Introduction
Cystic fibrosis (CF) is an autosomal recessive
genetic disorder caused by mutations of the gene that encodes the
cystic fibrosis transmembrane conductance regulator (CFTR) protein
(1). This protein is a chloride
(or bicarbonate)-conducting anion channel expressed on the membrane
of epithelial cells of the lung, intestines and pancreas, which
modulates electrolytic exchange. Therefore, mutations in CFTR,
leading to altered ion transport, induce a progressive
deterioration of the target organs and, consequently, premature
mortality of patients (2). CFTR
mutations may induce alterations in maturation, subcellular
localization (trafficking) and activity (gating) of the CFTR
protein (3,4). The predominant CFTR mutation is
termed Phe508del (F508del), and results in severely impaired
protein maturation with a consequent alteration of CFTR membrane
translocation (5,6). However, in the case that the
F508del-CFTR is able to reach the plasma membrane, it exhibits
reduced activity and stability in the membrane, in addition to a
greater tendency towards degradation by the ubiquitin/proteasome
system (7,8).
Research has primarily focused on the identification
and development of modulators that are able to resolve gating and
trafficking problems (9). The
‘corrector’ acts on trafficking and promotes CFTR membrane
localization (10,11) while the ‘potentiator’ acts on
gating and leads to an increase in CFTR activity (12). Therefore, any therapy should be
designed by choosing the most appropriate modulator which is able
to target the specific mutation. However, in patients with
F508del-CFTR, it has been demonstrated that treatment with a
potentiator is ineffective, since it is unable to induce CFTR
maturation and membrane translocation. Therefore, in these
patients, the co-administration of a corrector and a potentiator is
necessary to allow for the almost complete repair of CFTR
trafficking and gating (13).
At present, there are two molecules which are
frequently used: The potentiator VX770 (ivacaftor) and the
corrector VX809 (lumacaftor). Recently, the use of
Orkambi®, which is a combination of VX770 and VX809, was
approved (3). However, though an
improvement in the quality of life of patients treated with the
Orkambi was observed, the drug induced a modest improvement in lung
function (14). This limited
effect may be due to an inhibitory action exerted by VX770 on VX809
(15). In order to overcome this
problem, research is focusing on the analysis of natural molecules
that may resolve the problem at its origin; among them, matrine, an
alkaloid extracted from roots of Sophora flavescens, appears
to provide promising results. In particular, matrine, by
interacting with the heat shock cognate (HSC)/heat shock 70 kDa
protein 1A (HSP70) chaperone system, led to an increase in
F508del-CFTR membrane localization (16), and the corrector activity of
matrine was observed at high doses (0.4–0.8 mM) which may be toxic
for long-term treatment of patients with CF.
The aim of the present study was to investigate
whether lower concentrations (30 µM) of matrine (PubChem CID:
91466) may be able to render more effective the action of the known
corrector VX809 (PubChem CID: 16678941) and the potentiator VX770
(PubChem CID: 16220172), in addition to that of two
dihydropyridines, FD-1 (F508act-05) (17) and FD-2, the latter being a
newly-synthesized compound. The use of dihydropyridines may be
advantageous, since these molecules were established as
potentiators with notable activity, simple synthesis and a low
production cost, by Pedemonte et al (18), Cateni et al (19) and Giampieri et al (20). Due to the cited interference
between VX770 and VX809, it was deemed noteworthy to investigate
the behavior of this class of potentiators, coupled with the
corrector VX809. Therefore, FD-1, a compound with a moderate level
of activity against FD-2, was selected. A previous study on
asymmetrical dihydropyridines demonstrated the ability of the
benzyl group to maximize potentiator activity (20), which provided the basis for the
analysis of a newly-synthesized dihydropyridine (FD-2) bearing two
benzyl groups at the ester level.
Materials and methods
Cell culture and treatments
Fischer rat thyroid (FRT) cells, stably transfected
with F508del-CFTR and yellow fluorescent protein (YFP), were
provided by Dr L.J. Galietta (G. Gaslini Institute, Genoa, Italy).
The co-expressed YFP acts as a halide sensitive dye that may be
utilized to measure the anion permeability of CFTR (18,21).
Cells were cultured in Coon's modified F-12 medium (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) supplemented with 5% fetal bovine
serum (EuroClone SpA, Pero, Italy), 2 mM glutamine (EuroClone SpA),
1% penicillin/streptomycin (EuroClone SpA), 0.8 mg/ml zeocin
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1.5 mg/ml
G418 (Sigma-Aldrich; Merck KGaA). Cells were plated
(1×105 cells/well) into 96-well microplates and treated
with matrine (30 µM; Sigma-Aldrich; Merck KGaA) for 24, 48 and 72
h. In a series of experiments, cells were co-treated for 24, 48 or
72 h with 2 µM VX809 (Selleck Chemicals, Houston, TX, USA) and/or
with one of three potentiators: 10 µM VX770 (Selleck Chemicals), 10
µM FD-1 and 10 µM FD-2 (both synthesized in the Department of
Pharmacy, University of Genoa, Genoa, Italy).
