Introduction
Lumbar disk degeneration (LDD) results in bone
instability, compression of neural elements and reduction of spinal
canal dimensions, which seriously affect the quality of life of
patients (1). LDD is generally
caused by genetic, environmental, mechanical and other external
factors (2,3). At present, the treatment of LDD
includes non-surgical treatment and surgical intervention, the
purposes of which are to reduce pain (4). The surgical methods include the
removal of intervertebral disc lesions and intervertebral disc
arthroplasty, without addressing the inherent loss of disc
functions (5). Fusion surgery has
been identified as a viable treatment option for reducing chronic
low back pain due to LDD for patients refractory to nonsurgical
care (6). A previous study
indicated that LDD may be affected by genetic factors, and that
gene therapy may become the future direction of LDD treatment
(7).
Wild-type p53-induced protein phosphatase 1
(Wip1) is a serine/threonine protein phosphatase and a proto
oncogene, which belongs to the protein phosphatase 2C family
(8). Wip1, coded by
phosphatase Mg2+-dependent 1δ, is aberrantly amplified
in different types of primary cancer in humans, and its deletion in
mice has been found to result in a profound tumor-resistant
phenotype (8). In the presence of
Mg2+ and other divalent cations, Wip1 is directly
involved in the dephosphorylation and inactivation of proteins
downstream of p53, thereby inhibiting the DNA repair and
apoptosis functions mediated by p53 in damaged cells
(9,10). Adeno-associated virus (AAV) is a
human virus with low immunogenicity, which can attach to human
chromosomes precisely and steadily; therefore, AVV can be used as a
stable and safe carrier for exogenous genes (11,12).
AAV vectors have been used in the treatment of several diseases and
have become one of the most promising gene therapy vectors
(13). The present aimed to
examine the clinical efficacy of Wip1 delivered by an AAV
vector in targeted therapy for LDD.
Materials and methods
Ethical statement
The animal experiments were designed with the
approval of the Animal Ethics Committee of Shanghai Changzheng
Hospital (Shanghai, China) and all experiments were performed in
accordance with the international standard (14).
Experimental subjects
A total of 84 healthy adult New Zealand white
rabbits (Hubei Academy of Medical Sciences, Hubei, China) were
selected for the experiments, regardless of gender. Their weights
ranged between 2.5 and 3 kg, and their ages ranged between 4 and 6
months. The rabbits were adaptively fed for 3 days prior to the
experiments and were housed at 21–23°C and 60±5% humidity with a
12-h light/dark cycle. During feeding, the rabbits received a
rationed intake of food and water at regular intervals. The
temperature, humidity and ventilation conditions were recorded to
ensure the health of the rabbits.
Construction of Wip1 gene vector
A recombinant adenovirus vector for Wip1
(AdWip1) was constructed by the Transformation Research
Laboratory of the Second Military Medical University (Shanghai,
China), which included a green fluorescent protein (GFP) expression
cassette. The recombinant adenovirus vector for GFP (AdGFP)
expressed GFP only. The vectors were diluted with PBS to
5×105 pfu/µl.
In vitro culture of intervertebral disc
nucleus pulposus cells. A total of six rabbits were sacrificed
prior to the removal of the lumbar spine, which was soaked in 75%
of alcohol for 5 min and washed in 0.9% saline three times. The
fiber rings in the intervertebral disc, between L1-L2 and L6-L7
were exposed under sterile conditions, revealing the nucleus
pulposus tissues. The gel-like tissues were collected using metal
tweezers and transferred into 5 ml centrifuge tubes. The tissue
samples were washed by centrifugation in Dulbecco's minimum
essential medium (DMEM), at 200 × g and 4°C for 5 min (model 800;
SMEW, Shanghai, China). The samples were then digested in 0.25%
type II collagenase (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 20–25 min at 37°C, vibrated for 5 min and
washed again by centrifugation in DMEM three times at 200 × g and
4°C for 5 min. Finally, 1% of hyaluronic acid enzyme (Sigma; Merck
Millipore, Darmstadt, Germany) was added to the samples and the
cell numbers were counted in an automated cell counter (Vi-Cell XR;
Beckman Coulter, Inc., Brea, CA, USA). The cells were then seeded
at a density of 1.4×105 cells/ml into 24-well culture
plates, and cultured at 37°C with 5% CO2 in DMEM
containing 10% fetal bovine serum (FBS).
Determination of transfection
efficiency
The culture solution was replaced every 3 days until
the cells had completely fused; following which 0.25% trypsin was
used to digest the cells for subculture. When the cells reached 80%
confluence, the cells were removed from the culture medium. The
recombinant adenovirus vector AdWip1 was added to the cells
at 25, 50, 100, 200, 400 and 800 multiplicity of infection (MOI),
and incubated at 37°C with 5% CO2. After 1 h, the
supernatant liquid was discarded, and fresh cell culture medium
(DMEM+10% FBS) was added into each well with 10 mM sodium butyrate.
The transfection efficiency was measured using flow cytometry.
Detection of cell growth and
activity
The highest transfection efficiency and the optimal
transfection concentration were detected using flow cytometry. The
cells were divided into two groups: Recombinant vector Wip1
group and blank control group. In the Wip1 group, culture
medium was removed and 200 MOI of Wip1 vector was added into
each well. The cells were incubated at 37°C with 5% CO2
for 1 h. The liquid supernatant was then discarded, and fresh DMEM
containing 10% FBS medium was added with 10 mM sodium butyrate. In
the blank control group, the cells were cultured with DMEM+10% FBS
only. The growth of the cells was observed using a light
microscope. At 1, 3, 5, 7, 9, 11 and 14 days post-transfection, the
cells were counted and the cell growth curve was drawn.
