Introduction
Atrial fibrillation (AF) is a common and serious
type of arrhythmia, which is characterized by an irregular and
rapid heartbeat (1). Clinically,
pulmonary vein isolation (PVI) is a feasible, safe and effective
treatment method for patients with AF; however, it is associated
with the potential risk of peri-procedural stroke, despite
appropriate anticoagulation (2).
As a high risk factor in the treatment of AF, thrombosis can
seriously affect the effects of PVI treatment on AF, and
appropriated anticoagulation is always used to reduce the risk of
thrombosis (3,4). It has been reported that
P-selection+ platelets, platelet-derived microparticles
(PMPs) and leukocyte-derived MPs (LMPs) are increased immediately
following PVI and persist in the subsequent 24 h, which can
significantly increase the risk of thrombosis (5–7). In
terms of the predominant underlying mechanisms, increased
thromboembolic risk during PVI may be explained by endocardium
denaturation and hemodynamic alterations due to energy applied in
catheterization and electrical cardioversion (8–10).
However, associated mechanisms underlying how procoagulant activity
(PCA) is affected by PVI require further investigation.
As an anionic lipid, phosphatidylserine (PS) is an
important phospholipid membrane component located between the inner
and cytoplasmic leaflet of the bilayer (11). Exposure of PS on the outer leaflet
and microparticles (MPs) are found in the process of apoptosis
(12,13). In addition, PS exposure on the
outer membrane surface can function as a docking site for various
coagulation proteins, including factor (F)VII, FIX, FV, FVIII, FX
and prothrombin, and promote the formation of thrombin (14,15).
As a primary cellular initiator of blood coagulation via
interaction with coagulation FVII, tissue factor (TF) is generally
quiescent unless it resides on a cell membrane containing PS
(16). It has been reported that
TF is involved in the generation of MPs, cell-associated PCA in
diabetes mellitus and disseminated intravascular coagulation
associated with severe infections (17,18).
Therefore, PS and TF are considered to contribute to PCA and
outcomes for patients with AF following PVI. However, whether and
how PS exposure of blood cells and MPs contributes to PCA following
PVI requires further investigation.
The post-ablation recurrence of AF remains a major
clinical problem, occurring in 20–60% of patients during follow-up
(19,20). In addition, there is a paucity of
data on the correlations of early recurrence AF (ERAF) following
PVI with PS+ blood cells and MPs. In the present study,
the exposure of PS on blood cells (platelets, erythrocytes and
leukocytes) and MPs were detected in patients with AF treated with
PVI, and the PCA was evaluated by determining coagulation time, and
the formation of FXa and thrombin. Subsequently, independent
factors associated with PS+ blood cells and MPs, and the
predictors of ERAF following PVI were investigated. The findings
may reveal the mechanisms underlying the promoting effects of
PS+ blood cells and MPs on PCA following PVI, and its
clinical prognostic value on ERAF.
Materials and methods
Subjects
A total of 56 patients with AF, including 48
patients with paroxysmal AF and 8 with non-paroxysmal AF, who had
undergone a transseptal PVI procedure were selected from the Second
Hospital of Harbin Medical University (Harbin, China) between
November 2013 and April 2015. AF was identified according to the
Heart Rhythm Society expert consensus statement (21). An additional 40 healthy subjects
were recruited from the preoperative clinic, which were included as
the control group. Patients with a history of previous myocardial
infarction, surgery or ablation procedure within 3 months,
congenital heart disease, history of connective tissue diseases or
chronic inflammatory conditions, acute/chronic infection or chronic
renal/liver failure were excluded from the study. The study was
approved by the local ethics committee of the Second Hospital of
Harbin Medical University and performed with written informed
consent from the patients.
PVI
The PVI was performed on patients with AF, as
previously described (9). In
brief, warfarin was first titrated to an international normalized
ratio of 1.8–2.5 2 days prior to PVI by oral anticoagulation.
Trans-septal puncture was guided by transesophageal
echocardiography to exclude thrombi in the left atrium.
Subsequently, 50 IU/kg unfractionated heparin was administered to
maintain an activated clotting time between 300 and 350 sec.
Ablation of the pulmonary veins was performed with a delivered
power of 30–35 W and irrigation rates of 17–30 ml/min. All patients
were scheduled for 24 h Holter recording at baseline (prior to
PVI), and at follow-up at 1, 3 and 6 months. ERAF was defined as AF
occurring at any time beyond the 3-month blanking period following
ablation.
Flow cytometric analysis of PS-exposed
blood cells
Peripheral vein blood samples were collected from
the patients with AF prior to PVI (baseline), at the end of
transseptal puncture (0 h), 1 h post-PVI, and 1, 3 and 7 days
post-PVI. Platelets (300 × g for 15 min at 20°C), erythrocytes
(1,500 × g for 10 min at room temperature) and leukocytes (300 × g
for 5 min at room temperature) were isolated by centrifugation and
Percoll density gradients (22,23).
These blood cells, at a density of 1×106, were adjusted
to a final volume of 45 µl in Tyrode's buffer, and then incubated
with 4 nM Alex Fluor 488-lactadherin (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 15 min at room temperature
in the dark (diluted with 150 µl Tyrode's buffer). Finally, the
exposure of PS on the blood cells was detected using flow cytometry
(FACSAria, BD Biosciences, Franklin Lakes, NJ, USA).
Flow cytometric analysis of PS-exposed
MPs
The MPs were isolated from platelet-free plasma of
peripheral vein blood samples by centrifugation (350 × g for 15 min
at 4°C) and Tyrode's buffer (10).
The phenotype of the MPs was identified, as previously described
(22), and MPs were bound by
lactadherin (Al ex Fluor 488-lactadherin) to evaluate the exposure
of PS. In detail, platelet-derived MPs (PMPs), endothelial-derived
MPs (EMPs), leukocyte-derived MPs (LMPs), erythrocyte-derived MPs
(RMPs) and tissue factor-MPs (TF-MPs) were identified by
lactadherin+ CD41a+,
CD31+/CD41a−, CD45+,
CD235a+ and CD142, respectively (14,24).
The numbers of various MPs were calculated using a Trucount Tube
(BD Biosciences) with a precise number of fluorescent beads
(48,678; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
(22).
Subcellular localization of PS by
microscopy
The subcellular localizations of PS on platelets,
leukocytes and erythrocytes were observed via microscopic
fluorescence observation. Briefly, 50 µl suspensions of platelets,
leukocytes and erythrocytes at a density of 1×106 were
collected at baseline and 1 day post-PVI. These cells were then
incubated with 128 nM Alex Fluor 488-lactadherin and propidium
iodide (PI) for 10 min at room temperature in the dark. Following
removal of the unbound dye by PBS containing 0.02% Triton X-100
(v/v), images were captured using an LSM 510 Meta confocal
microscope (Carl Zeiss AG, Jena, Germany) (22).
PCA assays
The PCAs of the platelets, leukocytes, erythrocytes
and MPs at baseline, and 0, 1 h, 1 day, 3 days and 7 days post-PVI
were measured in 100 µl citrate plasma using a single-stage
recalcification time assay with an Amelung KC4A-coagulometer
(Labcon, Heppenheim, Germany) as previously described (14). The effects of 128 nM lactadherin
and 40 µg/ml anti-TF on the PCAs of the platelets, erythrocytes and
MPs (1 day post-ablation) and leukocytes (3 day post-ablation) were
also analyzed (22).
