepared for
immunohistochemistry. Following deparaffinization in xylene and
rehydration in a descending ethanol series (two changes in 100%
ethanol, two changes in 95% ethanol, one change in 70% ethanol, and
finally in distilled water), endogenous peroxidase was quenched by
3% hydrogen peroxide (H2O2) for 20 min, and
antigen retrieval was performed using 0.01 M citrate buffer [(pH
6.0), 20 min, 95°C; cat. no. C02-02001; Bioss, Beijing, China].
Following blocking of non-specific binding sites with a normal goat
serum (1:20; cat. no. C-0005; Bioss) for 20 min at room
temperature, sections were incubated overnight with mouse
monoclonal anti-Runx2 (Cbfa1; 1:100; cat. no. D130-3; MBL
International Co., Woburn, MA, USA), rabbit polyclonal
anti-osteocalcin (1 µg/ml; cat. no. M173; Takara Biotechnology Co.,
Ltd., Dalian, China), mouse monoclonal anti-VEGF (VG-1; 1:100; cat.
no. ab1316; Abcam, Cambridge, UK) and rabbit polyclonal anti-CD31
(1:50; cat. no. ARG52748; Arigo Biolaboratories, Hsinchu City,
Taiwan) primary antibodies. Peroxidase-conjugated goat anti-mouse
(1:100; cat. no. HA1006; Hangzhou HuAan Biotechnology Co., Ltd.,
Hangzhou, China) or peroxidase-conjugated goat anti-rabbit
(1:1,000; cat. no. GS1001; Shanghai Good-Science Biotech Company,
Shanghai, China) immunoglobulin G antibodies were used as secondary
antibodies, respectively. Diaminobenzidine (DAB) served as a
chromogen, and hematoxylin served as a counterstain (20 sec, at
room temperature). Tissue sections were examined under a light
microscope (Zeiss Axio Scope A1, Carl Zeiss Co, Ltd). A total of 4
randomly-selected fields from at least 3 different tissue sections
were selected for positive staining quantification. The mean
optical density defined as the ratio of integrated optical density
(IOD) to the corresponding cavity area was calculated using
Image-Pro Plus software.
In order to further investigate angiogenesis, the
analysis of microvessel density (MVD) based on CD31-positive
staining was performed, as previously described (15). Slides were examined (magnification,
×200) to identify the highest neovascularization area (hot spot)
and a count of individual microvessels was conducted
(magnification, ×400). The brown-staining single endothelial cells
or cell clusters that visibly separated from adjacent microvessels
were defined as a countable microvessels. The MVD was calculated in
five representative areas of each slide. The evaluation was
performed in a blinded manner by three independent
investigators.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
TUNEL assay was performed on bone sections using an
ApopTag Plus Peroxidase In Situ Apoptosis kit (cat. no.
S7101; EMD Millipore, Billerica, MA, USA) according to the
manufacturer's protocol. Sections were deparaffinized, pretreated
with proteinase K (20 µg/ml; cat. no. 21627; EMD Millipore) and
endogenous peroxidase was quenched using H2O2
(3%). Bone sections were incubated with a TdT enzyme (part#90418
from ApopTag Plus Peroxidase In Situ Apoptosis kit; EMD
Millipore), visualized with DAB detection system (5 min at room
temperature) and counterstained in 0.5% (w/v) methyl green (10 min
at room temperature). Stained sections were visualized under a
light microscope (Zeiss Axio Scope A1, Carl Zeiss Co., Ltd.). Four
randomly-selected fields from at least 3 different tissue sections
were selected for positive staining quantification and the
apoptosis rate (number of TUNEL-positive cells/number of all cells)
was calculated in each section in a blinded manner by three
independent investigators.
Statistical analysis
All data are presented as the mean ± standard
deviation. Following the verification of normality of distributions
and equal variance, comparisons between groups were performed by
Student's t-test. Statistics were performed using SPSS software
(version 13.0; SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
EPO treatment improves the
microstructure of the femoral head in ONFH
The micro-CT analysis from a 3-dimensional
reconstruction demonstrated that bone trabeculae of femoral heads
were more finely spread and intact in the EPO group compared with
the control group (Fig. 1A-J).
Trabeculae gradually lost their regular structure and connectivity
in the control group. On the contrary, subchondral trabeculae in
the EPO group maintained their normal structure and gradually
recovered at 4, 6 and 8 weeks. The quantitative analysis of
micro-CT parameters, including BV/TV and BMD, confirmed that the
microstructure of subchondral trabeculae in femoral heads from the
EPO group were improved compared with the control group following 6
and 8 weeks of treatment (Fig. 1K and
L). In particular, the BV/TV ratio and BMD of femoral heads at
6 and 8 weeks demonstrated significant differences between the two
groups of interest (P<0.05; Fig. 1K
and L). However, BV/TV and BMD did not differ significantly at
2 and 4 weeks following the EPO or saline treatment. These results
suggest that prolonged administration of EPO can prevent bone loss
of the femoral head in ONFH.
