Introduction
Breast cancer (BC) is the most common female
malignant tumors in the world and its incidence is rising annually
(1). In recent years, as
development of treatment modalities and early detections, overall
survival of BC patients has been improved to some extent. However,
for the occurrence of drug resistance, the prognosis of some
patients is still not good (2–5). So,
to find effective targets for inhibiting drug resistance is urgent
and will contribute to better therapy outcome of BC patients.
Vasohibin2 (VASH2) is a member of vasohibin family
and initially known as an angiogenic factor in different histology
and pathology conditions (6). In
recent years, its roles in different tumors have been widely
studied. It is reported that VASH2 can play important roles in
proliferation, angiogenesis and epithelial mesenchymal transition
(EMT) of hepatocellular carcinoma, ovarian adenocarcinoma,
pancreatic ductal adenocarcinoma and endometrial cancer cells
(7–11). In BC, it has been reported that
VASH2 could promote EMT and proliferation of BC cells through in
vivo and in vitro experiments (12,13).
However, its role in drug resistance of BC is still unknown.
In the present study, we detected VASH2 expression
and drug resistance of different BC cell lines. We proved that
VASH2 could promote drug resistance of BC cells through regulating
expression of ATP-binding cassette sub-family G member 2 (ABCG2),
at least partly. Moreover, we confirmed that VASH2 could promote
expression of ABCG2 via AKT signal pathway.
Materials and methods
Cell lines and cell culture
Human BC cell lines MCF-7 and MDA-MB-231 were both
purchased from American Type Culture Collection (Manassas, VA,
USA). Both cell lines were cultured in DMEM medium supplemented
with 10% fetal bovine serum (FBS), at 37°C, in 5%
CO2.
Stable transfection
Plasmids PcDNA3.1/VASH2-vector, p-GPU6/VASH2-shRNA
and control plasmids (Shanghai GenePharma Co., Ltd, Shanghai,
China) were transfected into BC cells using Lipo2000 (Invitrogen,
Carlsbad, CA, USA) to upregulate or silence VASH2 expression. After
48 h, cells were cultured in medium containing 1.0 ug/ml puromycin
for 3 weeks, then, monoclone was selected. VASH2 expression was
detected by RT-PCR and western blot analysis.
Transitent transfection
To silence ABCG2 expression, siRNA (Shanghai
GenePharma Co.) for ABCG2 was transfected into cells using Lipo2000
(Invitrogen). 48 h later, ABCG2 expression was detected by RT-PCR
and western blot.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Total RNA was extracted by TRIzol (Invitrogen)
according to the manufacturer's instruction. cDNAs were synthesized
using PrimeScript RT-PCR kit (TaKaRa, Dalian, China) as protocol.
The primers used were as follows: VASH2 forward,
5′-CTCTTCCAGCCTTCCTTCCT-3′ and reverse, 5′-AGCACTGTGTTGGCGTACAG-3′;
ABCG2 forward, 5′-CTGAGATCCTGAGCCTTTGG-3′ and reverse,
5′-TGCCCATCACAACATCATCT-3′. GAPDH was used as an internal
control.
Western blot analysis
Cell protein was extracted using RIPA lysis buffer.
Equal amount of protein was separated by SDS-PAGE and transferred
to polyvinylidene difluoride (PVDF) membrane. The membrane was
incubated in primary antibodies (Table
I) at 4°C overnight and in horseradish peroxidase-conjugated
secondary antibody (1:5,000; Proteintech Group, Inc, Wuhan, China)
in room temperature for 1 h, signal on the membrane was visualized
using enhanced chemiluminescence reagents (Pierce, Rockford, IL,
USA).
 | Table I.Primary antibodies used in western
blot analysis. |
Table I.
Primary antibodies used in western
blot analysis.
| Name | Company |
|---|
| VASH2 | Abcam, Cambridge, MA,
USA |
| ABCG2 | Abcam, Cambridge, MA,
USA |
| Phospho-ERK1/2 | Cell Signaling
Technology, Danvers, MA, USA |
| ERK1/2 | Cell Signaling
Technology, Danvers, MA, USA |
| Phospho-AKT | Cell Signaling
Technology, Danvers, MA, USA |
| AKT | Cell Signaling
Technology, Danvers, MA, USA |
| GAPDH | Epitomics,
Burlingame, CA, USA |
Establishment of cells with stable adriamycin (ADM)
resistance. MDA-MB-231 or MCF-7 cells were cultured in DMEM medium
containing 0.4 or 0.8 umol/l ADM for 48 h, then cells were cultured
in ADM free medium. The cells would not be passaged until they grow
up to 80% confluence. ADM concentration is elevated gradually until
cells could grow steadily in 1 or 3 umol/l. New drug-resistance
cells were named as MDA-MB-231-ADM or MCF-7-ADM.
