Introduction
Inflammation, which is the natural response of
living tissue to infection or injury, is able to inactivate toxins,
destroy microorganisms and ultimately to restore damaged tissue
(1). However, the majority of
inflammatory mediators exert adverse effects that can cause further
tissue and organ damage, chronic inflammation and hypersensitivity.
During onset of the inflammatory response, macrophages serve an
important role by releasing proinflammatory mediators and
cytokines.
Lipopolysaccharide (LPS) is a major constituent of
the outer membrane of Gram-negative bacteria, which induces
inflammation through the release of inflammatory mediators,
including tumor necrosis factor (TNF)-α and interleukin (IL)-1β
(2). When LPS acts on Toll-like
receptors, which are expressed on macrophages, various pathways are
activated. These activated macrophages often produce downstream
proinflammatory mediators and cytokines, which may ultimately
result in the development of anti-inflammatory agents.
IL-1β and TNF-α are multifunctional cytokines that
are involved in the regulation of the immune response,
hematopoiesis and inflammation (3). While numerous cytokines have
demonstrated beneficial effects on immune regulation, some have
also been implicated in the pathogenesis of acute and chronic
inflammatory disease (4).
High-mobility group box 1 (HMGB1) is a protein,
which is released by activated monocytes or macrophages, as well as
damaged and necrotic cells (5).
Within the nucleus, HMGB1 serves an important role in the
regulation of gene transcription (6). Upon release by phagocytes and
damaged/necrotic cells (7–9), extracellular HMGB1 has a critical
role in the initiation of inflammation; HMGB1 can activate
macrophages and upregulate the expression of cytokines such as
TNF-α and IL-1β (10), and
contribute to the pathogenesis of various inflammatory diseases
(11,12). As a late mediator of inflammation,
HMGB1 has been reported to be released days after endotoxin
exposure (13). HMGB1 amplifies
the inflammatory response by stimulating the release of various
proinflammatory cytokines in numerous types of cell, including
macrophages and monocytes (14).
Due to the critical role of HMGB1 in the process of inflammation,
HMGB1 represents a promising drug target for the clinical treatment
of inflammatory diseases (15).
Furthermore, it has previously been demonstrated that the mechanism
underlying inhibition of HMGB1 release is associated with the
nuclear factor (NF)-κB signaling pathway (16). Therefore, in order to develop a
therapeutic strategy to treat inflammatory conditions by inhibiting
HMGB1, it is important to determine whether HMGB1 secretion may be
attenuated via activation of the NF-κB pathway.
Acanthopanax gracilistylus (AGS) belongs to
the Araliaceae family. Its dried roots and stem bark are officially
listed in the Chinese Pharmacopoeia as Acanthopanacis Cortex, and
have been commonly used to treat paralysis, arthritis, rheumatism,
myasthenia gravis, bone pains, lameness and liver disease for
several centuries in China (17,18).
Furthermore, various Acanthopanax Miq. plants have been
widely used as a treatment for inflammatory diseases, due to their
anti-inflammatory activity. For example, extracts from A.
giraldii Harms, A. senticosus, A. henryi and
A. koreanum have previously been reported to inhibit the
production of inflammatory factors, including TNF-α, nitric oxide,
prostaglandin E2, IL-1β, and IL-6, in activated
inflammatory cells (19–23). However, the mechanism by which the
biologically active components of AGS elicit these
anti-inflammatory effects remains to be elucidated.
Previous studies have isolated numerous chemical
constituents from AGS (24–27).
In our initial screen of medicinal plants for anti-inflammatory
compounds, five saponins (compounds 1–5) were isolated from AGS. In
order to investigate the anti-inflammatory properties of these
saponins, the present study used the murine macrophage-like cell
line, RAW264.7. RAW264.7 cells were exposed to LPS to initiate an
inflammatory cascade, and the effects of AGS-isolated compounds 1–5
were assessed on the production of TNF-α, IL-1β and HMGB1 in
LPS-stimulated cells. In addition, the present study aimed to
determine whether the anti-inflammatory effects of these compounds
were mediated via the NF-κB signaling pathway.
Materials and methods
Plant material
The leaves of AGS were collected from Changsha,
China, in September 2012, and were botanically identified by
Professor Chang-Soo Yook (Kyung Hee University, Seoul, South
Korea).
Chemicals and regents
The RAW264.7 cell line was purchased from Shanghai
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). LPS, dimethyl sulfoxide (DMSO), and
dexamethasone (DEX) were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). The EZ4 U cell proliferation and cytotoxicity
assay kit (cat. no. BI-5000) was obtained from Biomedica
Medizinprodukte GmbH & Co KG (Vienna, Austria). Anti-HMGB1
antibody (cat no. ab18256) was purchased from Abcam (Cambridge,
UK). The secondary antibody conjugated with horseradish peroxidase,
conjugated goat anti-rabbit Immunoglobulin G (cat. no. A0208) and
Taq DNA Polymerase (cat. no. D7209) were purchased from Beyotime
Institute of Biotechnology (Jiangsu, China). TNF a Mouse ELISA kit
(cat. no. KMC3012) and IL-1β Mouse ELISA kit (cat. no. KMC0012)
were obtained from Invitrogen; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The anti-β-actin primary antibody (cat. no.
sc-130656) was obtained from Santa Cruz Bioechnology, Inc. (Dallas,
TX, USA). Total NF-κB p65 Sandwich ELISA kit was purchased from
(cat. no. 7174; Cell Signalling Technology, Inc. (Danvers, MA,
USA), RPMI 1640 medium, Dulbecco's modified Eagle's medium (DMEM),
OPTI-MEM I medium and fetal bovine serum (FBS) were obtained from
Gibco; Thermo Fisher Scientific, Inc. Other reagents used in the
present study were endotoxin-free.