The stock solutions of all tested compounds were
prepared in dimethyl sulfoxide (DMSO) and pilot studies
demonstrated that the final DMSO concentration did not alter any of
the cellular responses analyzed. In addition, under all conditions,
the data obtained in treated cells was compared to DMSO-treated
cells (Ctr). The pilot study was performed by treating cells with
the highest dose of DMSO used to dissolve all tested compounds for
24, 48 and 72 h. Then, cell viability was evaluated by MTT
assay.
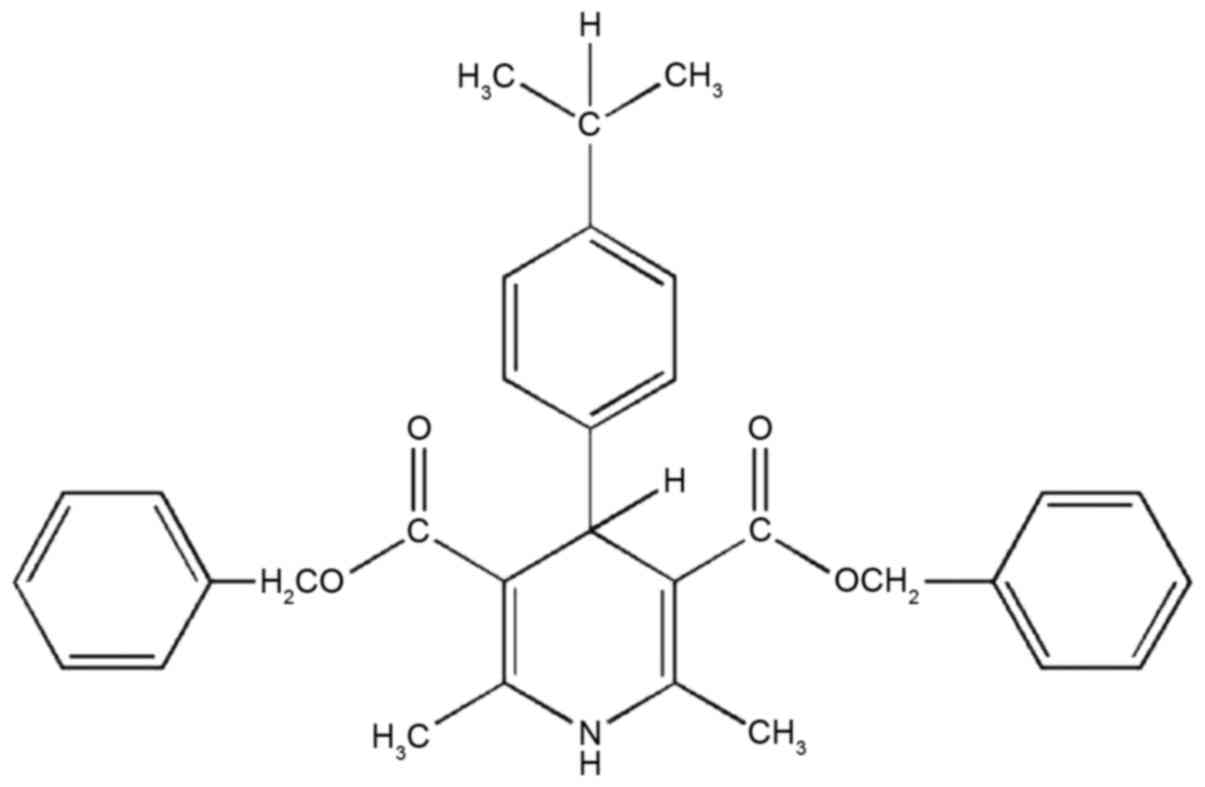
FD-2 was synthesized following the procedure
described in Cateni et al (19). Benzyl acetoacetate,
4-isopropylbenzaldehyde and ammonia were dissolved in isopropanol
and refluxed for 4 h. Subsequently, the crude product was subjected
to chromatography on a silica gel (n-hexane/diethylether) and the
residue was crystallized from cyclohexane. Yield, 25%; melting
point 107–109°C. 1H-nuclear magnetic resonance
(CDCl3): δ1.22–1.27 [m, 6H, CH
(CH3)2]; 2.34 (s, 6H, CH3); 2.80
[m, 1H, CH (CH3)2]; 5.09–5.13 (m, 5H,
2CH2 + H-4); 5.78 (br s, 1H, NH); and 7.04–7.32 (m, 14H
Ar). Infrared (KBr): 3439 (NH); and 1691 (CO) cm−1.
Combustion elemental analysis calculated for
C32H33NO4: C 77.55, H 6.71, N
2.83; observed: C 77.57, H 6.40, N 2.97 (Fig. 1).