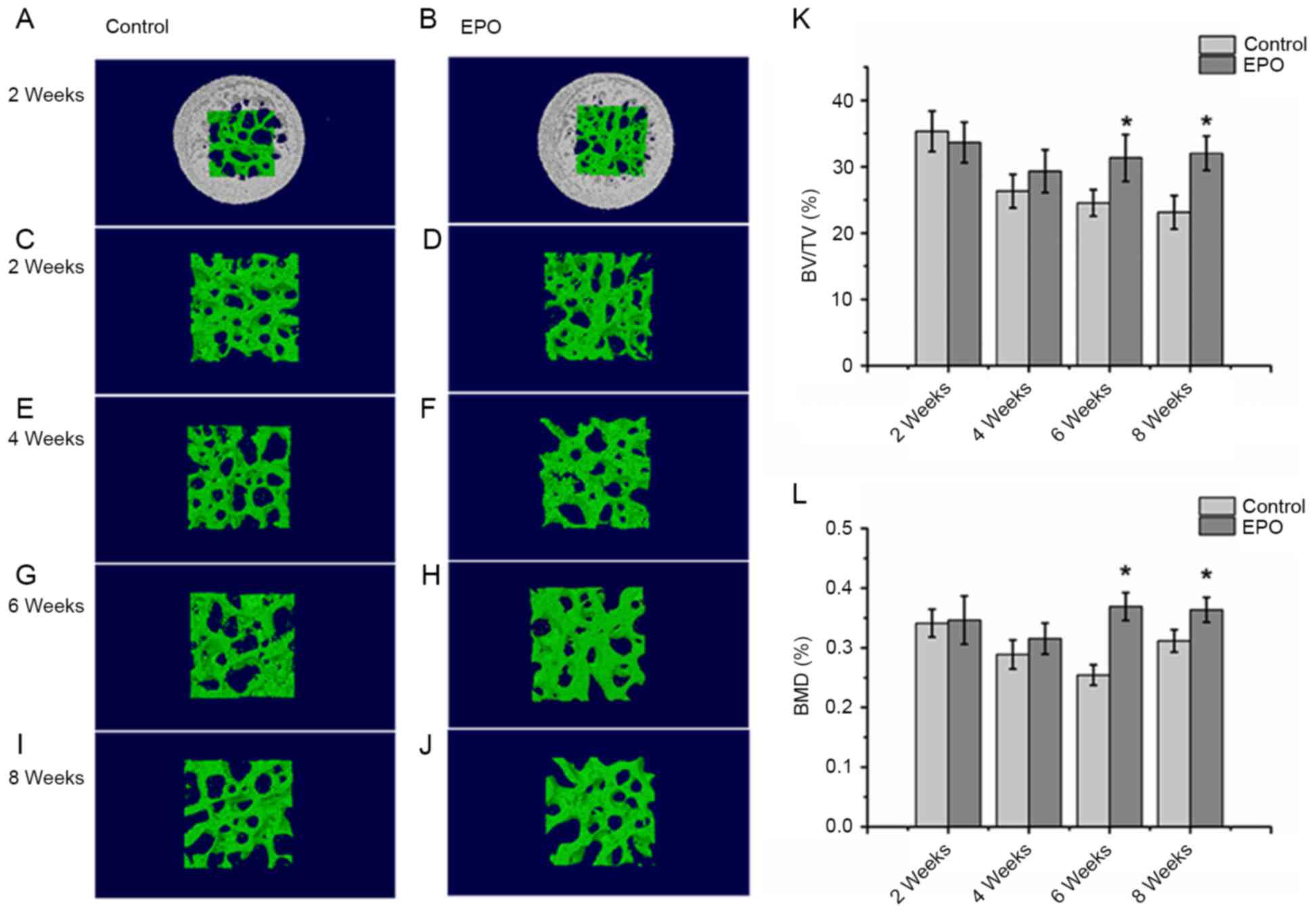 | Figure 1.Micro-CT analysis of the trabeculae of
femoral heads. At 2 weeks, the (A) control, (B) EPO, (C) control
and (D) EPO groups. At 4 weeks, the (E) control and (F) EPO groups.
At 6 weeks, the (G) control and (H) EPO groups. At 8 weeks, the (I)
control and (J) EPO groups. The trabecular bone in the EPO
treatment group recovered gradually compared with the control
group. (K) Quantitative analysis of the bone volume; and (L) bone
mineral density in the control and EPO treatment group at 2, 4, 6
and 8 weeks. The BV/TV and BMD of femoral heads increased in the
EPO treatment group at 6 and 8 weeks compared with the control
group. Data are presented as the mean ± standard deviation.
*P<0.05 vs. the control group. EPO, erythropoietin; BV/TV, bone
volume/total volume; BMD, bone mineral density; micro-CT, micro
computed tomography. |
EPO treatment reduces the rate of
empty lacunae and improves the trabecular bone volume in ONFH
As presented in Fig.
2, histological analysis of the H&E staining revealed
severely thinned and disordered trabeculae, large empty bone
lacunae and numerous pyknotic nuclei of osteocytes in the femoral
heads from the control group, which indicated that an animal model
of ONFH was successfully established (Fig. 2A, C, E and G). In contrast, the
histological analysis of femoral heads from the EPO group
demonstrated that the microstructure of trabeculae gradually
recovered (Fig. 2B, D, F and H).
