Introduction
Excessive erythropoiesis refers to an increase of
mature erythrocytes in peripheral circulation. Overburdened
hemoglobin (HB) increases blood viscosity, microcirculation
disturbance and worsens organ hypoxia, augmenting clinical risks of
multiple ischemic diseases including stroke, thrombosis (1). Excessive erythropoiesis could be
classified into idiopathic or acquired pathological symptoms for
different pathogenesis. Specifically, hypoxia has been considered
as a major stimulating factor for secondary polycythemia. It is
generally accepted that, in hypoxic environment, hypoxia-inducible
factor is upregulated and serves as an oxygen-dependent regulator
which stimulates erythropoietin production in hematopoietic organs,
enhances iron uptake/utilization, and facilitates maturation and
proliferation of erythroid progenitor, further lead to
erythropoiesis (2,3).
The syndrome with high HB (males, HB ≥21 g/dl;
females, HB ≥19 g/dl) in long living plateau residents, is called
high altitude polycythemia (HAPC) (4). As one of the typical models of
secondary erythrocytosis, HAPC model is widely employed in
scientific researches of hypoxia-induced excessive erythropoiesis,
with the performance of effective generalization and high precision
(5). In addition, epidemiological
study has shown that HAPC subjects accounted for nearly 10% of the
population residing in Tibet (6).
Although HAPC poses a great threat to these individuals' life
security and quality, no effective therapeutic methods could be
applied for these subjects except for long term oxygen therapy or
returning to plain (7). Therefore,
it is urgently needed to explore the mechanisms and potential
therapeutic biomarkers in relieving HAPC.
Metabolomics, as a promising approach in systemic
biology, offers a systematic description of low-molecular weight
molecules from samples and is typically used to monitor the
response of cells or organism to external stimuli (8). Metabolomics provides valuable methods
of scientific investigations in exploring global alterations
enrolled in the occurrence and development of diseases.
Importantly, derived from metabolic reactions of individuals
themselves, distinguishing molecules detected in metabolomics pose
greater clinical significances (9). To date, metabolomics has exhibited
enormous roles in exploring hypoxic diseases while only one study
focused on polycythemia which suggested that familial
VHLR200W homozygotes with elevated citrate and glycerol
showed a greater risk in suffering erythrocytes hyperplasia
(10). A comprehensive
understanding in metabolomics profiling of HAPC subjects is
urgently needed.
In this pilot study, the objective of our research
is to search for distinguishing molecules and related pathways in
the recovery phase of HAPC subjects. Hence, we conducted a
non-targeted UPLC-QTOF/MS method on serum samples from HAPC and
control individuals and described dynamic alterations of these
metabolites during re-oxygenation. To eliminate the influence of
environment and living habits, we introduced heterogeneous samples
from two different locations and employed Venn figures to search
for distinguishing molecules of HAPC. Additionally, we attempted to
explore if these potential biomarkers are involved in the
progression of excessive erythropoiesis. Our findings may
contribute to a better understanding of mechanisms and potential
therapeutic strategies for hypoxia-induced excessive
erythrocytosis.
Materials and methods
Subjects and experimental
procedures
A total of twenty-seven subjects were recruited in
this study, including 14 subjects from the location of 5300 m
(represented by letter K) and 13 residents from the area of 5170 m
(represented by letter T), respectively, which share a close range
of latitude, identical geographical and climatic environment. The
inclusion and exclusion criteria were as follows: i) age ≥18 years;
ii) at least 12-month experience in their respective residences;
iii) participants from plains; iv) none exposure history to high
altitude before; v) none medical intervention for excessive
erythropoiesis during recovery period; vi) controls were defined as
subjects with HB level <21 g/dl; vii) individuals with HB
reaching 21 g/dl were included in experimental group. None
statistical differences could be observed for age, oxygen
saturation, oxygen saturation, heart rate, diastolic blood
pressure, and systolic blood pressure, among subjects from these
two locations. Written informed consent was obtained from all
volunteers. The present study received ethical approval from the
medical ethical committee of the Third Military Medical University.
The entire experiment was complied with the principles of the
Declaration of Helsinki.