Extraction and isolation
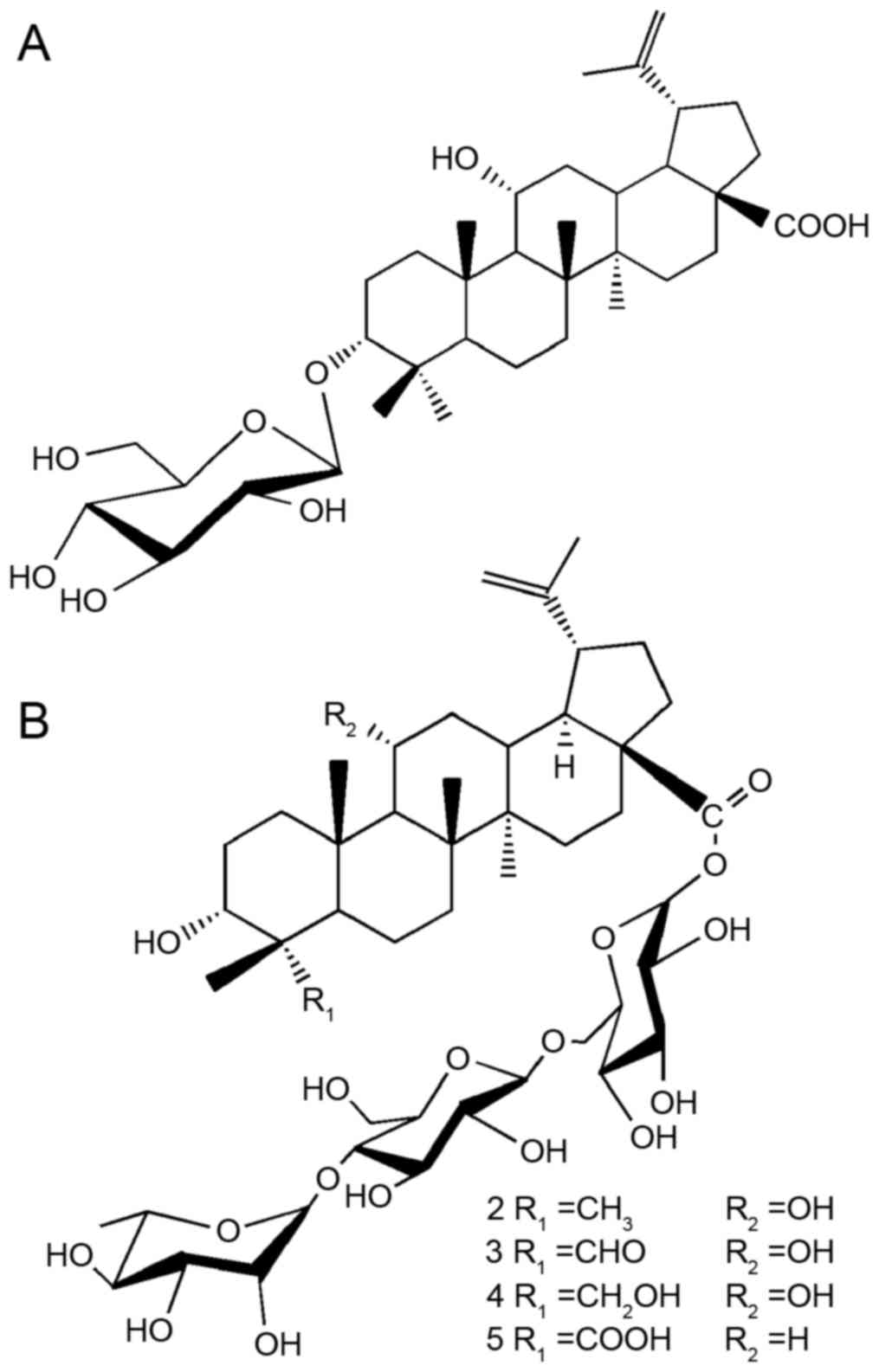
The isolation of compounds 1–5 (compound 1,
3-O-β-D-glucopyranosyl 3α,
11α-dihydroxylup-20(29)-en-28-oic acid; compound 2,
acantrifoside A; compound 3, acankoreoside D; compound 4,
acankoreoside B; and compound 5, acankoreoside A) was performed as
described previously (27).
Briefly, the dried leaves of AGS (1,000 g) were extracted three
times using hot methanol (3×10 l). The combined methanol extract
was evaporated under reduced pressure to obtain a residue (140 g),
which was dissolved in water and successively partitioned with
petroleum ether, ethyl acetate and n-butyl alcohol, resulting in
petroleum ether (6 g), ethyl acetate (42 g) and n-butyl alcohol (58
g) layers. The ethyl acetate fraction (4.0 g) underwent
chromatography on a silica gel column (Φ 25×100 mm) using
chloroform-methanol (10:1, v/v) to obtain three fractions
(E1-E3). E2 was recrystallized to
generate compound 1 (65 mg). In addition, 2.0 g n-butyl alcohol
fraction underwent chromatography on an minimum dead space (a
reversed-phase chromatography material manufactured by Beijing
Medicine Technology Center, Beijing, China) column (Φ 30×150 mm)
using V (MeOH):V (H2O)=5:5 to obtain five fractions
(B1-B5). B1 and B4 were
recrystallized to obtain compounds 2 (315 mg) and 5 (440 mg),
respectively. B2 and B3 underwent
chromatography on a silica gel, eluted with
chloroform-methanol-water (7:3:0.3, v/v), and were recrystallized
to obtain compounds 3 (35 mg) and 4 (30 mg), respectively. The
structures of compounds 1–5 were identified by analyzing the
signals of spectral data [mass spectrometry, 1H- and
13C-nuclear magnetic resonance, and their values were
reported in the authors' previous study (27)] and are presented in Fig. 1. The compounds used in this study
were checked by HPLC and were >98% pure.