Consistent with the findings of the micro-CT assay, histological
analyses of femoral heads demonstrated that trabecular bone volume
displayed an increasing trend in mice in the EPO treatment group.
Statistical analyses were performed between groups at multiple time
points. BV/TV at 6 and 8 weeks in the EPO group was increased
compared with the control group at the respective time points
(P<0.05; Fig. 2I). In addition,
in the EPO group, BV/TV at 8 weeks was increased compared with at 2
weeks (P<0.05; Fig. 2I).
Moreover, the proportion of empty bone lacunae at 2 and 4 weeks in
the control group was higher than in the EPO group at corresponding
time points (P<0.05; Fig. 2J).
These data are consistent with the hypothesis that administration
of EPO can prevent bone loss in ONFH.
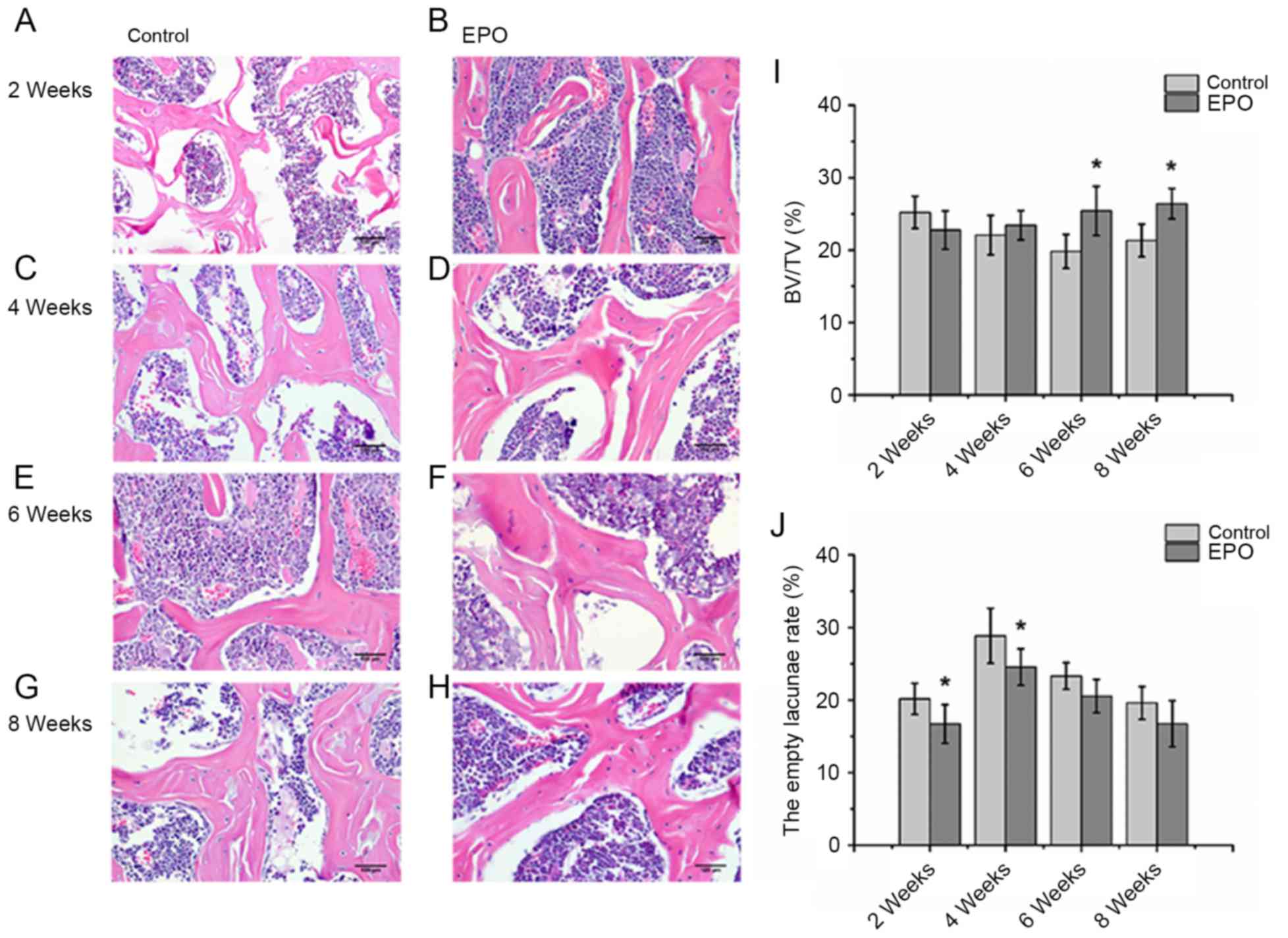 | Figure 2.Histopathological analysis of the
trabecular bone in the femoral head. At 2 weeks, the (A) control,
(B) EPO, (C) control and (D) EPO groups. At 4 weeks, the (E)
control and (F) EPO groups. At 6 weeks, the (G) control and (H) EPO
groups. At 8 weeks, the (I) control and (J) EPO groups. Severely
thinned and disordered trabeculae, large empty bone lacunae and
numerous pyknotic nuclei of osteocytes were evident in femoral
heads from the control group, whereas the morphological structure
of EPO treatment group relatively improved (magnification, ×400).
