Introduction
Prostate cancer is one of the most common
malignancies in men, and acts as one of leading cause of
cancer-related death in the world, especially in some developed
countries, although some therapy approaches have been used for
prostate cancer therapy (1,2).
Most prostate cancer patients have metastases at the time of
diagnosis leading to decreased treatment outcomes and poor survival
rates (3). Despite the advances in
treatment approaches over the past few decades, the treatment
outcome has not significantly improved (4,5),
proliferation and apoptosis is responsible for the main reason of
death, and many patients with prostate cancer can progress to
metastatic disease finally (6).
Thus uncovering the molecular mechanism of prostate cancer cell
proliferation and apoptosis is urgent and important. Therefore, the
regulatory mechanism of prostate cancer progression must be further
studied and gain a better understanding to identify novel
therapeutic targets.
Interleukin-8 (IL-8), also known as CXCL-8, a member
of the Glu-Leu-Arg (ELR) motifpositiveCysteine-X-Cysteine (CXC)
chemokine family that is secreted by multiple cell types, including
monocytes, neutrophils, endothelial and mesothelial cells, and
tumor cells (7,8). It was originally classified as a
potent neutrophil chemoattractant, has been shown to regulate
biological processes through interactions with relative receptors
(9). Studies have shown that tumor
progression and metastasis may be associated with overexpression of
IL-8 (10,11). In terms of tumor, IL-8 is known to
participate in cancer progression by promoting the angiogenic
response of endothelial cells, the recruitment of neutrophils to
the site of the tumor, and the proliferation, survival and
migration of tumor cells (12).
Numerous studies have suggested that IL-8 plays a critical role
during tumor angiogenesis, growth and metastasis in hepatocellular
and nasopharyngeal carcinoma, gastric, lungcancer, prostate cancer
(13–17), gastric and pancreatic carcinoma
(18,19). Induction of IL-8 expression is
mediated primarily by activator protein and/or nuclear factor kappa
B (NF-κB) (12).
STAT3/AKT/NF-κB play pivotal roles in various
aspects of the tumorigenic process in several cancer entities,
including colon, lung cancer, and hepatocellular carcinoma
(20–22). These are powerful activators of
malignancy and are pre-requisites for the expression of a variety
of target genes that are important for cell proliferation,
survival, angiogenesis, invasion, and metastasis (23). On the contrary, in immune cells,
NF-κB and STAT3 control the expression of other cytokines and
inflammatory/immune mediators that mediate NF-κB and STAT3
activation in cancer cells, including IL-1, IL-6, and TNFα
(24). NF-κB physically interacts
with STAT3, which may result in either specific transcriptional
synergy or repression of NF-κB/STAT3 regulated genes (25). STAT3 may interact with RelA/p65 in
the nucleus and prolong the presence of active NF-κB in the nucleus
(25). Despite these versatile
interactions, NF-κB and STAT3 cooperate to promote the development
and progression of tumors (26).
However, the mechanisms of action of IL-8 in
prostate cancer are largely unknown. The goal of the present study
was explored the effect of IL-8 on tumor progression in prostate
cancer, whether overexpression of endogenous IL-8 in prostate
cancer cells affects the malignant behavior, including in
vitro anchorage-independent growth, proliferation, migration,
invasion and apoptosis.
In addition, we explored the mechanisms by IL-8
overexpression to promotes proliferation and inhibition of
apoptosis via STAT3/AKT/NF-κB pathway in prostate cancer. These
findings shed new light on prostate cancer pathogenesis and are
helpful for developing innovative therapeutic strategy for prostate
cancer patients.
Materials and methods
Tissue samples
Thirty patients with biopsyproven diagnosis of
prostate cancer were enrolled in this study, tissues were collected
from Gynecology Department, Children's Hospital of Nanjing Medical
University. The corresponding adjacent non-neoplastic tissues from
the macroscopic tumor margin were isolated at the same time and
used as controls. All patients did not process chemotherapy or
radiotherapy prior to surgery. These tissues were frozen in liquid
nitrogen immediately after surgical removal and stored at 80°C
until use. The study protocol was approved by the ethics committee
of the Children's Hospital of Nanjing Medical University. The use
of the tissue samples for all experiments were obtained with
informed consent.
Cell culture
The human prostate cancer LNCap, PC-3, and DU-145
cells and normal prostate RPWE-1 cells were obtained from American
Type Culture Collection (ATCC, Manassas, VA). These cells were
cultured in RPMI-1640 media supplemented with 10% fetal bovine
serum (FBS) and incubated in a humidified atmosphere containing 5%
CO2 at 37°C.
Treatment with IL-8 of pancreatic
cancer cell lines
70% confluent prostate cancer cells were treated for
3 d with escalating concentrations of IL-8 (10, 30 and 50 µg/l) or
medium alone. IL-8 concentrations and the duration of cytokine
treatment were based on our pilot experiments and our previous
cytokine studies.
ELISA assay
Cells were growth in medium for 6 days, supernatants
of prostate cancer cell lines were collected and frozen at −20°C
until the assay was performed. Cells were seeded into six-well
plates and were starved overnight, then the cells were treated with
the indicated concentrations of embelin in DMEM medium for 48 h,
the cell culture supernatant was collected, and the concentration
of IL-8 was assessed using Legend Max human heterodimer IL-8 ELISA
kit (Biolegend) according to the manufacturer's protocol. The
OD405 was measured on a SpectraMax 340 counter using
Soft-MAX PRO 1.2.0 software (Molecular Devices). All assays were
performed in triplicate.
