Introduction
Atherosclerosis (AS), the underlying cause of
cardiovascular disease, is characterized by multiple key events,
including endothelial cell (EC) injury, conversion of
lesion-resident macrophages into foam cells, and smooth muscle cell
proliferation (1). EC injury is
the key initiating step in the formation of AS (2), which is induced by multiple
mechanisms, including oxidative stress, endoplasmic reticulum
stress, and insulin resistance. Oxidative stress has increasingly
been investigated to explain EC injury (3). Homocysteine (Hcy) is an independent
risk factor for AS and is involved as an early atherosclerotic
promoter, which enhances EC injury (4). However, the potential causative role
of Hcy in EC injury by oxidative stress remains to be fully
elucidated.
EC injury can be triggered by oxidized low-density
lipoprotein (ox-LDL) in AS, and associated investigations have
indicated that ox-LDL and its receptor, lectin-like ox-LDL
receptor-1 (LOX-1), are important in the development of EC injury
(5). LOX-1 is a scavenger
receptor, which allows the uptake of ox-LDL into ECs, and the
expression of this receptor is involved in the formation of
atherosclerotic vascular lesions; LOX-1 involved at various steps
in the pathogenesis of AS and expressed at high levels in
atherosclerotic lesions (6,7).
Simultaneously, it has been suggested that anti-LOX-1 antibody
significantly suppresses EC injury in the absence of hypertension
(8). Based on genetic and
functional investigations, LOX-1 may be a novel biomarker and
target in EC injury of AS. One of main mechanisms involved in AS
induced by Hcy involves DNA methylation (9), and our previous study suggested that
Hcy induced the hypomethylation of genes associated with AS,
including platelet-derived growth factor (10). Therefore, it was hypothesized that
Hcy injures ECs through mediating LOX-1 DNA methylation, however,
the mechanism remains to be elucidated.
DNA methylation is an epigenetic change, which
arises from the addition of a methyl group at the carbon-5 position
of cytosine residues, this process is mediated by DNA
methyltransferases (DNMTs), a family of enzymes, including DNMT1,
DNMT3a and DNMT3b (11). DNMT1,
the principal DNMT in mammalian cells, is a large dynamic enzyme
with multiple regulatory features, which can control DNA
methylation in cells (12). A
number of studies have reported that DNMT1 is an alternative
mechanism of DNA methylation, and nuclear factor-κB (NF-κB) is one
of the transcriptional factors regulating the transcription of
several genes involved in numerous critical pathways (13,14),
this transcriptional factor is potentially targeted at various
levels. An associated study has suggested that bortezomib results
in the downregulation of DNMT1 via the specificity protein-1
(SP1)/NF-κB pathway and induces genomic DNA hypomethylation in
leukemia cells (15). NF-κB is a
key transcription factor pathway in several key biological
processes, including inflammation, apoptosis and immune responses,
which is a key in the toll-like receptor 4 (TLR4) signaling pathway
(16). TLR4 is a
pattern-recognizing receptor, forming the first line of defense. A
previous study suggested that the lipopolysaccharide (LPS) -induced
inflammatory response may be mediated through the reduced
expression of TLR4 to suppress the activation of NF-κB (17,18).
Simultaneously, TLR4/NF-κB is important in monocyte-endothelium
adhesion, at least in part.
The aim of the present study was to investigate
whether elevated Hcy levels are associated with EC injury and to
examine whether Hcy-induced oxidative stress occurs through
TLR4/NF-κB/DNMT1-mediated LOX-1 DNA methylation in ECs.
Materials and methods
EC culture
The CRL-1730 EC line was purchased from American
type culture collection (Manassas, VA, USA). The cells were treated
with different concentrations (0, 50, 100, 200 and 500 µmol/l) of
Hcy (Sigma; Merck Millipore, Darmstadt, Germany) or 100 µmol/l Hcy
with 30 µmol/l vitamin B12 and 30 µmol/l folic acid at
37°C in an incubator with 5% CO2 for 72 h. The ECs were
divided into a further three groups: untreated cells, endothelial
cells treated with Hcy (100 µmol/l), endothelial cells treated with
Hcy (100 µmol/l) and 10 µmol/l pyrrolidine dithiocarbamate (PDTC;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Cell viability assessment
Methylthiazoletetrazolium (MTT) (Sigma-Aldrich;
Merck KGaA) was used for the evaluation of cell viability. The
cells were grown in 96-well microtiter plates at a density of
1×104 cells in 200 µl per well. When cells grew to 80%
confluence, 20 µl MTT (5 mg/ml) was added to each well and
incubated at 37°C for 4 h. The supernatant was discarded and 150 µl
dimethylsulfoxide was added to each well. Following incubation for
10 min, the plates were read on a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 490 nm.
Measurement of NF-κB, DNMT1 and ox-LDL
concentrations using ELISA
The cells were collected and samples were determined
using the following ELISA kits, NF-κB (cat. no. DY1795) and ox-LDL
(cat. no. DYC4299) obtained from R&D Systems, Inc.
(Minneapolis, MN, USA), and the DNMT1 ELISA kit (cat. no. ab113469;
Abcam, Cambridge, UK) according to the manufacturers'
protocols.
