Introduction
Head and neck cancer is the sixth most common cancer
worldwide, which affect 650,000 people and cause 350,000 deaths per
year (1). Oral squamous cell
carcinoma (OSCC) is the most common type of head and neck cancer,
which accounts for more than 80% of all forms of head and neck
cancer (2). In spite of some
advances in the treatment of OSCC, the five-year survival rate of
these patients is only 50% and has not improved in the past three
decades (3). The poor prognosis is
due to poor response to current therapy methods and high recurrence
rates (4). Therefore, it is
necessary to identify additional therapeutic options for OSCC.
Leflunomide (LEF) is an anti-inflammatory and
immunomodulatory drug which was introduced and licensed for the
treatment of rheumatoid arthritis (RA) in 1998 (5). Besides that, some other treatment
potential of LEF has been reported. LEF has been used in other
autoimmune diseases, like Psoriatic Arthritis, Wegener
granulomatosis, Sarcoidosis and others (6). LEF also been used as antiviral drug
in solid organ transplantation for polyomavirus type BK or
cytomegalovirus infection (5). In
addition, some reports showed that LEF had anti-proliferation
effect in some malignant tumors including glioma (7,8),
leukemia (9,10), melanoma (11), and neuroblastoma (12). These reports provided evidence that
LEF might be used as a novel drug for antitumoral treatment.
However, the effect of LEF on the growth of OSCC is not clear.
In this study, we showed that LEF inhibited cell
proliferation and blocked cell cycle in S phase in OSCC. We also
found that LEF inhibited colony formation in soft agar and reduced
tumor growth in a xenograft model. The result suggested that LEF
might be an optional agent for the treatment of OSCC.
Materials and methods
Cell culture
Human OSCC cell lines Tca8113 and KB were purchased
from Shanghai Cell Bank, Chinese Academy of Science. Both cells
were grown in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS) and 1% antibiotics penicillin and streptomycin (P/S). The
growth media, FBS and antibiotics were purchased from Gibco. The
cells were cultured at 37°C in a 5% CO2 humidified
incubators.
Cell growth assay
Leflunomide was dissolved in dimethyl sulfoxide
(DMSO) (both from Sigma) as 200 mM stock solutions. Tca8113 and KB
cells were seed in 6-well plate at 20,000 cells/well for overnight.
Then the cells were treated with 100 µM LEF or DMSO for 72 h.
Micrographs of cell morphology were taken by an inverted microscopy
(Olympus). Cells were collected and calculated by trypan blue
staining.
Cell viability was further determined using MTT
assay. Cells were seed in 96-well plate at 1,000 cells/well for
overnight. Then the cells were treated with LEF at 25, 50, 100 or
200 µM. After indicated time period, 20 µl MTT (5 mg/ml; Sigma) was
added to each well and incubated at 37°C for 4 h. Then the
supernatant was removed and 200 µl DMSO was poured to each well to
dissolve the cell pellets. After shaking for 15 min, the absorbance
was measured at a wavelength of 570 nm.
Cell cycle assay
Cells were plated in 10-cm plates and treated with
100 µM LEF or DMSO. After 72 h of treatment, cells were collected
and fixed with 70% ethanol, resuspended in 200 µl PBS and stained
with 1 µl propidium iodide (PI, 5 mg/ml) for 1 h, and analyzed by
flow cytometry with CellQuest analysis software (BD
Biosciences).
Apoptosis assay
Cells were plated in 10-cm plates and treated with
100 µM LEF or DMSO. After 72 h of treatment, cells were collected
and resuspended in 100 µl binding buffer, incubated with 2.5 µl
FITC-Annexin V and 5 µl PI (50 µg/ml) for 15 min, and analysed by
flow cytometry with CellQuest analysis software.
Western blot assay
Cells were plated in 10-cm plates and treated with
100 µM LEF or DMSO. After 48 or 72 h of treatment, cells were
collected and proteins were extracted with RIPA lysis buffer and
PMSF (Beyotime). Protein concentrations were determined with
enhanced BCA protein assay kit (Beyotime). Seventy micrograms of
proteins were separated in 10% SDS-PAGE and transferred to PVDF
membranes. After blocked with 5% nonfat milk for 2 h, the membrane
was washed in TBST and incubated with primary antibody for
overnight. Then the membrane was washed in TBST and incubated with
horseradish peroxidase (HRP)-labeled secondary antibody for 2 h.
The signal was visualized by the ECL reagent (Beyotime) and
captured by western blotting detection instruments (Clinx Science).
The primary antibodies mouse anti-CDK2 (sc-6248, 1:200), mouse
anti-dihydroorotate dehydrogenase (DHODH, sc-166377, 1:200) and
rabbit anti-CCNA (sc-751, 1:200) were purchased from Santa Cruz
Biotechnology. The primary antibody mouse anti-GAPDH (AG019,
1:1,000) was purchased from Beyotime Biotech. The second antibodies
including HRP-labeled goat anti-mouse IgG (H+L) (A0216, 1:5,000),
and HRP-labeled goat anti-rabbit IgG (H+L) (A0208, 1:5,000) were
purchased from Beyotime Biotech.