Western blot analysis
Total protein was collected and the protein
concentration was measured by BCA protein assay kit (Applygen
Technologies Inc., Beijing, China). Equal amount of protein was
added into SDS-PAGE gel, and then transferred to the polyvinylidene
difluoride (PVDF) membrane. The membraneswere further blocked in
TBST containing 5% BSA for 1 h, and then were incubated with
primary antibody against IL-8 and GAPDH at 4°C overnight. The
membranes were further washed for three times and were incubated
with secondary antibodies for 1 h. The bands were scanned and
analyzed with with chemiluminescence with SuperSignal West Femto
Chemiluminescent Substrate (Thermo Scientific, USA), and images
were captured with a Bio-Rad camera system (Bio-Rad, USA). GAPDH
were used as internal loading control.
Cell proliferation assay
Cells growth in vitro was detected by
analyzing proliferative expansion of human prostate cancer cells
(PC-3 and DU-145) with MTT assay and clonogenic survival assay.
Briefly, prostate cancer cells were seeded at 5×103
cells each well and incubated with or without recombinant human
IL-8 protein in 96-well plates and measured with MTT cell
proliferation kit (Cayman Chemical) according to the manual's
instructions. For clonogenic survival assay, cells were treated
with IL-8 after three days, the cells were detached and counted in
a hemocytometer. Clonogenic survival assay was performed as
described previously (27,28). The number of colonies was counted
and expressed as a percentage of total colonies in controls.
BrdU assay
Cell proliferation assay was measured by BrdU
incorporation. Cell cultures grown on 13 mm round coverslips were
incubated with a final concentration of 15 µl BrdU for 8 h, after
which time the cells were fixed with 4% paraformaldehyde for 10 min
at room temperature. Coverslips were washed with PBS and then
incubated for 10 min with 0.1% Triton-X100 to permeablize the
membranes. The BrdU epitope was exposed by incubating the cells in
2 N hydrochloric acid for 1 h at 37°C. Then endogenous peroxidase
activity was quenched with 0.3% hydrogen peroxide in PBS for 30 min
at room temperature. After being washed with PBS, cultures were
blocked with 1% Bovine serum albumin and 2% normal goat serum in
PBS (blocking solution). A primary antibody was added at a 1:200
dilution in blocking solution, and incubated at room temperature
for 1 h. Cells were washed three times with PBS and then incubated
with secondary antibody at a 1:200 dilution for 1 hr at room
temperature in a blocking solution. BrdU was then detected with
Tyramide-488 signal amplification (Invitrogen).
Cell migration and invasion
assays
Cell migration assays were performed with wound
healing assay. Briefly, cells were seeded into six-well plates and
were starved overnight when the cells reached at least 95%
confluence. A linear scratch was made using a 10 µl micropipette
tip. The cells were further cultured in DMEM medium containing 2%
fetal bovine serum. The width of the wound was photographed by
light microscopy at 0, 24 and 48 h. Each experiment was performed
in triplicate. The measurements were obtained by measuring the
distance of the wound using ImageJ software.
Furthermore, cell migration and invasion assays were
performed using transwell plates (Millipore). For migration assay,
1.0×105 cells in 200 µl of serum-free medium were placed
in the upper chamber, whereas RPMI-1640 containing 20% BSA was
placed in the lower chamber. The plates were incubated for 16 h at
37°C in 5% CO2. For invasion assay, the upper chamber
was coated with 30 µl matrigel before used. Then 1.8×105
cells in 200 µl of serum-free medium were placed in the upper
chamber, whereas RPMI-1640 containing 20% BSA was placed in the
lower chamber. Non-invading or non-migrating cells were removed
from the upper surface by a cotton swab. The membrane was fixed
with 4% formaldehyde and was stained with 0.5% crystal violet.
Cells were counted in 5 different fields at ×200 magnification, and
the mean number of cells per field was calculated.
Cellular apoptosis assay
Apoptosis was determined by TUNEL (terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling) assa.
Cells were treated with the indicated concentrations of embelin in
complete medium for 48 h. Apoptosis was assessedusing an Apoptag
kit (Chemicon) as previously described (27). To quantify the number of apoptotic
cells, all cells in 5–6 randomly selected high power fields
(magnification: 400) were manually counted using image analysis
software MetaMorph. TUNEL and cells were expressed as a percentage
of total cells.
Statistical analysis
All data were analyzed with SPSS 17.0 (SPSS Inc.,
Chicago, IL, USA), The results were represented as means ± standard
deviation (SD) at least three independent experiments. The
statistical analysis was performed using a two-tailed unpaired
t-test (between two groups) or a one-way analysis of variance
(ANOVA) (among three or more groups), using the computer software
SAS 9.2 (SAS Institute Inc., Cary, NC, USA). P<0.05 was
considered significant and <0.01 was considered statistically
significant.
