Introduction
Intervertebral disc (IVD) degeneration results in
the pathogenesis of spinal disorders and is one of the main causes
of lower back pain; it has also been associated with a high
socioeconomic burden (1,2). The treatment and prevention of
degenerative disc disease are restricted by a limited understanding
of the mechanisms that regulate the processes underlying the
development, maintenance and degeneration of the IVD (3). Previous studies have demonstrated in
humans that the population of senescent cells is markedly increased
in aged and degenerated discs in vitro and in vivo
(4–6). Cellular senescence, characterized by
irreversible growth arrest, is caused by a number of stressors,
including reactive oxygen species and DNA damage, and decreases the
cellular viability capacity for self-repair (7–9). In
addition, senescent cells secrete multiple proinflammatory
cytokines and matrix-degrading enzymes (5,10–13),
that induce inflammation-associated stress and promote
extracellular matrix degradation, which, in turn lead to the
deterioration of the microenvironment and the promotion of the
pathogenesis of degenerative diseases, such as IVD degeneration
(5,10). Previous studies have also revealed
that cellular senescence has a positive correlation with the
progressive degree of IVD degeneration (14,15).
These studies have indicated that cellular senescence may be a
critical underlying mechanism of IVD degeneration. However, the
precise mechanism by which cellular senescence accelerates disc
degeneration has not been elucidated.
Caveolae are 50 to 100 nm flask-shaped invaginations
of the plasma membrane (16).
Caveolin-1 is a structural protein component of caveolae in the
majority of cell types, and is thought to be involved in lipid
transport, membrane trafficking and the regulation of a variety of
signaling molecules (17,18). Caveolin-1 has also been associated
with the premature senescent phenotype of several cell types,
including human fibroblasts, articular chondrocytes and nucleus
pulposus (NP) cells (11,19,20).
Bartholomew et al (19)
demonstrated that caveolin-1 is a novel MDM2 proto-oncogene binding
protein and that it induced cellular senescence via the p53
signaling pathway. Volonte et al (11) also implicated caveolin-1 in
cellular senescence via the inhibition of sirtuin 1 and the
activation of the p53 signaling pathway in response to oxidative
stress. In addition, caveolin-1 gene and protein expression have
been detected in human IVD degeneration, and a role for caveolin-1
has been proposed in degenerative, as opposed to age-induced,
alterations in the NP (4). A
previous study reported that early IVD degeneration may be
associated with the downregulation of canonical Wnt signaling and
caveolin-1 expression, which, are thought to be essential to the
physiology and preservation of notochordal cells (21). These observations demonstrated that
the role of caveolin-1 in the development, maintenance and
degeneration of IVD is still unclear. As cellular senescence may be
involved in the mechanism of disc degeneration, elucidating the
effects and the underlying mechanism of caveolin-1 in NP cellular
senescence may provide promising strategies for the prevention of
premature cellular senescence, and in turn, the prevention IVD
degeneration.
The IVD is the largest avascular organ and in an
oxidative microenvironment, the disposal of cellular waste in the
IVD is hindered and cell viability is challenged (22–24).
In the present study, oxidative stress was utilized to introduce
cellular senescence in the rat NP in order to investigate the
expression and mechanism of caveolin-1 in NP cells in response to
this stress.
Materials and methods
Cell isolation and culture
All animal experiments were approved by the Ethics
Committee on Animal Experiments of Fudan University (Shanghai,
China). A total of 2 male Sprague-Dawley rats (age, ~12 weeks;
weight, 400 g) were used in the present study and were supplied by
the Shanghai Public Health Center (Shanghai, China). Animals were
housed with free access to food and water under a 12-h light/dark
cycle, with a constant temperature (20–23°C) and humidity (55±5%).
Rats were euthanized by cervical dislocation following anesthesia
with pelltobarbitalum natricum (1.5%) by intraperitoneal injection
(50 mg/kg; Shanghai Shangxiao New Asia Pharmaceutical Co., Ltd.,
Shanghai, China; http://www.xinyapharm.com). Lumbar spines were
obtained within 1 h of sacrifice, and the discs were carefully
dissected under a microscope using aseptic conditions to obtain the
NP. Tissues were sequentially treated with 0.25% trypsin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 2 h
followed by 0.02% collagenase (Sigma-Aldrich; Merck KGaA) at 37°C
for 24 h, then washed with phosphate-buffered saline (PBS).
Subsequently, the cells were released from the matrix by
centrifugation at 200 × g for 5 min at room temperature, seeded
into 6-well plates (2×104 cells/well) and maintained in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
of penicillin (Beyotime Institute of Biotechnology, Nantong, China)
and 100 µg/ml of streptomycin (Beyotime Institute of Biotechnology)
under 5% CO2 in a humidified incubator at 37°C. Primary
cells were maintained in a high-density monolayer culture for 2
weeks. The cells were then trypsinized again and subcultured into
6-well plates; these cells were used in the subsequent experiments
as secondary cells after reaching 80% confluence.