Soft agar colony formation assay
The lower gel including 0.6% soft agar (Sigma) and
culture media was used as support. The upper gel consisted of 0.3%
soft agar and culture media. The cells were suspended in upper gel,
seed in 6-well plates at 1,000 cells/well and treated with 100 µM
LEF or DMSO. After 20 days of treatment, the micrographs of cell
colonies were taken by an inverted microscopy. Then the cells were
stained with MTT and pictures were taken using a scanner
(Epson).
In vivo tumorigenic assay
Six severe combined immunodeficiency (SCID) mice (4
weeks old) were used and maintained under specific pathogen-free
conditions. Tca8113 cells were trypsinized and collected. Cells
(1×106) were subcutaneously injected in 200 µl culture
media into the flanks of SCID mice. After 7 days of tumor growth,
the mice were administered intraperitoneal injections of LEF (7.5
mg/kg) or control DMSO once daily for 7 days. Tumor diameter was
measured with digital calipers every day, and tumor volume was
calculated (volume = length × width2 × 0.5236). Mice
body weight was monitored every two days. After treatment, the mice
were sacrificed by CO2, and tumors were measured and
weighed. All animal experiments were pre-approved by the
Institutional Animal Care and Use Committee of our university.
Statistical analysis
Each experiment was repeated at least three times.
The results were presented as mean ± SD. The two-tailed Student's
t-test was performed for paired samples. P<0.05 was considered
statistically significant.
Results
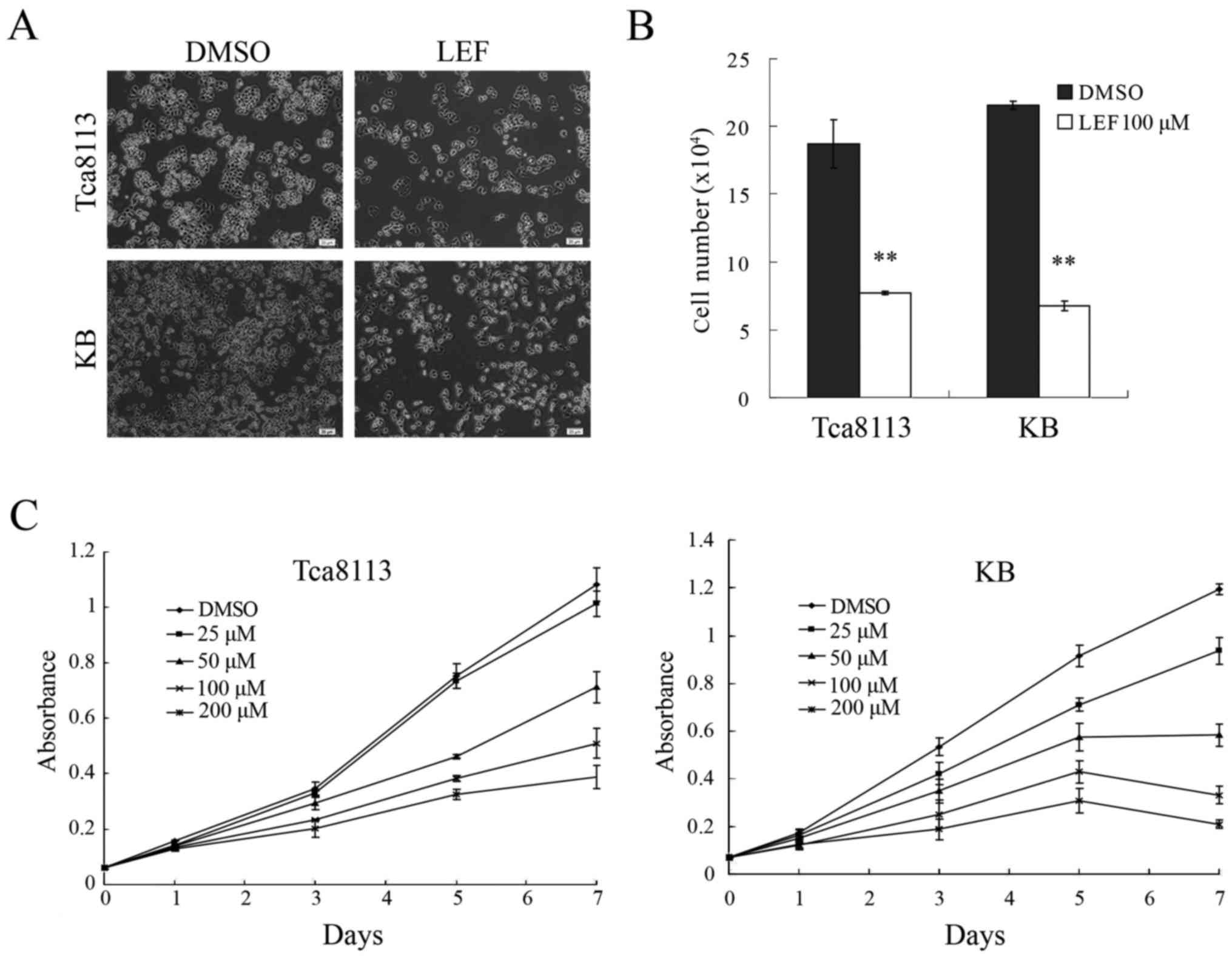
LEF inhibited cell growth and
proliferation in OSCC cells
Two OSCC cell lines Tca8113 and KB were treated with
LEF or control DMSO for 72 h. The result showed that LEF at 100 µM
dramatically inhibited cell growth compared with DMSO-treated
control (Fig. 1A). More than 50%
reduction in cell number was observed in both cell lines (Fig. 1B). To further investigate the
cytostatic effects of LEF, cell growth curve was determined by MTT
assay. The result showed that LEF could inhibit cell growth in a
dose and time-dependent manner in both cells (Fig. 1C). LEF at concentration higher than
100 µM could lead to dramatically decrease in cell number compared
with control, so 100 µM LEF was used in following experiments. This
dose was consistent with other studies (12,13).