Drug resistance assay
Cells were plated in 96-well plate in triplicate in
DMEM supplemented with 10% FBS at 8,000 cells per well. After 24 h,
the medium was replaced using DMEM containing different
concentrations of ADM (0.02, 0.08, 0.32, 1.28, 5.12 and 20.48
µmol/l). After 48 h, MTT assay was performed at 490 nm wavelengths.
The survival curves were constructed and 50% inhibitory
concentration (IC50) was calculated. The experiment was repeated at
least three times.
Statistical analysis
The data were stored and analyzed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA). The difference between two
groups was analyzed using Student two-tailed t test. IC50 was
gotten using regression analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
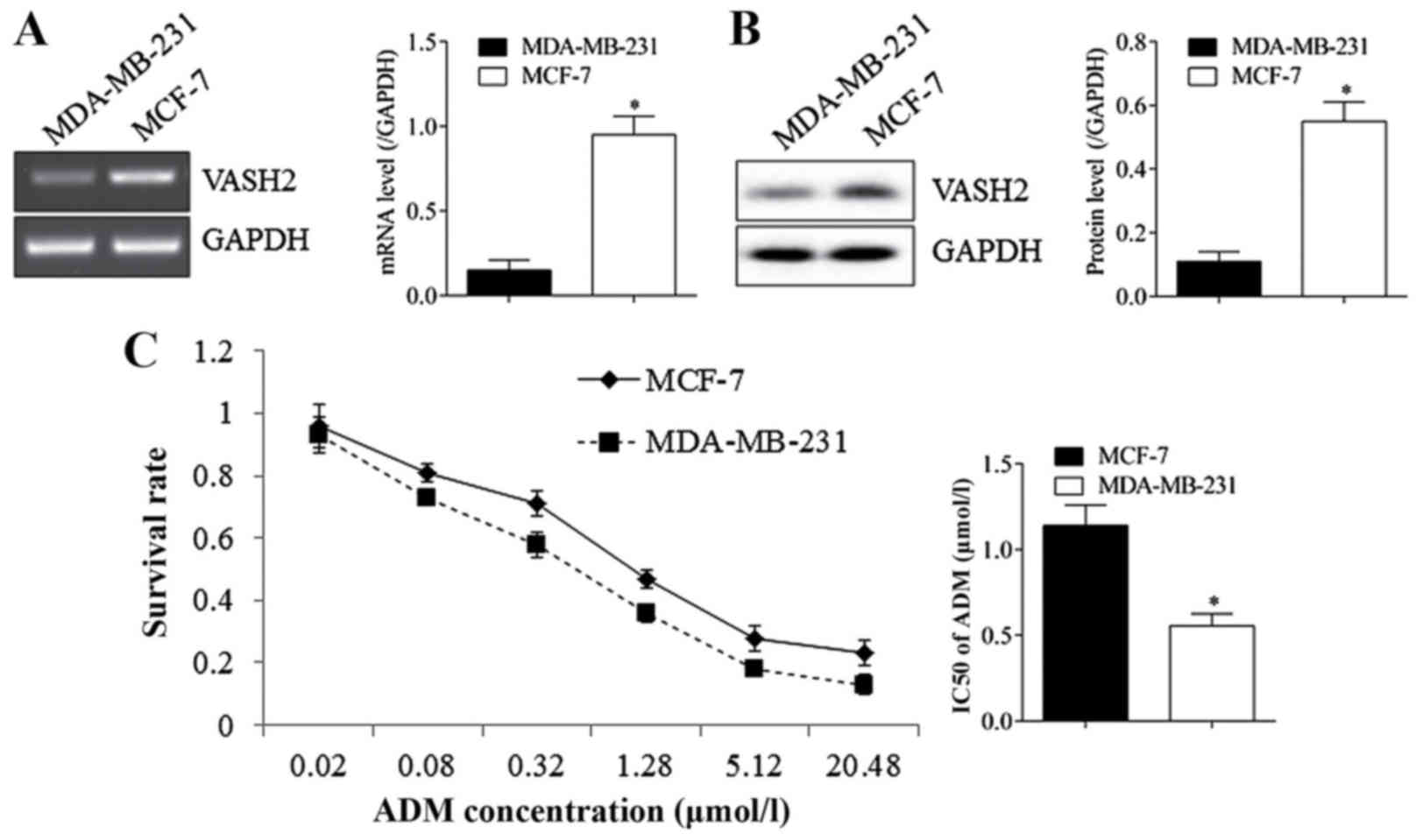
MCF-7 cells with higher VASH2 than
MDA-MB-231 cells and showed stronger ADM resistance
As shown in Fig.