Construction of lentivirus
vectors
A DNA template and oligonucleotides corresponding to
the caveolin-1 gene (Gene ID: NM_001753; www.ncbi.nlm.nih.gov/nuccore/NM_001753) were
targeted. The oligonucleotide sequences (Shanghai R&S
Biotechnology Co., Ltd., Shanghai, China) were designed and
synthesized as follows: Caveolin-1 small interfering (sh)RNA,
forward, 5′-GACGUGGUCAAGAUUGACUTT-3′ and reverse,
5′-AGUCAAUCUUGACCACGUCTT-3′. A control shRNA unassociated with
these gene sequences was used as the negative control (NC):
Caveolin-1 shRNA-NC, forward, 5′-CUGUGAUCCACUCUUUGAATT-3′ and
reverse, 5′-UUCAAAGAGUGGAUCACAGTT-3′. The combined sequences of the
enhanced green fluorescent protein (GFP) gene and the caveolin-1
shRNA were cloned into the AscI and PmeI sites of the pLenti6.3-MCS
vector (Shanghai R&S Biotechnology Co., Ltd., Shanghai, China)
containing a cytomegalovirus-driven GFP reporter (25). All of the constructed plasmids (1
µg/µl; 5 µg) were confirmed by sequencing analysis, as previously
described (26); prior to
transfection into 293T cells (iCell Bioscience, Inc., Shanghai,
China) using ViraPower lentiviral Packaging Mix (5 µg, Invitrogen;
Thermo Fisher Scientific, Inc.) at 40% confluence (5×10
5 cells/well) in 10 cm plate. Supernatants containing
lentiviruses were harvested at 96 h following transduction.
Subsequent purification was performed using ultracentrifugation at
1610 × g and 4°C for 10 min. The isolated lentiviruses were stored
at −80°C until use within 2 weeks; the lentivirus titre was
1.5×106.
Transfection of lentivirus
Secondary cells were transferred to 6-well plates at
a density of 5×105 cells/well in DMEM with 10% FBS
without antibiotics the day prior to transduction procedures. Once
cells had reached 80% confluency, cells were transfected with the
aforementioned recombinant experimental or control lentivirus at a
multiplicity of infection of 50 using polybrene (5 µg/ml; Sidansai
Biotechnology Co., Ltd., Shanghai, China) for 24 h at 37°C. All
cells were then refreshed with DMEM containing 10% FBS without
antibiotics and cultured in DMEM with 10% FBS for 48 h. The
transduction efficiency was determined by fluorescence microscopy,
which was the average rate of green fluorescent cells in five
fields (magnification, ×100).
Cell treatments
To establish the cellular senescence model,
secondary cells were treated with 70% tert-butyl hydroperoxide
(t-BHP; Sigma-Aldrich; Merck KGaA) for 6 h at 37°C, and the
sublethal final concentration was observed to be 100 µmol/l. Cells
treated with t-BHP were infected with either the caveolin-1 short
hairpin (sh)RNA vector or the NC vector, which, were termed NP-LV
and NP-LV-NC, respectively. An additional untransfected group
treated with t-BHP was used as the control group, which, was termed
NP-CTR. Untransfected cells treated under normal conditions without
t-BHP treatment formed the normal control group, termed NP-NOM. All
four treatment groups were incubated under DMEM with 10% FBS for 72
h at 37°C; the subsequent experiments were performed using cells at
different time points throughout this 72 h period.
Senescence-associated β-galactosidase
(SA-β-gal) staining
SA-β-gal staining was performed using a SA-β-gal
staining kit (Cell Signaling Technology, Inc., Danvers, MA, USA)
according to the manufacturer's instructions. Briefly, cells were
washed twice with PBS and fixed with 3% formaldehyde for 15 min at
37°C. Cells were then incubated overnight at 37°C with the kit
staining solution. Cells were photographed under reflected light
using a digital high-fidelity fluorescence microscope (VH-8000;
Keyence Corporation, Osaka, Japan). A total of 5 fields, that were
distributed throughout the well, were counted (magnification,
×200), and the average rate of positive staining was recorded.
Measurement of proliferation
The proliferation of NP cells exposed to t-BHP
treatment was assessed by cell counting kit-8 (CCK-8; Beyotime
Institute of Biotechnology) at 0, 24, 48 and 72 h. Briefly, cells
were replated at 1×104 cells/well in a 96-well plate,
and were then incubated with 10 µl CCK-8 solution at 37°C for 2 h.
The mixtures were analyzed with an Epoch Multi-Volume
Spectrophotometer System (BioTek Instruments, Inc., Winooski, VT,
USA) at an absorbance of 490 nm. Proliferative activities were each
calculated as the change in absorbance at 490 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect caveolin-1 mRNA in
the different treatment groups at 24, 36 and 72 h; GAPDH was used
as an internal standard control. Briefly, total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions. Single-strand cDNA
templates were prepared from 1 µg total RNA using the RT-for-PCR
kit (Clontech Laboratories, Inc., Mountainview, CA, USA.). Specific
cDNAs were then amplified by PCR using the following primers:
Caveolin-1, forward, 5′-AAGGAGATCGACCTG-3′ and reverse,
5′-GGAATAGACACGGCTG-3′; GAPDH, forward, 5′-CCCCAATGTATCCGTTGTG-3′
and reverse, 5′-CTCAGTGTAGCCCAGGATGC-3′. PCR amplification from
cDNA was performed using the Takara TP800 Thermal Cycler Dice
(Takara Bio, Inc., Otsu, Japan) with a final volume of 20 µl [2X
SYBR Green Mix 10 µl (Invitrogen; Thermo Fisher Scientific, Inc.),
1 µl primer mix, 1 µl template DNA and 8 µl diethylpyrocarbonate
water]. The thermocycling conditions were as follows: Initial
denaturation at 95°C for 2 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, annealing at 59°C for 20 sec and
elongation at 72°C for 20 sec, and then a final extension at 72°C
for 10 min. PCR products were subjected to amplification curve
analysis and quantified using SYBR Green (Invitrogen; Thermo Fisher
Scientific, Inc.). The data were normalized to GAPDH and quantified
using the 2−ΔΔCq method (27,28).