Plasma sample preparation
Enrolled participants shared identical diet and
activity arrangements during the whole experiment in these
separating locations. Plasmas samples were obtained at the
beginning of this trial on the 1st day assigned and 180th day after
their arriving at the plain. Sampling strategies and preparation
methods are identical to our previous report (11). Briefly, morning fasting venous
blood (100 µl) was collected with EDTA as an anticoagulant, and
centrifuged to separate serum at 14,000 × g for 15 min at 4°C.
After the process of fast frozen, these plasma samples were
delivered to Chongqing for further metabolomics analysis in the
courier filled with dry ice. Processing procedure in this study was
totally in accordance to the manufacturers' protocols of all
devices.
Metabolome analysis by
UPLC-QTOFMS
The experimental procedures of UPLC-QTOFMS were
identical to our published report with minor modifications
(11). Briefly, metabolomics
analysis was conducted on an Agilent 1290 Infinity LC system
(Agilent, Santa Clara, CA, USA). Chromatographic separations were
performed on an ACQUITY UHPLC HSS T3 C18 column (2.1×100 mm, 1.8
µm; Waters, Milford, Ireland) at 45°C. The electrospray ionization
source interface was also performed in the optimized conditions
after the exploration in our prior studies, and to monitor as many
ions as possible in both positive and negative modes.
Metabolomics data preprocessing
Data preprocessing and metabolomics analysis
followed our previously published paper with minor modifications
(11). Raw LC-MS data were
converted to mzData formats via Agilent MassHunter Qualitative
software. The program XCMS (version 1.40.0) was used to preprocess
the raw data, including peak detection, peak matching, matched
filtration, and nonlinear alignment of data, with the default
parameters (fwhm, 10; bw, 10; and snthresh, 5). The internal
standards were removed after the employment of these molecules in
data quality control (reproducibility) and data normalization. The
variables that did not present in at least 80% of groups were
filtered. The resulting matrix consisting of retention time,
mass-to-charge ratio (m/z) and normalized ion intensities, were
introduced to the subsequent analysis.
Data processing and analysis
The preprocessed data matrix was exported to Matlab
(MathWorks, Natick, MA) and SIMCA-P 13.0 (Umetrics Inc., Kinnelon,
NJ) software for further analysis. Partial squares discriminant
analysis (OPLS-DA) and principal component analysis (PCA) were
carried out to visualize separation of pre- and post-re-oxygenation
in metabolic profiling. Variable importance project (VIP) revealing
discriminatory metabolites which enrolled in the classification
effects of re-oxygenation intervention was calculated in this
study. In addition, a paired t-test and fold-change value (FC) were
executed to discovery metabolic features. To improve the
statistical robustness, Bonferroni correction for multiple testing
with an adjusted P-value were also performed. The screening
criteria of VIP >1, q <0.05 and fold-change value increased
or decreased metabolites that changed by 1.5-fold were considered
as most responsible molecules, which were applied for further
analysis in their potential implications in the HAPC. Experimental
values in this study were carried out as mean ± SD. Generally,
P-value less than 0.05 was regarded as significant statistically,
unless noted otherwise.
Metabolites identification and
metabolic pathway analysis
Accurate mass measurements were subjected to
database searching in the public databases METLIN (12) and HMDB (13), and subsequently matched with
commercially available standard metabolites. Pathway analysis
containing enrichment analysis and pathway topological analysis was
established based on MetaboAnalyst platform (http://www.metaboanalyst.ca) (14).
Results
Characteristics of enrolled
participants
HB levels from clinical routine blood test divided
participants into four groups that HAPC group in location K
contains 4 participants and HAPC group of location T contains 7
subjects, while others with HB levels below 21 g/dl were enrolled
in control groups of separating locations. Their recover conditions
after reaching plain for 180 day were also measured by blood
routine examination. Detailed description of HB in HAPC and control
groups from these two separate places are presented in Fig. 1. HB levels differed markedly in
HAPC and control groups of different locations, namely HAPC of
location K, HAPC of location T, control group of location K,
control group of location T, before and after re-oxygenation,
separately. All recruitments showed ideal recovery status with
significant decreasing HB in plain environment (P-value
<0.05).