FXa and thrombin formation assays
FXa and thrombin formation assays were performed, as
previously described (22). A
universal microplate spectrophotometer (PowerWave XS; Bio-Tek,
Instruments, Inc., Winooski, VT, USA) was used to detect the
quantities of FXa and thrombin at 405 nm. The effects of
lactadherin (128 nM) and anti-TF (40 µg/ml) on blood cells and MPs
were also evaluated.
Statistical analyses
Continuous variables were examined for normal
distribution using a Shapiro-Wilk test. Normally distributed
variables are expressed as the mean ± standard deviation and
significance was analyzed using Student's t-test or
repeated-measures analysis of variance. Non-normally distributed
variables are expressed as the median with interquartile range and
significance was analyzed using a Mann-Whitney U-test. Categorical
variables are expressed as numbers (frequency) and were compared
using a Χ2 test or Fischer's exact test. Stepwise
multivariate linear regression analyses were used to assess
independent factors associated with PS+ blood cell and
MPs. Receiver operating characteristic curve analysis (ROC) was
used to determine the optimum cut-off levels of PS+
blood cells and MPs in the prediction of EARF following PVI.
Univariate and multivariate Cox proportional hazard analyses were
performed to investigate the predictors of ERAF post-PVI. Among the
above analytical methods, P<0.05 was considered to indicate a
statistically different difference. All statistical analyses were
performed by SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Clinical characteristics of patients
with AF who underwent PVI
The clinical characteristics of the patients with AF
who underwent PVI were evaluated. As shown in Table I, no significant differences were
found in age, male/female, body mass index, diabetes mellitus,
coronary artery disease, hypertension, previous stroke/transient
ischemic attack, smoking, dyslipidemia, leukocyte counts,
prothrombin time or D-dimer in the patients with AF, compared with
the controls. However, significantly shorter activated partial
thromboplastin time and higher fibrinogen were found in the
patients with AF, compared with the control group. Among these
patients, the levels of high-sensitivity C-reactive protein
(Hs-CRP), CHA2DS2-VASc score, left
ventricular ejection fraction (LVEF) and left atrial diameter (LAD)
were 2.5 (1.3–5.3) mg/l, 1.2±0.3, 61.18±10.22% and 34.97±5.84 mm
(23.21% >40 mm), respectively. In addition, between one and
three types of antiarrhythmic medications were used by these
patients, including angiotensin converting enzyme
inhibitor/angiotensin receptor blockers (53.57%) and statins
(35.71%).
 | Table I.Clinical characteristics of patients
with AF treated with pulmonary veins isolation. |
Table I.
Clinical characteristics of patients
with AF treated with pulmonary veins isolation.
| Characteristic | Control (n=40) | AF (n=56) |
|---|
| Age (years) | 54.23±7.10 | 55.25±7.70 |
| Male/female | 25/15 | 34/22 |
| BMI
(kg/m2) | 23.43±3.23 | 23.75±3.82 |
| DM, n (%) | 4
(10%) | 6
(10.71%) |
| CAD, n (%) | 3
(7.5%) | 5 (8.92%) |
| Hypertension, n
(%) | 12 (30%) | 19 (33.92%) |
| Previous
stroke/TIA, n (%) | 3
(7.5%) | 4 (7.14%) |
| Smoking, n (%) | 4
(10%) | 7 (12.5%) |
| Dyslipidemia, n
(%) | 3
(7.5%) | 4 (7.14%) |
| Leukocyte counts
(103/µl) | 6.74±1.35 |
6.91±1.62 |
| PT (sec) | 11.71±1.02 | 11.62±0.93 |
| APTT (sec) | 44.23±9.15 |
42.61±7.82a |
| Fibrinogen
(g/l) | 2.73
[1.73–4.24] | 3.43
[2.13–5.31]a |
| D-dimer
(ng/ml) | 98 [45–172] | 110 [32–145] |
In the treatment of AF, segmental PVI (5.38%),
circumferential pulmonary vein ablation (48.39%), left atrium
linear ablation (16.13%), right atrium linear ablation (20.43%) and
electrogram-based ablation (9.68%) were performed. During these
procedures, the total ablation time was 86.20±39.01 min and the
maximum power was 28.82±6.71 W. Following ablation, the curative
rate was 74.73% and ERAF was detected in 42.65% patients at the 3
month follow-up. Late recurrence of AF occurred in 15.27% patients,
whereas 62.5% patients were confirmed to have delayed curing at
subsequent follow-up.
PS exposure of blood cells
The platelets, leukocytes and erythrocytes exposed
to PS were detected by lactadherin binding using flow cytometry. At
baseline, PS+ platelets, leukocytes and erythrocytes,
and TF+ leukocytes were significantly higher in patients
with AF, compared with those in the control (P<0.01), whereas no
significant differences were revealed in the patients with
paroxysmal and non-paroxysmal AF (data not shown). Following
treatment with PVI, the number of PS+ platelets
increased with time until a peak at 1 day
(17.53±5.23×109/l; P<0.01), as shown in Fig. 1A. PS+ erythrocytes and
leukocytes were increased with a peak at 1 h
(26.23±5.31×109/l; P<0.001) and 3 days
(6.63±2.90×108/l; P<0.01), respectively (Fig. 1B and C). TF+ leukocytes
were also increased with time, which peaked at 1 h
(7.23±3.20×108/l; P<0.01), as shown in Fig. 1D.