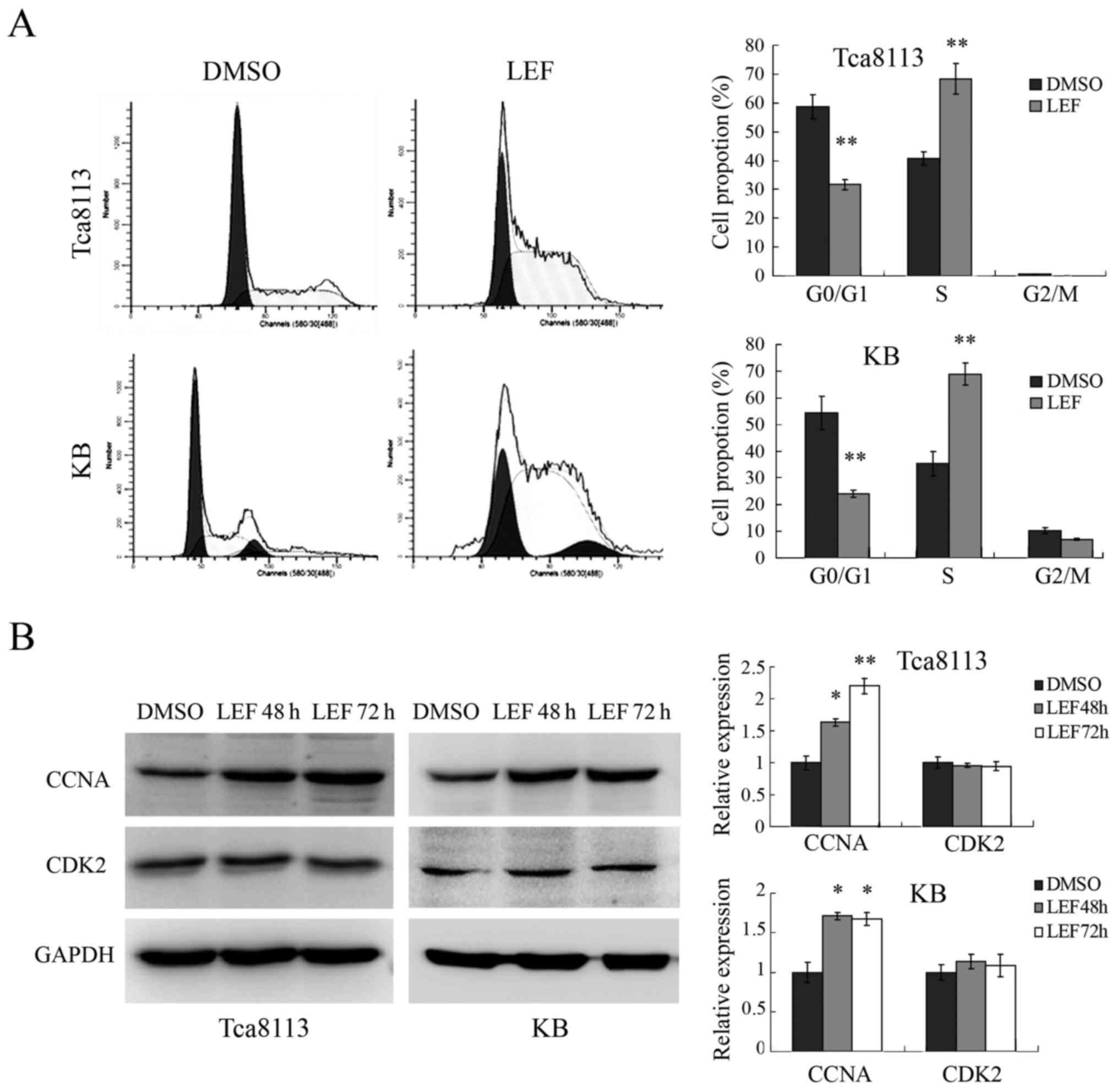
LEF induced cell cycle S phase arrest
in OSCC cells
Then we examined whether LEF could affect cell cycle
in Tca8113 and KB cells. As shown in Fig. 2A, 100 µM LEF dramatically increased
cell proportion in S phase and decreased cell proportion in G0/G1
phase. The percentage of S phase cells increased from 40.73 to
68.41% in Tca8113 and from 35.41 to 68.92% in KB. We further
checked the expression of cell cycle regulatory protein related to
S phase arrest. The results showed that the expression of CCNA was
obviously upregulated in both Tca8113 and KB cells after LEF
treatment, while the expression of CDK2 didn't change (Fig. 2B). Taken together, our results
demonstrated that LEF could inhibit OSCC cells growth through
regulating the expression of cell cycle protein and inducing S
phase arrest.