Results
Expression of IL-8 in prostate cancer
tissues and cell lines
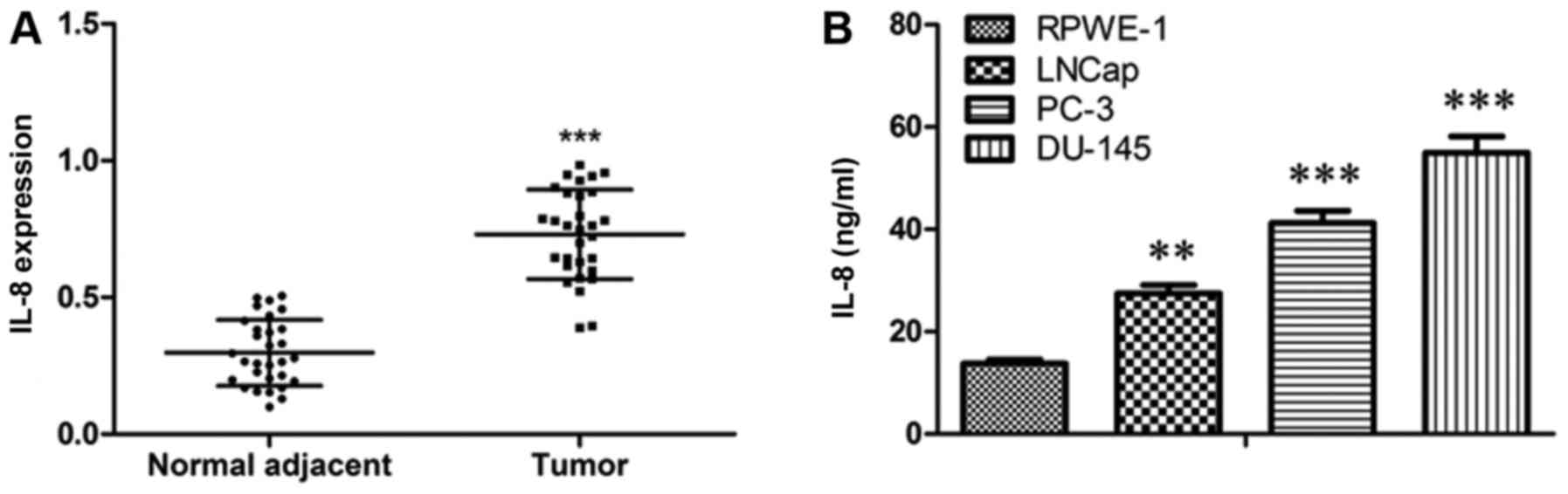
To detect the activity of IL-8 in prostate cancer
progression, firstly, we localized IL-8 expression in tumor tissues
and adjacent tissues from prostate cancer patients. IL-8 was
commonly shown to be pronouncedly up-regulated in tumor tissues
compared with paired normal control (Fig. 1A). Furthermore, we detected the
level of IL-8 in the supernatant of RPWE-1, LNCap, PC-3, and DU-145
cells. The results showed that the level of IL-8 was elevated in
prostate cancer cells as compared to that in RPWE-1 cells (Fig. 1B). On the whole, these data suggest
that IL-8 may play a role in prostate cancer.
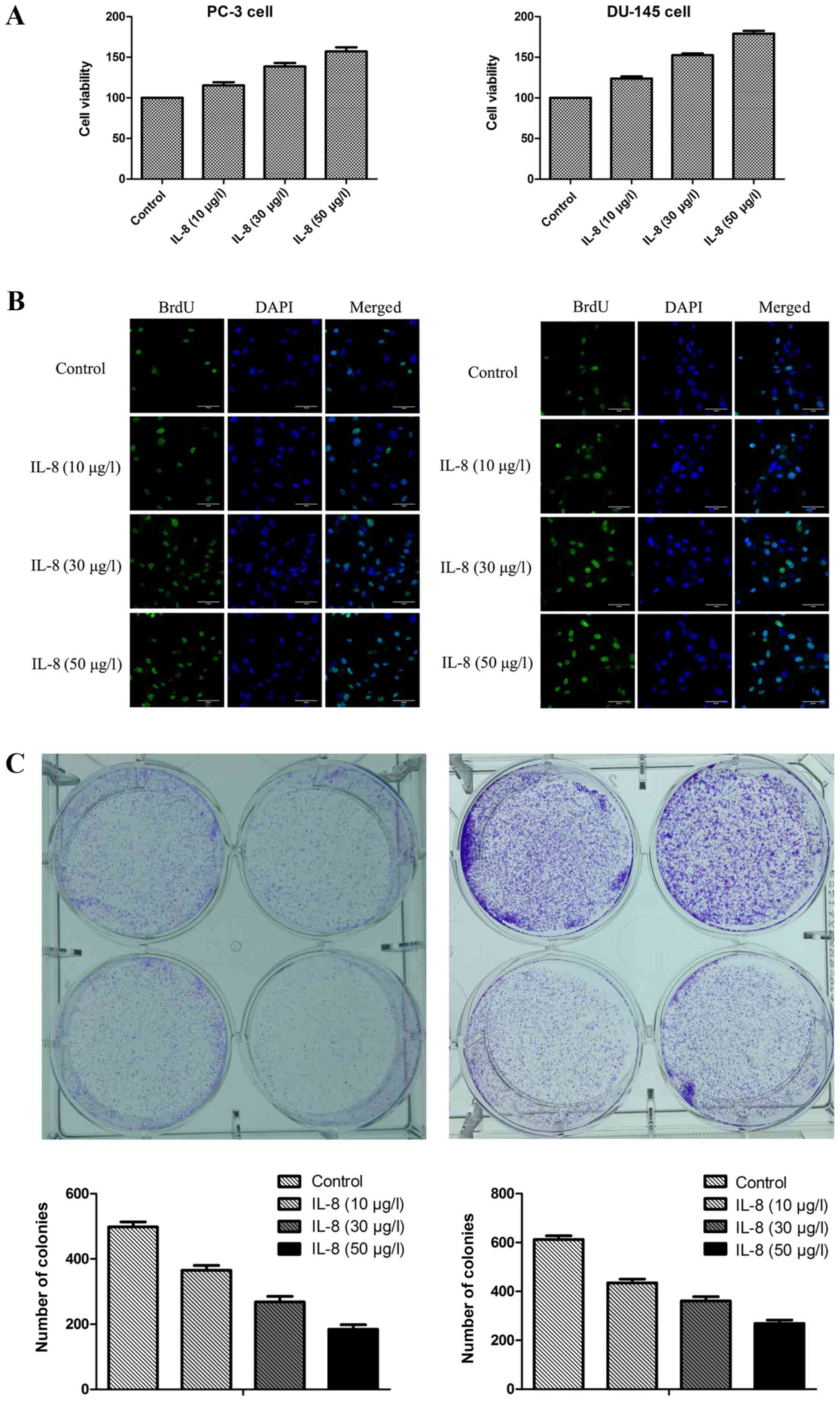
IL-8 promotes proliferation of
prostate cancer cells
To observe the effect of IL-8 over proliferation of
prostate cancer cells, MTT assay was conducted on PC-3 and DU-145
cell lines in respective after it were treated with escalating
doses of IL-8 (10, 30, or 50 µg/l) or medium alone for 3 days. It
was shown that IL-8 was found to be markedly capable of promoting
proliferation compared with control group in PC-3 and DU-145 cells
(Fig. 2A and B). To further
confirm the direct effect of IL-8 on prostate cancer cells
proliferation, cell survival was evaluated by BrdU cell
proliferation assay and clonogenic survival assay (Fig. 2C and D). The percentage of colonies
of PC-3 and DU-145 cells treated with IL-8 at the concentration of
10 µg/l was comparable with that in controls treated with medium
alone, while the percentage of colonies of cells was higher at 30
µg/l of IL-8 and the highest at the concentration of 50 µg/l. Thus,
our results indicate that IL-8 promotes survival and proliferation
of prostate cancer cells.