Measurement of superoxide dismutase
(SOD), malondialdehyde (MDA) and hydrogen peroxide
(H2O2) concentrations via colorimetry
The levels of SOD (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China), MDA (Nanjing Jiancheng Bioengineering
Institute) and H2O2 (Nanjing Jiancheng
Bioengineering Institute) were determined using colorimetry
according to the manufacturer's protocol. The absorbance was read
on a microplate reader at 550 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from the cultured cells using
a Tri-Reagent kit (Invitrogen; Thermo Fisher Scientific, Inc.). The
SYBR Green kit (Fermentas; Thermo Fisher Scientific, Inc.) was then
used for RT-qPCR analysis. Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was applied as an internal control for TLR-4, LOX-1 and
DNMT1 in the ECs. The primers are listed in Table I and the reaction system was: 2X
SYBR mixture 25 µl, Forward primer 1 µl, Reverse primer 1 µl, cDNA
2 µl and Rnase-free water up to 50 µl. The thermal cycler (Funglyn
Biotech, Inc., Toronto, ON, Canada) conditions comprised an initial
activation step at 95°C for 5 min, followed by a 2-step PCR program
of 95°C for 15 sec, annealing temperatures for 15 sec and at 72°C
for 30 sec for 30 cycles. Subsequently, the relative changes in the
mRNA expression levels of TLR-4, LOX-1 and DNMT1 were determined by
fold-change analysis, in which the degree of change was calculated
as 2−ΔΔCq, where Cq = (Cqgene -
CqGAPDH) treatment - (Cqgene -
CqGAPDH) control (19).
 | Table I.Primer sequences of TLR-4, LOX-1,
DNMT1 and GAPDH for reverse transcription-quantitative polymerase
chain reaction analysis. |
Table I.
Primer sequences of TLR-4, LOX-1,
DNMT1 and GAPDH for reverse transcription-quantitative polymerase
chain reaction analysis.
| Gene | Primer | Sequence
(5′-3′) | Temperature
(°C) | Length (bp) |
|---|
| TLR4 | Forward |
ATAAGTGTCGAACTCCCTC | 51 | 138 |
|
| Reverse |
GCTCATTCCTTACCCAGT |
|
|
| LOX-1 | Forward |
AATGATAGAAACCCTTGC | 46 | 132 |
|
| Reverse |
TTCCCAGTTAAATGAGCC |
|
|
| DNMT1 | Forward |
GGAGCCCAGCAAGAGTA | 51 | 141 |
|
| Reverse |
GGGAGACACCAGCCAAAT |
|
|
| GAPDH | Forward |
AGAAGGCTGGGGCTCATTT | 51 | 146 |
|
| Reverse |
AGGGGCCACAGTCTTCG |
|
|
Western blot analysis
Total proteins were isolated from the cells using
cell lysis buffer (Keygen Biotech Co., Ltd., Nanjing, China). Equal
amounts of protein (~80 µg) and known molecular weight marker were
loaded onto 12% sodium dodecyl sulfate-polyacrylamide gels
(SDS-PAGE) and were transferred to PVDF membrane by electrophoresis
at 300 mA for 50 min at 4°C, the membrane was then blocked in 10 ml
5% skimmed milk for 2 h at room temperature with gentle agitation
on a platform shaker. The LOX-1 and TLR-4 proteins were detected
using LOX-1 (cat. no. sc-66155) and TLR-4 (cat. no. sc-13593)
antibodies were obtained from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, US) diluted 1:500, and β-actin protein was detected
using a rabbit anti-human β-actin antibody (cat. no. sc-70319,
Santa Cruz Biotechnology, Inc.) diluted 1:2,000; all primary
antibodies were incubated at 4°C. The secondary antibody (goat
anti-mouse IgG-HRP, cat. no. sc-2031, 1:2,000; Santa Cruz
Biotechnology, Inc.) was added for 2 h at room temperature. The
protein bands were visualized and analyzed by the Gel Documentation
and Analysis System ChemiDoc XRS system with Image Lab software,
version 4.1 (Bio-Rad Laboratories, Inc.) and calculated by the gray
value of the bands.
Nested touchdown methylation-specific
PCR (ntMSP) analysis
Total genomic DNA was extracted from the cultured
cells using a DNA isolation kit (Sigma-Aldrich; Merck Millipore)
according to the manufacturer's protocol, and genomic DNA (1 µg)
was bisulfite modified (Sigma-Aldrich; Merck Millipore). The
detection of methylation levels was conducted as previously
described (20). The reaction
conditions of PCR were as follows: 94°C for 45 sec, 68.3°C for 45
sec, and 72°C for 45 sec for 20 cycles, followed by a 0.5°C
decrease of 53.3°C every cycle for 20 cycles. The second step of
PCR was performed with conventional PCR primers under the following
reaction conditions: 94°C for 45 sec, 67°C for 45 sec, and 72°C for
45 sec for 20 cycles, followed by a 0.5°C decrease of 52°C every
cycle for 20 cycles, and ending with extension at 72°C for 5 min.
Primer sequences are listed in Table
II.
 | Table II.Primer sequences of lectin-like
oxidized-low density lipoprotein receptor-1 for nested touchdown
methylation-specific polymerase chain reaction analysis. |
Table II.
Primer sequences of lectin-like
oxidized-low density lipoprotein receptor-1 for nested touchdown
methylation-specific polymerase chain reaction analysis.