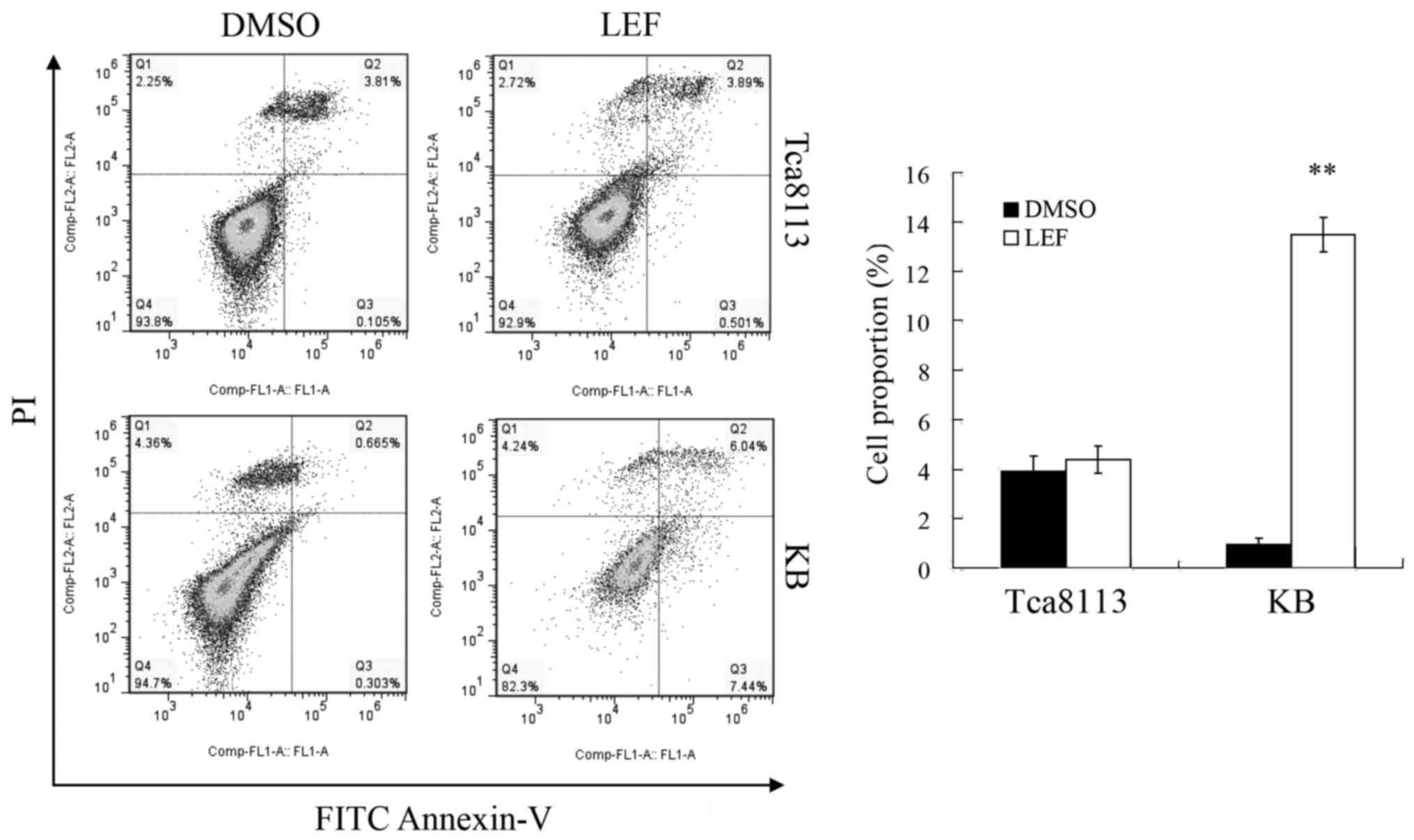
LEF induced cell apoptosis in KB
cells, not in Tca8113 cells
In order to determine whether LEF induced apoptosis
in Tca8113 and KB cells, we performed a flow cytometry assay by
FITC Annexin V and PI staining. The results demonstrated that 100
µM LEF slightly increased apoptotic cells proportion from 3.91 to
4.39% in Tca8113 cells after 72 h treatment. The difference was not
significant after statistical analysis. However, LEF treatment
obviously increased apoptotic cell proportion from 0.97 to 13.48%
in KB cells (Fig. 3). These
results showed that apoptosis was implicated in LEF-induced
inhibition of growth in KB cells, but not in Tca8113 cells.