Expression of AdWip1 in nucleus pulposus
tissues. A total of six rabbits were randomly selected for
establishment of the animal model by annulus fibrosus of
intervertebral disc injury (15).
The rabbits were placed under anesthesia using an intravenous
injection of 3% pentobarbital sodium, and the L2-3, L3-4 and L4-5
intervertebral disc regions were exposed by median incision of the
abdomen and peritoneum. An injection needle (size 7) was used to
puncture the annulus, following which the needle was withdrawn. If
the puncture site leaked out some nucleus pulposus fluid, the
surgery was complete. The intervertebral space was marked by suture
marks on lumbar muscles. The incision was closed at the end of
surgery. The normal diet and activities of the rabbits were
maintained in the postoperative period. At 4 weeks following
surgery, the AdWip1 vectors were injected. At 1, 7, 14, 21
and 28 days post-injection, nucleus pulposus tissues in the lumbar
intervertebral disc region were removed and pressed into slides.
The fluorescence intensity of the tissues was observed using a
fluorescence microscope, and the gene expression level of
Wip1 was analyzed using HPIAS 2000 image analysis software
(Tongji Qianping Company, Wuhan, China). The pathological sections
of the nucleus pulposus tissues were observed on the slides under
an optical microscope.
Model establishment and grouping
A total of 72 rabbits were randomly divided into two
primary groups, with each primary group further divided into three
sub-groups. The first primary group included rabbits subjected to
intervertebral disc injury by annulus fibrosus, and was further
divided into groups A-C. The second primary group was the blank
control group and was further divided into groups D-F. At 4 weeks
following model establishment, the intervertebral disc (L2-3, L3-4
and L4-5) was exposed through left extra-peritoneal puncture. The
AdWip1 vectors were injected into the rabbits in groups A
and D. Empty vectors were injected into the rabbits in groups B and
E. PBS was injected into the rabbits in groups C and F.
X-ray examination of the lumbar
spine
At 3, 6 and 9 weeks following the second surgical
procedure, the rabbits in each group underwent X-ray examinations.
The rabbits were maintained at the same depth of anesthesia during
the examinations. A computed radiograph machine (Siemens AG,
Munich, Germany) was used with the following scanning parameters:
50 kV, 100 mA and 50 mS. Following acquisition of X-ray images,
Centricity DICOM viewer 3.1.1 software (GE Healthcare Life
Sciences, Little Chalfont, UK) was used to enlarge the images and
measure the relevant parameters according to the referenced method
(16). The anterior and posterior
heights, and the intermediate heights of surgical segments L2-3,
L3-4 and L4-5 were measured, and the average of these three values
was calculated as the height of the intervertebral disc.
Determination of proteoglycan contents
in the nucleus pulposus
A total of six rabbits in each group were randomly
selected and sacrificed by ear venous air embolism. Their lumbar
spines were removed and intervertebral discs (L2-3, L3-4 and L4-5)
were separated. At each time point, the nucleus pulposus tissues
from six intervertebral discs in each group were obtained. The
tissues were placed in centrifuge tubes of 4 ml in size. In each
sample, 1 ml of 3% NaOH solution was added and incubated on an
oscillator (Yunyan Instrument, Shanghai, China) at 40°C for 3 h.
Subsequently, 4 ml of HCl was added into each sample to adjust the
pH value to 8–9, and 100 µl of 100 mg/ml trypsin was added and
incubated on the oscillator at 50°C for 2 h. In the subsequent
step, 30 ml of glacial acetic acid, and 10 ml of 50 mg/ml
trihydroxybenzene and ethanol solution were mixed with 40 ml of HCl
to prepare the trihydroxybenzene solutions. Following enzymolysis,
the samples were transferred into a 10 ml volumetric flask and were
diluted to the 10 ml constant volume. Using a dropper, 1 ml of the
diluted sample solution was transferred into another 10 ml
volumetric flask, to which 3 ml trihydroxybenzene solution was
added and mixed. The mixture was heated in boiling water for 30 min
prior to cooling in ice water for 5 min. The mixture was then mixed
with glacial acetic acid to the constant volume of 10 ml. Using the
blank reagent alone as the reference; the optical densities (OD) of
the samples were measured at a wavelength of 558 nm, and were
converted to the quantity of proteoglycan.
Detection of type II collagen via
immunohistochemistry using streptavidin-biotin complex (SABC)
method
The remaining six rabbits in each group were
sacrificed following X-ray imaging by ear injection of air. The
intervertebral discs (L2-3, L3-4 and L4-5) were separated from
lumbar spine. At each time point, nine intervertebral disc
specimens were continuously sectioned with a slicing machine
(Leike, Hubei, China). The thickness of the sections was 4 µm. The
slides were stained with 1:250 mouse anti-human collagen type II 1
(Col2a1) antibody (cat. no. sc-52658; 1:250; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), and stored at 4°C overnight.
The secondary antibody staining was performed using 1:1,000
biotinylated rabbit anti-mouse IgG (cat. no. ab6728; 1:1,000;
Abcam, Cambridge, UK) and incubated at 37°C for 2 h, and then
developed using the SABC kit and DAB reagent (Boster Systems, Inc.,
Wuhan, China), in accordance with the manufacturer's protocol. The
expression of Col2al was visualized using a marker index. In each
slide, five high magnification visual fields were randomly
selected, and the numbers of total cells and positive cells were
counted under a fluorescence microscope (GVM-55; Shanghai Precision
Instruments Co., Ltd., Shanghai, China). The percentage of positive
cells was calculated and averaged for the five visual fields.