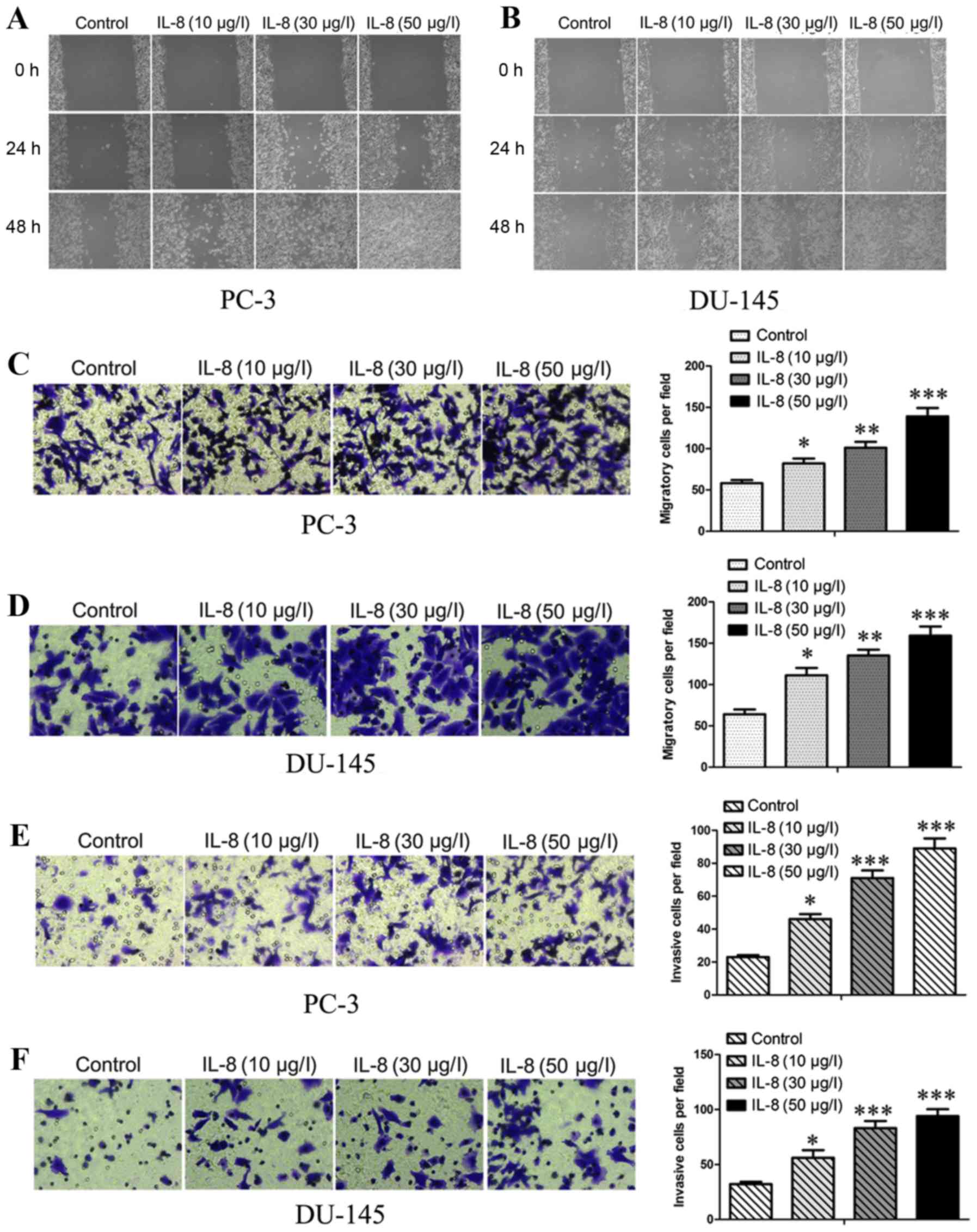
IL-8 promotes migration and invasion
of prostate cancer cells
Subsequently, to observe the effect over migration
and invasion of prostate cancer cells by IL-8, wound-healing and
transwell assays were carried out after treated with escalating
concentrations of IL-8 (10, 30 and 50 µg/l) or medium alone.
Firstly, we investigate the effects of IL-8 on migration about
prostate cancer cells by using scratch wound healing and in
vitro transwell assays. As shown in Fig. 3A and B, the extent of wound healing
was inversely proportional to the concentrations and duration of
embelin treatment. It was displayed that IL-8 was found to be
remarkably able to promote the migration (Fig. 3C and D) of PC-3 and DU-145 cells.
We then examined the role of IL-8 in prostate cancer cell invasion
by using transwell assays (Fig. 3E and
F), The migrating and invading cells on the lower surface of
the filter were stained and counted. The bar graphs show the number
of migrating and invading cells for each category of the cells.
Thus, IL-8 was shown to have capacity for abrogating the effect
over migratory and invasive abilities in PC-3 and DU-145 cells,
suggesting that IL-8 can migration and invasion of prostate cancer
cells in vitro.