(I) Quantitative analysis of bone volume; and (J) empty lacunae
rate in the control and EPO treatment group at 2, 4, 6 and 8 weeks.
The BV/TV of femoral heads increased in the EPO treatment group at
6 and 8 weeks compared with the control group. The empty lacunae
rate in femoral heads decreased in the EPO treatment group at 2 and
4 weeks compared with the control group. Data are presented as the
mean ± standard deviation. *P<0.05 vs. the control group. EPO,
erythropoietin; BV/TV, bone volume/total volume. |
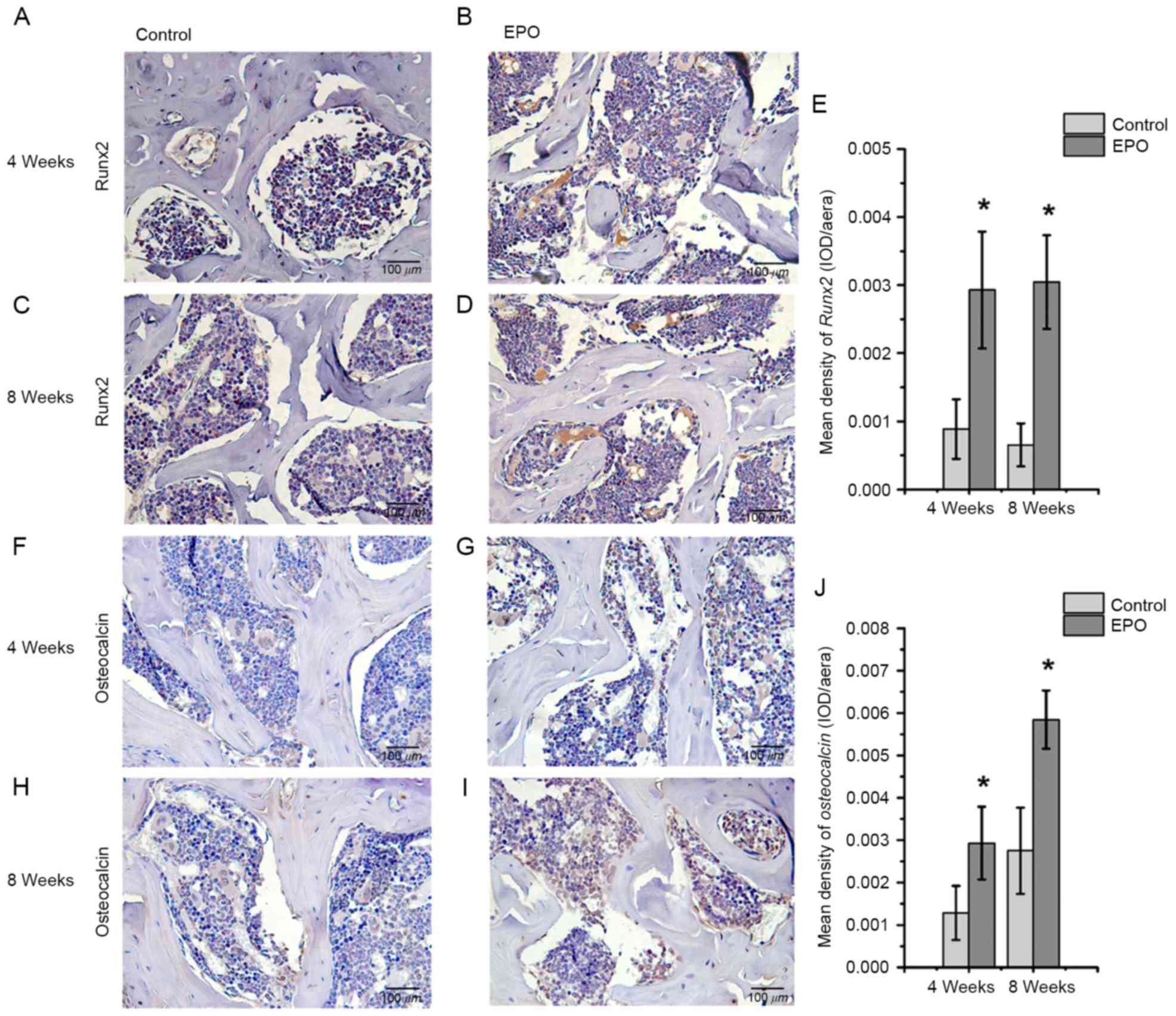
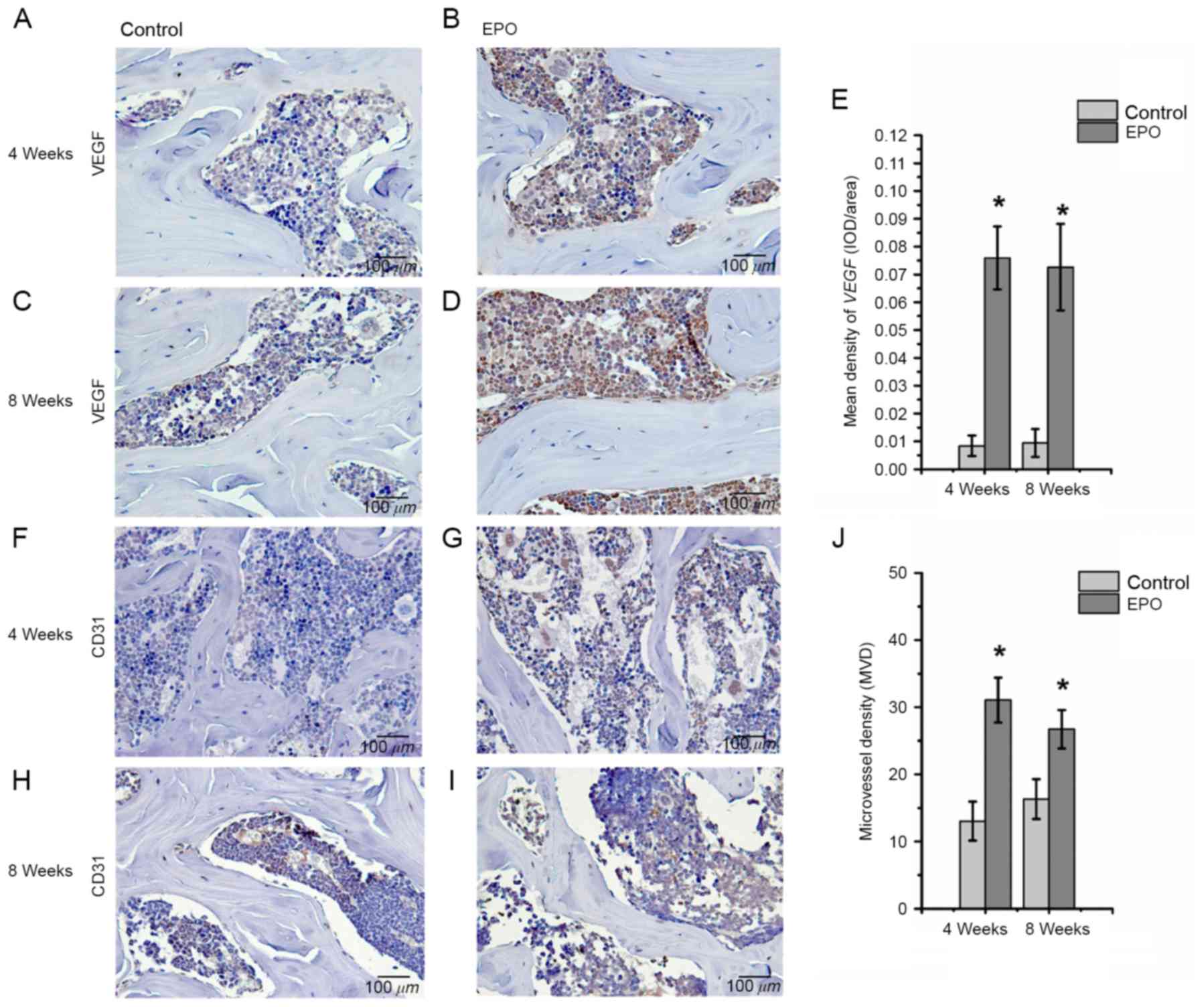
EPO treatment promotes expression of
Runx2, osteocalcin, VEGF and CD31 in ONFH
To further investigate the effect of EPO on ONFH at
4 and 8 weeks following treatment with EPO, Runx2, osteocalcin,
VEGF and CD31 were examined in the femoral heads by
immunohistochemistry. The authors of the present study identified
that positive signals for Runx2 and osteocalcin were significantly
higher in the EPO group than in the control group (P<0.05;
Fig. 3). In addition, an increased
abundance of VEGF-positive staining was observed predominantly in
the bone marrow cavity of femoral heads from the EPO group compared
with the control group (P<0.05; Fig. 4A-E) and the abundance of
CD31-positive staining was increased in subchondral trabecular bone
of necrotic femoral heads in the EPO group compared with the
control group (Fig. 4F-I). The
analysis of MVD, based on CD31-positive staining, demonstrated that
few microvessels were present in subchondral trabecular bones of
necrotic femoral heads in the control group at 4 and 8 weeks, while
the number of microvessels in the EPO group increased significantly
(P<0.05; Fig. 4J). These data
suggest that EPO can prevent bone loss in ONFH through enhancing
Runx2-mediated osteogenesis and VEGF-mediated angiogenesis.