| Primer | Sequence
(5′-3′) | Length (bp) |
|---|
| Left outer | TTAGTATTGTGGG | 251 |
|
| AGGTTGAGGTAG |
|
| Right outer | TAAAATTTCACCC |
|
|
| TTATTACCCAAA |
|
| Left M | TTGAAAATATAAA | 137 |
|
| ATAATTAGTCGG |
|
| Right M | TAAATTACAATAA |
|
|
| CATAATCTCG |
|
| Left U | TTGAAAATATAAA | 139 |
|
| ATAATTAGTTGG |
|
| Right U | AATAAATTACAATA |
|
|
| ACATAATCTCAAC |
|
Statistical analysis
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA) was used for data processing. Experiments were performed at
least in triplicate. Results were expressed as mean ± standard
error of the mean (x¯ ±S). Two-way analysis of
variance was used for comparisons between groups and additional
analysis was performed using Student-Newman-Keuls test for multiple
comparisons within treatment groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
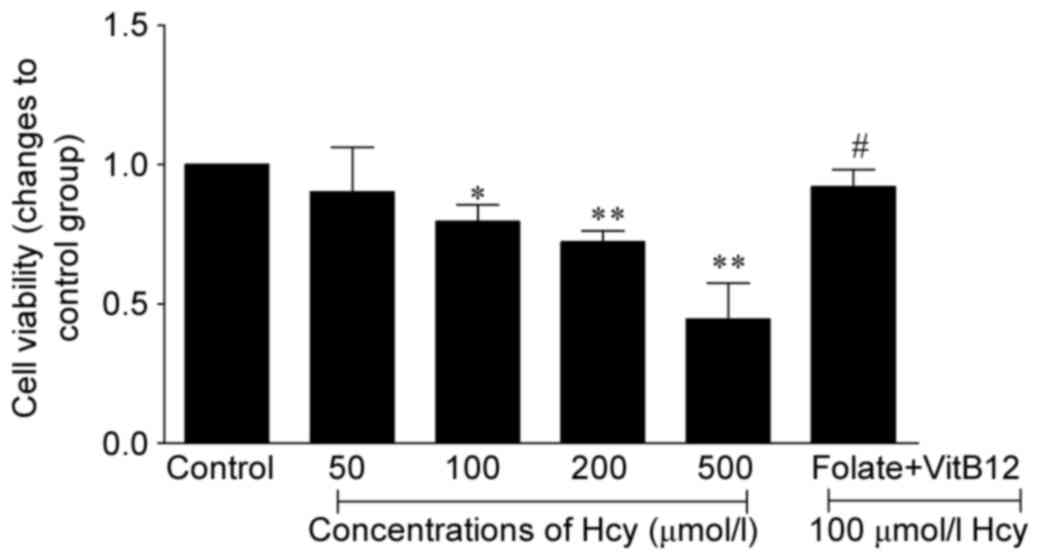
Hcy impairs EC viability
Following incubation of the ECs with Hcy for 72 h,
the viability of ECs was determined using an MTT assay. As shown in
Fig. 1, Hcy affected EC viability
in a dose-dependent manner, and found that cell activities were
decreased by 21, 28, and 56% in the 100, 200, and 500 µmol/l Hcy
group, respectively (P<0.05 or P<0.01). When treated with
folic acid and vitamin B12, the viability of the ECs was
increased by 1.15-fold, compared with that in the 100 µmol/l Hcy
group. Taken together, these results suggested that Hcy induced EC
injury.
Hcy decreases the activity of SOD and
increases levels of MDA, H2O2 and ox-LDL in
ECs
Hcy is an independent risk factor for several major
pathologies in cardiovascular disease, and elevated Hcy is
important in various pathologies by increasing the production of
H2O2, which affects the antioxidant defense
systems (21). In the present
study, H2O2 concentrations were increased in
the 100, 200 and 500 µmol/l Hcy groups (P<0.05 and P<0.01).
The results also demonstrated that the activity of the antioxidant
enzyme SOD was markedly decreased in parallel with the levels of
MDA in the Hcy-treated cells. Taken together, these results
suggested that Hcy injures ECs by oxidative stress. Ox-LDL is
considered to be a biomarker of in vivo oxidative stress in
AS, and elevated oxidative stress and superoxide anion formation in
vascular cells can promote the conversion of LDL to ox-LDL,
contributing to endothelial dysfunction and AS. In the present
study, the change in the levels of ox-LDL was consistent with the
trend observed for the oxidative stress indicators, increasing by
1.44-, 1.54- and 2.1-fold in the 100, 200 and 500 µmol/l Hcy
groups, respectively (P<0.01; Table III). The levels decreased by
20.2% in the folate and vitamin B12-treated cells.
 | Table III.Levels of SOD, MDA,
H2O2 and ox-LDL in endothelial cells. |
Table III.
Levels of SOD, MDA,
H2O2 and ox-LDL in endothelial cells.
| Group | SOD (U/mg
prot) | MDA (nmol/mg
prot) |
H2O2 (mmol/l) | ox-LDL (mg/l) |
|---|
| 0 µmol/l |
156.28±10.35 |
24.52±2.29 |
11.63±1.67 |
0.72±0.06 |
| 50 µmol/l |
148.23±7.40 |
27.90±2.00 |
16.49±1.58 |
0.87±0.09 |
| 100 µmol/l |
135.96±6.25a |
30.71±1.58a |
21.44±2.61b |
1.04±0.12b |
| 200 µmol/l |
111.89±6.18a |
37.49±5.09a |
36.81±3.42a |
1.11±0.18a |
| 500 µmol/l |
103.40±4.89a |
39.23±3.81a |
46.03±4.15a |
1.50±0.20a |
| 100 µmol/l+V+F |
146.06±3.60 |
28.66±2.62 |
15.01±2.23 |
0.83±0.06 |
Hcy promotes expression levels of TLR4
and LOX-1 in ECs
TLR4 is a central mediator of the innate immune
response, which is important in the defense mechanism against
microorganisms (22). In the
present study, the mRNA levels if TLR4 were upregulated by 16.6-,
18.7- and 30.3-fold in the 100, 200 and 500 µmol/l Hcy groups,
compared with that in the untreated group, respectively. In
addition, the protein expression of TLR4 was increased by 1.8-,
1.9- and 2.0-fold in the 100 and 200 and 500 µmol/l Hcy groups,
respectively (P<0.01; Fig. 2A and
B). The expression of TLR4 was decreased when the cells were
treated with folic acid and vitamin B12. As the level of
TLR4 increased with the increase in Hcy, TLR4 may be important in
EC injury induced by Hcy.