Statistical analysis
Statistical data analysis was performed using SPSS
19.0 statistical software (IBM SPPS, Armonk, NY, USA). The ranked
data were confirmed using rank sum tests. The count data were
compared using χ2 tests. The measurement data are
presented as the mean ± standard deviation and were verified using
the Kolmogorov-Smirnov test of normality. The data between two
groups were compared using an unpaired two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
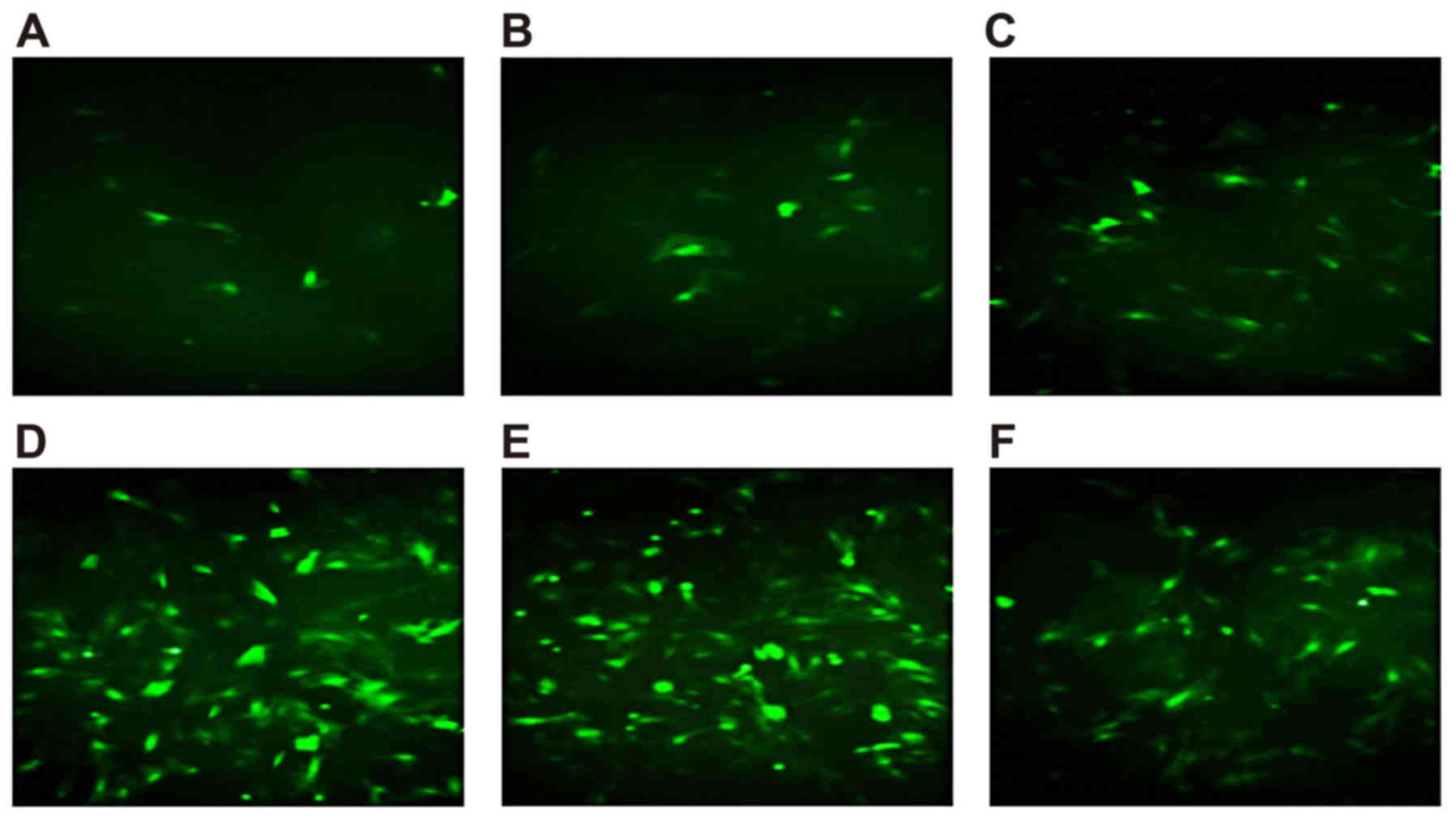
Determination of the optimal
concentration for Wip1 gene vectors
The expression of Wip1 was observed using a
fluorescence microscope as early as 12 h following transfection.
The expression peaked 48 h post-transfection. The nucleus pulposus
cells were transfected with six different virus concentrations (25,
50, 100, 200, 400 and 800 MOI) for 48 h, and the results from the
flow cytometry are shown in Table
I. The results from the fluorescence microscopy showed that the
transfection rate of the cells increased at higher concentrations
of the virus, with 200 MOI being the minimum dose required to reach
the maximum rate of transfection (Fig.
1A-F).
 | Table I.Flow cytometry results. |
Table I.
Flow cytometry results.