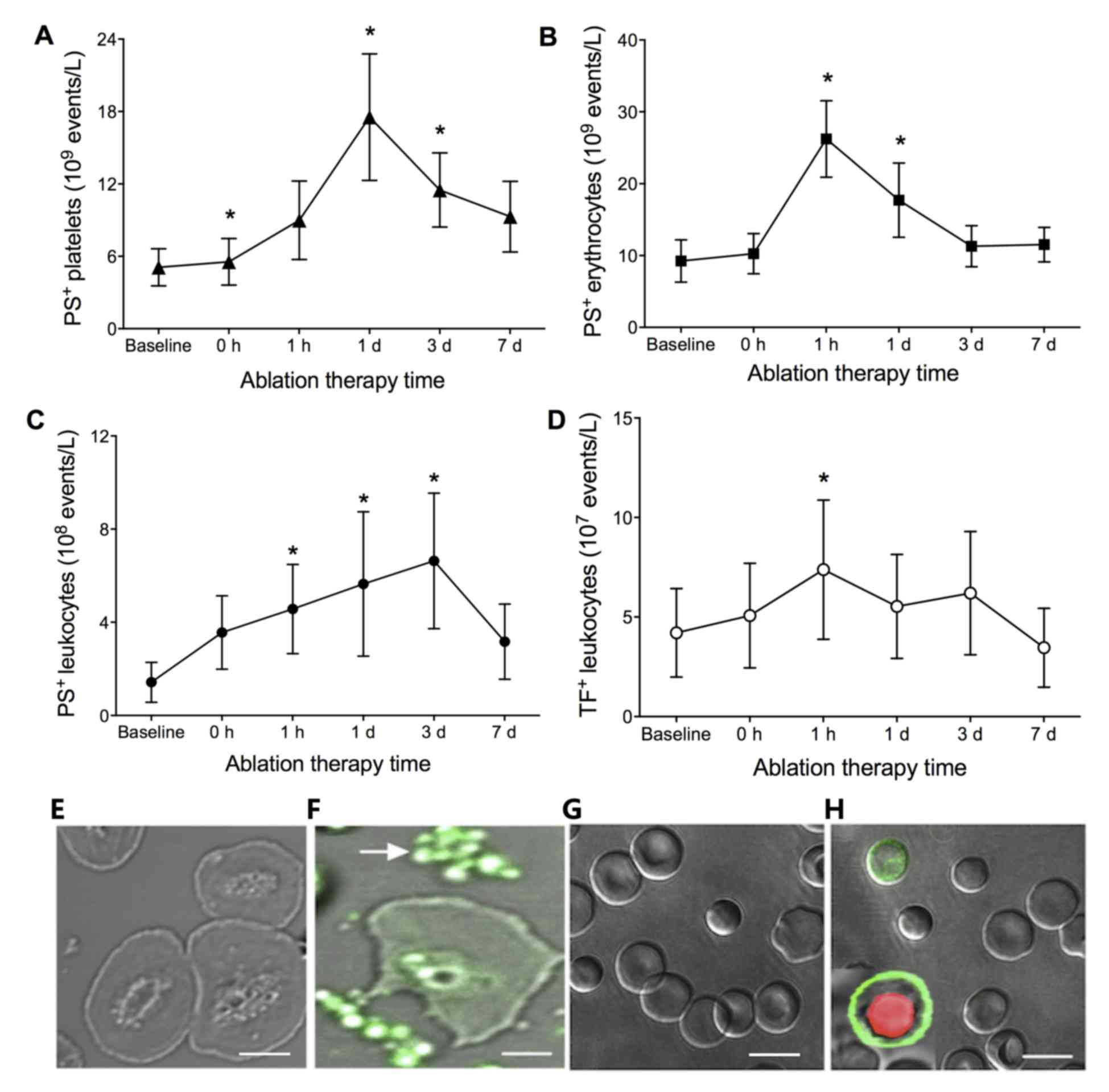 | Figure 1.Numbers of PS+ (A)
platelets, (B) erythrocytes and (C) leukocytes, and (D)
TF+ leukocytes in patients with AF at baseline (prior to
PVI), and 0, 1 h, 1 day, 3 days and 7 days post-PVI. *P<0.01,
compared with other time points. Fluorescence staining of
PS+ (E) leukocytes at baseline and (F) 1 day post-PVI,
and platelets and erythrocytes (G) at baseline and (H) 1 day
post-PVI in patients with AF. No staining was observed at baseline.
Green and red fluorescence represent positive staining of PS and
propidium iodide, respectively. Scale bar=5 µm in E and F and 10 µm
in G and H. AF, atrial fibrillation; PS, phosphatidylserine; TF,
tissue factor; PVI, pulmonary vein isolation. |
To further identify the presence of PS on platelets,
leukocytes and erythrocytes in the patients with AF following PVI,
the subcellular localizations of PS on these cells were observed.
No fluorescence was observed on the membranes of platelets,
leukocytes or erythrocytes in the patients with AF at baseline,
whereas fluorescence was observed in the platelets, leukocytes and
erythrocytes from the patients with AF at 1 day post-PVI treatment
(Fig. 1E-H).
Numbers of MPs
The effects of PVI on the numbers of MPs were
evaluated. As shown in Table II,
the PS+ MPs predominantly originated from platelets,
leukocytes and endothelial cells. The numbers of total MPs, PMPs,
LMPs, EMPs and TF-MPs were all significantly higher in the patients
with AF, compared with those in the control (P<0.01), whereas no
significant differences were found in the RMPs. No significant
differences were found in the patients with paroxysmal and
non-paroxysmal AF (data not shown). Following PVI, all types of
MPs, with the exception of TF-MPs (peak at 3 days) were
significantly elevated with a peak at 1 day, followed by a decrease
with intervention time (P<0.01; Table II).
 | Table II.Numbers of PS+ MPs in
patients with AF treated with pulmonary veins isolation. |
Table II.
Numbers of PS+ MPs in
patients with AF treated with pulmonary veins isolation.
|
|
| Patients with AF
(n=56) |
|---|
|
|
|
|
|---|
| PS+
(/µl) | Control (n=40) | Baseline | 0 h | 1 h | 1 day | 3 days | 7 days |
|---|
| Total MPs |
1,560±143 |
1,800±276b |
1,999±289a |
2.399±407a |
3,201±552a |
2,998±473a |
2,300±398a |
| PMPs |
835±170 |
1,143±245b |
1,276±247a |
1,528±279a |
2,023±376a |
1,846±287a |
1,485±268a |
| LMPs |
205±43 |
266±52b |
283±6a |
514±124a |
671±189a |
488±101a |
411±98a |
| EMPs |
136±23 |
174±34b |
226±56a |
302±74a |
367±99a |
340±86a |
277±67a |
| RMPs |
29±19 |
31±19 |
45±25a |
54±33a |
72±37a |
67±35 |
60±29a |
| TF-MPs |
33±12 |
43±13b |
54±14a |
68±22a |
84±32a |
104±78a |
66±27a |
PCAs of PS+ blood cells and
MPs
In order to reveal the PCA of PS in the patients
with AF, the coagulation time of PS+ platelets,
leukocytes, erythrocytes and MPs were evaluated. The coagulation
times of these factors were significantly shortened by PVI,
compared with those at baseline (P<0.01). The shortest
coagulation time of the PS+ platelets, erythrocytes and
MPs was observed at 1 day post-PVI, and at 3 days post-PVI for
leukocytes (Fig. 2A). The effects
of lactadherin and anti-TF on the coagulation times of these
factors were also evaluated. As shown in Fig. 2B and C, the coagulation times of
PS+ platelets, leukocytes, erythrocytes and MPs were all
prolonged by lactadherin (P<0.01), which was close to baseline
levels. Anti-TF also inhibited the coagulation times, although the
inhibitory efficacy was lower, compared with that of lactadherin
(P<0.01).