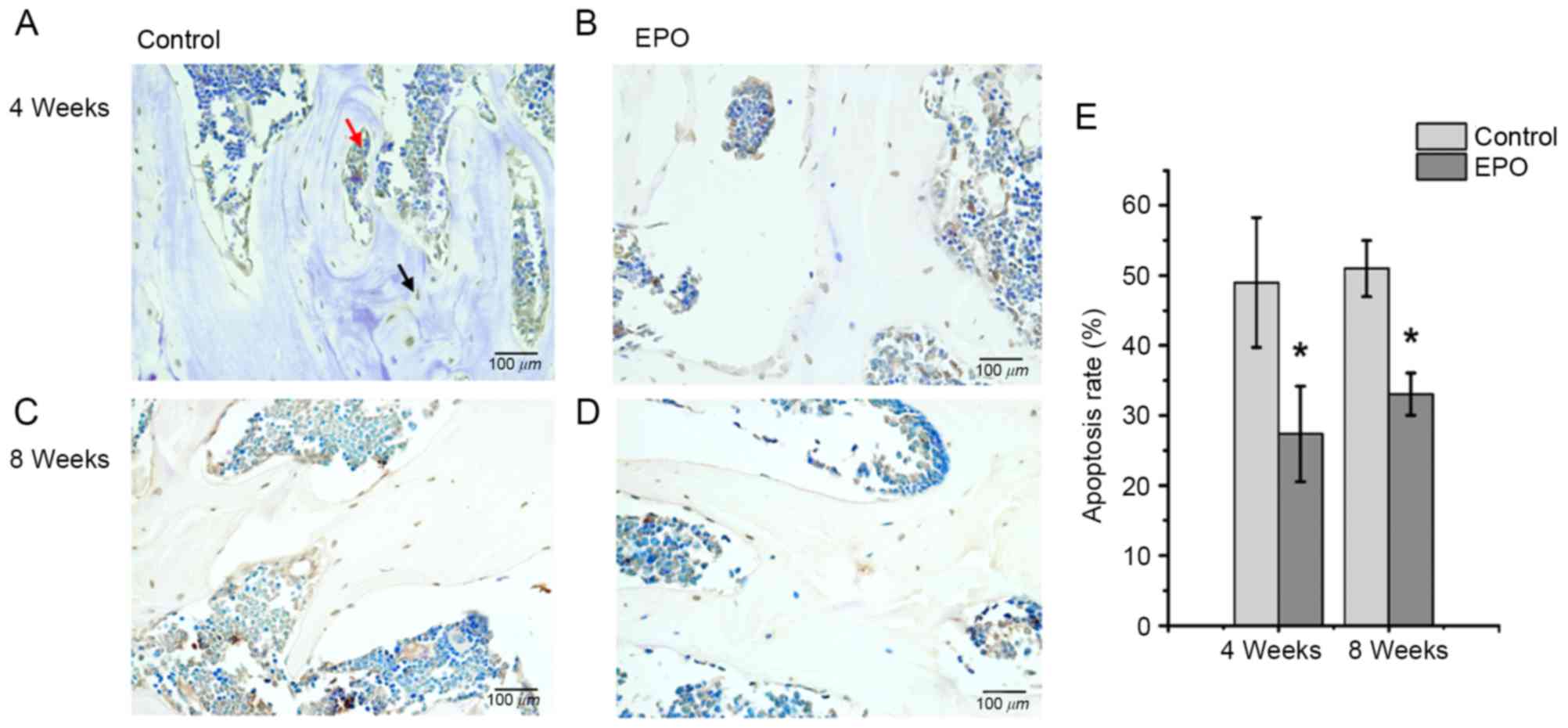
EPO treatment inhibits apoptosis
induced by ONFH
At 4 and 8 weeks following treatment with EPO, TUNEL
assays were performed to verify whether administration of EPO
affected apoptosis associated with ONFH. It was determined that the
treatment with EPO led to decreased abundance of apoptotic cells,
both in the trabecular bone and bone marrow cavity at the same time
points (Fig. 5A-D). In addition,
quantitative analysis confirmed a significantly increased apoptosis
rate in the control group compared with the EPO group at 4 and 8
weeks (P<0.05; Fig. 5E). These
results suggest that the administration of EPO can inhibit
apoptosis in the trabecular bone and bone marrow cavity in
ONFH.
Discussion
ONFH is a multifactorial disease and a leading cause
of mobility impairment. Long-term administration of GCs is
considered one of the predominant pathological factors stimulating
ONFH development and progression, leading to disruption of femoral
head blood supply in the majority of cases. Resulting insufficient
blood supply further affects the femoral head and eventually leads
to its collapse and the development of hip osteoarthritis in
patients (16). At present, there
are no effective treatments for ONFH and the current therapy
focuses on the management of symptoms. Hip replacement is the only
effective treatment of choice. Therefore, there is a need for
alternative interventions to treat ONFH and reduce patient
morbidity and economic impact.
In previous studies, angiogenic and growth factors
demonstrated promising effects on bone formation and regeneration
in ONFH, including VEGF, fibroblast growth factors and hepatocyte
growth factor (17–19). Several angiogenic factors could in
theory be used for clinical treatment, but they could also induce
severe complications, including an increased intraosseous pressure
due to vascular permeability, and arterial obliteration due to
vascular smooth muscle proliferation (19). EPO is one of the most suitable
candidates for the treatment of ONFH since it promotes bone
regeneration (3,4) and has been clinically used for the
treatment of chronic anemia with few side effects (20). However, it has been demonstrated
that systemic administration of EPO can deteriorate blood supply
and blood viscosity as it increases RBC mass, and induces
hypertension and thromboembolic events (21). In addition, a previous study
reported that administration of 5,000 U/kg/day EPO can
significantly increase the hematocrit, which can aggravate ONFH
through impaired blood flow and nutrition and perfusion (22). In order to avoid these
complications, the authors of the present study decided to
intramuscularly administer 500 U/kg/day EPO. During the
experimental period, no systemic side effects were observed.