| Virus concentration
(multiplicity of infection) | Positive cells
(%) |
|---|
| 25 | 21.32±2.71 |
| 50 | 45.56±2.91 |
| 100 | 66.11±1.06 |
| 200 | 89.65±2.47 |
| 400 | 80.41±3.32 |
| 800 | 74.19±3.11 |
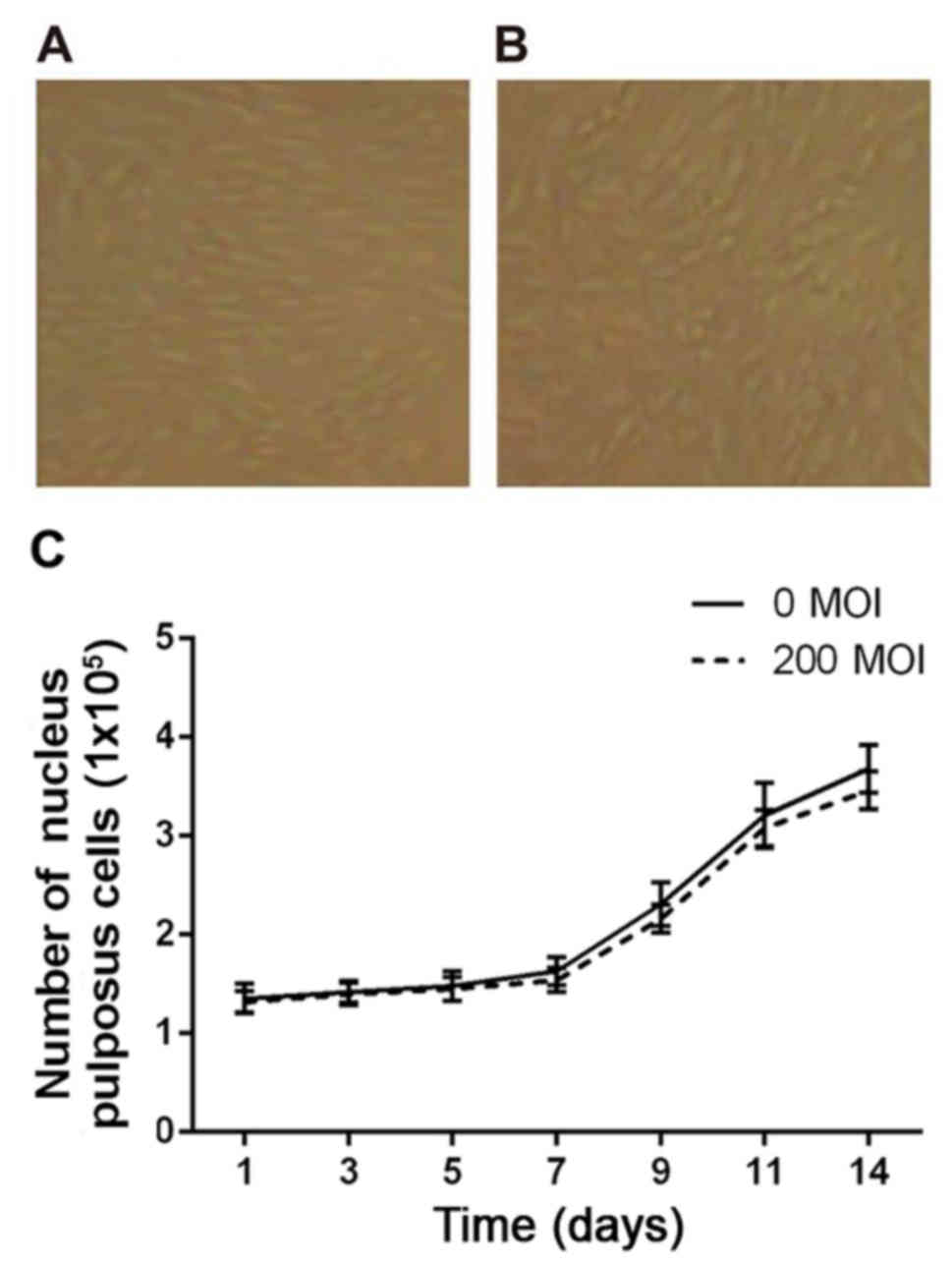
Effect of the Wip1 gene vector on the
growth of nucleus pulposus cells
Following separation of the nucleus pulposus cells
from the lumbar intervertebral disc, the numbers of living cells
were counted using trypan blue staining. The results showed that
the percentage of living cells was up to 93%. The cells were seeded
into 24-well culture plates and cultured for 15 days, until the
cells were fully fused and in good condition. The cell density
increased from 1.37×105 to 3.68×105 cells/ml,
and the percentage of living cells remained as high as 91%. As
shown in Fig. 2A and B, no
significant differences were found between the virus infection
group (200 MOI) and blank control group (0 MOI). As shown in
Fig. 2C, within 14 days of 200 MOI
AdWip1 transfection, the cells continued to grow, and the
rate of growth was almost identical with that of the blank control
group.
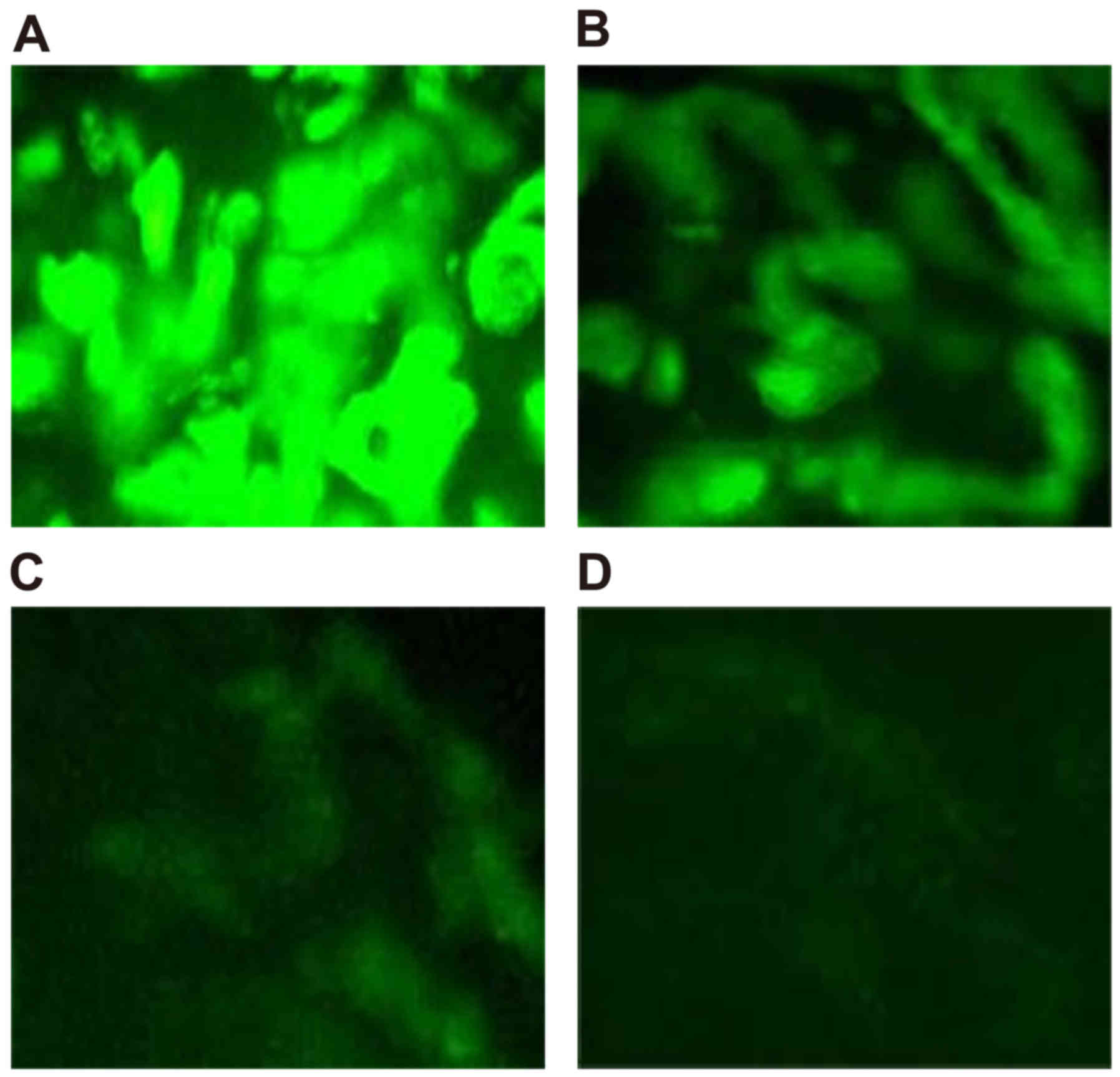
Expression of AdWip1 in rabbit nucleus
pulposus tissues
At 7 days post-transfection of the Wip1 gene
vectors into the nucleus pulposus of rabbit intervertebral discs,
the expression of Wip1 was highest, followed by a gradual
decrease. The OD values on days 7, 14, 21 and 28 were
0.0068±0.0031, 0.0057±0.0028, 0.0031±0.0011 and 0.0013±0.0008,
respectively. Although the OD value on day 14 was marginally lower,
compared with that on day 7, the difference was not significant
(P>0.05). However, the OD values on days 21 and 28 were