MTT assay
Cell viability was determined using MTT
(Sigma-Aldrich; Merck KGaA) staining (22,23).
Cells were seeded into 96-well microplates (Corning Incorporated,
Corning, NY, USA) at a density of 1×105 cells/well and
treated as described above. Subsequently, the cells were incubated
with 0.5 mg/ml MTT for 3 h at 37°C. Following incubation, the
supernatant was discarded, the insoluble formazan precipitates were
dissolved in HCl (0.1 M in isopropanol) and the absorbance at
570/630 nm was recorded using a microplate reader (EL-808; BioTek
Instruments Inc., Winooski, VT, USA).
Fluorescence assay
CFTR activity was determined using a fluorescence
assay (18,21,24).
Cells were plated (100,000 cells/well) into black 96-well
microplates with clear plastic bottoms (Corning Incorporated).
Following treatment as described above, cells were washed with PBS
(137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5
mM KH2PO4, 1 mM CaCl2 and 0.5 mM
MgCl2; pH 7.4) and stimulated for 20 min with 20 µM
forskolin. Microplates were subsequently transferred to a
microplate reader (TECAN Infinite® F200 PRO; Tecan
Group, Ltd., Männedorf, Switzerland) equipped with excitation
(485±20 nm) and emission (535±25 nm) filters. Each assay consisted
of a continuous 14 sec fluorescence reading with 2 sec prior to and
12 sec following the injection of 165 µl iodide-containing PBS (PBS
with Cl− replaced with I−). The I−
influx rate was evaluated by calculating the variation of
fluorescence intensity prior to and following the I−
injection.
Data analysis
Results are expressed as the mean ± standard error
of the mean from at least three independent experiments. The
statistical significance of any parametric differences among the
sets of experimental data was evaluated using one-way analysis of
variance and Dunnett's test for multiple comparisons. GraphPad
Prism software (version 4; GraphPad Software, Inc., La Jolla, CA,
USA) was used for analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
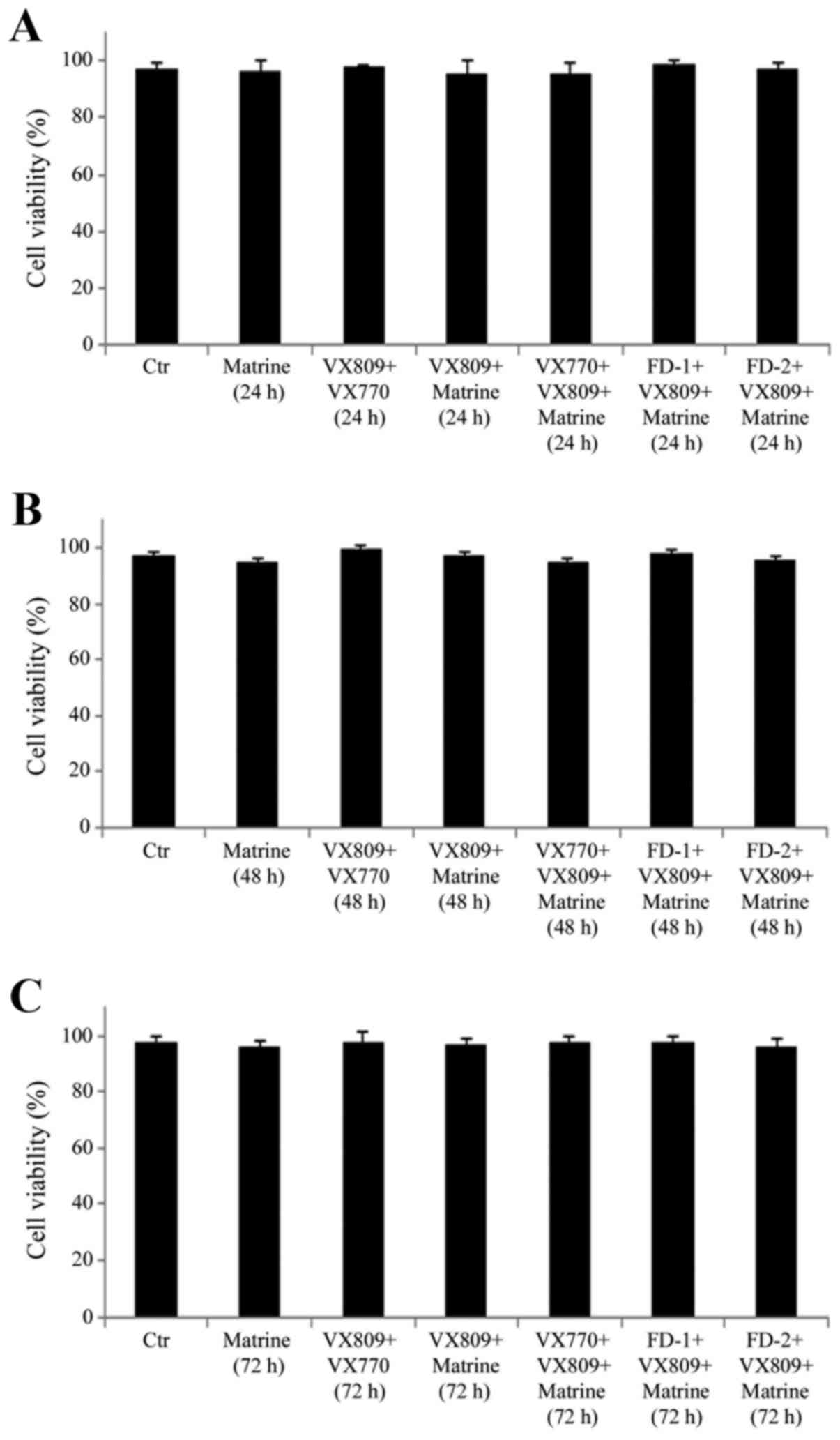
Treatment with matrine, VX809 or
potentiators (VX770, FD-1 and FD-2), alone or in combination, does
not affect the via-bility of F508del-CFTR FRT cells
F508del-CFTR FRT cells were exposed for 24, 48 and
72 h to matrine, VX809, VX770, FD-1 and FD-2, alone or in
combination, and MTT analysis revealed that none of the treatments
was cytotoxic (Fig. 2).