Cell culture and stimulation
RAW264.7 macrophages were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS, 100 U/ml penicillin and 100 U/ml
streptomycin in tissue culture dishes at 37°C, in a humidified
atmosphere containing 5% CO2. For most experiments,
cells were plated at a density of 1×106 cells/well in
12-well plates in 200 µl DMEM. Adherent RAW264.7 cells in 12-well
culture plates were gently washed, and cultured in serum-reduced
OPTI-MEM I medium (Gibco; Thermo Fisher Scientific, Inc.) for 8 h
prior to treatment with vehicle (0.1% DMSO) or various
concentrations of compounds 1–5 (5–150 µM, dissolved in 0.1% DMSO).
After 1 h of treatment with various concentrations of compounds 1–5
or vehicle, LPS was added at a final concentration of 100 ng/ml at
varying time intervals. Cells were incubated with LPS at 37°C until
further analysis.
Cytotoxicity assay
The cytotoxicity of compounds 1–5 was assessed in
RAW264.7 cells using an MTT-based test (EZ4 U cell proliferation
and cytotoxicity assay kit; Biomedica Medizinprodukte GmbH & Co
KG) according to the manufacturer's protocol. Briefly,
1×103 cells/well were seeded in quadruplicate in 96-well
microplates and were cultured with various concentrations of
compounds 1–5 (5–150 µM) for 48 h. Subsequently, 20 µl EZ4U reagent
was added to each well and the cells were incubated for 4 h at
37°C. Absorbance was recorded using a Spectrafluor fluorometer
(Tecan Group Ltd., Männedorf, Switzerland) at 450 nm; the reference
wavelength was 620 nm. The spectrophotometer was calibrated to 0
absorbance using cell-free culture medium. Cell viability (%)
relative to control was calculated as follows: (A) test/(A) control
×100, where A refers to absorbance. Data are presented as the mean
± standard deviation of three individual experiments performed in
triplicate.
Detection of TNF-α and IL-1β
production
Macrophages were pretreated with various
concentrations of compounds 1–5 or DEX (10 µM, as a positive
control) for 1 h, and were then exposed to LPS (100 ng/ml) for 6 h.
Subsequently, culture media were collected and assessed using
commercially available sandwich ELISA kits, according to the
manufacturer's protocols (Invitrogen; Thermo Fisher Scientific,
Inc.). Briefly, 50 µl incubation buffer, 50 µl standard diluent
buffer, and 50 µl standards, controls or samples were added in
triplicate to anti-TNF-α or anti-IL-1β-coated ELISA microplates,
and 50 µl biotin conjugate solution was added to the monoclonal
antibody-coated microtiter wells, with the exception of the
chromogen blank wells. Plates were covered and incubated at room
temperature for 90 min. Subsequently, the wells were aspirated and
washed four times with wash buffer, after which 100 µl
streptavidin-horseradish peroxidase working solution was added to
each well and incubated for 30 min at room temperature. Solutions
were aspirated and the wells were washed a further four times,
after which 100 µl stabilized chromogen was added to each well, and
the plates were incubated for 30 min at room temperature in the
dark. Finally, 100 µl stop solution was added to each well, and
absorbance was measured at 450 nm using a plate reader (Perkin
Elmer Cetus; PerkinElmer, Inc., Waltham, MA, USA). Absorbance
values were normalized using a standard curve.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Macrophages were pretreated with various
concentrations of compounds 1–5 (10, 20, 30, 40 or 50 µM) for 1 h
and were then exposed to LPS (100 ng/ml) for 2 h. After washing
twice with PBS, total RNA was extracted from the treated RAW264.7
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. RNA isolation was
conducted in an RNase-free environment. Subsequently, 4 µg RNA was
reverse transcribed at 42°C for 1 h using MuLV reverse
transcriptase (Promega Corporation, Madison, WI, USA), oligo (dT)16
primer, dNTP (0.5 µM) and 1 unit RNase inhibitor, and terminated by
heating at 70°C for 15 min. Then PCR analyses were performed on the
aliquots of cDNA to detect TNF-α, IL-1β and β-actin (as an internal
standard) gene expression using a DNA gene cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Reactions were carried out
in a volume of 25 µl containing 1 unit of Taq DNA polymerase (cat.
no. D7209; Beyotime Institute of Biotechnology), 0.2 mM dNTP, 10X
reaction buffer, and 100 pmol of the 5′ and 3′ primers. The PCR
cycle was as follows: 95°C for 10 min; 40 cycles of 95°C for 15 sec
and 60°C for 1 min. The PCR primer sequences used in the present
study were as follows (Takara Biotechnology Co., Ltd., Dalian,
China): TNF-α, sense 5′-GAATGGGTGTTCATCCATTCT-3′, anti-sense
5′-GCTTAAGTGACCTCGGAGCTTACA-3′; IL-1β, sense
5′-TTGACGGACCCCAAAGAGTG-3′, anti-sense 5′-ACTCCTGTACTCGTGGAAGA-3′;
and β-actin, sense 5′-ATGGTGGGAATGGGTCAGAAG-3′ and anti-sense
5′-GGAAGATGTTACTCGACGAGC-3′. After amplification, the PCR reaction
products were separated by 1.2% agarose gel electrophoresis, and
were visualized by ethidium bromide staining and ultraviolet
irradiation.
Western blot analysis
Macrophages were pretreated with compounds 1–5 for 1
h and were then exposed to LPS (100 ng/ml) for 24 h.
RAW264.7-conditioned medium was harvested, filtered and
concentrated through a Centricon YM-10 ultrafilter (pre-wetted with
distilled water; EMD Millipore, Billerica, MS, USA) according to
the manufacturer's protocol. Concentrated samples were stored in
aliquots at −80°C.
Cells were harvested and washed three times with
cold PBS, after which total protein extracts were isolated from the
RAW264.7 cells. Briefly, the cell pellet was resuspended in 100 ml
cell lysate buffer (50 mmol/l Tris, 150 mM NaCl, 1 mM
phenylmethylsulfonyl fluoride, 0.1% SDS, 0.02% sodium azide and 1%
Nonidet P-40). After mixing at 4°C for 30 min on a shaking platform
and undergoing ultrasonication at 20 KHz for 30 sec, the cellular
extracts were centrifuged at 12,000 × g for 30 min in a microfuge
at 4°C, and the supernatants were stored in aliquots at −80°C.