Western blot analysis
The protein expression levels of caveolin-1, p53,
p21 and p16 were detected by western blot analysis following 72 h
of cell treatment. Total protein was extracted with SDS-PAGE Sample
Loading Buffer 5X (cat. no. P0015, Beyotime Institute of
Biotechnology). The total protein concentration was determined by a
bicinchoninic acid assay (Sigma-Aldrich; Merck KGaA). Protein (20
µg/lane) extracts were separated by 8–12% SDS-PAGE and were then
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat dry
milk in Tris-buffered saline with 0.1% Tween (TBST) for 1 h at
37°C, and incubated overnight at 4°C in TBST with the following
primary antibodies: Mouse monoclonal anti-caveolin-1 (dilution
1:1,000; cat. no. AF0087; Beyotime Institute of Biotechnology),
mouse monoclonal anti-p53 (dilution 1:1,000; cat. no. AF0255;
Beyotime Institute of Biotechnology), mouse monoclonal anti-p21
(dilution 1:500; cat. no. AP021; Beyotime Institute of
Biotechnology), polyclonal rabbit anti-p16 (dilution 1:1,000; cat.
no. SAB4500072; Sigma-Aldrich; Merck KGaA) and mouse monoclonal
anti-β-actin (dilution 1:2,000; cat. no. AF0003; Beyotime Institute
of Biotechnology). The membranes were then further incubated with a
horseradish peroxidase-conjugated goat anti-rabbit IgG secondary
antibody (dilution 1:5,000; cat. no. A0208; Beyotime Institute of
Biotechnology) for 1 h at room temperature, prior to treatment with
Electrochemiluminescence Plus (Tanon Science and Technology Co.,
Ltd., Shanghai, China), according to the manufacturer's
protocol.
Statistical analysis
All measurements were carried out using the same
instrument under the same experimental conditions and were
independently performed at least three times to ensure consistency.
Statistical analyses were performed using SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA). Data are expressed as the mean ± standard
deviation. Significant differences were analyzed with a one-way
analysis of variance. Bonferroni was used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of oxidative stress on NP
cells
To evaluate the effects of t-BHP-induced oxidative
stress on NP cells, the percentage of SA-β-gal-positive cells and
the cellular proliferative rate were assessed. Premature senescence
of NP cells was investigated following treatment with t-BHP and
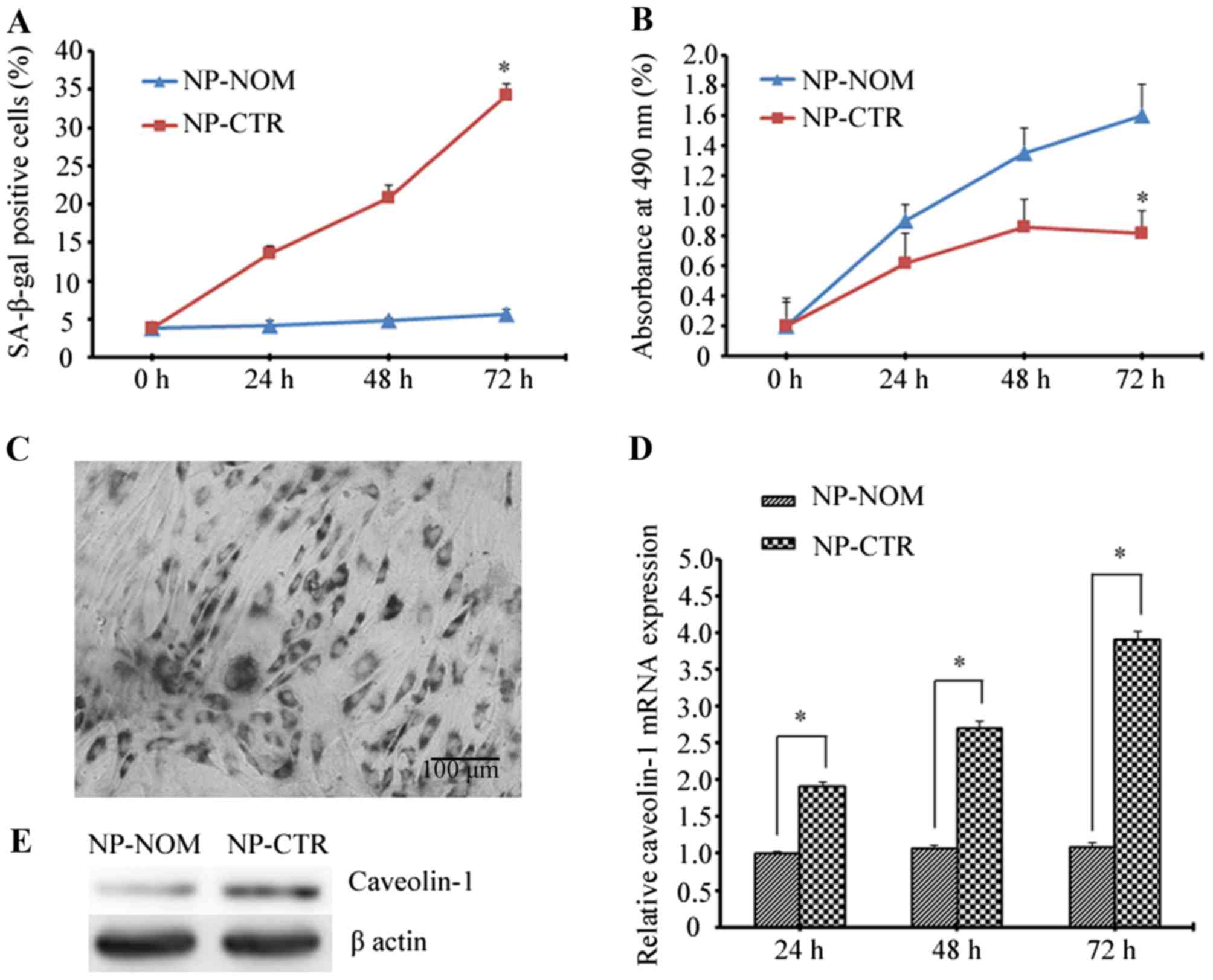
culture for 72 h. As is shown in Fig.