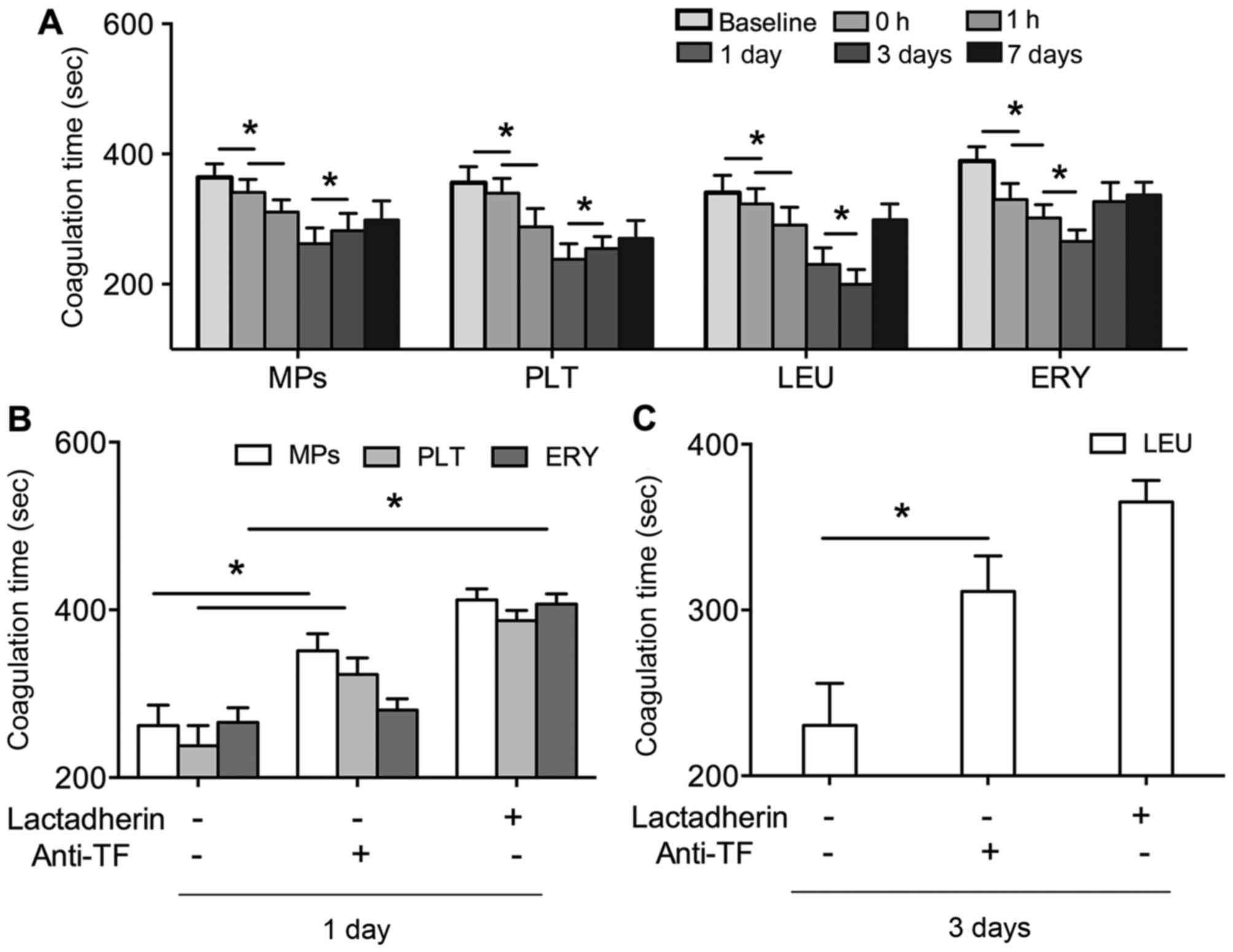 | Figure 2.(A) Coagulation times of PLT, LEU,
ERY and MPs in patients with AF at baseline (prior to PVI), and 0,
1 h, 1 day, 3 day and 7 days post PVI. (B) Inhibitory effects of
128 nM lactadherin and 40 µg/ml anti-TF on coagulation times of
PLT, ERY and MPs 1 day post-PVI, and (C) LEU 3 days post-PVI in
patients with AF. *P<0.01. AF, atrial fibrillation; PLT,
platelet; LEU, leukocyte; ERY, erythrocyte; MPs, microparticles;
TF, tissue factor; PVI, pulmonary vein isolation. |
The formation of FXa and thrombin in PS+
platelets, leukocytes, erythrocytes and MPs were found to reveal
alterations in PCA. The production of intrinsic and extrinsic FXa
and thrombin in the PS+ platelets, leukocytes,
erythrocytes and MPs were significantly increased and then reduced
with intervention time post-PVI (P<0.01). At peak
concentrations, the production of FXa and thrombin were 2–3-fold
higher than at baseline (Fig.
3A-C). However, the contents of FXa and thrombin in platelets,
leukocytes, erythrocytes and MPs were significantly inhibited by
lactadherin (Fig. 3D-F). Anti-TF
also inhibited the activity of extrinsic FXa in leukocytes and MPs
(Fig. 3E).
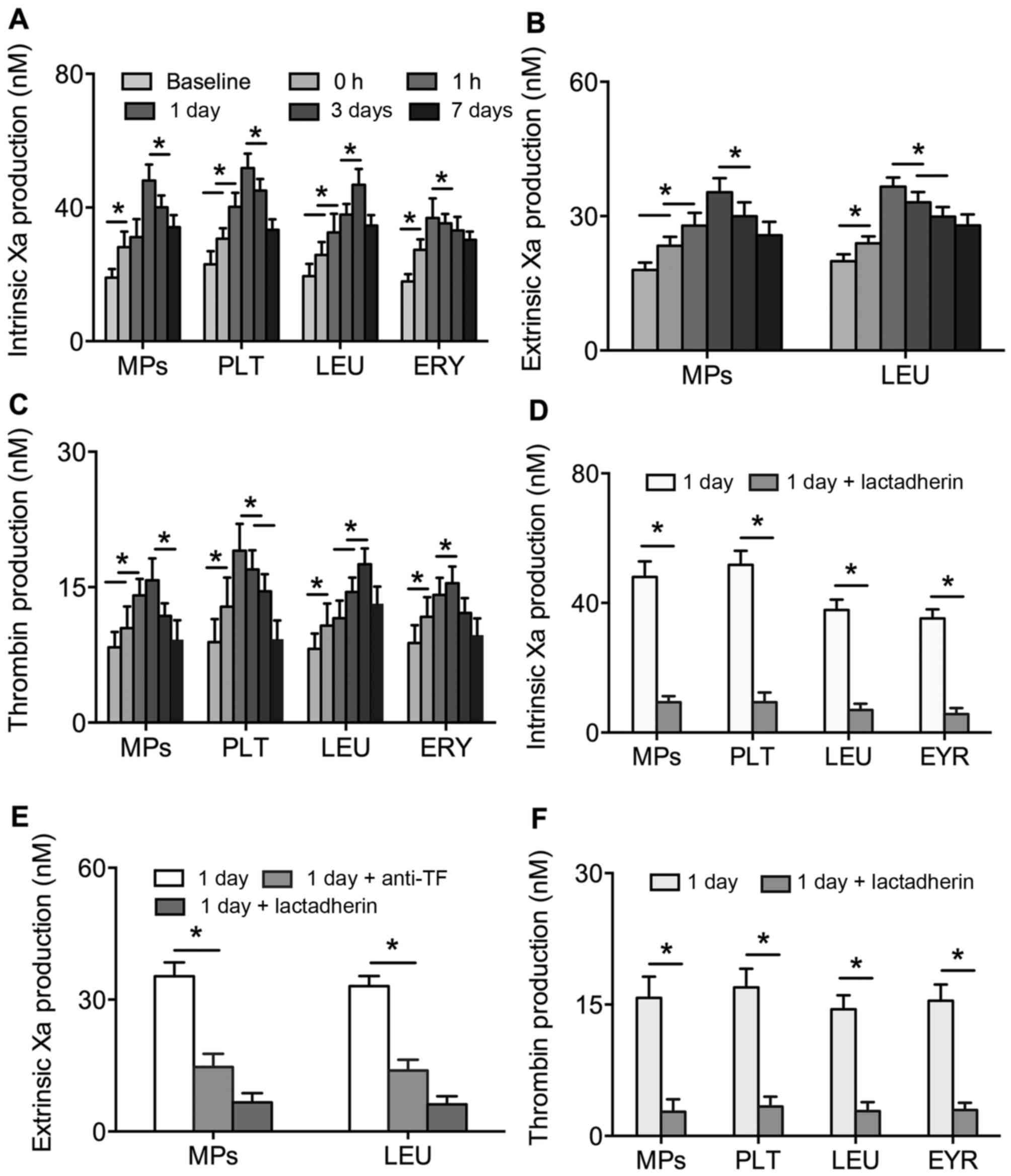 | Figure 3.Production of (A) intrinsic FXa, (B)
extrinsic FXa and (C) thrombin in PLT, LEU, ERY and MPs from
patients with AF at baseline (prior to PVI), and 0, 1 h, 1 day, 3
days and 7 days post-PVI. Inhibitory effects of 128 nM lactadherin
and 40 µg/ml anti-TF on the production of (D) intrinsic FXa, (E)
extrinsic FXa and (F) thrombin in PLT, LEU, ERY and MPs from
patients with AF at 1 day post-PVI. *P<0.01. AF, atrial
fibrillation; PLT, platelet; LEU, leukocyte; ERY, erythrocyte; MPs,
microparticles; FXa, factor Xa; TF, tissue factor; PVI, pulmonary
vein isolation. |
Associations between the clinical
characteristics of patients with AF and the numbers of
PS+ blood cells/MPs
The associations between the numbers of
PS+ blood cell/MPs and clinical characteristics of AF
were analyzed using multiple linear regression analysis. As shown
in Table III, the maximum power
was significantly associated with PMPs (β=0.101; P=0.0009), and
Hs-CRP was as a significant predictor of LMPs (β=0.325; P=0.038)
and EMPs (β=0.114; P=0.004).