Protein concentration was determined using the Enhanced
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology).
Subsequently, 25 µl processed conditioned medium or
15 µg total proteins from cellular extracts were loaded onto a 12%
SDS-PAGE gel. After electrophoretic separation, proteins were
transferred to a polyvinylidene fluoride membrane. The membrane was
blocked with 5% fat-free skim milk in Tris-buffered saline
containing 0.05% Tween-20 (TBST) at room temperature for 1 h, and
was then incubated with anti-HMGB1 (cat no. ab18256; 1:1,000;
Abcam) or anti-β-actin primary antibodies (cat. no. sc-130656;
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C
overnight. After washing with TBST, the membrane was incubated with
a horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin
G secondary antibody (cat. no. A0208; 1:5,000; Beyotime Institute
of Biotechnology) at room temperature for 1 h, followed by
extensive washing. The blot was visualized using enhanced
chemiluminescence detection reagent (Thermo Fisher Scientific,
Inc.) and was semi-quantified by densitometry using Quantity One
software (version 4.6.2; Bio-Rad Laboratories, Inc.). The protein
expression levels of in cellular extracts were normalized to
β-actin levels.
Determination of NF-κB activation
Macrophages were pretreated with compounds 1–5 (10,
20, 30, 40 or 50 µM) for 1 h and were then exposed to LPS (100
ng/ml) for 2 h. Following stimulation, nuclear extracts were
prepared using a Cayman Nuclear Extraction kit (Cayman Chemical
Company, Ann Arbor, MI, USA) according to the manufacturer's
protocol. The suspension was centrifuged at 16,000 × g, for 5 min
at 4°C and the supernatants containing cytosolic fractions were
stored at −80°C for subsequent analysis of cytoplasmic NF-κB.
Levels of NF-κB were measured using an NF-κB p65 ELISA kit (cat.
no. 7174; Cell Signalling Technology, Inc., Danvers, MA, USA),
according to the manufacturer's protocol.
Statistical analysis
All the experiments were repeated at least three
times. The results are expressed as the mean ± standard deviation.
One-way analysis of variance with Duncan's multiple range tests was
used to examine the difference between groups, and Student's t-test
was also used to examine the difference between vehicle and LPS
groups. In all comparisons, P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL,
USA).
Results
Effects of compounds 1–5 on cell
viability
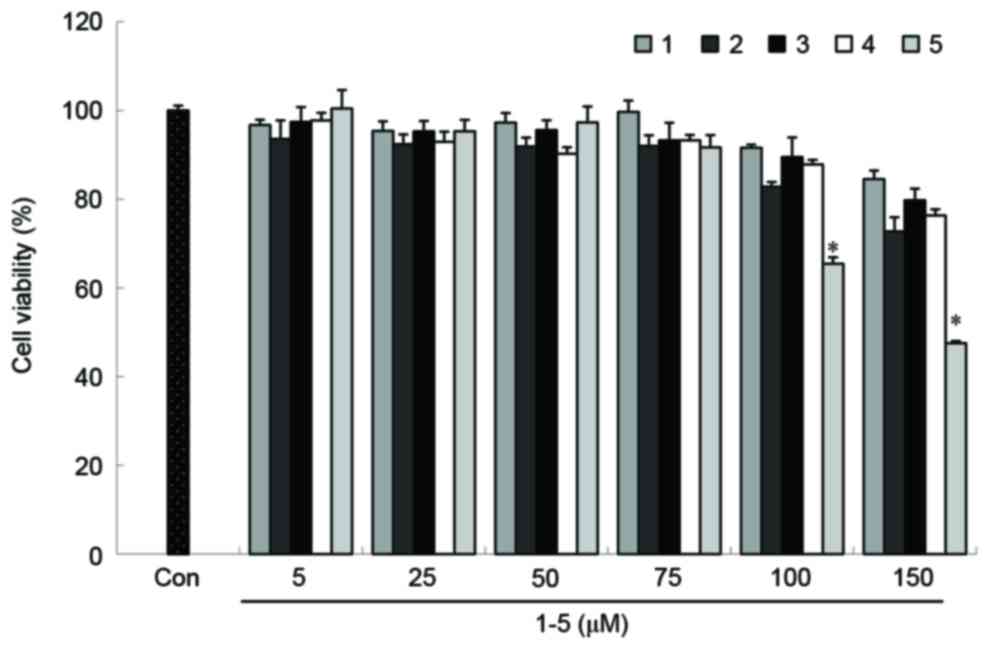
The viability of RAW264.7 cells was determined
following treatment with compounds 1–5 using the MTT-based EZ4 U
assay. Cytotoxicity was only observed in RAW264.7 cells treated
with concentrations of compound 5 >75 µM (Fig. 2). Therefore, subsequent experiments
were performed using concentrations ≤50 µM for all five
compounds.
AGS-derived compounds inhibit TNF-α
and IL-1β production in LPS-stimulated RAW264.7 macrophages
TNF-α and IL-1β are multifunctional proinflammatory
cytokines involved in the regulation of the immune response,
hematopoiesis and inflammation. To determine the effects of
AGS-derived compounds 1–5 on the release of TNF-α and IL-1β,
RAW264.7 cells were incubated with compounds 1–5 for 1 h and were
then exposed to LPS (100 ng/ml) for 6 h to induce inflammation. The
levels of TNF-α and IL-1β secreted from RAW264.7 cells were
determined using an ELISA assay. Dex (10 µM), which is a widely
used anti-inflammatory agent, was used as a positive control.