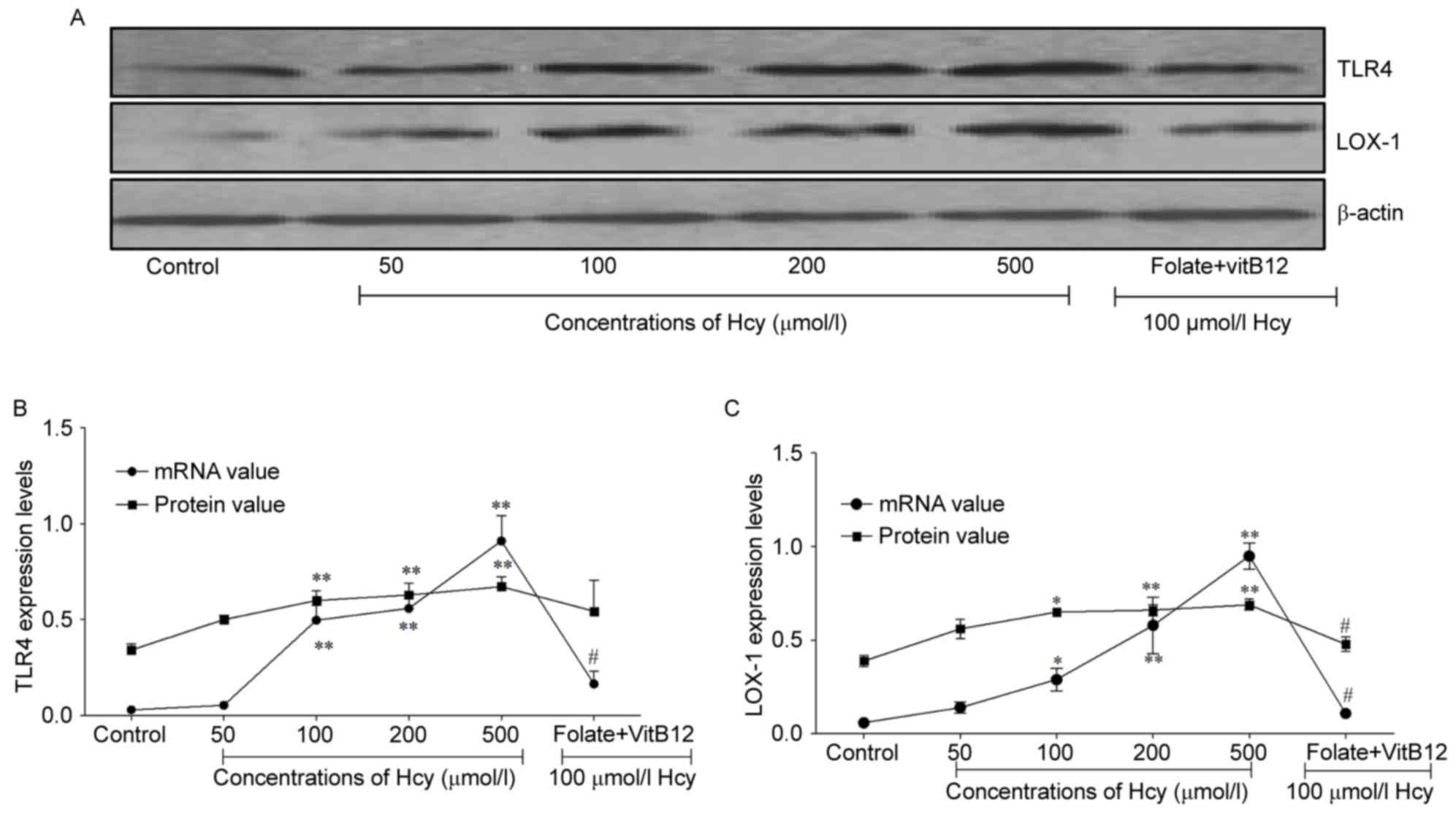 | Figure 2.Hcy promotes the expression of TLR4
and LOX-1 in ECs. (A) Protein expression of LOX-1 and TLR4,
detected using western blot analysis in ECs treated with different
concentrations of Hcy for 72 h. (B) Statistical analysis of
expression levels of TLR4, detected using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses in ECs treated with different concentrations
of Hcy for 72 h. (C) Statistical analysis of expression levels of
LOX-1 detected using RT-qPCR and western blot analysis in ECs
treated with different concentrations of Hcy for 72 h. The control
group contained untreated cells; the folate+VitB12 group contained
ECs treated with 30 µmol/l folate, 30 µmol/l VitB12 and 100 µmol/l
Hcy for 72 h. *P<0.05 and **P<0.01, compared with the control
group; #P<0.05, compared with the 100 µmol/l Hcy
group. EC, endothelial cell; Hcy, homocysteine; VitB12, vitamin
B12; TLR4, toll-like receptor 4; LOX-1, lectin-like
oxidized-low density lipoprotein receptor-1; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
LOX-1 is a type II membrane glycoprotein in ECs,
which has been implicated in atherosclerotic plaque formation,
progression and destabilization (23). When the cells were treated with
different concentrations of Hcy for 72 h, the mRNA expression of
LOX-1 was promoted in the Hcy groups (P<0.05 and P<0.01;
Fig. 2C), and decreased when the
cells were treated with folic acid and vitamin B12. The
protein levels of LOX-1 were increased by 1.7-fold in the 100
µmol/l Hcy group (Fig. 2A and C)
and when the cells were treated with folic acid and vitamin
B12, the expression levels of LOX-1 decreased by 26%.
These findings suggested that Hcy promoted the expression of LOX-1,
leading to EC injury.
Hcy induces LOX-1 DNA hypomethylation in ECs. In the
present study, Hcy suppressed the levels of LOX-1 DNA methylation
by in the 100, 200 and 500 µmol/l Hcy groups (Fig. 3A and B; P<0.01) while, when
treated with folate and vitamin B12, LOX-1 DNA
methylation levels were increased, compared with 100 µmol/l Hcy
treated cells. These data also suggested that the mRNA levels of
DNMT1 were decreased in the Hcy-treated cells (Fig. 3C), and the protein expression of
DNMT1 was suppressed in cells treated with increasing
concentrations of Hcy (Fig. 3D).
Taken together, Hcy appeared to decrease the levels of LOX-1 DNA
methylation by reducing the levels of DNMT1. Combined with the
results obtained on the expression levels of LOX-1, these results
suggested that Hcy may induce LOX-1 DNA hypomethylation to promote
the expression levels of LOX-1.