1A, the percentage of SA-β-gal-positive cells was significantly
higher in the NP-CTR group when compared with the NP-NOM group at
72 h (34.25±1.45% vs. 5.60±0.68%; P<0.05). In addition, the
proliferative rate of NP-CTR cells gradually decreased with
increasing time when compared with NP-NOM cells, and at 72 h the
proliferative activity decreased to nearly half that of the NP-NOM
control group (0.82±0.15% vs. 1.60±0.21%; P<0.05; Fig. 1B). Notably, caveolin-1 expression
at the mRNA and protein level was markedly increased following
treatment with t-BHP which, is in agreement with the increase
observed in the percentage of SA-β-gal-positive cells and the
reduction in cellular proliferative ability (Fig. 1C-E).
Silencing caveolin-1 expression by
lentiviral-mediated RNA interference
Lentiviral shRNA was successfully constructed and
confirmed by sequencing analysis; the transduction rate of the
lentivirus was ~90% at 48 h (Fig.
2A). Caveolin-1 expression was assessed by RT-qPCR and western
blot analysis following treatment with t-BHP. As shown in Fig. 2B, the mRNA levels of caveolin-1
decreased in the NP-LV group at 24, 36 and 48 h when compared with
the NP-CTR group; however, no marked difference was detected when
comparing the NP-CTR and NP-LV-NC groups. Similarly, the caveolin-1
protein level also decreased in the NP-LV group at 72 h and was
markedly decreased when compared with the NP-CTR group (Fig. 2C). These results demonstrated that
caveolin-1 gene expression was successfully knocked down by
lentivirus-mediated RNA interference.
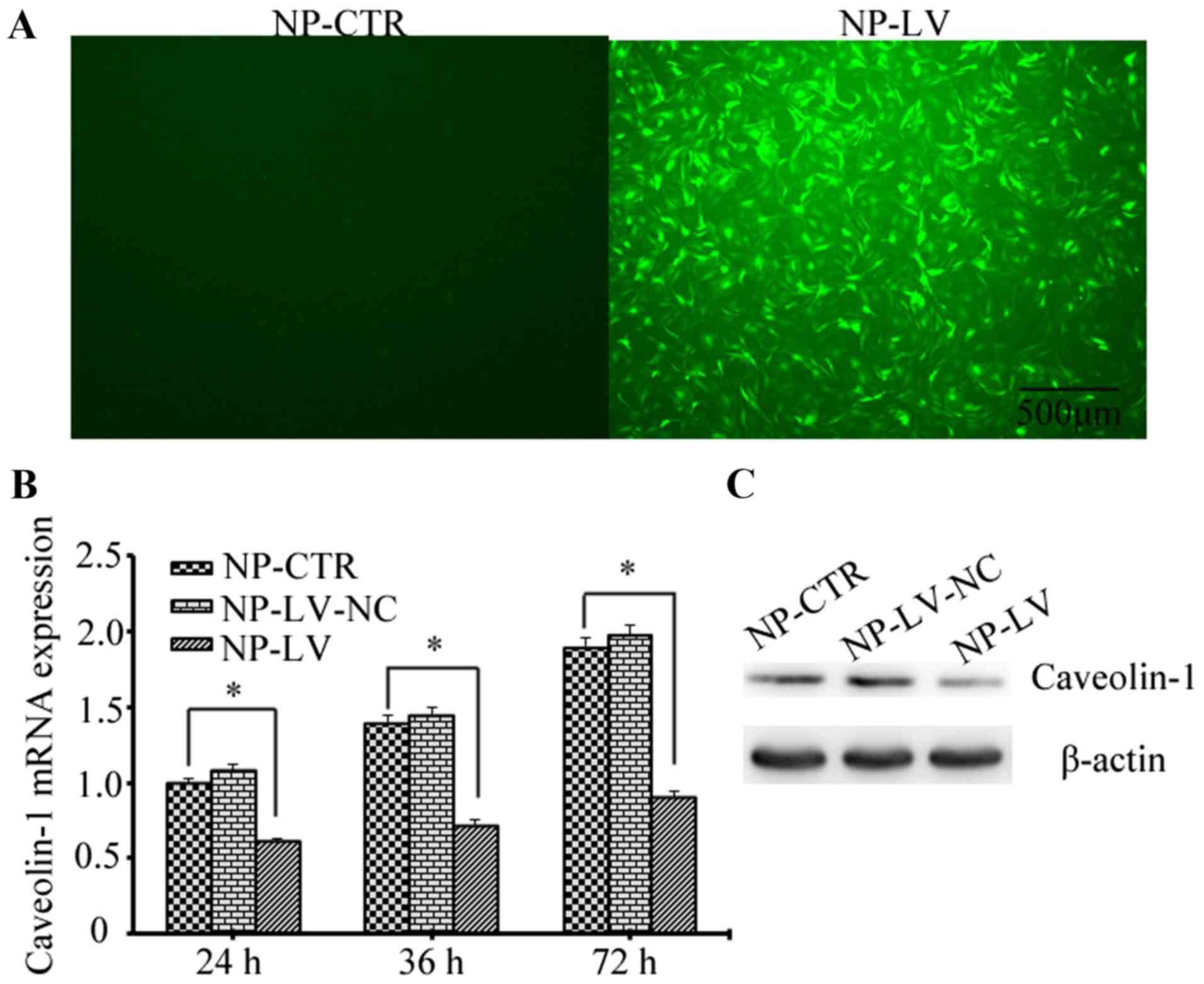 | Figure 2.Evaluation of the caveolin-1