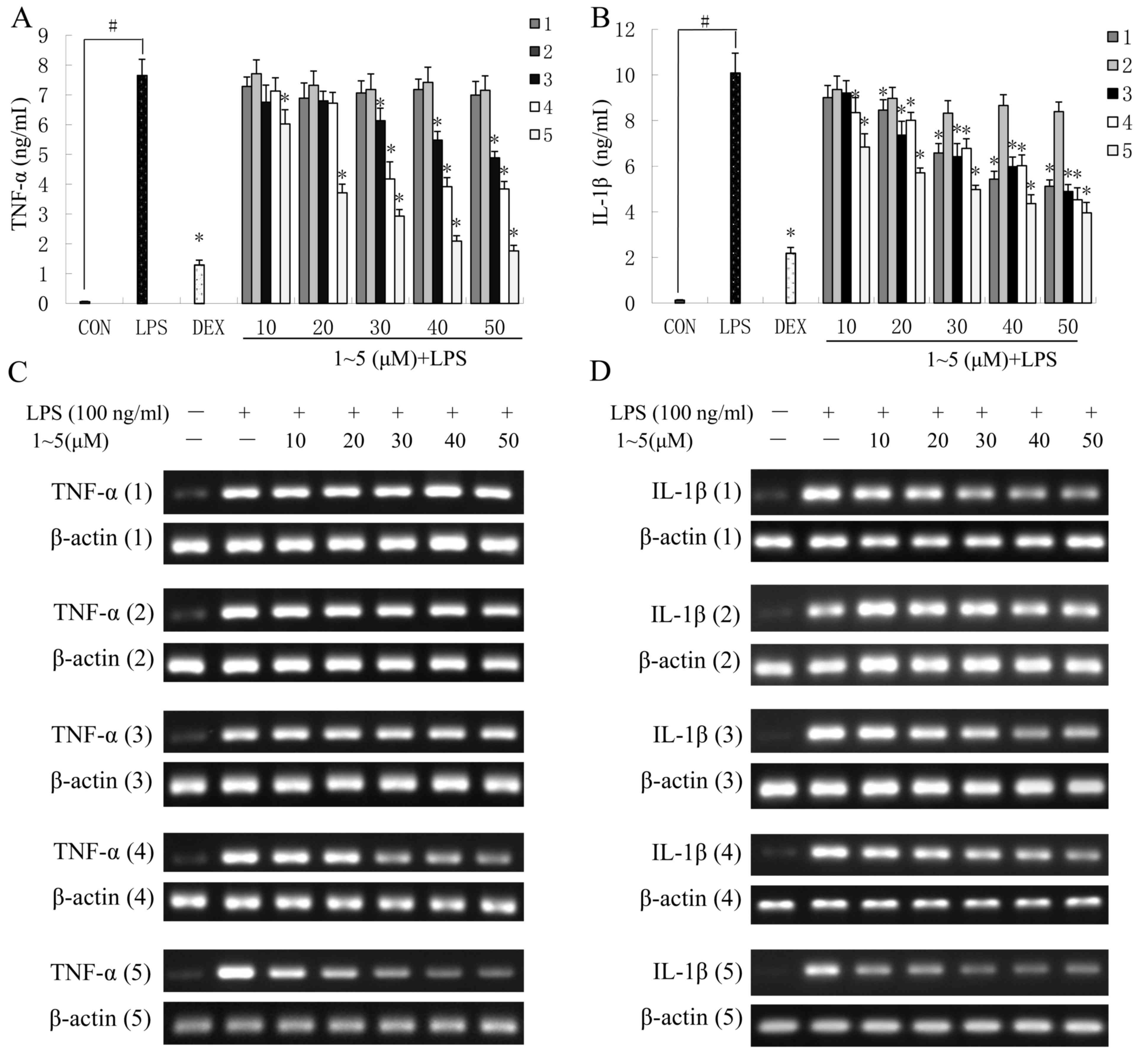
In LPS-stimulated RAW264.7 cells pretreated with
compounds 3–5, the secretion of TNF-α was significantly decreased
in a concentration-dependent manner compared with the
control-treated cells (P<0.05; Fig.
3A). Compound 5 elicited the greatest suppressive effect, and
50 µM compound 5 inhibited TNF-α production by 77%, which was
similar to the effects observed in cells treated with the positive
control DEX. However, compounds 1 and 2 exhibited no obvious
inhibitory effects on TNF-α release when used between 10 and 50 µM
(Fig. 3A).
In LPS-treated RAW264.7 cells treated with compounds
1–5, IL-1β secretion was significantly decreased in a
concentration-dependent manner (P<0.05; Fig. 3B). Similar to the results regarding
TNF-α secretion, compound 5 demonstrated the strongest inhibitory
effect with regards to IL-1β secretion. At a concentration of 50
µM, compound 5 significantly inhibited IL-1β production by 60.7%
compared with the LPS-stimulated RAW264.7 cells (Fig. 3B).
Compounds 1–5 suppress TNF-α and IL-1β
mRNA expression in LPS-stimulated RAW264.7 macrophages
To confirm whether the inhibition of TNF-α and IL-1β
production was due to decreased gene expression, the mRNA
expression levels of IL-1β and TNF-α were detected in
LPS-stimulated RAW264.7 cells. As presented in Fig. 3C and D, pretreatment with the
lupane-type triterpenes suppressed the mRNA expression levels of
TNF-α and IL-1β, which coincided with the protein levels of TNF-α
and IL-1β detected in the cell culture medium. These results
indicated that compounds 1–5 were able to inhibit the expression of
IL-1β and TNF-α at the transcriptional level, which, in turn,
reduced the production of IL-1β and TNF-α in the LPS-stimulated RAW
264.7 cells.