 | Table III.Multiple linear regression analysis
of the associations between the number of PS+ blood
cell/MPs and clinical characteristics of patients with AF. |
Table III.
Multiple linear regression analysis
of the associations between the number of PS+ blood
cell/MPs and clinical characteristics of patients with AF.
| Variable | Coefficient | PLT | LEU | ERY | PMPs | LMPs | RMPs | EMPs |
|---|
| Age |
β-coefficienta | 0.039 | 0.253 | 0.157 | 0.044 | 0.266 | −0.027 | 0.001 |
|
| P-value | 0.877 | 0.233 | 0.581 | 0.751 | 0.376 | 0.917 | 0.221 |
| Male | β-coefficient | 0.354 | 0.276 | 0.006 | −0.018 | −0.281 | 0.028 | −0.047 |
|
| P-value | 0.126 | 0.712 | 0.478 | 0.899 | 0.105 | 0.856 | 0.727 |
| DM | β-coefficient | 0.063 | −0.091 | −0.105 | −0.028 | 0.000 | −0.087 | −0.071 |
|
| P-value | 0.621 | 0.482 | 0.360 | 0.843 | 0.988 | 0.544 | 0.567 |
| CAD | β-coefficient | 0.020 | 0.142 | 0.010 | 0.047 | −0.029 | 0.009 | 0.152 |
|
| P-value | 0.881 | 0.292 | 0.894 | 0.290 | 0.831 | 0.143 | 0.239 |
| Non-paroxysmal
AF | β-coefficient | −0.063 | −0.128 | −0.028 | 0.021 | −0.087 | −0.152 | 0.189 |
|
| P-value | 0.621 | 0.336 | 0.143 | 0.198 | 0.554 | 0.239 | 0.155 |
|
CHA2DS2-VASc ≥2 | β-coefficient | 0.001 | 0.167 | −0.042 | −0.036 | 0.374 | −0.276 | −0.035 |
|
| P-value | 0.246 | 0.868 | 0.162 | 0.216 | 0.321 | 0.427 | 0.793 |
| Hs-CRP | β-coefficient | −0.126 | 0.115 | 0.103 | −0.172 | 0.325 | −0.190 | 0.114 |
|
| P-value | 0.416 | 0.456 | 0.428 | 0.457 | 0.038b | 0.169 | 0.004b |
| Total ablation
time | β-coefficient | 0.140 | 0.027 | 0.020 | −0.207 | −0.189 | −0.060 | 0.611 |
|
| P-value | 0.293 | 0.850 | 0.893 | 0.837 | 0.155 | 0.663 | 0.167 |
| Maximum power | β-coefficient | 0.103 | 0.158 | −0.039 | 0.101 | −0.014 | 0.096 | 0.003 |
|
| P-value | 0.428 | 0.227 | 0.754 | 0.009b | 0.902 | 0.495 | 0.982 |
| Baseline
PS+ PLT | β-coefficient | 0.142 | – | – | – | – | – | – |
|
| P-value | 0.292 | – | – | – | – | – | – |
| Baseline
PS+ LEU | β-coefficient | – | 0.128 | – | – | – | – | – |
|
| P-value | – | 0.322 | – | – | – | – | – |
| Baseline
PS+ ERY | β-coefficient | – | – | −0.006 | – | – | – | – |
|
| P-value | – | – | 0.968 | – | – | – | – |
| Baseline
PS+PMPs | β-coefficient | – | – | – | 0.178 | – | – | – |
|
| P-value | – | – | – | 0.130 | – | – | – |
| Baseline
PS+LMPs | β-coefficient | – | – | – | – | −0.195 | – | – |
|
| P-value | – | – | – | – | 0.183 | – | – |
| Baseline
PS+RMPs | β-coefficient | – | – | – | – | – | 0.036 | – |
|
| P-value | – | – | – | – | – | 0.812 | – |
| Baseline
PS+EMPs | β-coefficient | – | – | – | – | – | – | 0.206 |
|
| P-value | – | – | – | – | – | – | 0.108 |
Prediction of ERAF by PS+
blood cells and MPs
The ROC was used to identify the role of
PS+ blood cells and MPs in the prediction of ERAF at 1
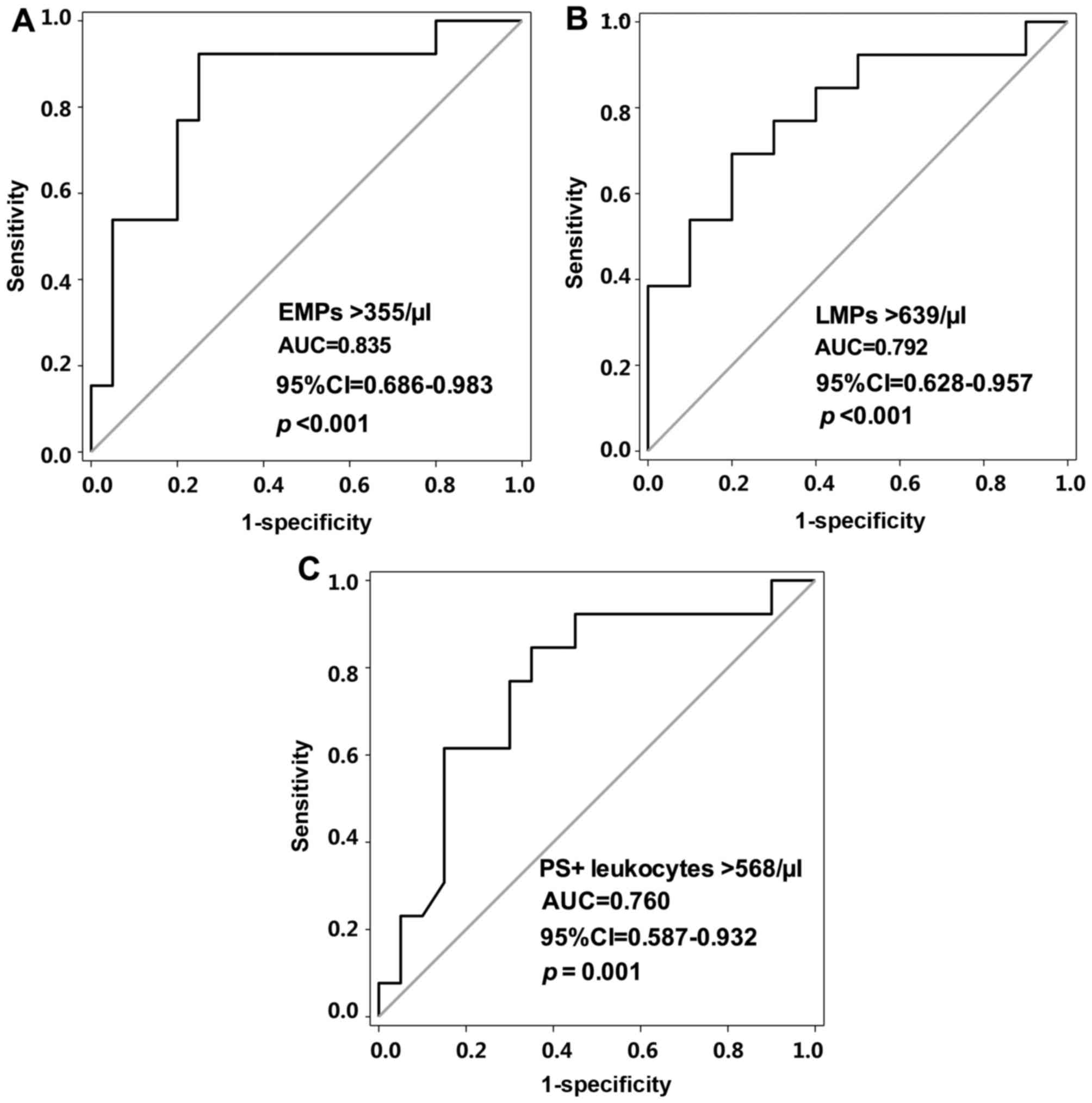
day post-PVI treatment. As shown in Fig. 4A and B, the area under the curve
(AUC) was >0.7 for PS+ leukocytes, LMPs and EMPs. The
optimal cut-off values for the PS+ leukocytes, LMPs and
EMPs were 568, 639 and 355/µl, respectively (Fig. 4A-C). In the prediction of ERAF, a
sensitivity of 76.92% and specificity of 75% were exhibited for the
PS+ leukocytes (AUC, 0.760; 95% CI, 0.587–0.932;
P=0.001). Sensitivities of 69.23 and 92.31%, and specificities of
80 and 75% were exhibited for the LMPs (AUC, 0.792; 95% CI,
0.628–0.957; P<0.001) and EMPs (AUC, 0.835; 95% CI, 0.686–0.983;
P<0.001), respectively.
The prognostic factors for ERAF were further
analyzed using univariate and multivariate analyses. The results
showed that hypertension (HR 1.96; 95% CI 0.75–5.14; P=0.047),