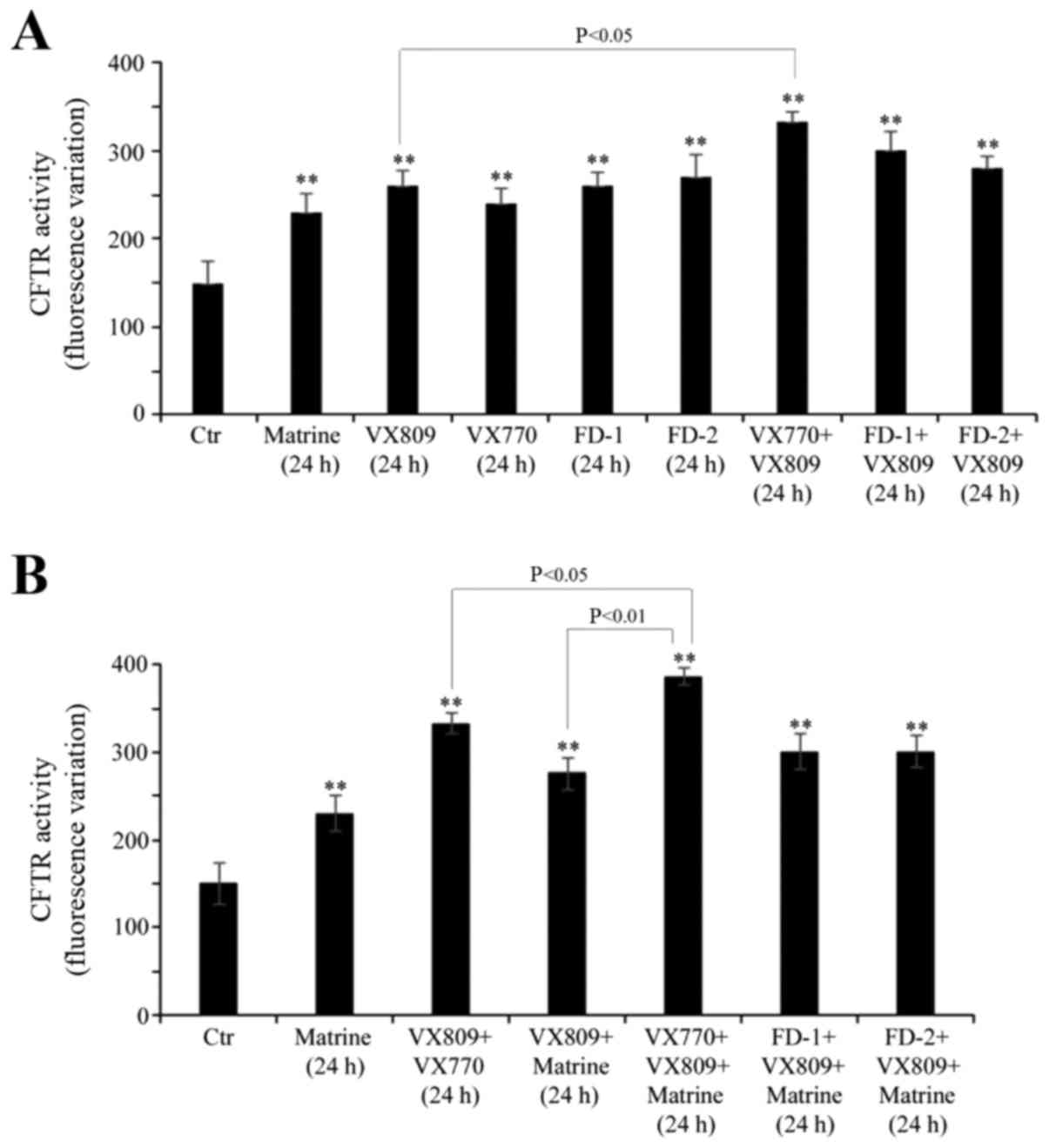
Treatment with matrine alone, and in
combination with VX809 plus VX770, is able to stimulate CFTR
activity
As exhibited in Fig.