decreased significantly (P<0.05), as shown in Fig. 3A-D.
General condition of the rabbits in
each group
Following transfection, all rabbits were fed for 4–6
h. At 24 h post-transfection, the rabbits resumed normal diets and
activities. All rabbits had normal hair color, adequate reactions
to external stimuli, and normal food intake and excrement. All
wounds healed in phase I with no animal death. In groups A-C, the
rabbits showed marginally slower activity following surgery,
however, no significant differences were observed between the
rabbits in groups D-F and the normal rabbits. With the passage of
time, the activities of rabbits in group A gradually returned to a
normal level, whereas the activities of rabbits in groups B and C
were slower, and responses to external stimulation were lower than
normal.
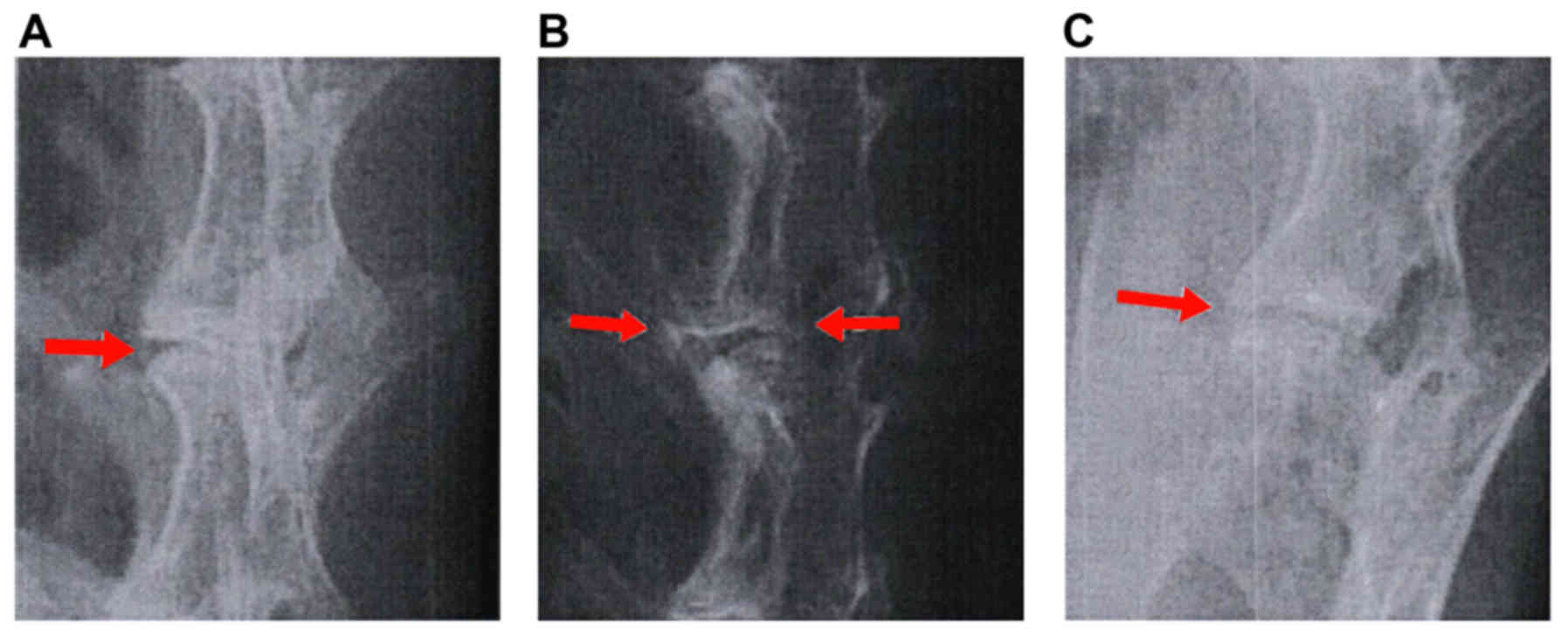
X-ray examination
Following surgery for model establishment, the
lumbar spine was assessed using X-ray examination. It was found
that the intervertebral space was relatively normal, the bones of
the upper and lower plates showed sclerosis, and the anterior
vertebral bone showed osteoproliferation, as shown in Fig. 4A-C. At different time points, the
heights of intervertebral disc spaces in groups A-C differed from
those in groups D-F (all P<0.05). The values among groups D-F
were similar (P>0.05). At 3 weeks following surgery, no
significant differences were found among groups A-C (all
P>0.05). After 6 and 9 weeks, the heights of the intervertebral
disc spaces in group A were significantly different, compared with
those in groups B and C (all P<0.05), as shown in Table II.