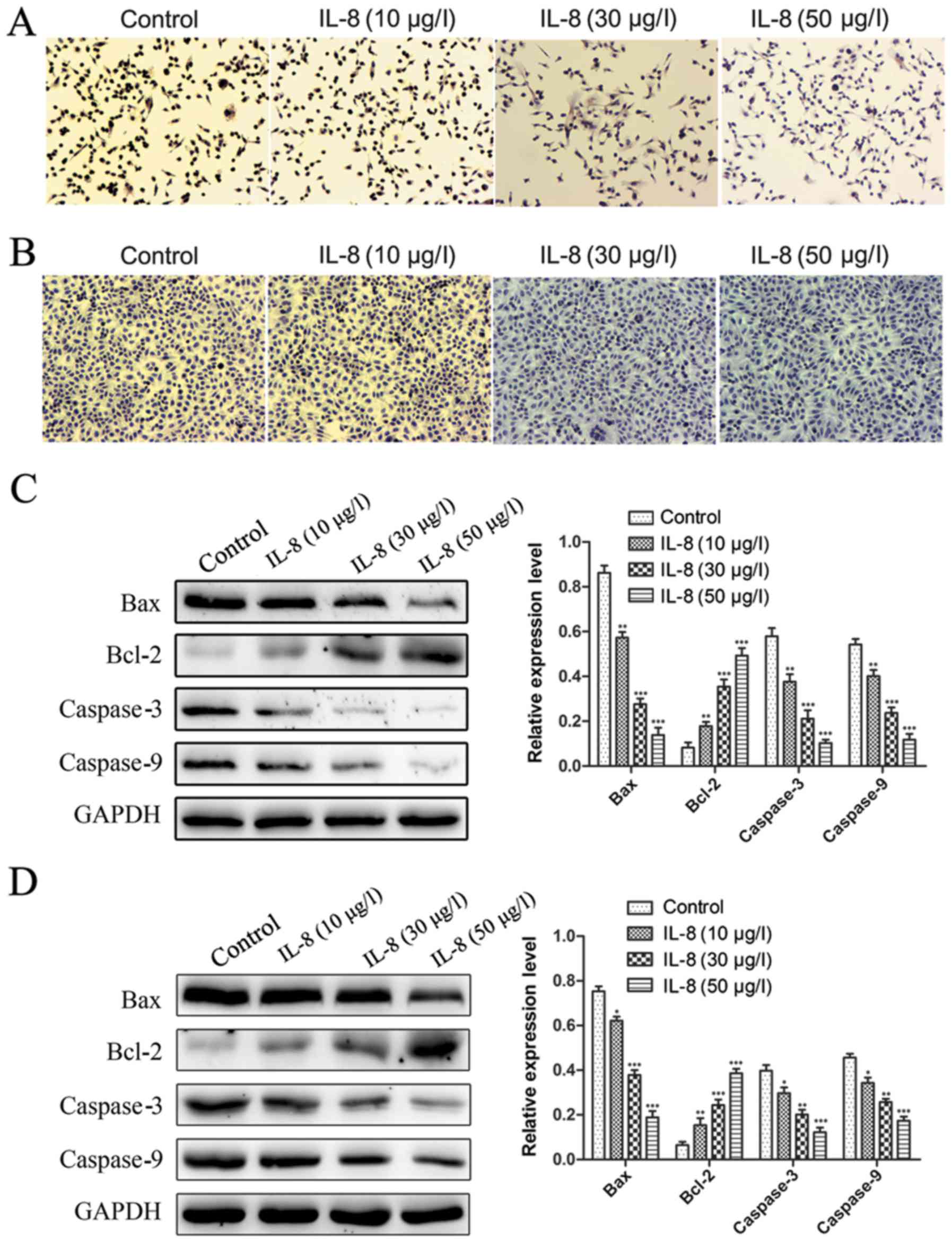
IL-8 decreases apoptosis of prostate
cancer cells
In addition to enhanced proliferation, decrease of
apoptosis could also result in IL-8 promotion of prostate cancer
cells growth. To address this possible effect of IL-8, we examined
apoptosis of PC-3 and DU-145 cells treated with IL-8. The cells
were treated with IL-8 (10, 30 and 50 µg/l) or medium alone for 3
days and apoptosis was evaluated by TUNEL staining. As shown in
Fig. 4A and B, cells were were
treated with IL-8, the number of apoptotic cells is visibly
decreased compared to the control group. Moreover, the number of
apoptotic cells was inversely proportional to the concentration of
IL-8. It was displayed that IL-8 was found to be remarkably able to
inhibit the apoptosis. Since the DU-145 cells proliferation ability
was faster than PC-3 cells, so the number of apoptotic cells in
DU-145 cells was more than of PC-3 cells under the same conditions.
The apoptosis-related proteins expression was detected by western
blot. Similar results were obtained, Bax, Caspase-3 and Caspase-9
expression was obviously decreased in cells were were treated with
IL-8 at the protein level compared to the control group. On the
contrary, Bcl-2 expression was obviously increased (Fig. 4C and D). These results suggest that
IL-8 inhibits apoptosis of prostate cancer cells.