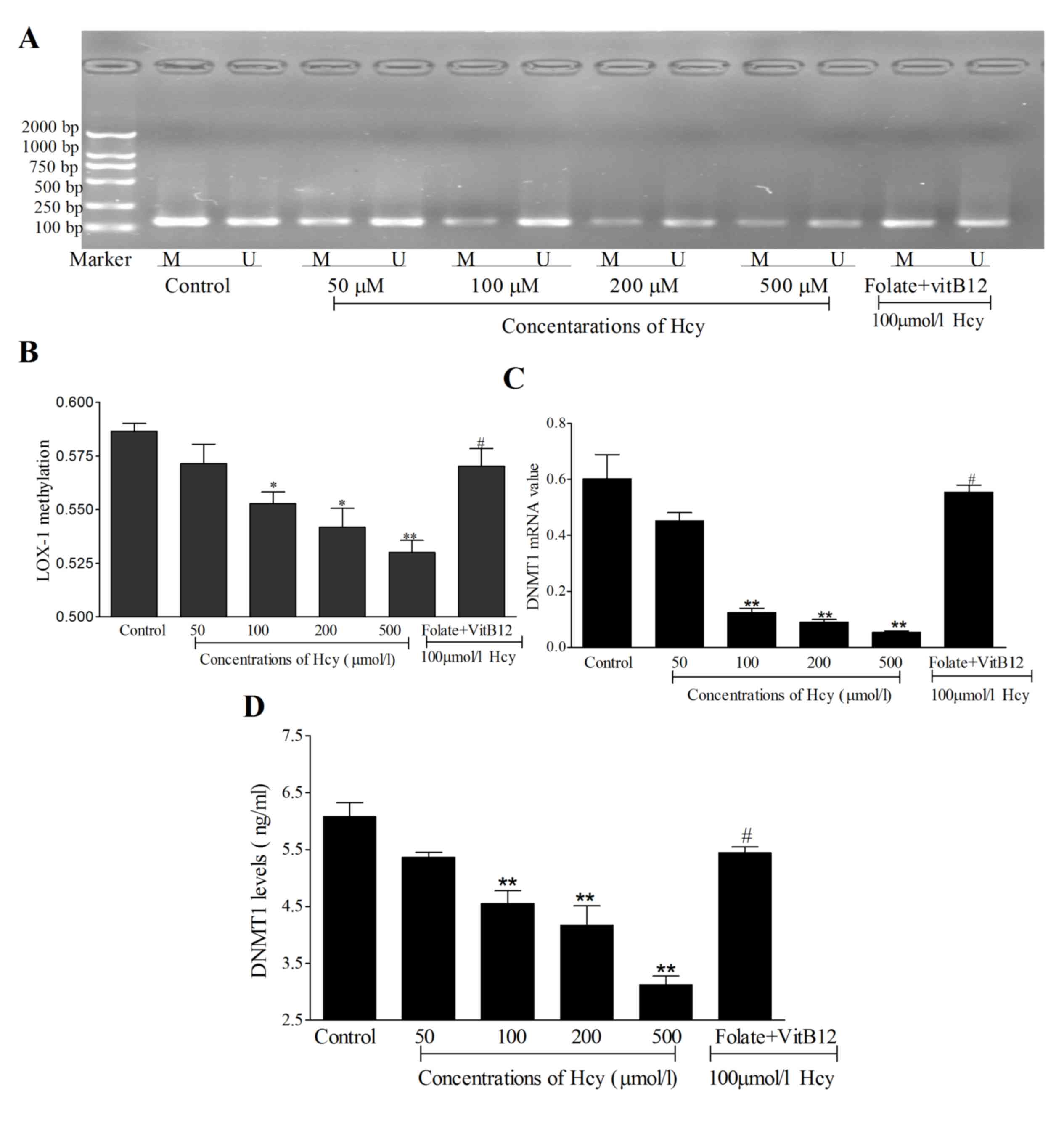 | Figure 3.Methylation of LOX-1 and expression
of DNMT1. (A) PCR product of LOX-1 DNA methylation. (B) Statistical
analysis of the levels of LOX-1 DNA methylation. (C) mRNA levels of
DNMT1 were detected using reverse transcription-quantitative PCR in
ECs following treatment with Hcy. (D) Protein expression levels of
DNMT1 were measured using ELISA following treatment of the ECs with
Hcy for 72 h. The control group contained untreated cells. In the
folate+VitB12 group, ECs were treated with 30 µmol/l
folate, 30 µmol/l BitB12 and 100 µmol/l Hcy for 72 h. The product
length of methylation-specific primer was 137 bp; the product
length of unmethylation-specific primer was 139 bp. *P<0.05 and
**P<0.01, compared with the control group;
#P<0.05, compared with the 100 µmol/l Hcy group.
Marker, DNA marker (top to bottom, 2,000, 1,000, 750, 500, 250 and
100 bp); EC, endothelial cell; Hcy, homocysteine; VitB12, vitamin
B12; DNMT1, DNA methyltransferase 1; M, amplified band
by methylation-specific primer; U, amplified band by
unmethylation-specific primer; PCR, polymerase chain reaction. |
NF-κB decreases the levels of DNMT1 in
ECs
Hcy can promote the secretion of NF-κB, and the
present study found that Hcy induced the levels of NF-κB in ECs by
1.6-, 1.9- and 2.3-fold in the 100, 200 and 500 µmol/l Hcy groups,
respectively (Fig. 4A; P<0.05
and P<0.01), whereas the level was decreased by 28.8% following
treatment of the cells with folate and vitamin B12
(P<0.05). Previous studies have suggested that the NF-κB
signaling pathway is potentially targeted at various levels,
including nuclear translocation, DNA binding and via methyl
transferases. The present study hypothesized that NF-κB may be
associated with DNMT1. Therefore, the cells were treated with
pyrrolidine dithiocarbamate (PDTC), which suppresses the activity
of NF-κB, to confirm the association between NF-κB and DNMT1. The
results suggested that, the PDTC-induced suppression of the
activity of NF-κB led to increased levels of DNMT1 (Fig. 4B and C). Taken together, these
findings suggested that Hcy may result in DNA hypomethylation by
inducing the activation of NF-κB and further decreasing the
expression of DNMT1.