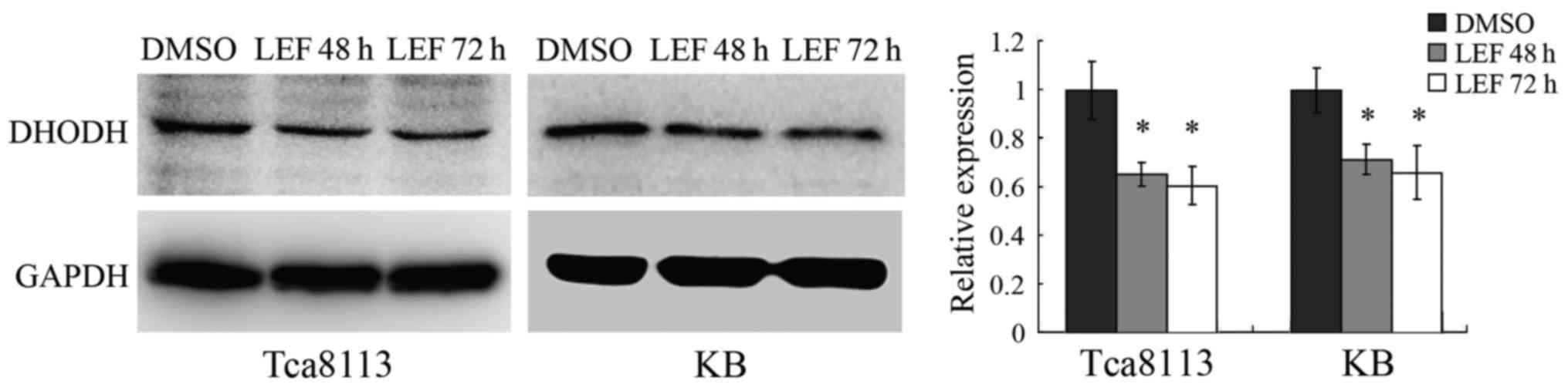
LEF reduced DHODH expression in OSCC
cells
DHODH is one of the essential enzymes in the de novo
pyrimidine biosynthetic pathway. LEF was reported to interfere with
the metabolism of pyrimidine nucleotides through directly blocking
the activity of DHODH (14,15).
Some studies have shown that DHODH inhibition through LEF was
effective for treatment of some cancers including melanoma
(11) and neuroblastoma (12).
To further explore the pathway by which LEF
inhibited cell growth in OSCC cells, we performed western blot
assay to detect the protein expression of DHODH after LEF
treatment. We found that LEF dramatically reduced DHODH expression
in Tca8113 and KB cells after 48 or 72 h treatment (Fig. 4). The results indicated that
downregulation of DHODH expression might be one of the mechanisms
implicated in LEF-induced inhibition of growth in OSCC cells.
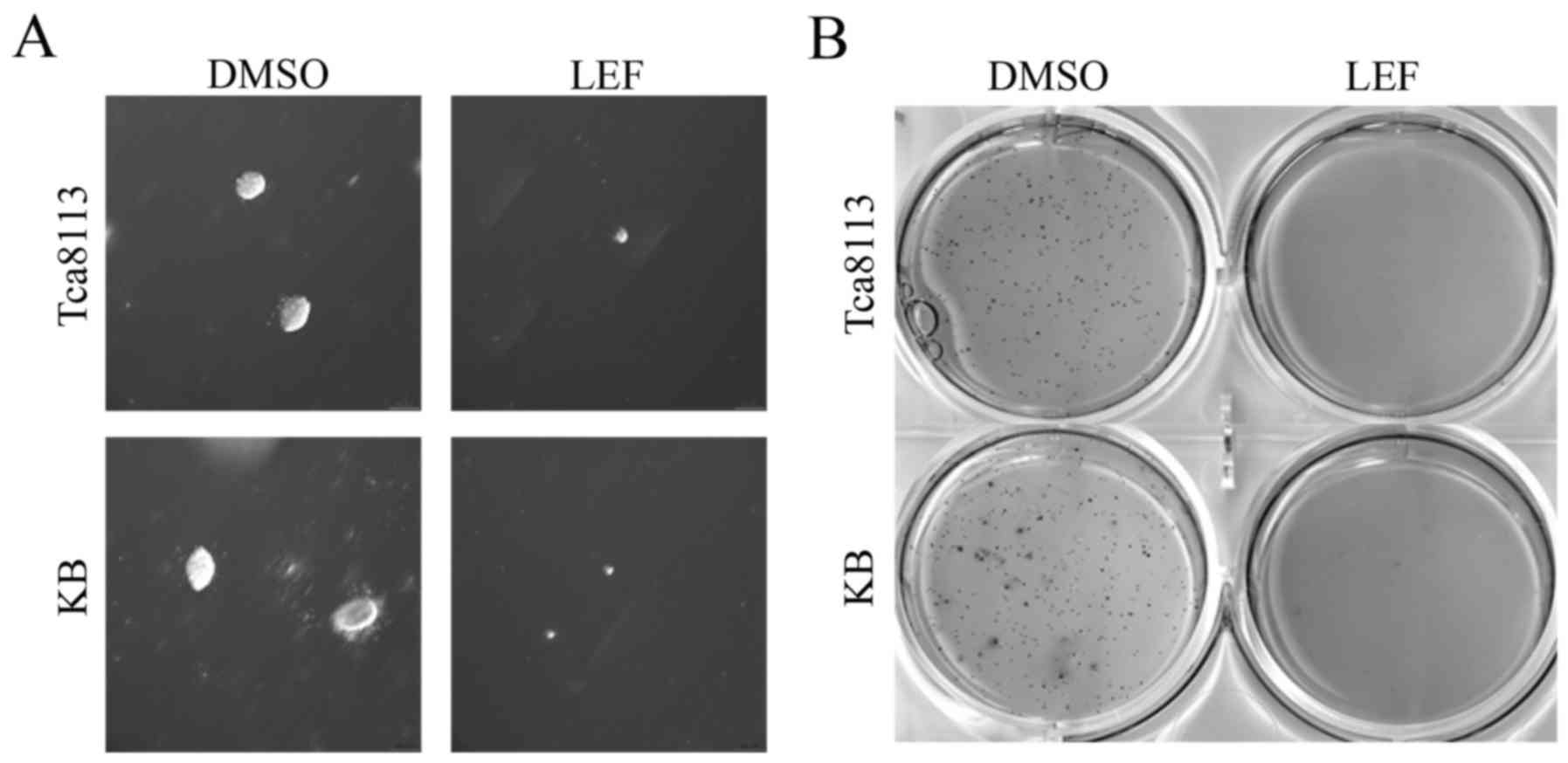
LEF inhibited soft agar colony
formation of OSCC cells
To determine whether LEF inhibits tumorigenic
ability of Tca8113 and KB cells in vitro, we performed a
soft agar colony formation assay. After 20 days of treatment, 100
µM LEF obviously inhibited the volume and number of colonies in
both cell lines (Fig. 5A and B).
These results suggested that LEF could inhibit
anchorage-independent growth and colony formation capacity in OSCC
cells.
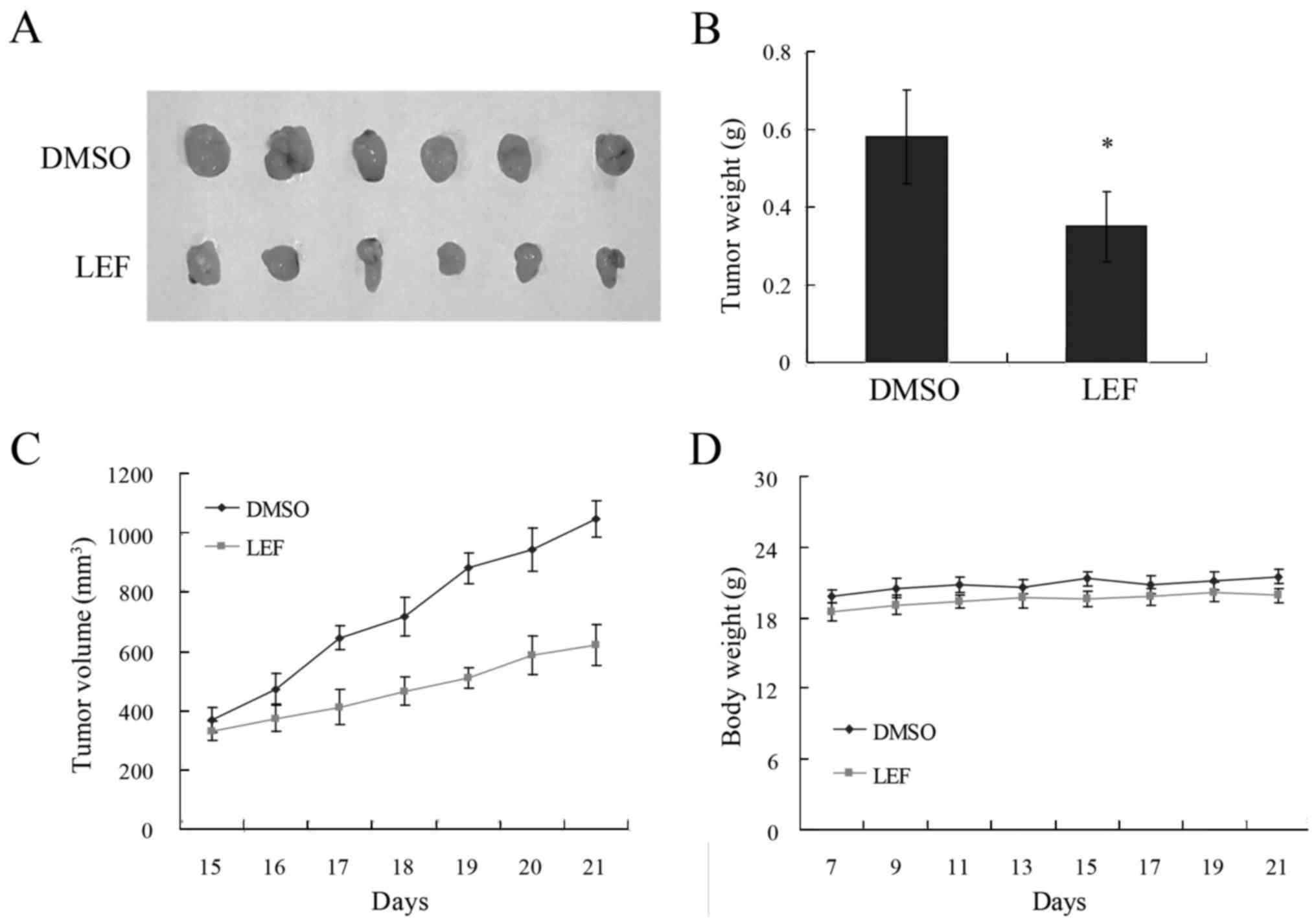
LEF inhibited tumor growth in mouse
xenograph model
We further examined the effect of LEF on OSCC tumor
growth in mouse xenograph model. Tca8113 cells were injected into