3A, matrine alone stimulated the activity of mutant CFTR by 53%
compared with untreated cells, which was a similar result to that
observed when the cells were treated with the other compounds
individually. In addition, a 24-h VX809/VX770 co-treatment
stimulated the effect of VX809 on CFTR activity by 30% (Fig. 3A).
In order to evaluate whether matrine was able to
increase the VX809-induced CFTR activity, other experiments were
performed by treating the cells for 24 h with a combination of
matrine, VX809 and/or potentiators. As illustrated in Fig. 3B, all the combinations tested were
able to stimulate CFTR activity by 80–100% compared with control
cells. In addition, the presence of matrine further stimulated the
effect of the VX809/VX770 combination on CFTR activity by 16%.
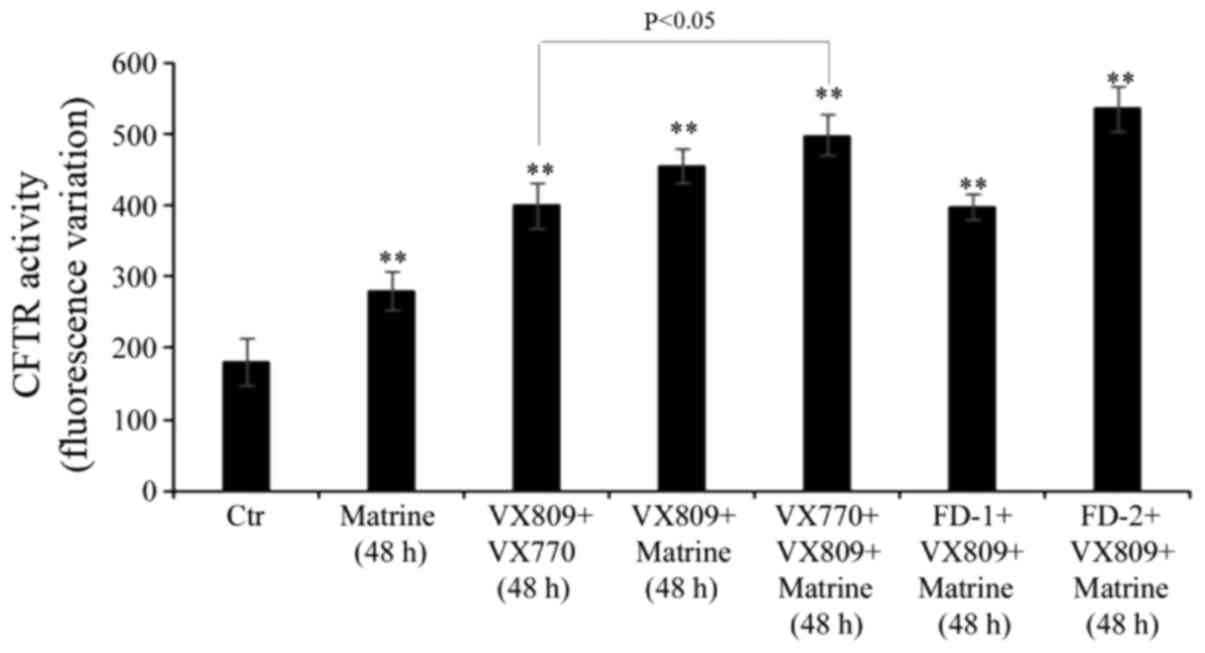
Matrine co-treatment increases the
effect of VX809 in combination with VX770
Following 48 h of treatment, the VX809/VX770
combination stimulated the activity of mutant CFTR by 120% compared
with control cells, and the co-administration of matrine further
increased CFTR activity by 25% (Fig.
4). Notably, the co-treatment of FD-1 with matrine/VX809 had a
similar effect to that observed in VX809/VX770-treated cells
(Fig. 4). FD-2, in combination
with matrine/VX809, stimulated the activity of mutant CFTR with a
similar efficiency to matrine/VX770/VX809 (Fig. 4).