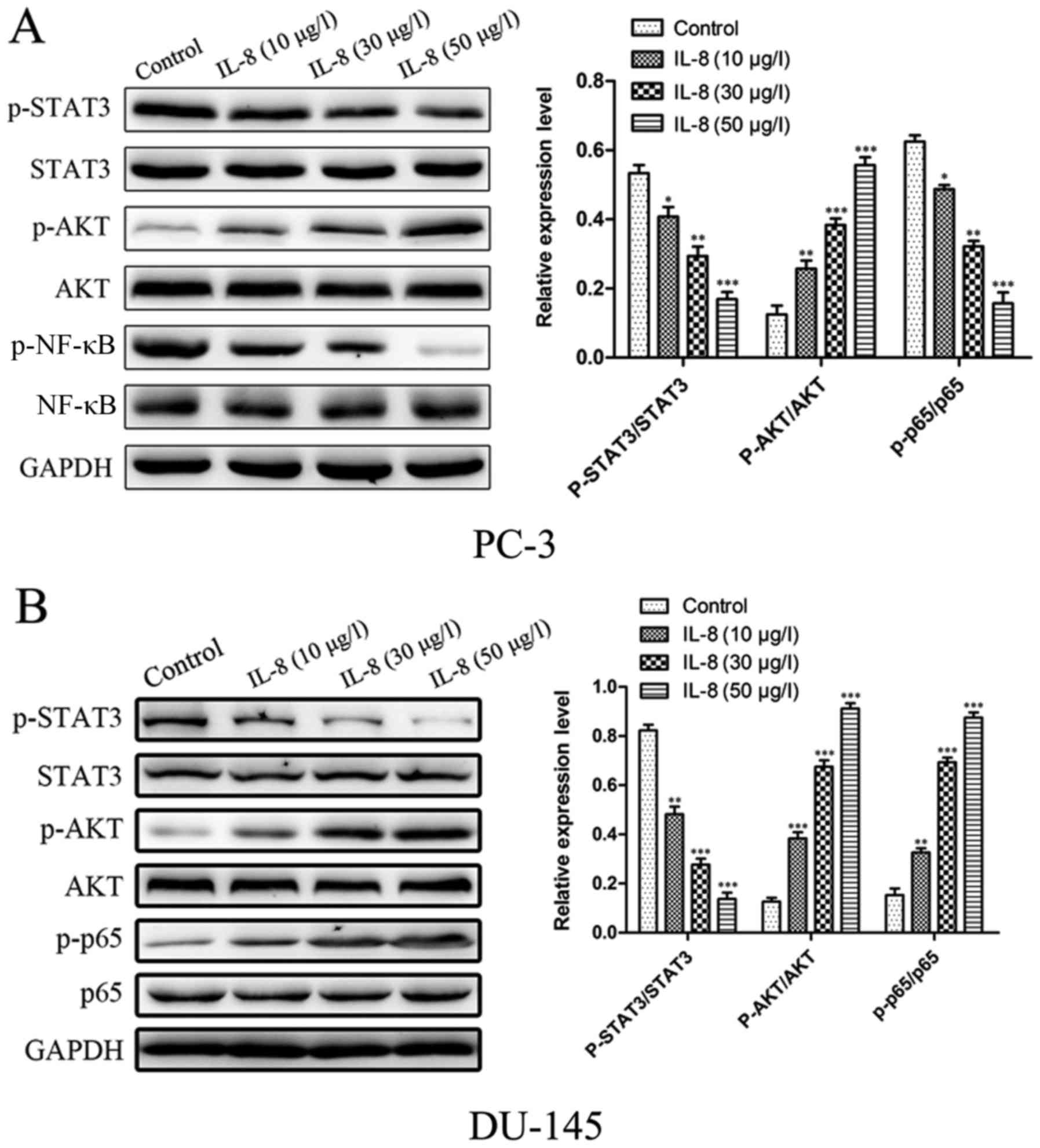
IL-8 induces activation of
STAT3/AKT/NF-κB pathway in prostate cancer cells
STAT3/AKT/NF-κB pathway plays an important role in
cancer progression. Here, after stimulating with different
concentration of IL-8 for 1 h, we found that IL-8 dose-dependently
induced activation of STAT3/AKT/NF-κB in prostate cancer cells.
Here, we investigated whether the STAT3 and Akt pathways
participate in the promoting proliferation capacity of IL-8 in PC-3
and DU-145 cells. As shown in Fig. 5A,
B, The phosphorylation of STAT3 was evaluated by the proportion
of p-STAT3 and STAT3. The ratio of p-STAT3 and STAT3 in cells was
significantly larger than the one in cells with control in PC-3
cells. We next examined whether IL-8 could induce the AKT pathway
in prostate cancer cells. Western blot analysis showed that IL-8
time-dependently induced activation of AKT in prostate cancer
cells. The cells which treat with IL-8 significantly increased the
phosphorylated level of AKT protein compared to the control. Gray
analysis showed that the ratio of p-AKT and AKT in cells treat with
IL-8 was significantly higher than the one in cells with control.
We further detected the effect of IL-8 on activation of NF-κB in
prostate cancer cells. Using western blotting, we found that IL-8
stimulation time-dependently induced phosphorylation of NF-κB P65.
The similiar results was obstained in DU-145 cells (Fig. 5C, D). For in all, our results
demonstrate that IL-8 could induce activation of STAT3/AKT/NF-κB in
prostate cancer cells.
Discussion
In current study, we determine a close correlation
of IL-8 expression with prostate cancer progression in clinical
patients. IL-8 was shown to be significantly up-regulated in
prostate cancer tissues in comparison with paired normal control
tissues. Over-expression of IL-8 can be a common phenomenon that
was readily seen in different types of cancer, including oral
squamous cell carcinoma, multiple myeloma, hepatocarcinoma, chronic
lymphocytic leukemia (29–33) melanoma, colon carcinoma, and
pancreatic cell lines (29,34).
Many studies have addressed the role of IL-8 in promoting cell
proliferation, migration, and invasion of cancer cells (17,35),
and more recently, has received attention in assisting cancer cells
to evade stress-induced apoptosis (36). Consistent with the preponderance of
effects indicated from in vitro studies, the expression of
IL-8 has been shown to correlate with the angiogenesis,
tumorigenicity, and metastatic potential of many solid cancers in
xenograft and orthotopic in vivo models (37).
However, the role of IL-8 about the detailed
mechanisms underlying cell proliferation and apoptosis in prostate
cancer is not been reported at present. In this study, we found
that abilities of cell proliferation was enhanced and cell
apoptosis was inhibited in IL-8 overexpressed cells compared with
their respective control cells, indicating that IL-8 might increase
proliferation and inhibite apoptosis of prostate cancer cells.