1A, expression of VASH2 was detected using RT-PCR and western
blot, the results showed that VASH2 in MCF-7 cells was much higher
than in MDA-MB-231 cells at RNA (A) and protein (B) levels, with
significant difference. Drug resistance analysis showed that MCF-7
cells (IC50=1.14±0.12 umol/l) exhibited higher survival rate in ADM
than MDA-MB-231 cells (IC50=0.55±0.07 umol/l) (C). The results
reminded us that VASH2 may affect ADM resistance of BC cells.
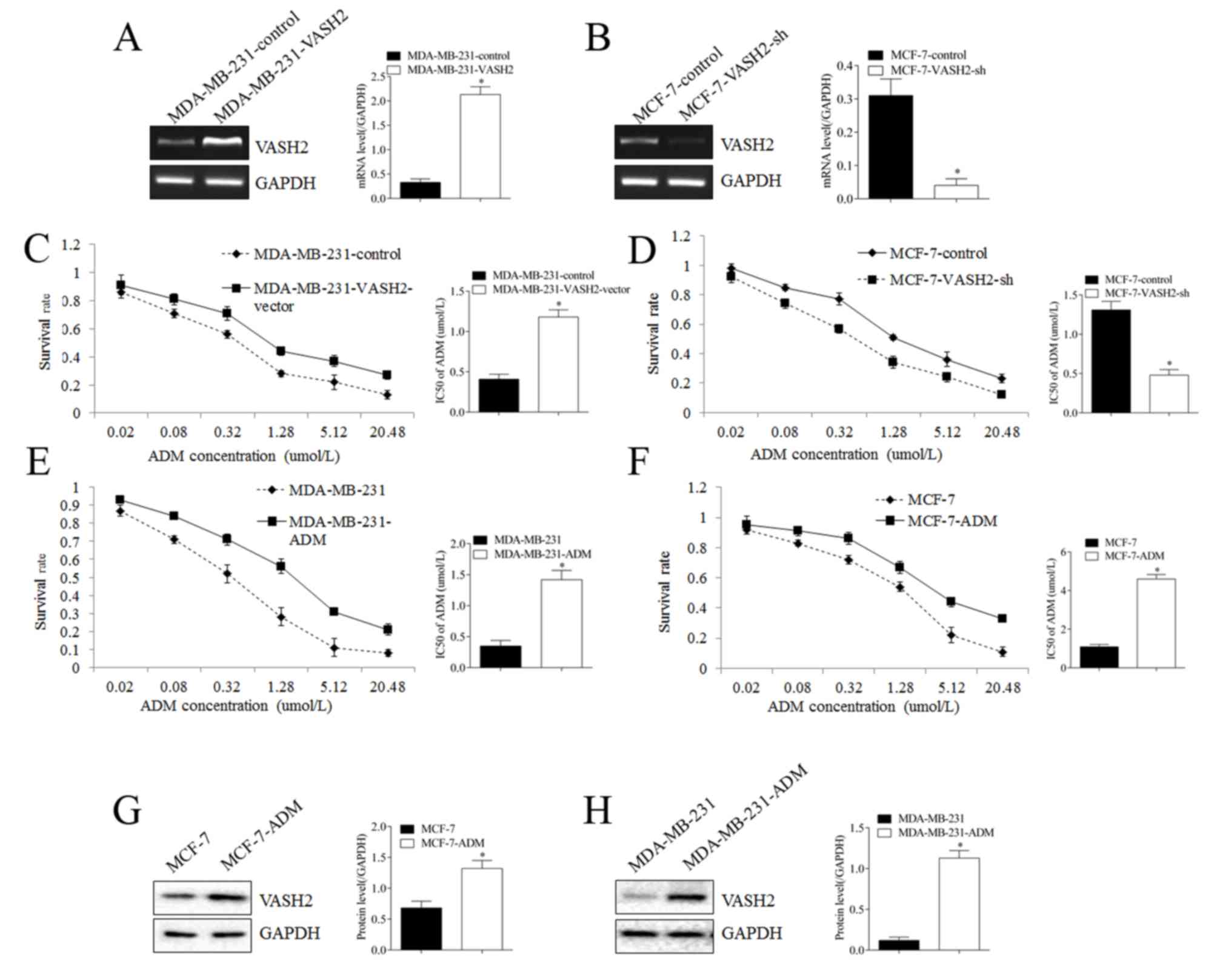
VASH2 promoted ADM resistance of BC
cells
To confirm whether VASH2 could affect ADM resistance
of BC cells, we changed expression of VASH2 through stable
transfection (Fig. 2). After VASH2
was upregulated in MDA-MB-231cells (Fig. 2A), survival rate of cells in ADM
increased significantly (Fig. 2C),
IC50 increased from 0.41±0.06 to 1.18±0.09 umol/l, with significant
difference. Reversely, after VASH2 was silenced in MCF-7 cells
(Fig. 2B), survival rate of cells
in ADM declined significantly, IC50 declined from 1.31±0.11 to
0.48±0.07 umol/l, with significant difference (Fig. 2D). Besides, after cultured in ADM
gradually, we got new drug resistance cells MCF-7-ADM and
MDA-MB-231-ADM with significantly increased survival rate and IC50
than parent cells (Fig. 2E, F).
Western blot showed that VASH2 in MCF-7-ADM cells was significantly