Results from Micro-CT and histological analyses
clearly demonstrated that administration of EPO restored
microstructure of the femoral head by improving the trabecular bone
volume and reducing the rate of empty lacunae in ONFH. These
observations are consistent with other studies which demonstrated
that EPO treatment can enhance bone regeneration (5). Therefore, EPO treatment can improve
ONFH in mice.
In order to further elucidate the mechanism
underlying EPO-induced stimulation of bone formation in
vivo, immunohistochemical analysis of Runx2 and osteocalcin was
performed. Runx2 is a key transcription factor associated with
osteoblast differentiation, and osteocalcin is a biochemical marker
secreted by mature osteoblasts (23). Immunohistochemistry of femoral
heads indicated that EPO treatment up-regulated the expression of
Runx2 and osteocalcin at 4 and 8 weeks. These results suggest that
EPO may stimulate bone formation through Runx2-mediated
osteogenesis. Other beneficial effects of EPO treatment include
increased VEGF-associated angiogenesis (4). As a significant component of skeletal
development and repair, angiogenesis contributes to the process of
bone development and repair through adequate formation of new
capillaries from existing vessels (24). In addition, GCs inhibit the
expression of VEGF in femoral heads and consequently limit
angiogenesis and osteogenesis in ONFH induced by GCs (7). Experiments in vitro
demonstrated that primary osteoblasts derived from clinically
osteoarthritic femoral heads displayed downregulation of VEGF
following co-incubation with GCs for 24 h (25). In the present study,
immunohistochemical analysis of VEGF demonstrated that the
expression VEGF can be upregulated in the femoral heads in ONFH
following treatment with EPO, compared with the control group.
CD31, a member of the Ig superfamily of cell adhesion molecules, is
commonly used to indicate the presence of endothelial cells
(26). An analysis of MVD was
performed based on CD31-positive staining in femoral heads and it
was demonstrated that microvessels were increased in the
subchondral trabecular bone of necrotic femoral heads following
treatment with EPO for 4 and 8 weeks, compared with the control
group. Taken together, administration of EPO prevented bone loss in
ONFH partially by increasing VEGF-mediated angiogenesis. It has
been reported that VEGF is important for bone remodeling and
regeneration as it effectively couples angiogenesis and
osteogenesis in the bone microenvironment (27), but whether EPO-induced osteogenesis
is coupled with VEGF remains to be elucidated.
Apoptosis of osteoblasts and osteocytes is a common
pathogenic pathway of GC-induced ONFH (28). A previous study suggested that
apoptosis partially causes cell death in the femoral head of
patients with ONFH (29). In
addition, O'Brien et al (30) reported that GCs could directly
induce apoptosis of osteoblasts and osteocytes. Moreover, once the
blood supply from collateral circulation is restricted without
enough capillary arterialization in GC-induced ONFH, the lack of
available oxygen and nutrients may cause apoptosis of osteoblasts
and osteocytes (31). Results of
the TUNEL assay performed in the present study demonstrated that
apoptosis occurred in osteocytes and bone marrow cells in the mice
model of ONFH, while the apoptosis index in the EPO treatment group
was significantly reduced at 4 and 8 weeks, suggesting that EPO
could interfere with apoptosis and prevent bone loss in
steroid-associated ONFH.
In conclusion, the present study demonstrated that
the administration of EPO prevents bone loss by up-regulation of
Runx2-mediated osteogenesis, an increase in VEGF-mediated
angiogenesis and inhibition of cell apoptosis in the ONFH mouse
model. Therefore, EPO is a promising agent for the treatment of
ONFH. Further research will elucidate the mechanism underlying the
effect of EPO treatment on the prevention of bone loss during ONFH
while avoiding induction of side effects.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grants nos. 81273770 and
81573994), the Natural Science Foundation of Zhejiang Province
(grant no. LY16H270010), Traditional Chinese Medical Administration
of Zhejiang Province (grant no. 2016ZA048) and the Cultivation
Program for Innovative Talent Graduate Students (grant no.
311100G00901). The present study was also supported by the Program
for Zhejiang Leading Team of S&T Innovation and Key Laboratory
of Zhejiang Province.
References
|
1
|
Erlebacher A, Filvaroff EH, Gitelman SE
and Derynck R: Toward a molecular understanding of skeletal
development. Cell. 80:371–378. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lombardero M, Kovacs K and Scheithauer BW:
Erythropoietin: A hormone with multiple functions. Pathobiology.