silencing mediated by lentiviral short hairpin RNA. (A) The
transduction efficiency was ~90%, as determined by fluorescence
microscopy (magnification, ×40; scale bars, 500 µm). (B) Assessment
of caveolin-1 mRNA expression by reverse transcription-quantitative
polymerase chain reaction at 24, 48 and 72 h following
t-BHP-induced oxidative stress. In the NP-LV group caveolin-1 mRNA
levels were reduced to ~46% of those produced by the NP-CTR group
at 72 h. *P<0.05, as indicated. (C) Protein levels were assessed
by western blotting. The expression of caveolin-1 was markedly
decreased in the NP-LV group at 72 h when compared with the other
two groups. t-BHP, tert-butyl hydroperoxide; NP, nucleus pulposus;
NP-CTR group, control cells treated with t-BHP only; NP-LV group,
cells treated with t-BHP and lentivirus; NP-LV-NC, cells treated
with t-BHP and negative control lentivirus. |
Caveolin-1-mediated premature
senescence induced by oxidative stress
To determine whether caveolin-1 mediated oxidative
stress-induced cellular senescence, the present study
quantitatively assessed the percentage of SA-β-gal-positive cells
and the proliferative rate following silencing of caveolin-1
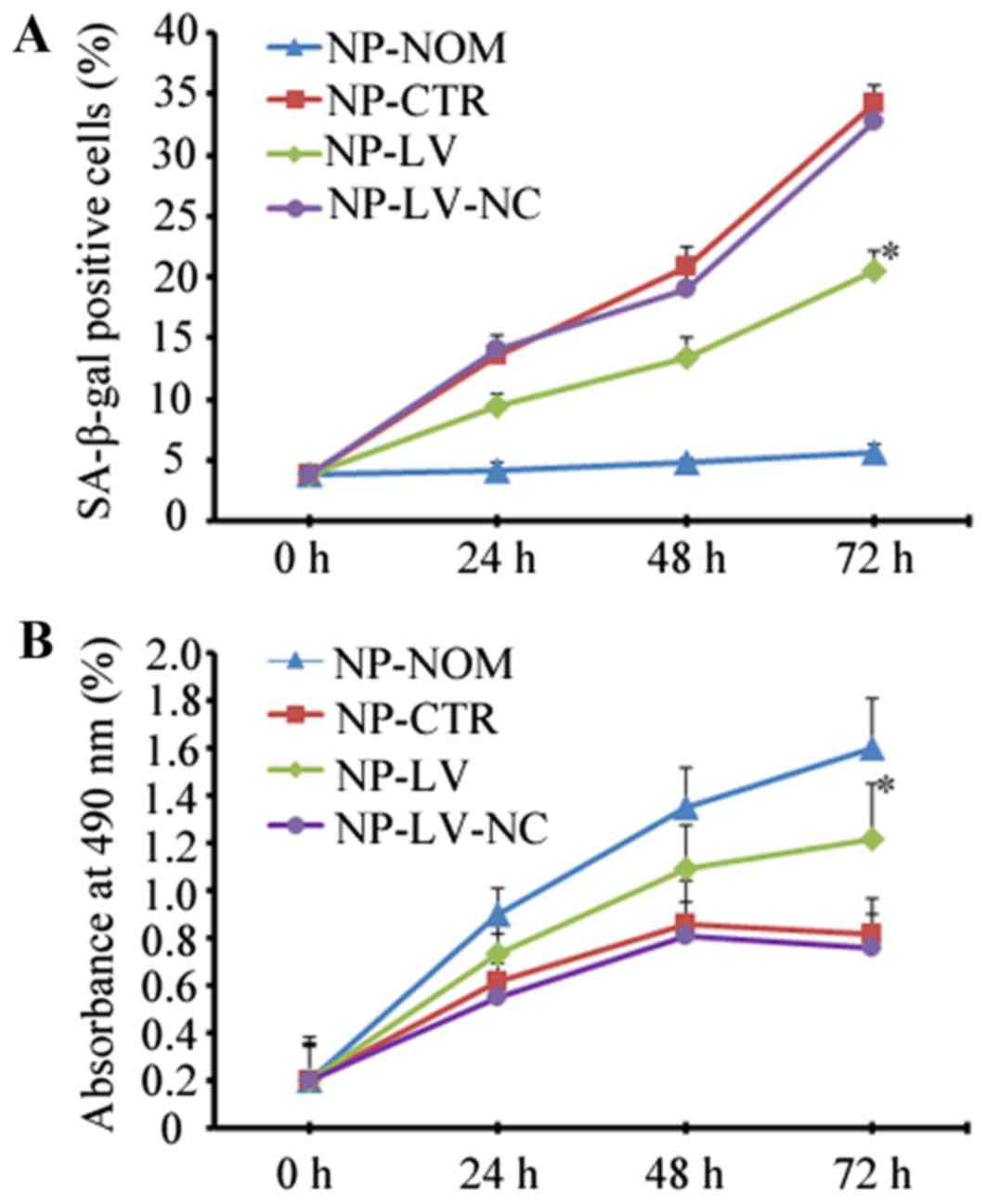
expression. As shown in Fig. 3,
when caveolin-1 was knocked down in the NP-LV group, the increase
in SA-β-gal-positive cells at 72 h was not as marked when compared
with the NP-CTR group (20.56±1.28% vs. 34.25±1.45%; P<0.05;
Fig. 3A). At 72 h, more
proliferative cells were observed in the NP-LV group than in the
NP-CTR group when caveolin-1 expression decreased (1.22±0.20% vs.