Firstly, we find that IL-8 expression promotes growth of prostate
cancer, as evidenced by MTT assay and soft agar colony formation
assay. In addition, we also find that IL-8 expression inhibit
apoptosis of prostate cancer cells, as evidenced by TUNEL staining
and western blot. These findings provide direct evidence that the
IL-8 promotes cell proliferation and inhibit apoptosis in prostate
cancer.
According to previous published results for IL-6 and
IL-8, we found that IL-8 up-regulation in stromal cells because of
interaction with IM9 cells was partially mediated by the
transcription factor NF-κB (38)
and other mechanisms including AP-1, NF-κB, STAT-3 and β-catenin
signaling pathways could also be involved in IL-8-induced tumor
migration (12). Research
previously demonstrated that IL-27 could inhibit tumor growth
directly dependent of the STAT1 signalpathway in mouse melanoma.
Moreover, STAT3 and STAT5 signal pathways also participated in the
antitumor activity of IL-27. However, there have been few studies
reporting the correlation between IL-8 and the STAT3, AKT signal
pathway as well as NF-κB expression in tumor studies. To
investigate the underlying mechanism, we've explored the role of
IL-8 in vitro in prostate cancer cells. Our study showed
that IL-8 could promote prostate cancer cells proliferation and
inhibit apoptosis by activate the STAT3, AKT and NF-κB expression.
NF-κB is a transcription factor that plays an important role in
carcinogenesis as well as in the regulation of immune and
inflammatory response. Aberrant or constitutive NF-κB activation
has been linked to cell transformation, proliferation, survival,
invasion, angiogenesis, and metastasis (12).
The AKT pathway is a major cascade that promotes the
activation of the NF-κB signaling pathway in human cancer cells
(40). In various cells,
phospho-AKT and NF-κB work as antiapoptotic factors by
phosphorylating bad to prevent its binding to Bcl-2. In
unstimulated cells, NF-κB exists in the cytoplasm as an inactive
form bound to an inhibitor protein called IκB, which can be
phosphorylated by IκB kinase (41). The various stimuli activate NF-κB,
causing phosphorylation of IκB. Then IκBα is phosphorylated and
separates from the p50-p65-IκBα heterotrimer. Finally,
phosphorylation and nuclear translocation of p65 to the nucleus
leads to activation of NF-κB (42).
In this context, We here found that IL-8 axis
induced the activation of NF-κB via upregulation of phosphorylated
AKT. Then we demonstrated that IL-8 activated the canonical NF-κB
pathway, increased nuclear p65. Our work deeply clarified that IL-8
in vitro can prevent the release of cytochrome c from
mitochondria and prevent cleavage of caspase 3. This is another
demonstration that IL-8 treatment stabilizes the mitochondrial
membrane and prevents of release of cytochrome c, effects that are
likely to be because of the increased expression of Bcl-2.
In summary, our study demonstrated that IL-8
increased cell proliferation and inhibit apoptosis via the
STAT3/AKT/NF-κB pathways in prostate cancer cell lines. It is
crucial to prevent prostate cancer, the discovery of an
IL-8-mediated signaling pathway helps to better understand the
mechanism of prostate cancer metastasis and may contribute to the
development of therapeutic strategies in the future.
Acknowledgements
The present study was supported by the topic of
Nanjing Municipal Health Bureau (no. YKK14162).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watson PA, Arora VK and Sawyers CL:
Emerging mechanisms of resistance to androgen receptor inhibitors
in prostate cancer. Nat Rev Cancer. 15:701–711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edlind MP and Hsieh AC: PI3K-AKT-mTOR
signaling in prostate cancer progression and androgen deprivation
therapy resistance. Asian J Androl. 16:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
González-Alonso P, Cristóbal I, Manso R,
Madoz-Gúrpide J, García-Foncillas J and Rojo F: PP2A inhibition as
a novel therapeutic target in castration-resistant prostate cancer.
Tumor Biol. 36:5753–5755. 2015. View Article : Google Scholar
|
|
6
|
Bratt O and Schumacher MC: Natural history
of prostate cancer, chemoprevention and active surveillance. Acta
Oncol. 50(Suppl 1): S116–S119. 2011. View Article : Google Scholar
|
|
7
|
Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma
J, Zou B, Gu Q, Wang J, Pang R, et al: Peroxisome
proliferator-activated receptor-gamma contributes to the inhibitory
effects of Embelin on colon carcinogenesis. Cancer Res.
69:4776–4783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zmijewski MA and Slominski AT:
Neuroendocrinology of the skin: An overview and selective analysis.
Dermatoendocrinol. 3:3–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SW, Kim SM, Bae H, Nam D, Lee JH, Lee
SG, Shim BS, Kim SH, Ahn KS, Choi SH, et al: Embelin inhibits
growth and induces apoptosis through the suppression of
Akt/mTOR/S6K1 signaling cascades. Prostate. 73:296–305. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka T, Bai Z, Srinoulprasert Y, Yang
BG, Hayasaka H and Miyasaka M: Chemokines in tumor progression and
metastasis. Cancer Sci. 96:317–322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu J, Ren X, Chen Y, Liu P, Wei X, Li H,
Ying G, Chen K, Winkler H and Hao X: Dysfunctional activation of
neurotensin/IL-8 pathway in hepatocellular carcinoma is associated
with increased inflammatory response in microenvironment, more
epithelial mesenchymal transition in cancer and worse prognosis in
patients. PLoS One. 8:e560692013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XJ, Peng LX, Shao JY, Lu WH, Zhang JX,
Chen S, Chen ZY, Xiang YQ, Bao YN, Zheng FJ, et al: As an
independent unfavorable prognostic factor, IL-8 promotes metastasis
of nasopharyngeal carcinoma through induction of
epithelial-mesenchymal transition and activation of AKT signaling.