SCID mice subcutaneously. After 7 days of tumor growth, LEF was
used for 7 days. The result showed that LEF treatment inhibited
tumor growth dramatically (Fig.
6A-C). The tumor volume and weight was decreased by 42.7 and
37.6% respectively. In addition, the injection of LEF did not
affect the animal weight (Fig. 6D)
and behaviour. These results suggested that LEF could inhibit OSCC
growth in vivo.
Discussion
In recent years, LEF have been reported to display
some antitumoral activity in several kinds of malignant tumors by
inhibiting cell growth and proliferation. In this study, we
investigated the effect of LEF on OSCC growth in vitro and
in vivo. The result showed that LEF obviously reduced OSCC
cell growth, colony formation and xenograph tumor growth.
It was reported that LEF could inhibit cell growth
by G0/G1 phase cell cycle arrest in multiple myeloma cells
(16) and chronic lymphocytic
leukemia cells (9) or S phase cell
cycle arrest in neuroblastoma (12) and prostate cancer (17). In this study we confirmed that LEF
induced S phase cell cycle arrest in OSCC cells. The percentage of
S phase cells increased obviously in both Tca8113 and KB cells.
Then we examined the expression of cell cycle regulatory protein
related to S phase arrest. We found that the expression of CCNA was
obviously upregulated in both cells after LEF treatment. Therefore,
the inhibition of cell cycle progression might be an important
mechanism by which LEF controlled OSCC cells growth.
LEF was also reported to promote apoptosis in a
variety of tumor cells (9,12,16,17).
In this study we found that LEF induced apoptosis in KB cells, but