0.82±0.15%; P<0.05; Fig.
3B).
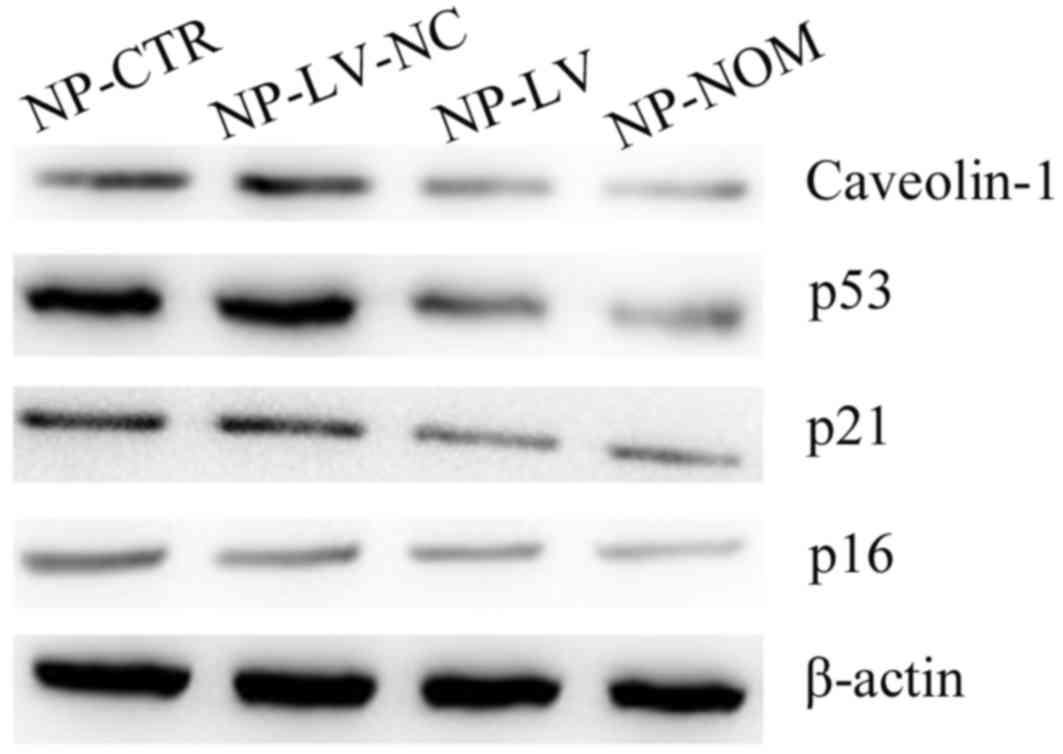
Caveolin-1 induced senescence via the
p53/p21 signaling pathway under oxidative stress
Cellular senescence primarily occurs via two
signaling pathways: The p16 pathway and the p53/p21 pathway
(16). In the present study, the
expression of the p53 protein increased under oxidative stress in
the NP-CTR and NP-LV-NC groups when compared with NP-NOM (Fig. 4). The levels of the p21 protein, a
pro-senescence modulator of p53 activity, were also increased under
t-BHP-induced oxidative stress in the two groups (29). In addition, inhibition of
caveolin-1 expression markedly decreased p53 and p21 protein
expression levels in the NP-LV group. Notably, the decrease in p53
and p21 protein expression were associated with the decrease in
SA-β-gal-positive cells and the recovery of cellular proliferative
ability. By contrast, there was no marked difference in p16 protein
levels among the three groups when compared with NP-NOM.
Discussion
A previous study has reported that cellular
senescence is widespread in degenerated IVD and is positively
associated with degenerative grade (15). In addition, higher levels of cell
senescence have been observed in degenerated discs than in
age-matched non-degenerated discs (30,31).
Senescence results in the deterioration of the microenvironment due
to the secretion of inflammatory cytokines and catabolic enzymes,
which, in turn accelerate IVD degeneration (10). The IVD microenvironment is unique
and is characterized by a limited nutrient supply, hypoxia
(32), hypertonicity, increased
acidity, varied mechanical loading, and continuous and unavoidable
exposure to reactive oxygen species (ROS) (24). However, whether excessive oxidative
stress is the main result of premature senescence in the NP remains
unclear. In addition, the potential mechanism underlying senescence
in the NP also requires elucidation. Therefore, the aim of the
present study was to investigate the associations and mechanisms
underlying oxidative stress-associated senescence in NP cells.
There are two types of cellular senescence:
Replicative senescence (RS) and stress-induced premature senescence
(SIPS) (31). RS is dependent on
the gradual shortening of telomeres at the ends of chromosomes
(29). By contrast, certain
extrinsic stresses, such as ultraviolet irradiation (33) and oxidative stress (34,35),
trigger the type of premature senescence termed SIPS. In the
present study, the percentage of SA-β-gal-positive cells increased
following treatment with t-BHP, and inhibition of cellular
proliferation was also observed. This result indicated that more
cellular senescence was triggered by oxidative stress in NP cells;
this was consistent with previous findings (22). These results suggested that the
induction of cellular SIPS by excess ROS may be the reason for the
accelerated degeneration of discs.