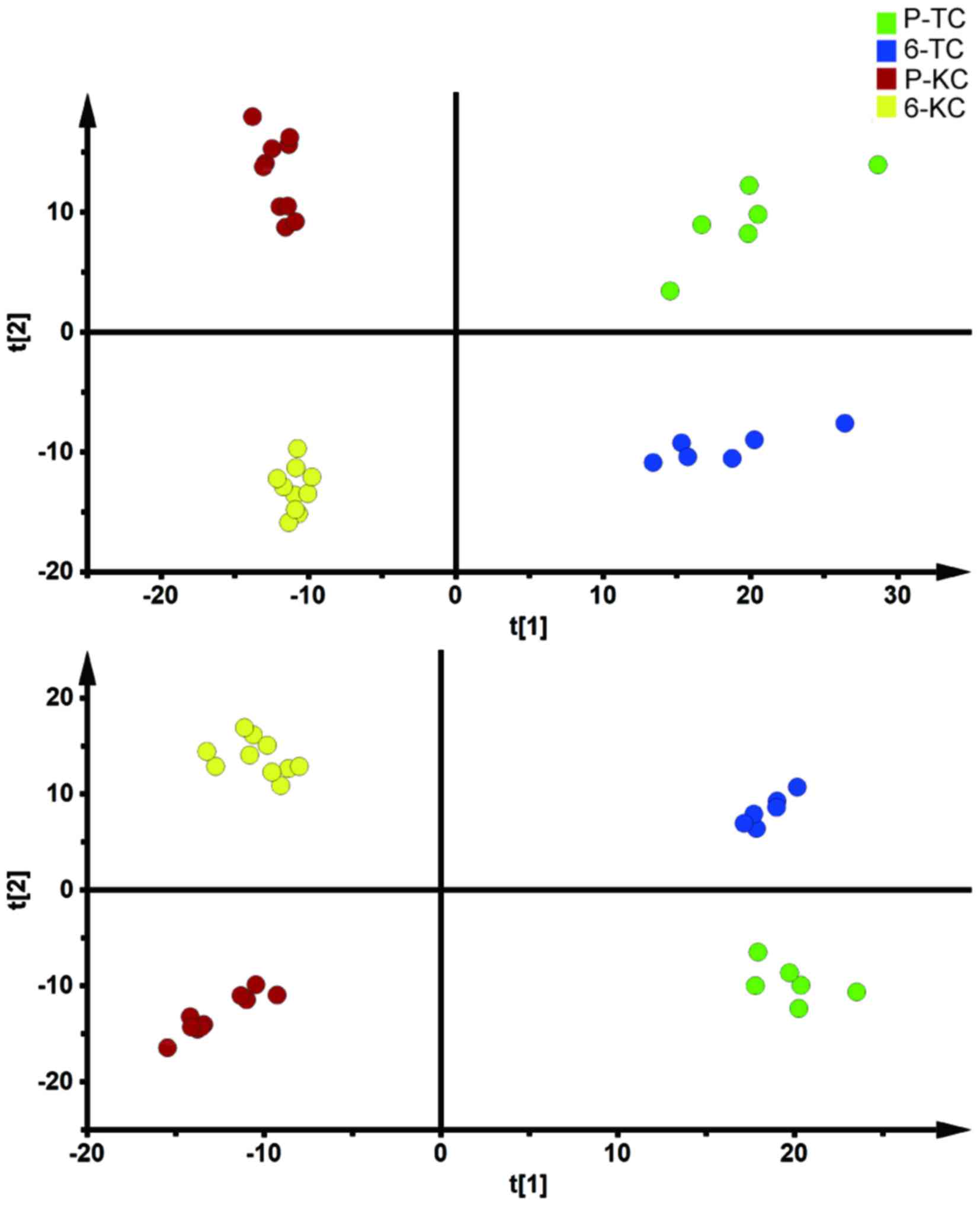
Serum metabolomics alterations in HAPC
groups
For global metabolomic profiling in HAPC group,
multivariate statistical analysis was performed to classify
metabolic phenotypes and identify differentiating metabolites. As
shown in the Fig. 2, a clear
separation was achieved on PCA and OPLSDA scores plot based on
spectral data in HAPC subjects in separating locations before and
after restoring HB value to significantly lower level. Detailed
information about multivariate model is listed in Table I.
 | Table I.Summary of parameters for OPLS-DA and
PCA models. |
Table I.