Carcinogenesis. 33:1302–1309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kido S, Kitadai Y, Hattori N, Haruma K,
Kido T, Ohta M, Tanaka S, Yoshihara M, Sumii K, Ohmoto Y and
Chayama K: Interleukin 8 and vascular endothelial growth
factor-prognostic factors in human gastric carcinomas? Eur J
Cancer. 37:1482–1487. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan A, Yang PC, Yu CJ, Chen WJ, Lin FY,
Kuo SH and Luh KT: Interleukin-8 messenger ribonucleic acid
expression correlates with tumor progression, tumor angiogenesis,
patient survival, and timing of relapse in non-small-cell lung
cancer. Am J Respir Crit Care Med. 162:1957–1963. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Araki S, Omori Y, Lyn D, Singh RK,
Meinbach DM, Sandman Y, Lokeshwar VB and Lokeshwar BL:
Interleukin-8 is a molecular determinant of androgen independence
and progression in prostate cancer. Cancer Res. 67:6854–6862. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuai WX, Wang Q, Yang XZ, Zhao Y, Yu R and
Tang XJ: Interleukin-8 associates with adhesion, migration,
invasion and chemosensitivity of human gastric cancer cells. World
J Gastroenterol. 18:979–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li M, Zhang Y, Feurino LW, Wang H, Fisher
WE, Brunicardi FC, Chen C and Yao Q: Interleukin-8 increases
vascular endothelial growth factor and neuropilin expression and
stimulates ERK activation in human pancreatic cancer. Cancer Sci.
99:733–737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shalapour S and Karin M: Immunity,
inflammation, and cancer: An eternal fight between good and evil. J
Clin Invest. 125:3347–3355. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Didonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He G and Karin M: NF-κB and STAT3-key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan Y, Mao R and Yang J: NF-κB and STAT3
signaling pathways collaboratively link inflammation to cancer.
Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grivennikov SI and Karin M: Inflammation
and oncogenesis: A vicious connection. Curr Opin Genet Dev.
20:65–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terlizzi M, Casolaro V, Pinto A and
Sorrentino R: Inflammasome: Cancer's friend or foe? Pharmacol Ther.
143:24–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang Y, Demarco VG and Nicholl MB:
Resveratrol enhances radiation sensitivity in prostate cancer by
inhibiting cell proliferation and promoting cell senescence and
apoptosis. Cancer Sci. 103:1090–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang Y, Herrick EJ and Nicholl MB: A
possible role for perforin and granzyme B in resveratrol-enhanced
radiosensitivity of prostate cancer. J Androl. 33:752–760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujita Y, Okamoto M, Goda H, Tano T,
Nakashiro K, Sugita A, Fujita T, Koido S, Homma S, Kawakami Y and
Hamakawa H: Prognostic significance of interleukin-8 and
CD163-positive cell-infiltration in tumor tissues in patients with
oral squamous cell carcinoma. PLoS One. 9:e1103782014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Punyani SR and Sathawane RS: Salivary
level of interleukin-8 in oral precancer and oral squamous cell
carcinoma. Clin Oral Investig. 17:517–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herrero AB, Garcíagómez A, Garayoa M,
Corchete LA, Hernández JM, San Miguel J and Gutierrez NC: Effects
of IL-8 up-regulation on cell survival and osteoclastogenesis in
multiple myeloma. Am J Pathol. 186:2171–2182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perbellini O, Cioffi F, Malpeli G, Zanolin
E, Lovato O, Scarpa A, Pizzolo G and Scupoli MT: Up-regulation of
CXCL8/interleukin-8 production in response to CXCL12 in chronic
lymphocytic leukemia. Leuk Lymphoma. 56:1897–1900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brew R, Erikson JS, West DC, Kinsella AR,
Slavin J and Christmas SE: Interleukin-8 as a growth factor for
human colorectal carcinoma cells in vitro. Cytokine. 12:78–85.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh S, Singh AP, Sharma B, Owen LB and
Singh RK: CXCL8 and its cognate receptors in melanoma progression
and metastasis. Future Oncol. 6:111–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W,
Zhu YF and Wang SM: Interleukin-8 modulates growth and invasiveness
of estrogen receptor-negative breast cancer cells. Int J Cancer.
121:1949–1957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Singh RK and Lokeshwar BL: Depletion of
intrinsic expression of Interleukin-8 in prostate cancer cells
causes cell cycle arrest, spontaneous apoptosis and increases the
efficacy of chemotherapeutic drugs. Mol Cancer. 8:572009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karashima T, Sweeney P, Kamat A, Huang S,
Kim SJ, Bar-Eli M, McConkey DJ and Dinney CP: Nuclear factor-kappaB
mediates angiogenesis and metastasis of human bladder cancer
through the regulation of interleukin-8. Clin Cancer Res.
9:2786–2797. 2003.PubMed/NCBI
|
|
38
|
Kline M, Donovan K, Wellik L, Lust C, Jin
W, Moon-Tasson L, Xiong Y, Witzig TE, Kumar S, Rajkumar SV and Lust
JA: Cytokine and chemokine profiles in multiple myeloma;
significance of stromal interaction and correlation of IL-8
production with disease progression. Leuk Res. 31:591–598. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shrimali D, Shanmugam MK, Kumar AP, Zhang
J, Tan BK, Ahn KS and Sethi G: Targeted abrogation of diverse
signal transduction cascades by emodin for the treatment of
inflammatory disorders and cancer. Cancer Lett. 341:139–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Profita M, Bonanno A, Siena L, Ferraro M,
Montalbano AM, Pompeo F, Riccobono L, Pieper MP and Gjomarkaj M:
Acetylcholine mediates the release of IL-8 in human bronchial
epithelial cells by a NFkB/ERK-dependent mechanism. Eur J
Pharmacol. 582:145–153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nguyen DP, Li J, Yadav SS and Tewari AK:
Recent insights into NF-κB signalling pathways and the link between
inflammation and prostate cancer. BJU Int. 114:168–176. 2013.
View Article : Google Scholar
|