Effects of compounds 1–5 on HMGB1
secretion in LPS-induced RAW264.7 macrophages
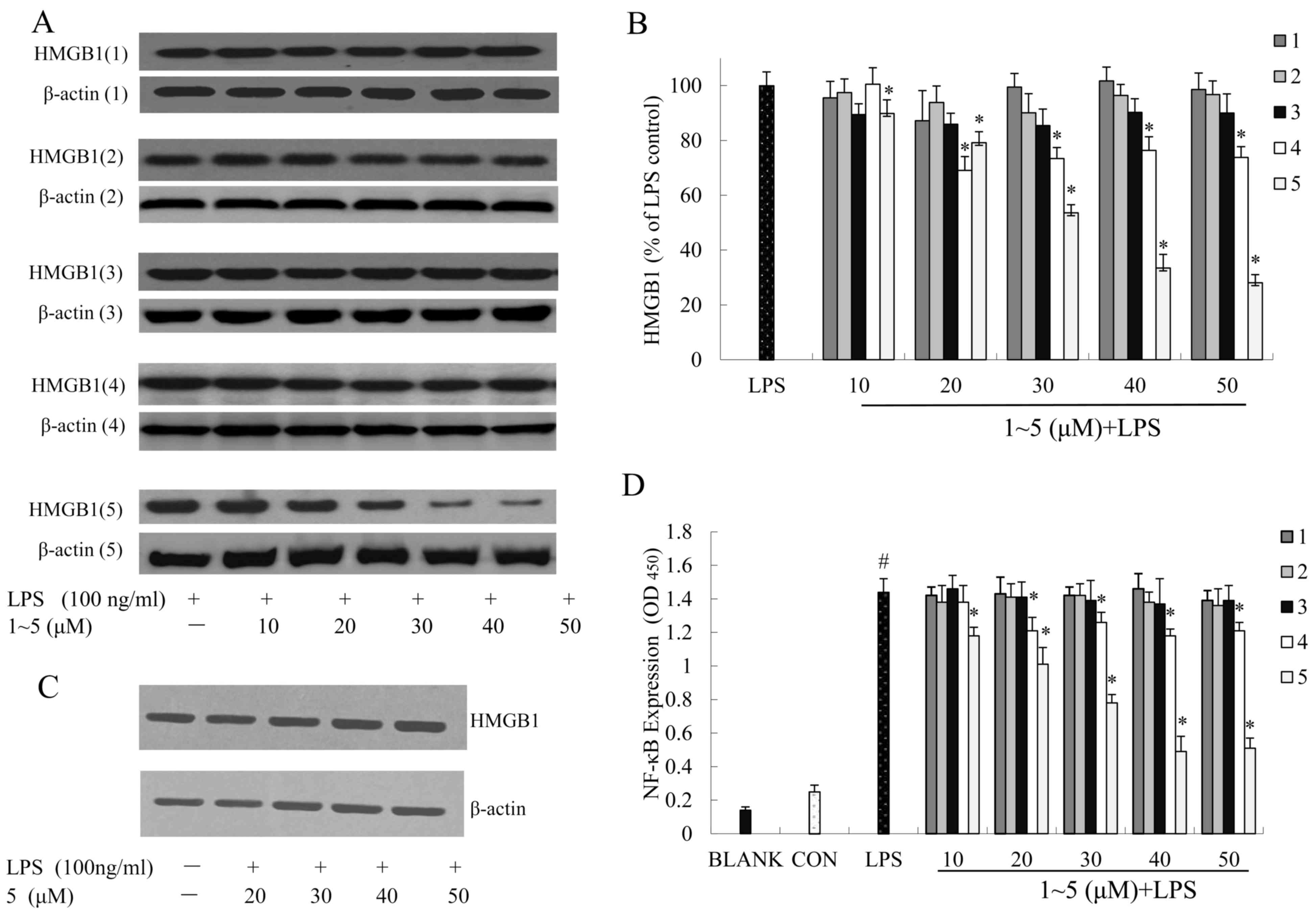
To examine the effects of compounds 1–5 on the
expression of HMGB1, RAW264.7 cells were incubated with compounds
1–5 and were then exposed to LPS (100 ng/ml). The protein
expression levels of HMGB1 in cell supernatants or whole cell
lysates were analyzed by western blot analysis.
HMGB1 is an intracellular protein that, when present
in the extracellular matrix, acts as a ‘necrotic marker’ for the
immune system. Studies indicate that damaged or necrotic cells can
release HMGB1 into the extracellular matrix, where it triggers
inflammatory responses (28). The
HMGB1 protein was low in the RAW264.7 cell supernatants without
LPS-stimulation. However, HMGB1 protein levels in the RAW264.7 cell
supernatants were significantly increased following LPS
stimulation, and preincubation with compound 5 reversed this
effect. The relative increase in the expression of secreted HMGB1
in the supernatant following LPS stimulation was significantly
reduced by treatment with compound 5 (Fig. 4A and B).
Following western blot analysis of whole cell
lysates, it was demonstrated that in LPS-treated RAW264.7 cells
pretreated with compound 5 at 20, 30, 40 or 50 µM, the expression
of HMGB1 protein was not obviously altered. These findings
suggested that treatment with compound 5 induced a significant
decrease in the secretion of HMGB1; however, it did not alter the
steady-state levels of HMGB1 protein in RAW264.7 cells (Fig. 4C).
Effects of compounds 1–5 on NF-κB
transcriptional activity in LPS-stimulated RAW264.7
macrophages
RAW264.7 cells were stimulated with or without LPS
(100 ng/ml) in the absence or presence of compounds 1–5 (10, 20,
30, 40 or 50 µM) for 24 h. Nuclear proteins were extracted, and
NF-κB activity was determined using the NF-κB p65 ELISA kit.
Control values were obtained in the absence of LPS and compounds
1–5. LPS values were obtained in the presence of LPS (100 ng/ml)
and absence of compounds 1–5. Our aforementioned results
demonstrated that compound 5 inhibited HMGB1 secretion in
LPS-induced RAW264.7 macrophages. Therefore, the present study
aimed to determine whether the upstream NF-κB signal transduction
pathway was involved. The results indicated that NF-κB activity was
increased in RAW264.7 cells stimulated with LPS (100 ng/ml), and
that compound 5 significantly reduced NF-κB activity in a
dose-dependent manner (Fig.