 | Table II.X-ray results showing the height of
intervertebral disc spaces at different time points. |
Table II.
X-ray results showing the height of
intervertebral disc spaces at different time points.
|
|
| Height of
intervertebral disc space (mm) |
|---|
|
|
|
|
|---|
| Group | Sub-group | 3 weeks | 6 weeks | 9 weeks |
|---|
| AdWip1 | A | 0.75±0.09 | 0.63±0.08 | 0.73±0.08 |
|
| B | 0.61±0.08 | 0.42±0.05 | 0.56±0.05 |
|
| C | 0.67±0.08 | 0.46±0.06 | 0.52±0.06 |
| Control | D | 0.91±0.10 | 0.83±0.10 | 0.90±0.08 |
|
| E | 0.92±0.13 | 0.89±0.09 | 0.93±0.10 |
|
| F | 0.97±0.12 | 0.90±0.12 | 0.94±0.11 |
Determination of proteoglycan content
in the nucleus pulposus
At 3, 6 and 9 weeks following the injection of
AdWip1, the proteoglycan content in the nucleus pulposus of
the intervertebral discs (L2-3, L3-4 and L4-5) were measured. The
content of proteocylcan in group A remained higher, compared with
the content in groups B and C (P<0.05). The content among groups
D-F was similar (P>0.05). The results in group A were similar to
those in groups D-F (all P>0.05). However, the results in groups
B and C differed from those in groups E and F (all P<0.05;
Table III).
 | Table III.Proteoglycan content in nucleus
pulposus cells of the intervertebral discs in each group. |
Table III.
Proteoglycan content in nucleus
pulposus cells of the intervertebral discs in each group.
| Group | Weeks
post-injection | L2-3 (mg/g) | L3-4 (mg/g) | L4-5 (mg/g) |
|---|
| A | 3 | 5.06±0.31 | 5.19±0.22 | 5.13±0.19 |
|
| 6 | 4.99±0.28 | 5.07±0.18 | 5.09±0.16 |
|
| 9 | 4.84±0.31 | 4.91±0.21 | 5.03±0.22 |
| B | 3 | 4.62±0.17 | 4.78±0.12 | 4.68±0.14 |
|
| 6 | 4.58±0.18 | 4.65±0.20 | 4.59±0.12 |
|
| 9 | 4.49±0.11 | 4.38±0.17 | 4.45±0.11 |
| C | 3 | 4.58±0.28 | 4.69±0.16 | 4.62±0.15 |
|
| 6 | 4.45±0.21 | 4.58±0.19 | 4.49±0.14 |
|
| 9 | 4.42±0.17 | 4.49±0.21 | 4.40±0.13 |
| D | 3 | 4.88±0.25 | 4.92±0.26 | 4.86±0.25 |
|
| 6 | 4.76±0.31 | 4.81±0.29 | 4.72±0.26 |
|
| 9 | 4.68±0.21 | 4.74±0.31 | 4.63±0.26 |
| E | 3 | 5.10±0.24 | 5.21±0.32 | 5.18±0.28 |
|
| 6 | 5.01±0.29 | 5.09±0.28 | 5.06±0.31 |
|
| 9 | 4.92±0.24 | 4.98±0.31 | 4.97±0.33 |
| F | 3 | 5.17±0.26 | 5.23±0.32 | 5.22±0.30 |
|
| 6 | 5.06±0.23 | 5.11±0.26 | 5.10±0.33 |
|
| 9 | 4.98±0.32 | 5.04±0.24 | 5.03±0.29 |
Type II collagen
At 3, 6 and 9 weeks following the injection of
AdWip1, the levels of type II collagen were measured in the
nucleus pulposus of the intervertebral discs (L2-3, L3-4 and L4-5).
The results are shown in Table
IV. The content of type II collagen differed in group A,
compared with the content in groups B and C (P<0.05). The
results from groups B and C were similar (P>0.05). The results
among groups D-F were similar (P>0.05). The type II collagen
contents in groups A-C were lower compared with those in groups D-F
(all P<0.05).
 | Table IV.Type II collagen content in nucleus
pulposus cells of intervertebral discs in each group. |
Table IV.