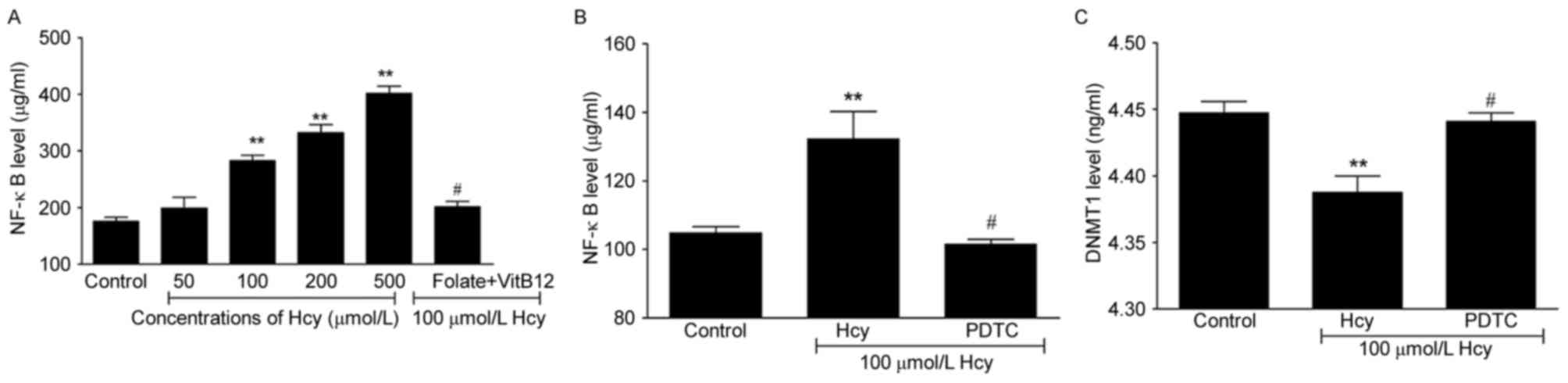 | Figure 4.Hcy regulates the expression of DNMT1
through NF-κB. (A) Hcy increased expression levels of NF-κB in ECs
following co-incubation of cells with Hcy. (B) Levels of NF-κB were
analyzed in ECs following co-incubation with PDTC. (C) Following
co-incubation of ECs with PDTC, levels of DNMT1 were analyzed.
Control group, untreated cells; Hcy group, ECs co-incubated with
100 µmol/l Hcy for 72 h; PDTC group, ECs treated with 100 µmol/l
Hcy and 10 µmol/l PDTC for 72 h. **P<0.01, compared with the
control group; #P<0.05, compared with the 100 µmol/l
Hcy group. EC, endothelial cell; Hcy, homocysteine; VitB12, vitamin
B12; NF-κB, nuclear factor-κB; DNMT1; DNA
methyltransferase 1; PDTC, pyrrolidine dithiocarbamate. |
Discussion
Studies have confirmed that elevated levels of Hcy
are a cause of EC injury and can promote the formation of AS
(24,25). In the present study, it was
demonstrated that Hcy induced EC injury, which was the main
mechanism in the development of AS induced by Hcy.
Oxidative stress is a condition in which the balance
between the production of reactive oxygen species (ROS) and level
of antioxidants is significantly disturbed and results in damage to
cells by excessive ROS production. O2•-,
H2O2 and OH•, as the ROS produced, are
sensitively controlled by several antioxidant enzymes, including
SOD and MDA (26). Accumulating
evidence suggests that elevated plasma Hcy affects the
oxidant-antioxidant balance in the body following endothelial
injury (27). In the present
study, H2O2 concentrations were higher in the
Hcy groups and the activity of antioxidant enzyme SOD was
decreased, whereas the levels of MDA were increased in the cells
under hyperhomocysteinemia. Taken together, these results suggested
that oxidative stress induced the imbalance of redox reactions and
that Hcy may injure ECs, which produce high levels of ROS through
oxidative stress imbalance. This evidence provides scope for
subsequent investigations.
LOX-1 was originally identified as the major
receptor for ox-LDL in ECs. A feedback exists involving ox-LDL and
LOX-1, in which ox-LDL can induce the secretion of LOX-1, then more
ox-LDL can be uptaken by LOX-1 in early phase of AS (28). The present study suggested that Hcy
promoted ox-LDL and LOX-1 expressions in ECs. Hcy is a sulfur amino
acid that can induce oxidative stress by trans-sulfuration and
increasing evidence suggests that oxidative stress occurs in
response to EC injury, which can oxidize LDL to produce ox-LDL.
LOX-1 is one of the scavenger receptors and Hcy has been shown to
upregulate scavenger receptors, as reported for the expression of
CD36 at atherogenic lesion sites in apoE−/− female mice
(29). However, the regulation of
gene expression is affected by various factors under elevated Hcy,
including DNA methylation, which is a focus with AS.
DNA methylation serves as an important mechanism
which controls gene expression in AS (30). As shown in the present study, the
levels of LOX-1 DNA methylation were significantly decreased in the
Hcy groups. Hcy is involved in a one-carbon transfer reaction,
which is also important for DNA methylation. In Hcy metabolism,
S-adenosylmethionine (SAM) is a metabolic intermediate, which is
synthesized from methionine catalyzed by methionine
adenosyltransferase, providing methyl group moieties in several
transmethylation reactions (31).
SAM is converted into S-adenosylhomocysteine (SAH), which is the
sole metabolic precursor of Hcy in a reversible reaction catalyzed
by SAH hydrolase and can inhibit DNMT1. It has been confirmed that
DNA methylation patterns depend on DNMT1, and the results of the
present study found that Hcy decreased the levels of DNMT1.