4D).
Discussion
Macrophages can be stimulated by various agents,
including LPS, to produce TNF-α, IL-1β and HMGB1 (29), which are known to serve important
roles in the immune response. Previous studies regarding sepsis
have largely focused on the suppression of early inflammatory
cytokines during the super-acute inflammatory response. The use of
antibodies and antagonists specific to these early inflammatory
cytokines has had some success in avoiding the development of
septic shock in animal models (30–32).
However, clinical trials using these antagonists to treat patients
with sepsis did not produce satisfactory results (33,34).
This may be because the intervention of early inflammatory
cytokines is not feasible. HMGB1 is considered a late inflammatory
cytokine, which appears relatively late in the inflammatory
response, and has a long duration. Serum concentrations of HMGB1
have been revealed to increase late (16–24 h) in patients with
sepsis (5,35). In addition, HMGB1 protein
inhibitors or antagonists have been reported to significantly
reduce the incidence of lethal endotoxemia and resulting acute
tissue damage, even when given 24 h following the occurrence of
endotoxemia and sepsis in mice (36). Furthermore, HMGB1 may enhance the
inflammatory response by stimulating various cells to synthesize
other proinflammatory cytokines. Therefore, inhibition of HMGB1 is
considered a potential target for reducing mortality and
complications in patients with sepsis.
Previous studies regarding herbal medicine have been
conducted to identify potential natural anti-inflammatory
properties in various in vitro and in vivo systems.
AGS is an important constituent of traditional Chinese medicine,
which has been used since ancient times to treat various diseases.
Although numerous pharmacological and biochemical pharmacokinetic
studies of AGS-derived compounds have previously been conducted,
the potential existence of anti-inflammatory properties of
lupane-type triterpenoids has not been explored. The present study
demonstrated that saponin compunds 1–5 reduced the production of
TNF-α and IL-1β. Acankoreoside A (compound 5) and acankoreoside B
(compound 4) were also able to suppress HMGB1 secretion and reduce
NF-κB activity induced by LPS in RAW 264.7 macrophages. These
results indicated that the anti-inflammatory effects of AGS were
due to the saponins present in this plant.
Based on the results of present study, it was
hypothesized that the anti-inflammatory effects of these saponins
related to their structures. The functional groups at C-3 and C-28
may have no effect on anti-inflammatory activity. However, the
presence of functional groups at C-23 or an hydroxyl group at C-11,
may affect the anti-inflammatory activity. The authors' of the
present study hypothesize that the order of the anti-inflammatory
activity of functional groups at C-23 was revealed to be:
-COOH>-CH2OH>-CHO>-CH3.
Acankoreoside A (5), which
possesses a carboxyl group at C-23 and no hydroxyl group at C-11,
was able to significantly inhibit the expression levels of TNF-α,
IL-1β and HMGB1. Further studies are necessary to research the
structure-activity relationship of lupane-type triterpenoid with
groups at C-3, C-11, C-23, or C-28.
In conclusion, the present results demonstrated that
AGS-derived lupane-type triterpenoid acankoreoside A (compound 5),
may exert anti-inflammatory effects by inhibiting NF-κB activation
in macrophages, and thus, preventing the expression of TNF-α, IL-1β
and HMGB1. Accordingly, these results suggested that acankoreoside
A is a promising therapeutic agent for the treatment of
inflammatory diseases, including rheumatoid arthritis,
scapulohumeral periarthritis, cervical spondylosis and slipped
disk. Furthermore, the discovery of the anti-inflammatory
properties of acankoreoside A (compound 5) indicate that future
studies are required to identify other potentially beneficial
pharmacological mechanisms underlying AGS-derived compounds.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of Hunan Province, China (grant no.
11JJ2042) and the Key Projects of Changsha City Science and
Technology Bureau (grant no. k1403122-31).
References
|
1
|
Morson BC: Pathology of inflammatory
disease. Proc R Soc Med. (63 Suppl): pp. S631970;
|
|
2
|
Gholijani N, Gharagozloo M, Farjadian S
and Amirghofran Z: Modulatory effects of thymol and carvacrol on
inflammatory transcription factors in lipopolysaccharide-treated
macrophages. J Immunotoxicol. 13:157–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akira S, Hirano T, Taga T and Kishimoto T:
Biology of multifunctional cytokines: IL 6 and related molecules
(IL 1 and TNF). FASEB J. 4:2860–2867. 1990.PubMed/NCBI
|
|
4
|
Beutler B and Cerami A: Cachectin and
tumour necrosis factor as two sides of the same biological coin.
Nature. 320:584–588. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et
al: HMG-1 as a late mediator of endotoxin lethality in mice.
Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bustin M: Regulation of DNA-dependent
activities by the functional motifs of the high-mobility-group
chromosomal proteins. Mol Cell Biol. 19:5237–5246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abraham E, Arcaroli J, Carmody A, Wang H
and Tracey KJ: HMG-1 as a mediator of acute lung inflammation. J
Immunol. 165:2950–2954. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersson U and Tracey KJ: HMGB1 is a
therapeutic target for sterile inflammation and infection. Annu Rev
Immunol. 29:139–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harris HE and Raucci A: Alarmin (g) news
about danger: Workshop on innate danger signals and HMGB1. EMBO
Rep. 7:774–778. 2006.PubMed/NCBI
|
|
12
|
Bianchi ME: DAMPs, PAMPs and alarmins: All
we need to know about danger. J Leukoc Biol. 81:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andersson U, Wang H, Palmblad K, Aveberger
AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M,
Yang H and Tracey KJ: High mobility group 1 protein (HMG-1)
stimulates proinflammatory cytokine synthesis in human monocytes. J
Exp Med. 192:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sha Y, Zmijewski J, Xu Z and Abraham E:
HMGB1 develops enhanced proinflammatory activity by binding to
cytokines. J Immunol. 180:2531–2537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wang LK, Wang LW, Han XQ, Yang F and
Gong ZJ: Cisplatin protects against acute liver failure by
inhibiting nuclear HMGB1 release. Int J Mol Sci. 14:11224–11237.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang Y, Huang X, Liu Z, Han G, Huang L,
Xiong YC and Wang Z: Dexmedetomidine inhibits the secretion of high
mobility group box 1 from lipopolysaccharide-activated macrophages
in vitro. J Surg Res. 181:308–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan BE, Yoshita Y, Sugiur T and Yamashita
U: Suppressive effect of Chinese medicinal herb, Acanthopanax
gracilistylus, extract on human lymphocytes in vitro. Clin Exp
Immunol. 118:41–48. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
China Pharmacopoeia Commission:
Pharmacopoeia of People's Republic of China. 1. Chinese Medical
Science and Technology Press; Beijing: pp. 612010
|
|
19
|
Cai XF, Lee IS, Shen G, Dat NT, Lee JJ and
Kim YH: Triterpenoids from Acanthopanax koreanum root and their
inhibitory activities on NFAT transcription. Arch Pharm Res.