non-paroxysmal AF (HR 2.35; 95% CI, 1.08–5.11; P=0.031), hs-CRP (HR
5.47; 95% CI, 3.26–9.20; P=0.0001), PS+ leukocytes
>568/µl (HR 2.17; 95% CI, 1.06–4.41; P=0.033), LMPs >639/µl
(HR 1.29; 95% CI, 1.10–1.53; P=0.002) and EMPs >355/µl (HR 4.28;
95% CI, 2.03–9.03; P=0.0001) were risk factors for ERAF in the
multivariate model. Furthermore, hs-CRP (HR 4.89; 95% CI,
2.37–6.28; P=0.0001), non-paroxysmal AF (HR 1.64; 95% CI,
1.03–2.61; P=0.03) and EMPs >355/µl (HR 4.92; 95% CI,
2.25–10.74; P=0.0001) were identified as independent predictors of
ERAF (Table IV).
 | Table IV.Univariate and multivariate analyses
of predictors of early AF recurrence in patients with AF treated
with pulmonary veins isolation. |
Table IV.
Univariate and multivariate analyses
of predictors of early AF recurrence in patients with AF treated
with pulmonary veins isolation.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | Hazard ratio (95%
confidence interval) | P-value | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Age | 0.99
(0.96–1.02) | 0.464 | – | – |
| Male | 1.25
(0.63–2.51) | 0.526 | – | – |
| History |
|
Hypertension | 1.96
(0.75–5.14) | 0.047a | 0.98
(0.96–1.01) | 0.167 |
|
CAD | 0.63
(0.26–1.90) | 0.461 | – | – |
|
Non-paroxysmal AF | 2.35
(1.08–5.11) | 0.031a | 1.64
(1.03–2.61) |
0.030a |
|
CHA2DS2-VASc
≥2 | 1.00
(1.00–1.01) | 0.255 | – | – |
| Transthoracic
echocardiography |
| LAD
>40 mm | 1.04
(0.98–1.11) | 0.156 | – | – |
|
LVEF | 1.62
(0.83–3.17) | 0.161 | – | – |
| Laboratory
data |
|
Hs-CRP | 5.47
(3.26–9.20) |
<0.001a | 4.89
(2.37–6.28) |
<0.001a |
|
PS+ leukocytes
>568/µl | 2.17
(1.06–4.41) | 0.033a | 0.84
(0.32–2.21) |
0.721 |
|
PS+ LMPs
>639/µl | 1.29
(1.10–1.53) | 0.002a | 3.06
(0.62–14.98) |
0.168 |
|
PS+ EMPs
>355/µl | 4.28
(2.03–9.03) |
<0.001a | 4.92
(2.25–10.74) |
<0.001a |
Discussion
PS is known to be important in the process of
coagulation, and thrombosis is a risk factor in the treatment of AF
by ablation (25,26). It has been reported that
PS+ MPs exhibit marked procoagulant effects in patients
with AF following PVI (27).
Similarly, the PCA of MPs in the patients with AF treated with PVI
were significantly promoted by PS in the present study, which
exhibited decreased coagulation time, and increased levels of FXa
and thrombin. The present study is the first, to the best of our
knowledge, to evaluate the procoagulant role of PS on blood cells.
The results showed that PS exposure significantly reduced the
coagulation time, and increased the production of FXa and thrombin
in the blood cells of patients with AF treated with PVI. In
addition, 80% of the PCA of the blood was inhibited by the
intervention of lactadherin. These findings demonstrated that
exposure of blood cells to PS increased the risk of thrombosis, and
this effect may be caused by an additional procoagulant
phospholipid surface for the assembly of thrombase complexes and
thrombin generation in the circulation (28). In the clinical treatment of AF by
PVI, the inhibition of PS+ blood cells and MPs may bean
an effective method in the prevention of hypercoagulable states. TF
on blood cells and MPs has also been suggested to be associated
with increased PCA during PVI (29). In the present study, TF+
leukocytes were significantly increased at 1 h post-PVI, and
anti-TF treatment significantly prolonged the coagulation time of
the blood cells and MPs. This result indicated that thermally
injured leukocytes induced by ablation increased the expression of
TF (30). TF was also be activated
by PS residing on cell membranes and promoted the coagulation
reaction (31).