NF-κB is a key transcription factor which is
responsible for several biological processes, and it has been
identified as a member of the structurally-related eukaryotic
transcription factor family and regulates inducible gene expression
(32). In the present study, data
showed that Hcy increased the levels of NF-κB, whereas DNMT1 was
suppressed in the Hcy groups. In order to further examine the
association between NF-κB and DNMT1, PDTC, an antioxidant
suppressing the activity of NF-κB, was used, and it was found that
NF-κB downregulated DNMT1. A possible mechanism for this involves
the translocation of NF-κB to the nucleus, binding to specific DNA
sequences and promoting the transcription of target genes. DNMT1
has been shown to interact with the transcription factors Sp1, Sp3
and signal transducer and activator of transcription (STAT) 3, with
an STAT3-DNMT1-HDAC1 complex binding to the promoter of
phosphatase-1 (33), and Sp1/NF-κB
DNA-binding decreasing the expression of DNMT1. Investigations of
DNA hypomethylation have suggested that Hcy induces oxidative
stress to activate NF-κB, possibly by generating ROS (34). Taken together, NF-κB/DNMT1 may be
key in Hcy-induced DNA methylation. NF-κB is capable of migrating
into the nucleus and activating the transcription of target genes,
contemporaneously. It is also involved in the pro-inflammatory
response, a first line of defense against infectious diseases,
whereas TLR4 is involved in the induction of innate immune and
inflammatory responses. A previous study has demonstrated that
TLR4-mediated signaling pathways mainly stimulate the activation of
NF-κB (35) and the present study
showed that Hcy promoted the mRNA and protein expression of TLR4.
According to previous studies and the present study,
TLR4/NF-κB/DNMT1 may be involved in Hcy-induced LOX-1 DNA
hypomethylation.
In our previous study, it was demonstrated that
folic acid and vitamin B12 are important in regulating
the metabolic process of Hcy (20), and Ma et al (36) showed that supplementation of folic
acid and vitamin B12 in patients with
hyperhomocysteinemia (HHcy) reduced the levels of Hcy, suggesting
folic acid supplementation may be useful in reducing Hcy levels in
high risk patients with HHcy, but may also significantly improve
endothelial dysfunction in patients with coronary artery disease.
In the present study, it was found that, following supplementation
with folate and vitamin B12, the damaging effect of Hcy
on the ECs was inhibited, and this may be an important method for
the remittance of AS caused by Hcy.
In conclusion, the accumulated evidence suggests
that Hcy injures ECs via oxidative stress and that TLR4/NF-κB/DNMT1
may be involved in Hcy-induced EC injury by mediating LOX-1 DNA
hypomethylation. These findings may be significant in the treatment
of EC injury associated with gene expression due to the
hypomethylation of gene regulatory regions, with TLR4/NF-κB/DNMT1
identified as a novel component in the mechanism. Taken together,
the findings reveal a novel role of Hcy in the pathogenesis of
AS.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81570452, 81460121,
81560120, 81560084, 81560086, 81660047, 81660088 and 81760139).
References
|
1
|
Lynn EG and Austin RC: Hydrogen sulfide in
the pathogenesis of atherosclerosis and its therapeutic potential.
Expert Rev Clin Pharmacol. 4:97–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Puddu P, Puddu GM, Cravero E, Muscari S
and Muscari A: The involvement of circulating microparticles in
inflammation, coagulation and cardiovascular diseases. Can J
Cardiol. 26:140–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolattukudy PE and Niu J: Inflammation,
endoplasmic reticulum stress, autophagy, and the monocyte
chemoattractant protein-1/CCR2 pathway. Circ Res. 110:174–189.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suhara T, Fukuo K, Yasuda O, Tsubakimoto
M, Takemura Y, Kawamoto H, Yokoi T, Mogi M, Kaimoto T and Ogihara
T: Homocysteine enhances endothelial apoptosis via upregulation of
Fas-mediated pathways. Hypertension. 43:1208–1213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ando K and Fujita T: Role of lectin-like
oxidized low-density lipoprotein receptor-1 (LOX-1) in the
development of hypertensive organ damage. Clin Exp Nephrol.
8:178–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujita Y, Yamaguchi S, Kakino A, Iwamoto
S, Yoshimoto R and Sawamura T: Lectin-like oxidized LDL receptor 1
is involved in CRP-mediated complement activation. Clin Chem.
57:1398–1405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Enneman AW, Van der Velde N, de Jonge R,
Heil SG, Stolk L, Hofman A, Rivadeneira F, Zillikens MC,
Uitterlinden AG and van Meurs JB: The association between plasma
homocysteine levels, methylation capacity and incident osteoporotic
fractures. Bone. 50:1401–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoo J, Kim JH, Robertson KD and
Medina-Franco JL: Molecular modeling of inhibitors of human DNA
methyltransferase with a crystal structure: Discovery of a novel
DNMT1 inhibitor. Adv Protein Chem Struct Biol. 87:219–247. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson AA, Akman K, Calimport SR, Wuttke
D, Stolzing A and de Magalhães JP: The role of DNA methylation in
aging, rejuvenation, and age-related disease. Rejuvenation Res.
15:483–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhargava S, Parakh R and Srivastava LM:
Studies on homocysteine demonstrating its significance as a
possible tool for differential diagnosis in occlusive vascular
disease. Indian J Clin Biochem. 19:76–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han XB, Zhang HP, Cao CJ, Wang YH, Tian J,
Yang XL, Yang AN, Wang J, Jiang YD and Xu H: Aberrant DNA
methylation of the PDGF gene in homocysteine-mediated VSMC
proliferation and its underlying mechanism. Mol Med Rep.
10:947–954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laing LV, Viana J, Dempster EL, Trznadel
M, Trunkfield LA, Webster TM Uren, van Aerle R, Paull GC, Wilson
RJ, Mill J and Santos EM: Bisphenol A causes reproductive toxicity,
decreases dnmt1 transcription, and reduces global DNA methylation
in breeding zebrafish (Danio rerio). Epigenetics. 11:526–538. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye EA and Steinle JJ: miR-146a attenuates
inflammatory pathways mediated by TLR4/NF-κB and TNFα to protect
primary human retinal microvascular endothelial cells grown in high
glucose. Mediators Inflamm. 2016:39584532016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang BG, Hu L, Zang MD, Wang HX, Zhao W,
Li JF, Su LP, Shao Z, Zhao X and Zhu ZG: Helicobacter pylori CagA
induces tumor suppressor gene hypermethylation by upregulating
DNMT1 via AKT-NFκB pathway in gastric cancer development.