Type II collagen content in nucleus
pulposus cells of intervertebral discs in each group.
| Group | Weeks
post-injection | L2-3 (mg/g) | L3-4 (mg/g) | L4-5 (mg/g) |
|---|
| A | 3 | 119.56±4.62 | 121.25±4.37 | 123.33±5.69 |
|
| 6 | 112.33±5.15 | 120.24±5.63 | 120.66±4.44 |
|
| 9 | 104.43±4.18 | 105.76±4.38 | 106.75±4.48 |
| B | 3 | 87.33±4.26 | 84.75±3.77 | 84.42±3.23 |
|
| 6 | 85.41±3.93 | 83.59±3.74 | 80.12±3.03 |
|
| 9 | 79.56±3.02 | 82.35±3.21 | 78.52±3.16 |
| C | 3 | 86.81±3.95 | 85.77±4.11 | 85.52±4.12 |
|
| 6 | 84.38±3.86 | 83.58±3.81 | 84.87±3.97 |
|
| 9 | 79.67±2.99 | 82.86±3.05 | 81.58±3.14 |
| D | 3 | 140.13±6.34 | 141.36±6.35 | 140.37±6.42 |
|
| 6 | 137.43±5.63 | 139.74±5.28 | 138.49±6.54 |
|
| 9 | 136.58±6.15 | 137.45±6.36 | 136.32±5.85 |
| E | 3 | 138.43±6.14 | 140.31±5.42 | 140.34±6.15 |
|
| 6 | 136.15±6.32 | 138.21±6.54 | 137.69±5.81 |
|
| 9 | 134.36±5.82 | 134.14±6.32 | 134.43±6.94 |
| F | 3 | 141.51±5.64 | 142.36±6.58 | 141.11±6.84 |
|
| 6 | 138.37±6.21 | 141.22±6.28 | 139.54±5.76 |
|
| 9 | 137.46±6.36 | 138.62±6.83 | 137.44±6.42 |
Discussion
LDD is primarily manifested as bone hyperplasia, end
plate sclerosis and intervertebral disc space narrowing, resulting
in back pain (17). Currently,
surgery and non-surgical treatments are used in parallel to
alleviate pain, however, it is difficult to always achieve
satisfactory results. In addition, there is a risk associated with
LDD surgery, which may worsen the degeneration of adjacent tissues
(18). As current understanding
the cause of LDD at the genetic level has improved, innovative
treatment strategies may become available for LDD.
The experimental results in the present study showed
that the transfection of AdWip1 into New Zealand white
rabbits caused significant recovery from LDD, but could not
completely eliminate the disease. Intervertebral discs contain
three major components: A gel-like nucleus pulposus to resist
internal pressure, fiber rings to resist external pressure, and
cartilage end plates (19). It has
been shown that LDD is closely associated with reduced height of
the intervertebral disc (20).
During the degeneration of lumbar intervertebral discs, modic
changes generally occur in patients, including degeneration, damage
to cartilage endplates in the regeneration and vascular granulation
regions, fractures, and increased signal intensity in bone tissues
and granulation tissues (21).
These external stimuli can trigger the DNA damage response (DDR) in
cells, which has a dual effect: It prevents the propagation of DNA
damage intracellularly, as DDR inhibits DNA replication and
promotes the decomposition of damaged DNA; however, DDR tends to
coordinate the internal repair of DNA in order to maintain the
integrity of the genome (22).
Therefore, in addition to the DDR repairing damage, it can also
lead to the aging of intervertebral disc cells by inhibiting DNA
replication. Wip1 is an important molecule, which inhibits
the activity of DDR-associated molecules through dephosphorylation,
causing the timely termination of DDR (23–25).
The experimental results of the present study showed that the
intervertebral disc height in the groups transfected with
AdWip1 increased, indicating that the expression of
Wip1 improved the prognosis of LDD.
The results of the present study also showed that
the type II collagen and proteoglycan contents were increased in
the groups transfected with AdWip1. The extracellular matrix
of the intervertebral disc primarily contains collagen
(predominantly type II collagen), proteoglycan, elastin and water.
In each layer of the fiber rings, grids are formed by elastin and
collagen fitting closely, and proteoglycan is integrated into these
grids (26). It has also been
shown that the aging of nucleus pulposus cells is the primary cause
of intervertebral disc degeneration (27). Following the occurrence of disc
degeneration, the separation lines between the fiber rings and
nucleus pulposus becomes less clear, the ability of the nucleus
pulposus to synthesize proteins and sugar is decreased, the
arrangements and types of collagen are altered, and the
corresponding biological functions of the nucleus pulposus is
significantly weakened (28). It
has been shown that the expression of Wip1 prevented the
premature senescence of cells (29). Therefore, transfection of the
Wip1 gene into cells may inhibit the senescence of nucleus
pulposus cells, and the increased levels of proteoglycan and type
II collagen contents may assist in the treatment of LDD.
In conclusion, Wip1 gene vectors showed
promising clinical efficacy for the treatment of LDD in the present
study. The optimum concentration of Wip1 gene vectors was
200 MOI. The AAV delivery of the Wip1 gene may become a
novel targeted approach to treat LDD. However, the sample size in
the present study was limited, therefore, the clinical significance
of the results requires further verification in future experiments
with larger sample sizes.
Acknowledgements
This study was supported in part by the National
Natural Science Foundation of China (grant no. 8127135).
References
|
1
|
Lee SY, Kim TH, Oh JK, Lee SJ and Park MS:
Lumbar stenosis: A recent update by review of literature. Asian
Spine J. 9:818–828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian W and Qi H: Association between
intervertebral disc degeneration and disturbances of blood supply
to the vertebrae. Chin Med J (Engl). 123:239–243. 2010.PubMed/NCBI
|
|
3
|
Eskola PJ, Lemmelä S, Kjaer P, Solovieva
S, Männikkö M, Tommerup N, Lind-Thomsen A, Husgafvel-Pursiainen K,
Cheung KM, Chan D, et al: Genetic association studies in lumbar
disc degeneration: A systematic review. PLoS One. 7:e499952012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Natarajan RN and Andersson GB: Lumbar disc
degeneration is an equally important risk factor as lumbar fusion
for causing adjacent segment disc disease. J Orthop Res.