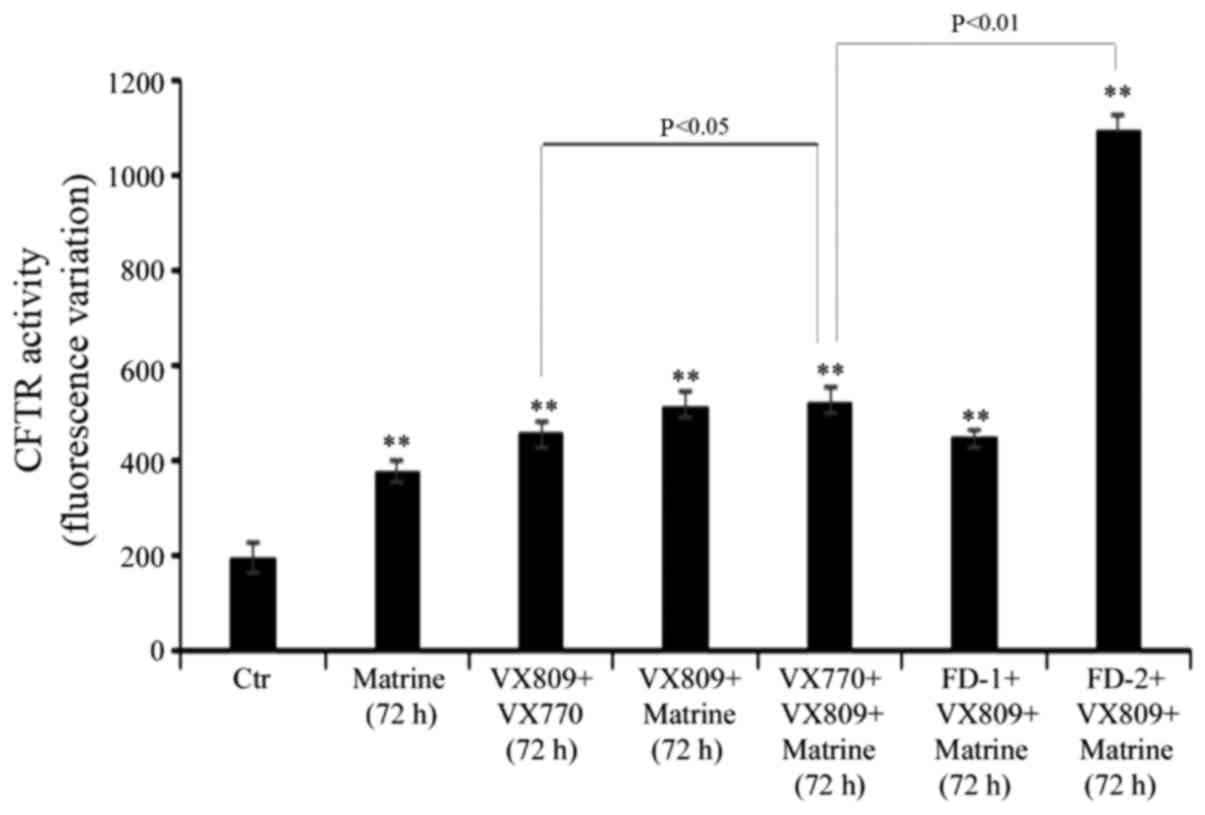
Matrine-FD-2 co-treatment markedly
increases the effect of VX809
Following 72 h of treatment, the combination of
matrine with FD-2 and VX809 stimulated mutant CFTR activity by 450%
compared with control cells (Fig.
5). Notably, when VX770 was administered in place of FD-2, the
rate of mutant CFTR activity was increased only by 165% compared
with that observed in untreated cells (Fig. 5). However, it is necessary to note
that, as already observed at 48 h, the VX809/VX770 combination
stimulated the activity of mutant CFTR by 130% (Fig. 5) and the co-administration of
matrine further increased CFTR activity by 15% (Fig. 5).
In addition, the effect of FD-1 co-treatment with
matrine and VX-809 produced similar effects to those observed in
cells co-treated with VX770 instead of FD-1 (Fig. 5).
Discussion
CF is the most common autosomal recessive disease
with a fatal outcome (8). The
disease is characterized by alterations in the maturation and
function of CFTR, a channel protein responsible for anion
transport, particularly chloride ions. To date, >2,000 CFTR
mutations have been identified, and it has been observed that
consequent electrolyte imbalances lead to a progressive loss of
function of a number of organs, resulting in the mortality
(25). Considering the high
incidence of this disease, it has become necessary to identify
novel therapies and to improve the effectiveness of existing
treatments. The current therapeutic approaches are primarily based
on the use of antibiotics, pancreatic enzymes, anti-inflammatory
drugs and mucolytics which, while improving the symptoms, do not
alter the outcome of the pathology.
However, novel pharmacotherapeutic approaches are
based on the use of correctors and/or potentiators. Among the known
correctors, VX809 (lumacaftor) is presently used to treat patients
with the F508del-CFTR mutation (a class II mutation) in which there
is an alteration in CFTR trafficking (10), and acts by inducing CFTR protein
maturation in the endoplasmic reticulum/Golgi apparatus and by
increasing CFTR translocation to the cell membrane. Potentiators
are able to counteract gating defects due to mutations, including
G551D (a class III mutation). The F508del-CFTR protein, though able
to translocate to the plasma membrane, is not functionally active
(12), while VX770 (ivacaftor),
acting as a potentiator, is able to stimulate the activity of the
mutant CFTR when the mutation is class III.