Oncotarget. 7:9788–9800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Mao L, Zhang L, Zhang L, Yang M,
Zhang Z, Li D, Fan C and Sun B: Adoptive regulatory T-cell therapy
attenuates subarachnoid hemor-rhage-induced cerebral inflammation
by suppressing TLR4/NF-B signaling pathway. Curr Neurovasc Res.
13:121–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Si Y, Wu C, Sun L, Ma Y, Ge A and
Li B: Lipopolysaccharide promotes lipid accumulation in human
adventitial fibroblasts via TLR4-NF-κB pathway. Lipids Health Dis.
11:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yilmaz N: Relationship between paraoxonase
and homocysteine: Crossroads of oxidative diseases. Arch Med Sci.
8:138–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satoh M, Ishikawa Y, Minami Y, Takahashi Y
and Nakamura M: Role of Toll like receptor signaling pathway in
ischemic coronary artery disease. Front Biosci. 13:6708–6715. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Y, Ma S, Zhang H, Yang X, Lu GJ,
Zhang H, He Y, Kong F, Yang A, Xu H, et al: FABP4-mediated
homocysteine-induced cholesterol accumulation in THP-1
monocyte-derived macrophages and the potential epigenetic
mechanism. Mol Med Rep. 14:969–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu S, Ogura S, Chen J, Little PJ, Moss J
and Liu P: LOX-1 in atherosclerosis: Biological functions and
pharmacological modifiers. Cell Mol Life Sci. 70:2859–2872. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi M: Oxidative stress and redox
regulation on in vitro development of mammalian embryos. J Reprod
Dev. 58:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bian D, Liu M, Li Y, Xia Y, Gong Z and Dai
Y: Madecassoside, a triterpenoid saponin isolated from Centella
asiatica herbs, protects endothelial cells against oxidative
stress. J Biochem Mol Toxicol. 26:399–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ou HC, Song TY, Yeh YC, Huang CY, Yang SF,
Chiu TH, Tsai KL, Chen KL, Wu YJ, Tsai CS, et al: EGCG protects
against oxidized LDL-induced endothelial dysfunction by inhibiting
LOX-1-mediated signaling. J Appl Physiol. 108:1745–1756. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai JJ, Wen J, Jiang WH, Lin J, Hong Y and
Zhu YS: Androgen actions on endothelium functions and
cardiovascular diseases. J Geriatr Cardiol. 13:183–196.
2016.PubMed/NCBI
|
|
26
|
Bonnefont-Rousselot D: Resveratrol and
cardiovascular diseases. Nutrients. 8:pii: E2502016. View Article : Google Scholar
|
|
27
|
Thampi P, Stewart BW, Joseph L, Melnyk SB,
Hennings LJ and Nagarajan S: Dietary homocysteine promotes
atherosclerosis in apoE-deficient mice by inducing scavenger
receptors expression. Atherosclerosis. 197:620–629. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Y, Zhang H, Sun T, Wang J, Sun W,
Gong H, Yang B, Shi Y and Wei J: The comprehensive effects of
hyperlipidemia and hyperhomocysteinemia on pathogenesis of
atherosclerosis and DNA hypomethylation in ApoE−/− mice.
Acta Biochim Biophys Sin (Shanghai). 44:866–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma S, Zhang H, Sun W, Gong H, Wang Y, Ma
C, Wang J, Cao C, Yang X, Tian J and Jiang Y: Hyperhomocysteinemia
induces cardiac injury by up-regulation of p53-dependent Noxa and
Bax expression through the p53 DNA methylation in ApoE (−/-) mice.
Acta Biochim Biophys Sin (Shanghai). 45:391–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rushworth SA, Murray MY, Barrera LN,
Heasman SA, Zaitseva L and Macewan DJ: Understanding the role of
miRNA in regulating NF-κB in blood cancer. Am J Cancer Res.
2:65–74. 2012.PubMed/NCBI
|
|
31
|
Menon R, Di Dario M, Cordiglieri C, Musio
S, La Mantia L, Milanese C, Di Stefano AL, Crabbio M, Franciotta D,
Bergamaschi R, et al: Gender-based blood transcriptomes and
interactomes in multiple sclerosis: Involvement of SP1 dependent
gene transcription. J Autoimmun. 38:J144–J155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pang X, Liu J, Zhao J, Mao J, Zhang X,
Feng L, Han C, Li M, Wang S and Wu D: Homocysteine induces the
expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal
pathway in rat vascular smooth muscle cells. Atherosclerosis.
236:73–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Q, Wang HY, Marzec M, Raghunath PN,
Nagasawa T and Wasik MA: STAT3- and DNA methyltransferase
1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor
suppressor gene in malignant T lymphocytes. Proc Natl Acad Sci USA.
102:pp. 6948–6953. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Au-Yeung KK, Woo CW, Sung FL, Yip JC, Siow
YL and O K: Hyperhomocysteinemia activates nuclear factor-kappaB in
endothelial cells via oxidative stress. Circ Res. 94:28–36. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Su J, Wan F, Yang N, Jiang H, Fang
M, Xiao H, Wang J and Tang J: Tissue kallikrein protects against
ischemic stroke by suppressing TLR4/NF-κB and activating Nrf2
signaling pathway in rats. Exp Ther Med. 14:1163–1170.
2017.PubMed/NCBI
|
|
36
|
Ma Y, Peng D, Liu C, Huang C and Luo J:
Serum high concentrations of homocysteine and low levels of folic
acid and vitamin B12 are significantly correlated with the
categories of coronary artery diseases. BMC Cardiovasc Disord.
17:372017. View Article : Google Scholar : PubMed/NCBI
|