higher than in parent cells (Fig.
2G), and it was also the case for MDA-MB-231-ADM cells
(Fig. 2H). These results proved
the promotion roles of VASH2 in ADM resistance of BC cells.
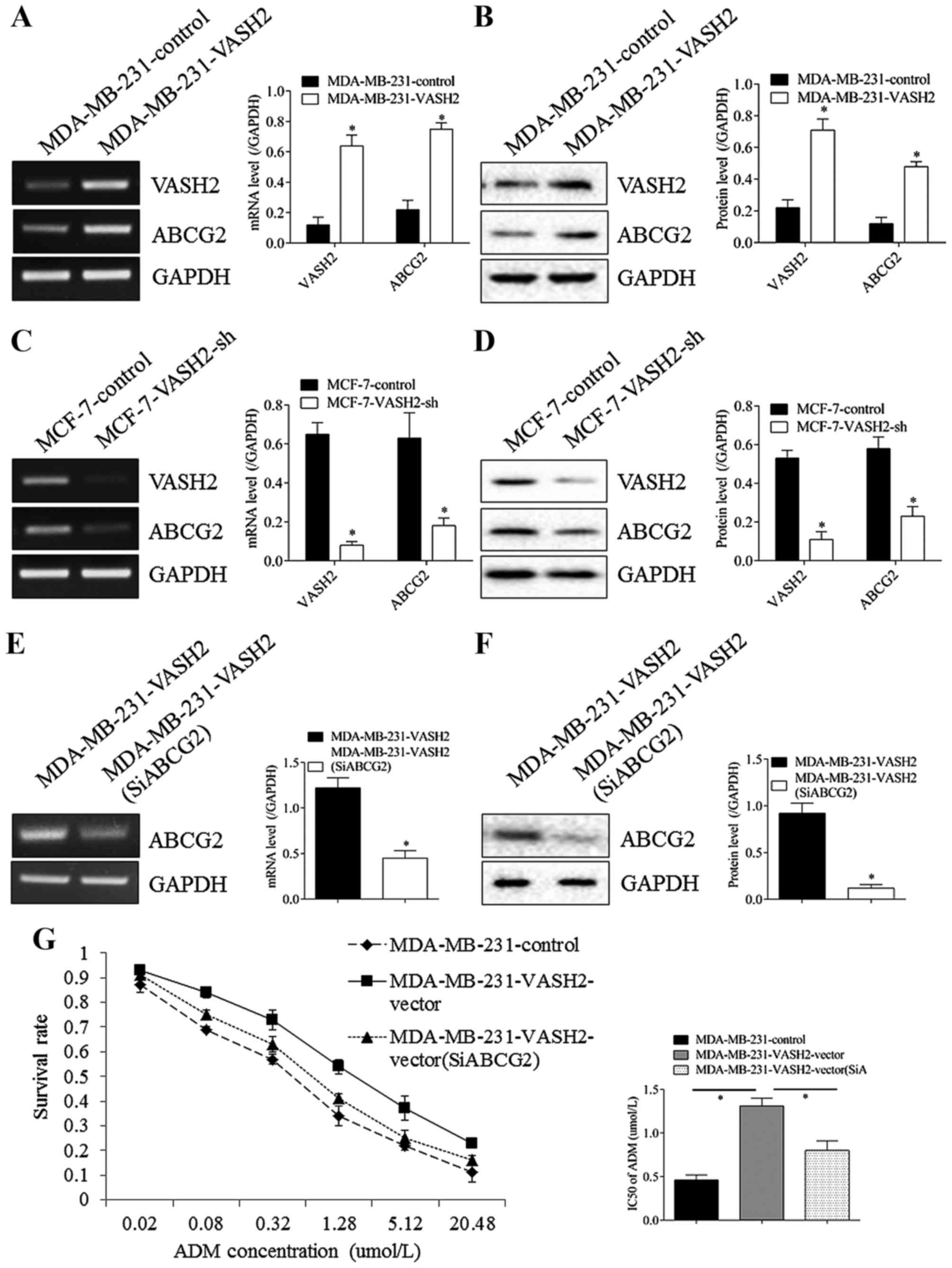
ABCG2 took part in drug resistance
induced by VASH2 in BC cells
As shown in Fig. 3,
after VASH2 in MDA-MB-231 cells was overexpressed, expression of
ABCG2 was increased both at RNA (Fig.
3A) and protein (Fig. 3B)
levels, with significant difference. Reversely, after VASH2 in
MCF-7 cells was silenced, expression of ABCG2 was decreased both at
RNA (Fig. 3C) and protein
(Fig. 3D) levels, with significant
difference. Moreover, after ABCG2 in MDA-MB-231-VASH2 cells was
silenced by transfection at RNA (Fig.
3E) and protein (Fig. 3F)
levels, survival rate declined significantly, but still higher than
MDA-MB-231 cells, IC50 declined from 1.31±0.09 to 0.81±0.11 umol/l,
with significant difference, but still higher than MDA-MB-231 cells
(0.46±0.06 umol/l), with significant difference (Fig. 3G). These results proved that VASH2
could promote ADM resistance of BC cells through regulating ABCG2,
at least partly.
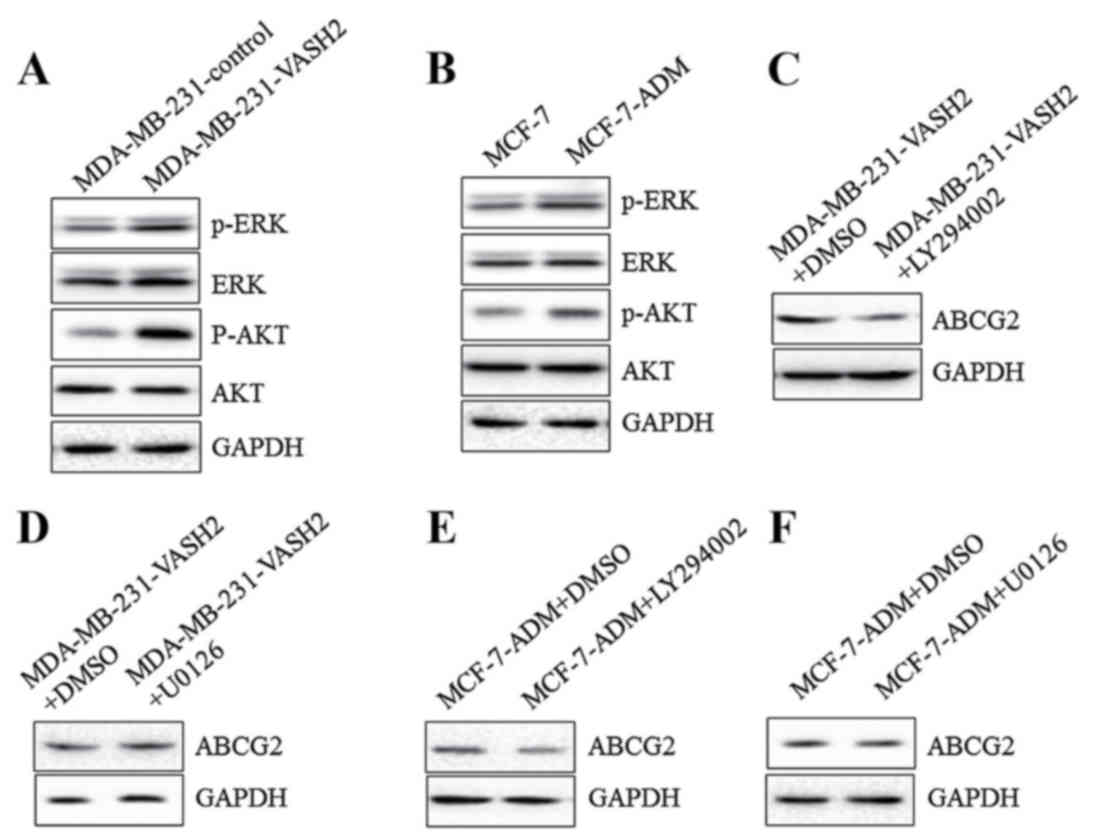
VASH2 could promote expression of
ABCG2 via AKT signal pathway
To confirm potential molecular mechanism, further