In addition, the mRNA and protein levels of
caveolin-1 were markedly increased following treatment with t-BHP,
indicating that caveolin-1 may be involved in the process of
cellular premature senescence. A previous report demonstrated that
caveolin-1 serves a major role in RS and SIPS (16). A recent study revealed that
caveolin-1 increased mouse embryonic fibroblast senescence by
inhibiting sirtuin 1 (11). The
importance of caveolin-1 in RS was supported by a previous study
that demonstrated that senescent bone marrow stromal cells
expressed higher levels of caveolin-1 than younger cells (36). In addition, mouse embryonic
fibroblasts prematurely induced by oxidative stress were inhibited
in caveolin-1 null mice (19). In
the present study, the results indicated that caveolin-1 expression
and cellular senescence may be associated in NP cells. Caveolin-1
was successfully silenced by lentiviral shRNA-mediated RNA
interference, and mRNA and protein expression decreased following
the transfection of the NP-LV cells. In addition, cellular
senescence induced by oxidative stress was decreased in the NP-LV
group, as demonstrated by the decrease in the percentage of
SA-β-gal-positive cells and the recovery of proliferative ability.
These results indicated that caveolin-1 may be involved in the
increased premature senescence of NP cells induced by oxidative
stress in vitro. Silencing of caveolin-1 protected cells
against premature senescence and increased cellular proliferative
ability.
Cellular senescence primarily occurs via two
pathways: The p16 pathway and the p53/p21cip pathway
(16). In the present study, the
potential pathway associated with caveolin-1 and oxidative
stress-induced senescence in NP cells was evaluated. Previous
studies have revealed that caveolin-1 may be involved in the two
pathways in SIPS in different cells (11,16,37).
Human cells from the degenerated NP have provided evidence of
cellular senescence and express high levels of caveolin-1; they
have also revealed a positive association with the SIPS biomarker
16INK4a (4). Volonte et al
(11) demonstrated that
overexpression of caveolin-1 generated stress-induced premature
senescence in the p53 wild-type, though not the p53 knockout, mouse
embryonic fibroblasts. Notably, in the present study caveolin-1 was
positively associated with p53 and p21, and silencing caveolin-1
resulted in the reduction of p53 and p21 expression in the NP-LV
group. By contrast, no marked decrease in p16 expression was
observed in the NP-LV group. These results indicated that
caveolin-1 may be primarily involved in NP cell senescence via the
p53/p21 signaling pathway under oxidative stress in vitro;
however, these results differ to those reported by Heathfield et
al (4). Pathogenesis of IVD
degeneration is a chronic process in vivo and the two
different pathways can overlap, thus, p16 expression may also be
associated with chronic SIPS and RS (16). In the present study, the use of
transient oxidative stress may have produced different results.
In conclusion, the results of the present study
provide potential strategies for the prevention premature NP
cellular senescence. However, additional experiments involving the
overexpression of caveolin-1 were not conducted and future research
including in vivo experiments are required in order to
determine whether the silencing the caveolin-1 expression is able
to delay the degeneration of the IVD.
Acknowledgements
The present study was supported by the Shanghai
Municipality Health Bureau (grant nos. 201640101 and 2014-399) and
the Jinshan Science and Technology Committee (grant no.
2016-3-06).
References
|
1
|
Hoy D, Brooks P, Woolf A, Blyth F, March
L, Bain C, Baker P, Smith E and Buchbinder R: Assessing risk of
bias in prevalence studies: Modification of an existing tool and
evidence of interrater agreement. J Clin Epidemiol. 65:934–939.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Schepper EI, Damen J, van Meurs JB,
Ginai AZ, Popham M, Hofman A, Koes BW and Bierma-Zeinstra SM: The
association between lumbar disc degeneration and low back pain: The
influence of age, gender, and individual radiographic features.
Spine (Phila Pa 1976). 35:531–536. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raj PP: Intervertebral disc:
Anatomy-physiology-pathophysiology-treatment. Pain Pract. 8:18–44.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heathfield SK, Le Maitre CL and Hoyland
JA: Caveolin-1 expression and stress-induced premature senescence
in human intervertebral disc degeneration. Arthritis Res Ther.
10:R872008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang F, Cai F, Shi R, Wang XH and Wu XT:
Aging and age related stresses: A senescence mechanism of
intervertebral disc degeneration. Osteoarthritis Cartilage.
24:398–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Maitre CL, Freemont AJ and Hoyland JA:
Accelerated cellular senescence in degenerate intervertebral discs:
A possible role in the pathogenesis of intervertebral disc
degeneration. Arthritis Res Ther. 9:R452007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Deursen JM: The role of senescent
cells in ageing. Nature. 509:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharpless NE and Sherr CJ: Forging a
signature of in vivo senescence. Nat Rev Cancer. 15:397–408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dimozi A, Mavrogonatou E, Sklirou A and
Kletsas D: Oxidative stress inhibits the proliferation, induces
premature senescence and promotes a catabolic phenotype in human
nucleus pulposus intervertebral disc cells. Eur Cell Mater.
30:89–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volonte D, Zou H, Bartholomew JN, Liu Z,
Morel PA and Galbiati F: Oxidative stress-induced inhibition of
Sirt1 by caveolin-1 promotes p53-dependent premature senescence and
stimulates the secretion of interleukin 6 (IL-6). J Biol Chem.
290:4202–4214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kletsas D: Senescent cells in the
intervertebral disc: Numbers and mechanisms. Spine J. 9:677–678.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Acosta JC, Banito A, Wuestefeld T,
Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka
F, Andrulis M, et al: A complex secretory program orchestrated by
the inflammasome controls paracrine senescence. Nat Cell Biol.