not in Tca8113 cells. The result suggested that the pro-apoptotic
effect of LEF was restricted to certain kinds of OSCC cells. LEF
might interfere with different pathways in Tca8113 and KB cells and
lead to different apoptosis effect. The similar result was found by
Ringshausen et al (9). They
found that teriflunomide (an active metabolite of LEF) treatment
induced apoptosis in ZAP70-positive chronic lymphocytic leukemia
cells, whereas it failed to induce cell death in ZAP70-negative
cells. Thus the apoptosis effect of LEF in OSCC cells might depend
on different cell kinds.
LEF had been reported to inhibit tumor growth by
interfering with the enzymatic activity of DHODH and inhibiting
pyrimidine biosynthesis (11), or
by inhibiting the tyrosine kinase activity of platelet-derived
growth factor (PDGF) receptor (7,18) or
epidermal growth factor (EGF) receptor (19). The biological activity of LEF was
dependent of its active metabolite, A77 1726. Previous studies have
demonstrated that A77 1726 was capable of inhibiting the activities
of DHODH and tyrosine kinases (20). In this study we found that LEF
dramatically reduced DHODH expression in both Tca8113 and KB cells.
The results suggested that DHODH pathway might be one of the
mechanisms implicated in LEF-induced inhibition of growth in OSCC
cells. Whether LEF could inhibit the growth of OSCC cells via
inhibiting the activities of tyrosine kinases will be investigated
in our next study.
In summary, our study demonstrated that LEF could
inhibit cell proliferation and tumor growth of OSCC cells. LEF has
been used in clinical treatment of RA for more than ten years.