35:123–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tschugg A, Michnacs F, Strowitzki M,
Meisel HJ and Thomé C: A prospective multicenter phase I/II
clinical trial to evaluate safety and efficacy of NOVOCART Disc
plus autologous disc chondrocyte transplantation in the treatment
of nucleotomized and degenerative lumbar disc to avoid secondary
disease: Study protocol for a randomized controlled trial. Trials.
17:1082016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phillips FM, Slosar PJ, Youssef JA,
Andersson G and Papatheofanis F: Lumbar spine fusion for chronic
low back pain due to degenerative disc disease: A systematic
review. Spine (Phila Pa 1976). 38:E409–E422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Woods BI, Vo N, Sowa G and Kang JD: Gene
therapy for intervertebral disk degeneration. Orthop Clin North Am.
42:563–574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le Guezennec X and Bulavin DV: WIP1
phosphatase at the crossroads of cancer and aging. Trends Biochem
Sci. 35:109–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pechackova S, Burdova K, Benada J,
Kleiblova P, Jenikova G and Macurek L: Inhibition of WIP1
phosphatase sensitizes breast cancer cells to genotoxic stress and
to MDM2 antagonist nutlin-3. Oncotarget. 7:14458–14475. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clausse V, Goloudina AR, Uyanik B,
Kochetkova EY, Richaud S, Fedorova OA, Hammann A, Bardou M, Barlev
NA, Garrido C and Demidov ON: Wee1 inhibition potentiates
Wip1-dependent p53-negative tumor cell death during chemotherapy.
Cell Death Dis. 7:e21952016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Wang Z, Zhang F, Zhu R, Bi J, Wu
J, Zhang H, Wu H, Kong W, Yu B and Yu X: Enhancing transgene
expression from recombinant AAV8 vectors in different tissues using
woodchuck hepatitis virus post-transcriptional regulatory element.
Int J Med Sci. 13:286–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ling C, Yin Z, Li J, Zhang D, Aslanidi G
and Srivastava A: Strategies to generate high-titer, high-potency
recombinant AAV3 serotype vectors. Mol Ther Methods Clin Dev.
3:160292016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nicolson SC, Li C, Hirsch ML, Setola V and
Samulski RJ: Identification and validation of small molecules that
enhance recombinant Adeno-associated virus transduction following
high throughput screen. J Virol. 90:7019–7031. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orlans FB: Ethical decision making about
animal experiments. Ethics Behav. 7:163–171. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paul R, Haydon RC, Cheng H, Ishikawa A,
Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P, et al:
Potential use of Sox9 gene therapy for intervertebral degenerative
disc disease. Spine (Phila Pa 1976). 28:755–763. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KS, Yoon ST, Li J, Park JS and Hutton
WC: Disc degeneration in the rabbit: A biochemical and radiological
comparison between four disc injury models. Spine (Phila Pa 1976).
30:33–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Schepper EI, Damen J, van Meurs JB,
Ginai AZ, Popham M, Hofman A, Koes BW and Bierma-Zeinstra SM: The
association between lumbar disc degeneration and low back pain: The
influence of age, gender, and individual radiographic features.
Spine (Phila Pa 1976). 35:531–536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Yu T, Ma XX, Xiang HF, Hu YG and
Chen BH: Lentivirus-mediated TGF-β3, CTGF and TIMP1 gene
transduction as a gene therapy for intervertebral disc degeneration
in an in vivo rabbit model. Exp Ther Med. 11:1399–1404. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YS: Pathophysiology of degenerative
disc disease. Asian Spine J. 3:39–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryu R, Techy F, Varadarajan R and
Amirouche F: Effect of interbody fusion on the remaining discs of
the lumbar spine in subjects with disc degeneration. Orthop Surg.
8:27–33. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albert HB and Manniche C: Modic changes
following lumbar disc herniation. Eur Spine J. 16:977–982. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
d'Adda di Fagagna F: Living on a break:
Cellular senescence as a DNA-damage response. Nat Rev Cancer.
8:512–522. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hat B, Kochańczyk M, Bogdał MN and
Lipniacki T: Feedbacks, bifurcations, and cell fate decision-making
in the p53 system. PLoS Comput Biol. 12:e10047872016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirzayans R, Andrais B, Scott A, Wang YW,
Weiss RH and Murray D: Spontaneous γH2AX foci in human solid
tumor-derived cell lines in relation to p21WAF1 and WIP1
expression. Int J Mol Sci. 16:11609–11628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwamoto K, Hamada H, Eguchi Y and Okamoto
M: Stochasticity of intranuclear biochemical reaction processes
controls the final decision of cell fate associated with DNA
damage. PLoS One. 9:e1013332014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang X, Jing L, Richardson WJ, Isaacs RE,
Fitch RD, Brown CR, Erickson MM, Setton LA and Chen J: Identifying
molecular phenotype of nucleus pulposus cells in human
intervertebral disc with aging and degeneration. J Orthop Res.
34:1316–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Accelerated cellular senescence in degenerate intervertebral discs:
A possible role in the pathogenesis of intervertebral disc
degeneration. Arthritis Res Ther. 9:R452007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakai H, Fujigaki H, Mazur SJ and Appella
E: Wild-type p53-induced phosphatase 1 (Wip1) forestalls cellular
premature senescence at physiological oxygen levels by regulating
DNA damage response signaling during DNA replication. Cell Cycle.
13:1015–1029. 2014. View
Article : Google Scholar : PubMed/NCBI
|