Clinically, higher levels of MPs are found in
patients with AF, and ablation is considered to affect the
distribution of MPs (32). It has
been reported that GPIb+ PMPs and CD11a+ LMPs
are increased in patients with AF within 48 h following
radiofrequency ablation or cryoablation (6). Consistent with previous findings, the
present study showed that the numbers of all types of MPs were
significantly increased by PVI in the patients with AF, which
further indicated the activation of apoptosis in blood cells.
However, associated studies on the associations between elevated
PS+ MPs and clinical characteristics of patients with AF
during PVI are limited. In the present study, the maximum power of
PVI was an independent predictor of PS+ PMPs, which
indicated the role of PMPs on the extent of tissue damage. In
addition, the level of hs-CRP was correlated with PS+
LMPs and EMPs independently. This correlation suggested that the
procoagulant state was associated with inflammation (33), potentially due to the activation of
associated complements. For example, the activation of cyclosporine
3 has been shown to induce MP shedding (34). However, non-paroxysmal AF and
CHA2DS2-VASc scores ≥2, which are
particularly sensitive to disease severity in AF, were not found to
be associated with MPs.
Although PVI is regarded as an effective and safe
therapeutic option for patients with symptomatic and
drug-refractory AF, ERAF occurs during follow-up (19). In the present study, PS+
EMPs >355/µl was revealed to be a significant predictor of ERAF.
This result supports the hypothesis that transient inflammation and
procoagulant state following PVI-induced tissue damage contribute
to the occurrences of ERAF (35).
The preoperative hs-CRP level was also found to be an independent
predictor of ERAF within 3 months following PVI. A high hs-CRP
level has been associated with abnormal left atrial substrate and a
high incidence of nonpulmonary vein AF sources, which contribute to
the recurrence of AF (36), and
the preoperative hs-CRP level has been associated with ERAF within
3 days following catheter ablation in patients with paroxysmal or
persistent AF (37). The results
of the present were consistent with previous studies, further
demonstrating the predictive value of hs-CRP on ERAF during
follow-up, and its association with inflammation and procoagulant
activity. Therefore, the high prognostic values of hs-CRP and
PS+ EMPs in ERAF were considered to be beneficial to the
determination of AF in those not benefiting from PVI.
In conclusion, the present study demonstrated
significant procoagulant effects exhibited by PS+ blood
cells and MPs in patients with AF treated with PVI. Hs-CRP and EMPs
>355/µl were significant prognostic factors of ERAF during the
follow-up period in patients with AF treated with PVI. However,
limitations of the study included insufficient subjects, whereas
bolus heparin and continuous aspirin treatment in patients may
underestimate the activation of the coagulating cascade post-PVI.
Therefore, further investigations on the procoagulant effect and
long-term prognostic implications of PS+ blood cells and
MPs in AF are required.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81270588 and
81470301), the Natural Science Foundation of Heilongjiang Province
(grant no. ZD2015020) and the Graduate Innovation Fund of Harbin
Medical University (grant no. YJSCX2014-41HYD). The authors would
like to thank Professor Bo Yu, Professor Jie Yuan, Professor Yong
Sun, Ms. Xueqin Gao, Mr. Qinlong Tao and Ms. Na Han from the
Department of Cardiology, The First Hospital, Harbin Medical
University (Harbin, China) for sample collection.
References
|
1
|
Lip GY and Lane DA: Stroke prevention in
atrial fibrillation: A systematic review. JAMA. 313:1950–1962.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calkins H, Kuck KH, Cappato R, Brugada J,
Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J,
et al: 2012 HRS/EHRA/ECAS expert consensus statement on catheter
and surgical ablation of atrial fibrillation: Recommendations for
patient selection, procedural techniques, patient management and
follow-up, definitions, endpoints, and research trial design. J
Interv Card Electrophysiol. 33:171–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noel P, Gregoire F, Capon A and Lehert P:
Atrial fibrillation as a risk factor for deep venous thrombosis and
pulmonary emboli in stroke patients. Stroke. 22:760–762. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiedrowicz RM, Kazmierczak J and
Wielusinkski M: Left atrial massive thrombus formation on the
transseptal sheath despite adequate anticoagulation with warfarin
and heparin during pulmonary vein isolation. J Cardiovasc
Electrophysiol. 24:11852013.PubMed/NCBI
|
|
5
|
Lim HS, Schultz C, Dang J, Alasady M, Lau
DH, Brooks AG, Wong CX, Roberts-Thomson KC, Young GD, Worthley MI,
et al: Time course of inflammation, myocardial injury, and
prothrombotic response after radiofrequency catheter ablation for
atrial fibrillation. Circ Arrhythm Electrophysiol. 7:83–89. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siklódy C Herrera, Arentz T, Minners J,
Jesel L, Stratz C, Valina CM, Weber R, Kalusche D, Toti F, Morel O
and Trenk D: Cellular damage, platelet activation, and inflammatory
response after pulmonary vein isolation: A randomized study
comparing radiofrequency ablation with cryoablation. Heart Rhythm.
9:189–196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stazi A, Scalone G, Laurito M, Milo M,
Pelargonio G, Narducci ML, Parrinello R, Figliozzi S, Bencardino G,
Perna F, et al: Effect of remote ischemic preconditioning on
platelet activation and reactivity induced by ablation for atrial
fibrillation. Circulation. 129:11–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haeusler KG, Kirchhof P and Endres M: Left
atrial catheter ablation and ischemic stroke. Stroke. 43:265–270.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaita F, Caponi D, Pianelli M, Scaglione
M, Toso E, Cesarani F, Boffano C, Gandini G, Valentini MC, De Ponti
R, et al: Radiofrequency catheter ablation of atrial fibrillation:
A cause of silent thromboembolism? Magnetic resonance imaging
assessment of cerebral thromboembolism in patients undergoing
ablation of atrial fibrillation. Circulation. 122:1667–1673. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bulava A, Slavik L, Fiala M, Heinc P,
Skvarilova M, Lukl J, Krcová V and Indrák K: Endothelial damage and
activation of the hemostatic system during radiofrequency catheter
isolation of pulmonary veins. J Interv Card Electrophysiol.