cell experiments were performed. As shown in Fig. 4A, overexpressing VASH2 stimulated
phosphorylation of AKT and ERK in MDA-MB-231 cells significantly.
Moreover, phosphorylation of AKT and ERK in MCF-7-ADM cells was
also significantly higher than in parent cells (Fig. 4B). Moreover, after AKT inhibitor
LY294002 was added, the increase of ABCG2 in MDA-MB-231-VASH2
(Fig. 4C) and MCF-7-ADM (Fig. 4E) cells was inhibited
significantly. However, after ERK inhibitor U0126 was added, the
increase of ABCG2 in MDA-MB-231-VASH2 (Fig. 4D) or MCF-7-ADM (Fig. 4F) cells did not change. All the
results proved that VASH2 could promote expression of ABCG2 in BC
cells via AKT signal pathway.
Discussion
Human VASH2 gene is located on chromosome 1q32.3 and
VASH2 protein is composed of 355 amino acid residues (6). It is reported that VASH2 can not only
promote tumor growth and metastasis through supporting
angiogenesis, but also regulate malignancies by direct effects on
tumor cells. For the multiple functions in tumor progression, it
has attracted more and more attention in recent years. In 2012,
Takahashi et al proved that VASH2 could accelerate ovarian
adenocarcinoma growth by promoting angiogenesis (14). In 2015, Kim et al reported
that VASH2 was positively correlated with clinical stage, tumor
proliferation and micro vessel density (MVD), as well as poor
outcome of pancreatic cancer patients (9). For BC cancer, Tu et al
reported that VASH2 could promote proliferation and EMT of BC cells
(12,13). But, the role of VASH2 in drug
resistance of BC cells has not been reported.
ADM is a classical chemotherapy drug and has been
extensively used in BC patients. On one hand, ADM can inhibit DNA
transcription and replication by intercalating between DNA base
pairs; on the other hand, ADM can induce breakage of DNA double
strands by generating oxygen free radicals (15). So, in the present study, we chose
ADM to study drug resistance of BC cells. MDA-MB-231 and MCF-7 are
both BC cell lines, but the original characteristics are not same.
Previous studies proved that MDA-MB-231 cells were ER (−) and grew
rapidly. Otherwise, MCF-7 cells were ER (+) and grew relatively
slowly (16). Both of MDA-MB-231
and MCF-7 cell lines were used in our research. In this study, we
found that BC cells with higher expression of VASH2 exhibited
stronger ADM resistance. Then, overexpressing VASH2 increased ADM
resistance, but silencing VASH2 inhibited ADM resistance in BC
cells. Moreover, newly established ADM resistance cell line also
showed stronger expression of VASH2 than parent cells. This
confirmed promotion roles of VASH2 in ADM resistance of BC
cells.