78:41–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hankenson KD, Dishowitz M, Gray C and
Schenker M: Angiogenesis in bone regeneration. Injury. 42:556–561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holstein JH, Orth M, Scheuer C, Tami A,
Becker SC, Garcia P, Histing T, Mörsdorf P, Klein M, Pohlemann T
and Menger MD: Erythropoietin stimulates bone formation, cell
proliferation, and angiogenesis in a femoral segmental defect model
in mice. Bone. 49:1037–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bakhshi H, Rasouli MR and Parvizi J: Can
local Erythropoietin administration enhance bone regeneration in
osteonecrosis of femoral head? Med Hypotheses. 79:154–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Childs SG: Osteonecrosis: Death of bone
cells. Orthop Nurs. 24:295–303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weinstein RS, Wan C, Liu Q, Wang Y,
Almeida M, O'Brien CA, Thostenson J, Roberson PK, Boskey AL,
Clemens TL and Manolagas SC: Endogenous glucocorticoids decrease
skeletal angiogenesis, vascularity, hydration, and strength in aged
mice. Aging Cell. 9:147–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uslu M, Kaya E, Yaykasli KO, Oktay M,
Inanmaz ME, Işık C, Erdem H, Erkan ME and Kandiş H: Erythropoietin
stimulates patellar tendon healing in rats. Knee. 22:461–468. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Zou YL, Ma J, Dang XQ and Wang
KZ: Apoptosis associated with Wnt/β-catenin pathway leads to
steroid-induced avascular necrosis of femoral head. BMC
Musculoskelet Disord. 16:1322015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weinstein RS, Nicholas RW and Manolagas
SC: Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis
of the hip. J Clin Endocrinol Metab. 85:2907–2912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryoo S, Lee S, Jo S, Lee S, Kwak A, Kim E,
Lee J, Hong J, Jhun H, Lee Y, et al: Effect of lipopolysaccharide
(LPS) on mouse model of steroid-induced avascular necrosis in the
femoral head (ANFH). J Microbiol Biotechnol. 24:394–400. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Müller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin L, Zhang G, Sheng H, Yeung KW, Yeung
HY, Chan CW, Cheung WH, Griffith J, Chiu KH and Leung KS: Multiple
bioimaging modalities in evaluation of an experimental
osteonecrosis induced by a combination of lipopolysaccharide and
methylprednisolone. Bone. 39:863–871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okazaki S, Nishitani Y, Nagoya S, Kaya M,
Yamashita T and Matsumoto H: Femoral head osteonecrosis can be
caused by disruption of the systemic immune response via the
toll-like receptor 4 signalling pathway. Rheumatology (Oxford).
48:227–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Babis GC, Sakellariou V, Parvizi J and
Soucacos P: Osteonecrosis of the femoral head. Orthopedics.
34:392011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Young S, Patel ZS, Kretlow JD, Murphy MB,
Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M and
Mikos AG: Dose effect of dual delivery of vascular endothelial
growth factor and bone morphogenetic protein-2 on bone regeneration
in a rat critical-size defect model. Tissue Eng Part A.
15:2347–2362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuroda Y, Akiyama H, Kawanabe K, Tabata Y
and Nakamura T: Treatment of experimental osteonecrosis of the hip
in adult rabbits with a single local injection of recombinant human
FGF-2 microspheres. J Bone Miner Metab. 28:608–616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wen Q, Ma L, Chen YP, Yang L, Luo W and
Wang XN: Treatment of avascular necrosis of the femoral head by
hepatocyte growth factor-transgenic bone marrow stromal stem cells.
Gene Ther. 15:1523–1535. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haroon ZA, Amin K, Jiang X and Arcasoy MO:
A novel role for erythropoietin during fibrin-induced wound-healing
response. Am J Pathol. 163:993–1000. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maiese K, Chong ZZ and Shang YC: Raves and
risks for erythropoietin. Cytokine Growth Factor Rev. 19:145–155.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rezaeian F, Wettstein R, Amon M, Scheuer
C, Schramm R, Menger MD, Pittet B and Harder Y: Erythropoietin
protects critically perfused flap tissue. Ann Surg. 248:919–929.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD,
Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al:
Endocrine regulation of energy metabolism by the skeleton. Cell.
130:456–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Winet H: The role of microvasculature in
normal and perturbed bone healing as revealed by intravital
microscopy. Bone. 19(1 Suppl): 39S–57S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varoga D, Drescher W, Pufe M, Groth G and
Pufe T: Differential expression of vascular endothelial growth
factor in glucocorticoid-related osteonecrosis of the femoral head.
Clin Orthop Relat Res. 467:3273–3282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sumpio BE, Yun S, Cordova AC, Haga M,
Zhang J, Koh Y and Madri JA: MAPKs (ERK1/2, p38) and AKT can be
phosphorylated by shear stress independently of platelet
endothelial cell adhesion molecule-1 (CD31) in vascular endothelial
cells. J Biol Chem. 280:11185–11191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clarkin CE and Gerstenfeld LC: VEGF and
bone cell signalling: An essential vessel for communication? Cell
Biochem Funct. 31:1–11. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weinstein RS: Glucocorticoid-induced
osteonecrosis. Endocrine. 41:183–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calder JD, Buttery L, Revell PA, Pearse M
and Polak JM: Apoptosis-a significant cause of bone cell death in
osteonecrosis of the femoral head. J Bone Joint Surg Br.
86:1209–1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Brien CA, Jia D, Plotkin LI, Bellido T,
Powers CC, Stewart SA, Manolagas SC and Weinstein RS:
Glucocorticoids act directly on osteoblasts and osteocytes to
induce their apoptosis and reduce bone formation and strength.
Endocrinology. 145:1835–1841. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaushik AP, Das A and Cui Q: Osteonecrosis
of the femoral head: An update in year 2012. World J Orthop.
3:49–57. 2012. View Article : Google Scholar : PubMed/NCBI
|