Because of its prominent antitumoral effect in OSCC and its known
toxicology and pharmacology in humans and animals, LEF could be an
optional candidate for OSCC treatment.
Acknowledgements
This study was supported by China Postdoctoral
Science Foundation (2013M542248) and open project of State Key
Laboratory of Silkworm Genome Biology (20120015).
References
|
1
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Landis SH, Murray T, Bolden S and Wingo
PA: Cancer statistics, 1999. CA Cancer J Clin. 49:8–31. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bettendorf O, Piffkò J and Bànkfalvi A:
Prognostic and predictive factors in oral squamous cell cancer:
Important tools for planning individual therapy? Oral Oncol.
40:110–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Teschner S and Burst V: Leflunomide: A
drug with a potential beyond rheumatology. Immunotherapy.
2:637–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pinto P and Dougados M: Leflunomide in
clinical practice. Acta Reumatol Port. 31:215–224. 2006.PubMed/NCBI
|
|
7
|
Xu X, Shen J, Mall JW, Myers JA, Huang W,
Blinder L, Saclarides TJ, Williams JW and Chong AS: In vitro and in
vivo antitumor activity of a novel immunomodulatory drug,
leflunomide: Mechanisms of action. Biochem Pharmacol. 58:1405–1413.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strawn LM, Kabbinavar F, Schwartz DP, Mann
E, Shawver LK, Slamon DJ and Cherrington JM: Effects of SU101 in
combination with cytotoxic agents on the growth of subcutaneous
tumor xenografts. Clin Cancer Res. 6:2931–2940. 2000.PubMed/NCBI
|
|
9
|
Ringshausen I, Oelsner M, Bogner C,
Peschel C and Decker T: The immunomodulatory drug Leflunomide
inhibits cell cycle progression of B-CLL cells. Leukemia.
22:635–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dietrich S, Krämer OH, Hahn E, Schäfer C,
Giese T, Hess M, Tretter T, Rieger M, Hüllein J, Zenz T, et al:
Leflunomide induces apoptosis in fludarabine-resistant and
clinically refractory CLL cells. Clin Cancer Res. 18:417–431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
White RM, Cech J, Ratanasirintrawoot S,
Lin CY, Rahl PB, Burke CJ, Langdon E, Tomlinson ML, Mosher J,
Kaufman C, et al: DHODH modulates transcriptional elongation in the
neural crest and melanoma. Nature. 471:518–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu S, Yan X, Xiang Z, Ding HF and Cui H:
Leflunomide reduces proliferation and induces apoptosis in
neuroblastoma cells in vitro and in vivo. PLoS One. 8:e715552013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alhefdhi A, Burke JF, Redlich A,
Kunnimalaiyaan M and Chen H: Leflunomide suppresses growth in human
medullary thyroid cancer cells. J Surg Res. 185:212–216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greene S, Watanabe K, Braatz-Trulson J and
Lou L: Inhibition of dihydroorotate dehydrogenase by the
immunosuppressive agent leflunomide. Biochem Pharmacol. 50:861–867.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruneau JM, Yea CM, Spinella-Jaegle S,
Fudali C, Woodward K, Robson PA, Sautès C, Westwood R, Kuo EA,
Williamson RA and Ruuth E: Purification of human dihydro-orotate
dehydrogenase and its inhibition by A77 1726, the active metabolite
of leflunomide. Biochem J. 336:299–303. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baumann P, Mandl-Weber S, Völkl A, Adam C,
Bumeder I, Oduncu F and Schmidmaier R: Dihydroorotate dehydrogenase
inhibitor A771726 (leflunomide) induces apoptosis and diminishes
proliferation of multiple myeloma cells. Mol Cancer Ther.
8:366–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hail N Jr, Chen P and Bushman LR:
Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and
apoptotic effects in transformed prostate epithelial cells:
Evidence supporting a role for teriflunomide in prostate cancer
chemoprevention. Neoplasia. 12:464–475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shawver LK, Schwartz DP, Mann E, Chen H,
Tsai J, Chu L, Taylorson L, Longhi M, Meredith S, Germain L, et al:
Inhibition of platelet-derived growth factor-mediated signal
transduction and tumor growth by
N-[4-(trifluoromethyl)-phenyl]5-methylisoxazole-4-carboxamide. Clin
Cancer Res. 3:1167–1177. 1997.PubMed/NCBI
|
|
19
|
Mattar T, Kochhar K, Bartlett R, Bremer EG
and Finnegan A: Inhibition of the epidermal growth factor receptor
tyrosine kinase activity by leflunomide. FEBS Lett. 334:161–164.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Williams JW, Gong H, Finnegan A and
Chong AS: Two activities of the immunosuppressive metabolite of
leflunomide, A77 1726. Inhibition of pyrimidine nucleotide
synthesis and protein tyrosine phosphorylation. Biochem Pharmacol.
52:527–534. 1996. View Article : Google Scholar : PubMed/NCBI
|