ABCG2 is also known as BC Resistance Protein (BCRP)
and can function as one of the major factors inducing drug
resistance of cancer cells. It can transport different chemotherapy
drugs from intracellular region to extracellular space, thus
providing drug resistance. So, ABCG2 has been treated as an
important target for improving sensitivity of tumor cells to
chemotherapy (17,18). In the present study, we found that
changes of VASH2 could induce consistent changes of ABCG2 after
transfection. Moreover, silencing ABCG2 abrogated increase of ADM
resistance induced by VASH2 partly, this proved that VASH2 could
regulate ADM resistance of BC cells through regulating ABCG2, at
least partly.
Deep understanding about molecular mechanism will
contribute to find new therapy targets. AKT and ERK signal pathways
can be stimulated in many types of tumors and can play important
roles in tumor proliferation, migration, drug resistance, and radio
resistance (19–21). In 2016, Hu CF reported that acidic
microenvironment could induce drug resistance through upregulating
expression of ABCG2 in lung cancer cells via PI3K-AKT-mTOR-S6
pathway (22). He et al
reported that HIF-1α could regulate ABCG2 activity through the
activation of ERK1/2 pathway and contribute to chemoresistance in
pancreatic cancer cells (23). In
the present study, we confirmed that VASH2 could promote expression
of ABCG2 in BC cells via AKT signal pathway. This is in consistence
with report from Hu et al (23), but different to report from He et
al. This may be attributed to different function factors or
different tumor types.
In conclusion, we confirmed the roles of VASH2 in
ADM resistance of BC for the first time. Moreover, we proved that
AKT-ABCG2 pathway was responsible for drug resistance induced by
VASH2, at least partly. This contributes to our further
understanding about VASH2 in tumor progression and suggests that
VASH2 may be a novel target in BC treatment.
References
|
1
|
Clarke CA, Glaser SL, Leung R,
Davidson-Allen K, Gomez SL and Keegan TH: Prevalence and
characteristics of cancer patients receiving care from single vs.
multiple institutions. Cancer epidemiol. 46:27–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narayanan R and Dalton JT: Androgen
receptor: A complex therapeutic target for breast cancer. Cancers
(Basel). 8:pii: E1082016. View Article : Google Scholar
|
|
3
|
Friese CR, Li Y, Bondarenko I, Hofer TP,
Ward KC, Hamilton AS, Deapen D, Kurian AW and Katz SJ: Chemotherapy
decisions and patient experience with the recurrence score assay
for early-stage breast cancer. Cancer. 123:43–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cata JP, Chavez-MacGregor M, Valero V,
Black W, Black DM, Goravanchi F, Ifeanyi IC, Hernandez M,
Rodriguez-Restrepo A and Gottumukkala V: The impact of
paravertebral block analgesia on breast cancer survival after
surgery. Reg Anesth Pain Med. 41:696–703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mikkola TS, Savolainen-Peltonen H,
Tuomikoski P, Hoti F, Vattulainen P, Gissler M and Ylikorkala O:
Reduced risk of breast cancer mortality in women using
postmenopausal hormone therapy: A Finnish nationwide comparative
study. Menopause. 23:1199–1203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sato Y: The vasohibin family.
Pharmaceuticals (Basel). 3:433–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Tu M, Han B, Gu Y, Xue X, Sun J, Ge
Q, Miao Y, Qian Z and Gao W: Vasohibin 2 decreases the cisplatin
sensitivity of hepatocarcinoma cell line by downregulating p53.
PLoS One. 9:e903582014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xue X, Zhang Y, Zhi Q, Tu M, Xu Y, Sun J,
Wei J, Lu Z, Miao Y and Gao W: MiR200-upregulated Vasohibin 2
promotes the malignant transformation of tumors by inducing
epithelial-mesenchymal transition in hepatocellular carcinoma. Cell
Commun Signal. 12:622014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JC, Kim KT, Park JT, Kim HJ, Sato Y
and Kim HS: Expression of vasohibin-2 in pancreatic ductal
adenocarcinoma promotes tumor progression and is associated with a
poor clinical outcome. Hepatogastroenterology. 62:251–256.