In order to improve the efficacy of the therapy, a
novel approach is to combine a corrector with a potentiator, and,
among the new drugs, Orkambi (which consists of the combination of
VX809 that facilitates F508del-CFTR maturation, and VX770 which
improves its function) was the first approved therapy to treat
homozygous patients for the F508del-CFTR mutation (26). In these patients, the use of a
potentiator alone is ineffective as it does not stimulate CFTR
trafficking, only stimulating the activity of the CFTR present in
the cell membrane. However, a previous study has demonstrated that
this combined approach reduces the efficacy of VX809 (15). These previous results are in line
with a recent study reporting that, although Orkambi induces an
improvement in symptomatology, long-term treatment leads to a
progressive reduction of its pharmacological efficacy, attributed
to the VX770-mediated destabilization of VX809-rescued F508del-CFTR
(15). In order to reduce this
inhibitory effect, other molecules with a different mechanism of
action compared with that of currently-used drugs are being
evaluated.
Matrine, a natural molecule used in traditional
Chinese medicine, may be effective in reducing the aforementioned
inhibitory effect. Matrine, interacting with the HSC/HSP70
chaperone system, downregulates the expression of HSC70 and
increases the protein levels of F508del-CFTR (16). Therefore, the results of the
present study are promising. Matrine was able to stimulate CFTR
activity, further increase the functionality of the channel in the
presence of VX809 and moderately affect the action of VX77/VX809.
In particular, this action of matrine was observed at 72 h and may
be due to the fact that this compound requires time to stimulate
CFTR activity. The inhibitory effect of VX770 on VX809, not
detectable at 24 h, was evident at 48 h and at 72 h. In addition,
the results of the present study suggested that matrine may
partially counteract the inhibitory effect of VX770 on VX809 under
all the time conditions assayed.
Previous research has led to the development and
study of novel potentiators and, among those tested, the results of
the present study demonstrated that FD-2, in the presence of
matrine, was able to markedly increase the CFTR activity induced by
VX809. This effect was time-dependent and was particularly evident
at 72 h. The different degrees of action of matrine on VX770/VX809
and FD2/VX809 is likely to be due to the different mechanisms of
action of the two potentiators. This hypothesis will be matter of
investigation in our future studies.
In conclusion, the results of the present study
proposed FD-2 to be a novel and possibly more efficacious
potentiator, compared with VX770. Although it is necessary to
perform these treatments on other cellular models and to also
validate these data in in vivo systems, the present results
may be useful in proposing novel and more effective therapeutic
approaches in CF.
Acknowledgements
The authors of the present study would like to
acknowledge Mr. Giuseppe Catalano (DIMES, University of Genoa) for
technical assistance and Ms. Suzanne Patten for language editing.
The present study was supported by grants from Genoa University (B.
Marengo) and funds from the Italian Cystic Fibrosis Research
Foundation, Verona, Italy (M. Mazzei).
Glossary
Abbreviations
Abbreviations:
|
CF
|
cystic fibrosis
|
|
CFTR
|
cystic fibrosis transmembrane
conductance regulator
|
|
FRT
|
Fischer rat thyroid
|
References
|
1
|
Rommens JM, Iannuzzi MC, Kerem B, Drumm
ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et
al: Identification of the cystic fibrosis gene: Chromosome walking
and jumping. Science. 245:1059–1065. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corradi V, Vergani P and Tieleman DP:
Cystic fibrosis transmembrane conductance regulator (CFTR): Closed
and open state channel models. J Biol Chem. 290:22891–22906. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elborn JS: Cystic fibrosis. Lancet.
388:2519–2531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caputo A, Hinzpeter A, Caci E, Pedemonte
N, Arous N, Di Duca M, Zegarra-Moran O, Fanen P and Galietta LJ:
Mutation-specific potency and efficacy of cystic fibrosis
transmembrane conductance regulator chloride channel potentiators.
J Pharmacol Exp Ther. 330:783–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng SH, Gregory RJ, Marshall J, Paul S,
Souza DW, White GA, O'Riordan CR and Smith AE: Defective
intracellular transport and processing of CFTR is the molecular
basis of most cystic fibrosis. Cell. 63:827–834. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalemans W, Barbry P, Champigny G, Jallat
S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP and
Lazdunski M: Altered chloride ion channel kinetics associated with
the delta F508 cystic fibrosis mutation. Nature. 354:526–528. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riordan JR: CFTR function and prospects
for therapy. Annu Rev Biochem. 77:701–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pedemonte N and Galietta LJ:
Pharmacological Correctors of Mutant CFTR Mistrafficking. Front
Pharmacol. 3:1752012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nieddu E, Pollarolo B, Mazzei MT, Anzaldi
M, Schenone S, Pedemonte N, Pesce E, Galietta LJ and Mazzei M: The
search for a common structural moiety among selected
pharmacological correctors of the mutant CFTR chloride channel.