15:978–990. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim KW, Chung HN, Ha KY, Lee JS and Kim
YY: Senescence mechanisms of nucleus pulposus chondrocytes in human
intervertebral discs. Spine J. 9:658–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gruber HE, Ingram JA, Davis DE and Hanley
EN Jr: Increased cell senescence is associated with decreased cell
proliferation in vivo in the degenerating human annulus. Spine J.
9:210–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zou H, Stoppani E, Volonte D and Galbiati
F: Caveolin-1, cellular senescence and age-related diseases. Mech
Ageing Dev. 132:533–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parton RG and Simons K: The multiple faces
of caveolae. Nat Rev Mol Cell Biol. 8:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smart EJ, Graf GA, McNiven MA, Sessa WC,
Engelman JA, Scherer PE, Okamoto T and Lisanti MP: Caveolins,
liquid-ordered domains, and signal transduction. Mol Cell Biol.
19:7289–7304. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartholomew JN, Volonte D and Galbiati F:
Caveolin-1 regulates the antagonistic pleiotropic properties of
cellular senescence through a novel Mdm2/p53-mediated pathway.
Cancer Res. 69:2878–2886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai SM, Shan ZZ, Nakamura H, Masuko-Hongo
K, Kato T, Nishioka K and Yudoh K: Catabolic stress induces
features of chondrocyte senescence through overexpression of
caveolin 1: Possible involvement of caveolin 1-induced
down-regulation of articular chondrocytes in the pathogenesis of
osteoarthritis. Arthritis Rheum. 54:818–831. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smolders LA, Meij BP, Onis D, Riemers FM,
Bergknut N, Wubbolts R, Grinwis GC, Houweling M, Koerkamp MJ Groot,
van Leenen D, et al: Gene expression profiling of early
intervertebral disc degeneration reveals a down-regulation of
canonical Wnt signaling and caveolin-1 expression: Implications for
development of regenerative strategies. Arthritis Res Ther.
15:R232013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou G, Lu H, Chen M, Yao H and Zhao H:
Oxidative stress participates in age-related changes in rat lumbar
intervertebral discs. Arch Gerontol Geriatr. 59:665–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nasto LA, Robinson AR, Ngo K, Clauson CL,
Dong Q, St Croix C, Sowa G, Pola E, Robbins PD, Kang J, et al:
Mitochondrial-derived reactive oxygen species (ROS) play a causal
role in aging-related intervertebral disc degeneration. J Orthop
Res. 31:1150–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang F, Zhao X, Shen H and Zhang C:
Molecular mechanisms of cell death in intervertebral disc
degeneration (review). Int J Mol Med. 37:1439–1448. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding L, Wu JP, Xu G, Zhu B, Zeng QM, Li DF
and Lu W: Lentiviral-mediated RNAi targeting caspase-3 inhibits
apoptosis induced by serum deprivation in rat endplate chondrocytes
in vitro. Braz J Med Biol Res. 47:445–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sanger F and Coulson AR: A rapid method
for determining sequences in DNA by primed synthesis with DNA
polymerase. J Mol Biol. 94:441–448. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding L, Wu J, Li D, Wang H, Zhu B, Lu W
and Xu G: Effects of CCN3 on rat cartilage endplate chondrocytes
cultured under serum deprivation in vitro. Mol Med Rep.
13:2017–2022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ben-Porath I and Weinberg RA: The signals
and pathways activating cellular senescence. Int J Biochem Cell
Biol. 37:961–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeong SW, Lee JS and Kim KW: In vitro
lifespan and senescence mechanisms of human nucleus pulposus
chondrocytes. Spine J. 14:499–504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng C, Liu H, Yang M, Zhang Y, Huang B
and Zhou Y: Disc cell senescence in intervertebral disc
degeneration: Causes and molecular pathways. Cell Cycle.
15:1674–1684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen JW, Li B, Yang YH, Jiang SD and Jiang
LS: Significance of hypoxia in the physiological function of
intervertebral disc cells. Crit Rev Eukaryot Gene Expr. 24:193–204.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou BR, Guo XF, Zhang JA, Xu Y, Li W, Wu
D, Yin ZQ, Permatasari F and Luo D: Elevated miR-34c-5p mediates
dermal fibroblast senescence by ultraviolet irradiation. Int J Biol
Sci. 9:743–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shin JH, Jeon HJ, Park J and Chang MS:
Epigallocatechin-3-gallate prevents oxidative stress-induced
cellular senescence in human mesenchymal stem cells via Nrf2. Int J
Mol Med. 38:1075–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kiyoshima T, Enoki N, Kobayashi I, Sakai
T, Nagata K, Wada H, Fujiwara H, Ookuma Y and Sakai H: Oxidative
stress caused by a low concentration of hydrogen peroxide induces
senescence-like changes in mouse gingival fibroblasts. Int J Mol
Med. 30:1007–1012. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun C, Wang N, Huang J, Xin J, Peng F, Ren
Y, Zhang S and Miao J: Inhibition of phosphatidylcholine-specific
phospholipase C prevents bone marrow stromal cell senescence in
vitro. J Cell Biochem. 108:519–528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kortum RL, Fernandez MR, Costanzo-Garvey
DL, Johnson HJ, Fisher KW, Volle DJ and Lewis RE: Caveolin-1 is
required for kinase suppressor of Ras 1 (KSR1)-mediated
extracellular signal-regulated kinase 1/2 activation,
H-RasV12-induced senescence, and transformation. Mol Cell Biol.
34:3461–3472. 2014. View Article : Google Scholar : PubMed/NCBI
|