10:271–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leventis PA and Grinstein S: The
distribution and function of phosphatidylserine in cellular
membranes. Annu Rev Biophys. 39:407–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeung T, Gilbert GE, Shi J, Silvius J,
Kapus A and Grinstein S: Membrane phosphatidylserine regulates
surface charge and protein localization. Science. 319:210–213.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rysavy NM, Shimoda LM, Dixon AM, Speck M,
Stokes AJ, Turner H and Umemoto EY: Beyond apoptosis: The mechanism
and function of phosphatidylserine asymmetry in the membrane of
activating mast cells. Bioarchitecture. 4:127–137. 2014.PubMed/NCBI
|
|
14
|
Tan X, Shi J, Fu Y, Gao C, Yang X, Li J,
Wang W, Hou J, Li H and Zhou J: Role of erythrocytes and platelets
in the hypercoagulable status in polycythemia vera through
phosphatidylserine exposure and microparticle generation. Thromb
Haemost. 109:1025–1032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tormoen GW, Recht O, Gruber A, Levine RL
and McCarty OJ: Phosphatidylserine index as a marker of the
procoagulant phenotype of acute myelogenous leukemia cells. Phys
Biol. 10:0560102013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen VM and Hogg PJ: Encryption and
decryption of tissue factor. J Thromb Haemost. 11 Suppl
1:S277–S284. 2013. View Article : Google Scholar
|
|
17
|
Diamant M, Nieuwland R, Pablo RF, Sturk A,
Smit JW and Radder JK: Elevated numbers of tissue-factor exposing
microparticles correlate with components of the metabolic syndrome
in uncomplicated type 2 diabetes mellitus. Circulation.
106:2442–2447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geisbert TW, Young HA, Jahrling PB, Davis
KJ, Kagan E and Hensley LE: Mechanisms underlying coagulation
abnormalities in ebola hemorrhagic fever: Overexpression of tissue
factor in primate monocytes/macrophages is a key event. J Infect
Dis. 188:1618–1629. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Joshi S, Choi AD, Kamath GS, Raiszadeh F,
Marrero D, Badheka A, Mittal S and Steinberg JS: Prevalence,
predictors, and prognosis of atrial fibrillation early after
pulmonary vein isolation: Findings from 3 months of continuous
automatic ECG loop recordings. J Cardiovasc Electrophysiol.
20:1089–1094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takahashi Y, Takahashi A, Kuwahara T,
Fujino T, Okubo K, Kusa S, Fujii A, Yagishita A, Miyazaki S, Nozato
T, et al: Clinical characteristics of patients with persistent
atrial fibrillation successfully treated by left atrial ablation.
Circ Arrhythm Electrophysiol. 3:465–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calkins H, Kuck KH, Cappato R, Brugada J,
Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J,
et al: 2012 HRS/EHRA/ECAS expert consensus statement on catheter
and surgical ablation of atrial fibrillation: Recommendations for
patient selection, procedural techniques, patient management and
follow-up, definitions, endpoints, and research trial design: A
report of the heart rhythm society (HRS) task force on catheter and
surgical ablation of atrial fibrillation. Developed in partnership
with the European heart rhythm association (EHRA), a registered
branch of the European society of cardiology (ESC) and the European
cardiac arrhythmia society (ECAS); and in collaboration with the
American college of cardiology (ACC), American heart association
(AHA), the Asia pacific heart rhythm society (APHRS) and the
society of thoracic surgeons (STS). Endorsed by the governing
bodies of the American college of cardiology foundation, the
American heart association, the European cardiac arrhythmia
society, the European heart rhythm association, the society of
thoracic surgeons, the Asia pacific heart rhythm society and the
heart rhythm society. Heart Rhythm. 9:632–696.e21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao C, Xie R, Yu C, Wang Q, Shi F, Yao C,
Xie R, Zhou J, Gilbert GE and Shi J: Procoagulant activity of
erythrocytes and platelets through phosphatidylserine exposure and
microparticles release in patients with nephrotic syndrome. Thromb
Haemost. 107:681–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao C, Xie R, Li W, Zhou J, Liu S, Cao F,
Liu Y, Ma R, Si Y, Liu Y, et al: Endothelial cell phagocytosis of
senescent neutrophils decreases procoagulant activity. Thromb
Haemost. 109:1079–1090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Ierssel SH, van Craenenbroeck EM,
Conraads VM, van Tendeloo VF, Vrints CJ, Jorens PG and Hoymans VY:
Flow cytometric detection of endothelial microparticles (EMP):
Effects of centrifugation and storage alter with the phenotype
studied. Thromb Res. 125:332–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spronk HM, ten Cate H and van der Meijden
PE: Differential roles of tissue factor and phosphatidylserine in
activation of coagulation. Thromb Res. 133 Suppl 1:S54-S562014.
View Article : Google Scholar
|
|
26
|
Kakkar AK, Mueller I, Bassand JP,
Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Lip GY,
Mantovani LG, et al: Risk profiles and antithrombotic treatment of
patients newly diagnosed with atrial fibrillation at risk of
stroke: Perspectives from the international, observational,
prospective GARFIELD registry. PLoS One. 8:e634792013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jesel L, Morel O, Pynn S, Radulescu B,
Grunebaum L, Freyssinet JM, Ohlmann P, Bareiss P, Toti F and
Chauvin M: Radiofrequency catheter ablation of atrial flutter
induces the release of platelet and leukocyte-derived procoagulant
microparticles and a prothrombotic state. Pacing Clin
Electrophysiol. 32:193–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Puddu P, Puddu GM, Cravero E, Muscari S
and Muscari A: The involvement of circulating microparticles in
inflammation, coagulation and cardiovascular diseases. Can J
Cardiol. 26:140–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lwaleed BA, Breish MO, Birch BR, Chowdhary
AP, Saad RA, Perigo O, Kazmi RS, Dusse LM and Cooper AJ: Tissue
factor and tissue factor pathway inhibitor in women with a past
history of preeclampsia: Implication for a hypercoagulable state
postpregnancy. Blood Coagul Fibrinolysis. 25:671–674. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rao LV and Pendurthi UR: Regulation of
tissue factor coagulant activity on cell surfaces. J Thromb
Haemost. 10:2242–2253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bach RR: Tissue factor encryption.
Arterioscler Thromb Vasc Biol. 26:456–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jesel L, Abbas M, Toti F, Cohen A, Arentz
T and Morel O: Microparticles in atrial fibrillation: A link
between cell activation or apoptosis, tissue remodelling and
thrombogenicity. Int J Cardiol. 168:660–669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kallergis EM, Manios EG, Kanoupakis EM,
Mavrakis HE, Kolyvaki SG, Lyrarakis GM, Chlouverakis GI and Vardas
PE: The role of the post-cardioversion time course of hs-CRP levels
in clarifying the relationship between inflammation and persistence
of atrial fibrillation. Heart. 94:200–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Renner B, Klawitter J, Goldberg R,
McCullough JW, Ferreira VP, Cooper JE, Christians U and Thurman JM:
Cyclosporine induces endothelial cell release of
complement-activating microparticles. J Am Soc Nephrol.
24:1849–1862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grubman E, Pavri BB, Lyle S, Reynolds C,
Denofrio D and Kocovic DZ: Histopathologic effects of
radiofrequency catheter ablation in previously infarcted human
myocardium. J Cardiovasc Electrophysiol. 10:336–342. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin YJ, Tsao HM, Chang SL, Lo LW, Tuan TC,
Hu YF, Udyavar AR, Tsai WC, Chang CJ, Tai CT, et al: Prognostic
implications of the high-sensitive C-reactive protein in the
catheter ablation of atrial fibrillation. Am J Cardiol.
105:495–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koyama T, Sekiguchi Y, Tada H, Arimoto T,
Yamasaki H, Kuroki K, Machino T, Tajiri K, Zhu XD, Kanemoto M, et
al: Comparison of characteristics and significance of immediate
versus early versus no recurrence of atrial fibrillation after
catheter ablation. Am J Cardiol. 103:1249–1254. 2009. View Article : Google Scholar : PubMed/NCBI
|