Futur Med Chem. 6:1857–1868. 2014. View Article : Google Scholar
|
|
10
|
Wang X, Venable J, LaPointe P, Hutt DM,
Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H,
et al: Hsp90 cochaperone Aha1 downregulation rescues misfolding of
CFTR in cystic fibrosis. Cell. 127:803–815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mu TW, Ong DS, Wang YJ, Balch WE, Yates JR
III, Segatori L and Kelly JW: Chemical and biological approaches
synergize to ameliorate protein-folding diseases. Cell.
134:769–781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zegarra-Moran O, Monteverde M, Galietta LJ
and Moran O: Functional analysis of mutations in the putative
binding site for cystic fibrosis transmembrane conductance
regulator potentiators. Interaction between activation and
inhibition. J Biol Chem. 282:9098–9104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sondo E, Tomati V, Caci E, Esposito AI,
Pfeffer U, Pedemonte N and Galietta LJ: Rescue of the mutant CFTR
chloride channel by pharmacological correctors and low temperature
analyzed by gene expression profiling. Am J Physiol Cell Physiol.
301:C872–C885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Langron E, Simone MI, Delalande CM,
Reymond JL, Selwood DL and Vergani P: Improved fluorescence assays
to measure the defects associated with F508del-CFTR allow
identification of new active compounds. Br J Pharmacol.
174:525–539. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bali V, Lazrak A, Guroji P, Matalon S and
Bebok Z: Mechanistic approaches to improve correction of the most
common disease-causing mutation in cystic fibrosis. PLoS One.
11:e01558822016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basile A, Pascale M, Franceschelli S,
Nieddu E, Mazzei MT, Fossa P, Turco MC and Mazzei M: Matrine
modulates HSC70 levels and rescues DeltaF508-CFTR. J Cell Physiol.
227:3317–3323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verkman A, LJ V Galietta and Guy RK:
Compounds having activity in increasing ion transport by
mutant-cftr and uses thereof. US Patent WO2004110352. Filed July
14, 2004; issued December 23. 2004.
|
|
18
|
Pedemonte N, Diena T, Caci E, Nieddu E,
Mazzei M, Ravazzolo R, Zegarra-Moran O and Galietta LJ:
Antihypertensive 1,4-dihydropyridines as correctors of the cystic
fibrosis transmembrane conductance regulator channel gating defect
caused by cystic fibrosis mutations. Mol Pharmacol. 68:1736–1746.
2005.PubMed/NCBI
|
|
19
|
Cateni F, Zacchigna M, Pedemonte N,
Galietta LJ, Mazzei MT, Fossa P, Giampieri M and Mazzei M:
Synthesis of 4-thiophen-2′-yl-1,4-dihydropyridines as potentiators
of the CFTR chloride channel. Bioorg Med Chem. 17:7894–7903. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giampieri M, Vanthuyne N, Nieddu E, Mazzei
MT, Anzaldi M, Pedemonte N, Galietta LJ, Roussel C and Mazzei M:
Asymmetric 4-Aryl-1,4-dihydropyridines potentiate mutant cystic
fibrosis transmembraneconductance regulator (CFTR). Chem Med Chem.
7:1799–1807. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galietta LJ, Haggie PM and Verkman AS:
Green fluorescent protein-based halide indicators with improved
chloride and iodide affinities. FEBS Lett. 499:220–224. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marengo B, De Ciucis C, Ricciarelli R,
Passalacqua M, Nitti M, Zingg JM, Marinari UM, Pronzato MA and
Domenicotti C: PKCdelta sensitizes neuroblastoma cells to
L-buthionine-sulfoximine and etoposide inducing reactive oxygen
species overproduction and DNA damage. PLoS One. 6:e146612011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colla R, Izzotti A, De Ciucis C, Fenoglio
D, Ravera S, Speciale A, Ricciarelli R, Furfaro AL, Pulliero A,
Passalacqua M, et al: Glutathione-mediated antioxidant response and
aerobic metabolism: two crucial factors involved in determining the
multi-drug resistance of high-risk neuroblastoma. Oncotarget.
7:70715–70737. 2016.PubMed/NCBI
|
|
24
|
Galietta LV, Jayaraman S and Verkman AS:
Cell-based assay for high-throughput quantitative screening of CFTR
chloride transport agonists. Am J Physiol Cell Physiol.
281:C1734–C1742. 2001.PubMed/NCBI
|
|
25
|
McAuley DF and Elborn JS: Cystic fibrosis:
Basic science. Paediatr Respir Rev. 1:93–100. 2000.PubMed/NCBI
|
|
26
|
Deeks ED: Lumacaftor/Ivacaftor: A review
in cystic fibrosis. Drugs. 76:1191–1201. 2016. View Article : Google Scholar : PubMed/NCBI
|