2015.PubMed/NCBI
|
|
10
|
Koyanagi T, Suzuki Y, Saga Y, Machida S,
Takei Y, Fujiwara H, Suzuki M and Sato Y: In vivo delivery of siRNA
targeting vasohibin-2 decreases tumor angiogenesis and suppresses
tumor growth in ovarian cancer. Cancer Sci. 104:1705–1710. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koyanagi T, Saga Y, Takahashi Y, Suzuki Y,
Suzuki M and Sato Y: Downregulation of vasohibin-2, a novel
angiogenesis regulator, suppresses tumor growth by inhibiting
angiogenesis in endometrial cancer cells. Oncol Lett. 5:1058–1062.
2013.PubMed/NCBI
|
|
12
|
Tu M, Liu X, Han B, Ge Q, Li Z, Lu Z, Wei
J, Song G, Cai B, Lv N, et al: Vasohibin-2 promotes proliferation
in human breast cancer cells via upregulation of fibroblast growth
factor-2 and growth/differentiation factor-15 expression. Mol Med
Rep. 10:663–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tu M, Lu C, Lv N, Wei J, Lu Z, Xi C, Chen
J, Guo F, Jiang K, Li Q, et al: Vasohibin 2 promotes human luminal
breast cancer angiogenesis in a non-paracrine manner via
transcriptional activation of fibroblast growth factor 2. Cancer
Lett. 383:272–281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi Y, Koyanagi T, Suzuki Y, Saga Y,
Kanomata N, Moriya T, Suzuki M and Sato Y: Vasohibin-2 expressed in
human serous ovarian adenocarcinoma accelerates tumor growth by
promoting angiogenesis. Mol Cancer Res. 10:1135–1146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao M, Yu S and Zhang M: Differential
expression of multidrug resistance-related proteins in
adriamycin-resistant (pumc-91/ADM) and parental (pumc-91) human
bladder cancer cell lines. Mol Med Rep. 14:4741–4746. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cetin I and Topcul MR: In vitro
antiproliferative effects of nab-paclitaxel with liposomal
cisplatin on MDA-MB-231 and MCF-7 breast cancer cell lines. J BUON.
22:347–354. 2017.PubMed/NCBI
|
|
17
|
Sui H, Zhou LH, Zhang YL, Huang JP, Liu X,
Ji Q, Fu XL, Wen HT, Chen ZS, Deng WL, et al: Evodiamine suppresses
ABCG2 mediated drug resistance by inhibiting p50/p65 NF-κB pathway
in colorectal cancer. J Cell Biochem. 117:1471–1481. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, Gu M, Zhu L, Liu J, Xiong Y, Wei Y
and Li F: Gemcitabine upregulates ABCG2/BCRP and modulates the
intracellular pharmacokinetic profiles of bioluminescence in
pancreatic cancer cells. Anticancer Drugs. 27:183–191. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dahlmann M, Okhrimenko A, Marcinkowski P,
Osterland M, Herrmann P, Smith J, Heizmann CW, Schlag PM and Stein
U: RAGE mediates S100A4-induced cell motility via MAPK/ERK and
hypoxia signaling and is a prognostic biomarker for human
colorectal cancer metastasis. Oncotarget. 5:3220–3233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Cui J, Duan X, Chen H and Fan F:
Suppression of type I collagen expression by miR-29b via PI3K, Akt,
and Sp1 pathway in human Tenon's fibroblasts. Invest Ophthalmol Vis
Sci. 53:1670–1678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao B and Degterev A: Targeting
phospshatidylinositol 3-kinase signaling with novel
phosphatidylinositol 3,4,5-triphosphate antagonists. Autophagy.
7:650–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu CF, Huang YY, Wang YJ and Gao FG:
Upregulation of ABCG2 via the PI3K-Akt pathway contributes to
acidic microenvironment-induced cisplatin resistance in A549 and
LTEP-a-2 lung cancer cells. Oncol Rep. 36:455–461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He X, Wang J, Wei W, Shi M, Xin B, Zhang T
and Shen X: Hypoxia regulates ABCG2 activity through the
activivation of ERK1/2/HIF-1α and contributes to chemoresistance in
pancreatic cancer cells. Cancer Biol Ther. 17:188–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|