27:825–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park SH, Nhiem NX, Kiem PV, Choi EM, Kim
JA and Kim YH: A new norlupane triterpene from the leaves of
Acanthopanax koreanum increases the differentiation of osteoblastic
MC3T3-e1 cells. Arch Pharm Res. 33:75–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu YL, Jiang YZ, Jin XJ, Lian LH, Piao JY,
Wan Y, Jin HR, Lee J Joon and Nan JX: Acanthoic acid, a diterpene
in Acanthopanax koreanum, protects acetaminophen-induced hepatic
toxicity in mice. Phytomedicine. 17:475–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nhiem NX, Kiem PV, Minh CV, Tai BH, Tung
NH, Ha do T, Soung KS, Kim JH, Ahn JY, Lee YM and Kim YH:
Structure-activity relationship of lupane-triterpene glycosides
from Acanthopanax koreanum on spleen lymphocyte IL-2 and IFN-gamma.
Bioorg Med Chem Lett. 20:4927–4931. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang WC, Li Z, Li XJ, Gong LM, Liu XQ,
Kwon OK, Ye HX and Lee HK: Chemical composition, anti-inflammatory
activity and cytotoxic activity of the liposoluble constituents
from different parts of Acanthopanax evodiaefolius by the Herbal
Blitzkrieg Extractor. Environ Toxicol Pharmacol. 38:406–411. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yook CS, Liu XQ, Chang SY, Park SY and
Nohara T: Lupane triterpene glycosides from the leaves of
Acanthopanax gracilistylus. Chem Pharm Bull (Tokyo). 50:1383–1385.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XQ, Yook CS and Chang SY: Chemical
constituents of Acanthopanax gracilistylus. Chin Tradit Herb Drugs.
35:250–252. 2004.(In Chinese).
|
|
26
|
Liu XQ, Chang SY, Park SY, Nohara T and
Yook CS: A new lupane triterpene glycosides from the leaves of
Acanthopanax gracilistylus. Arch Pharm Res. 25:831–836. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zou QP, Liu XQ, Lee HK and Oh OJ: Lupane-
triterpenoids from the methanol extracts of leaves of Acanthopanax
gracilistylus W.W. Smith. J Lanzhou Univ (Natural Sciences).
47:120–126. 2011.
|
|
28
|
Ulloa L and Messmer D: High-mobility group
box 1 (HMGB1) protein: Friend and foe. Cytokine Growth Factor Rev.
17:189–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Otterlei M, Ostgaard K, Skjåk-Braek G,
Smidsrød O, Soon-Shiong P and Espevik T: Induction of cytokine
production from human monocytes stimulated with alginate. J
Immunother (1991). 10:286–291. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fiedler VB, Loof I, Sander E, Voehringer
V, Galanos C and Fournel MA: Monoclonal antibody to tumor necrosis
factore alpha prevents lethal endotoxin sepsis in adult rhesus
monkeys. J Lab Clin Med. 120:574–588. 1992.PubMed/NCBI
|
|
31
|
Van der Poll T, Levi M, Hack CE, ten Cate
H, van Deventer SJ, Eerenberg AJ, de Groot ER, Jansen J, Gallati H,
Büller HR, et al: Elimination of interleukin 6 attenuates
coagulation activation in experimental endotoxemia in chimpanzees.
J Exp Med. 179:1253–1259. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van der Poll T, Levi M, van Deventer SJ,
ten Cate H, Haagmans BL, Biemond BJ, Büller HR, Hack CE and ten
Cate JW: Differential effects of anti-tumor necrosis factor
monoclonal antibodies on systemic inflammatory responses in
experimental endotoxemia in chimpanzees. Blood. 83:446–451.
1994.PubMed/NCBI
|
|
33
|
Fisher CJ Jr, Dhainaut JF, Opal SM,
Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ,
Greenman RL, et al: Recombinant human interleukin 1 receptor
antagonist in the treatment of patients with sepsis syndrome:
Results from a randomized, double-blind, placebo-controlled trial.
Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA.
271:1836–1843. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abraham E, Anzueto A, Gutierrez G, Tessler
S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S,
Cooney R, et al: Double-blind randomised controlled trial of
monoclonal antibody to human tumour necrosis factor in treatment of
septic shock. NORASEPT II Study Group. Lancet. 351:929–933. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao H, Wu L, Kuroyanagi M, Harada K,
Kawahara N, Nakane T, Umehara K, Hirasawa A and Nakamura Y:
Antitumor-promoting constituents from Chaenomeles sinensis KOEHNE
and their activities in JB6 mouse epidermal cells. Chem Pharm Bull
(Tokyo). 51:1318–1321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fogo AS, Antonioli E, Calixto JB and
Campos AH: Tormentic acid reduces vascular smooth muscle cell
proliferation and survival. Eur J Pharmacol. 615:50–54. 2009.
View Article : Google Scholar : PubMed/NCBI
|