Summary of parameters for OPLS-DA and
PCA models.
| Model name | N | R2X | R2Y | Q2 |
|---|
| OPLSDA |
|
|
|
|
|
KC-pos | 20 | 0.578 | 0.99 | 0.974 |
|
KC-neg | 20 | 0.622 | 0.991 | 0.98 |
|
TC-pos | 12 | 0.687 | 0.983 | 0.959 |
|
TC-neg | 12 | 0.516 | 0.994 | 0.972 |
|
KH-pos | 8 | 0.611 | 0.997 | 0.968 |
|
KH-neg | 8 | 0.782 | 0.998 | 0.978 |
|
TH-pos | 14 | 0.559 | 0.992 | 0.976 |
|
TH-neg | 14 | 0.651 | 0.995 | 0.986 |
| PCA |
|
|
|
|
|
KC-pos | 20 | 0.688 |
| 0.473 |
|
KC-neg | 20 | 0.625 |
| 0.498 |
|
TC-pos | 12 | 0.71 |
| 0.484 |
|
TC-neg | 12 | 0.683 |
| 0.49 |
|
KH-pos | 8 | 0.759 |
| 0.242 |
|
KH-neg | 8 | 0.702 |
| 0.427 |
|
TH-pos | 14 | 0.627 |
| 0.419 |
Based on previous OPLS-DA models, variable
importance in project (VIP) was extracted to facilitate the
classification of re-oxygenation intervention. A series of methods
including paired t-test, FDR and FC, were also performed to
validate the significance of these discriminated variables.
Physiological alterations after
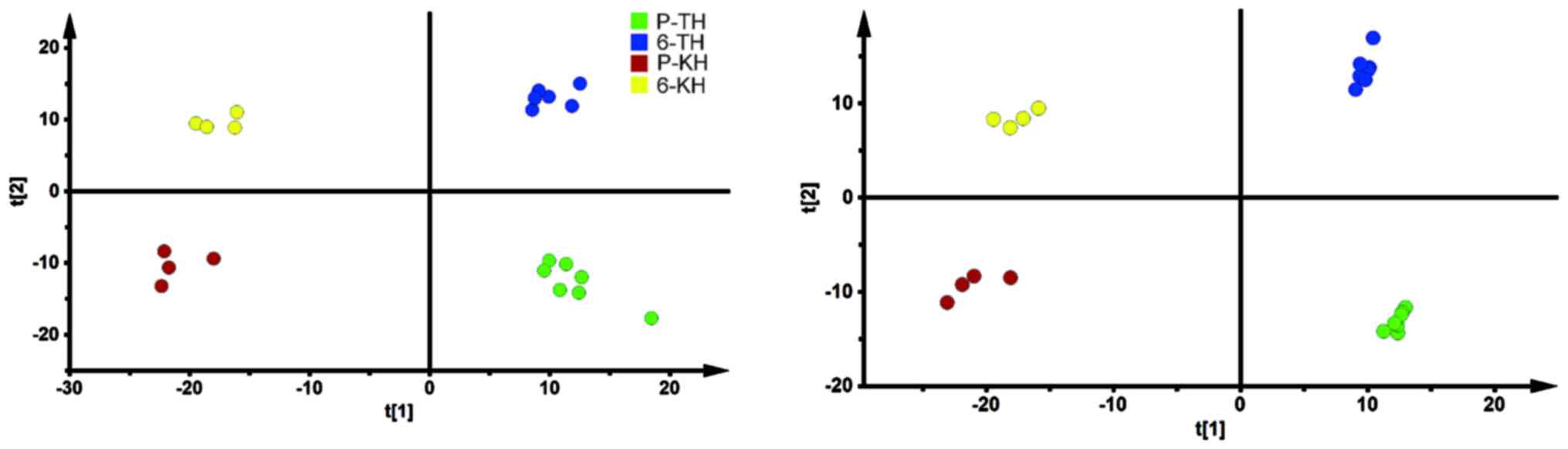
re-oxygenation in metabolic profiles
To exclude influence of re-oxygenation on
physiological alterations and gain a better understanding of
mechanisms after re-oxygenation, the metabolic profiling in control
groups from two locations were assessed separately. Fig. 3 illustrated the PCA and OPLSDA
score plots for subjects in control groups based on spectral data
of UPLC-QTOFMS. Detailed information about multivariate model is
also listed in Table I. All these
score plots showed clearly separation before and after the exposure
to re-oxygenation.
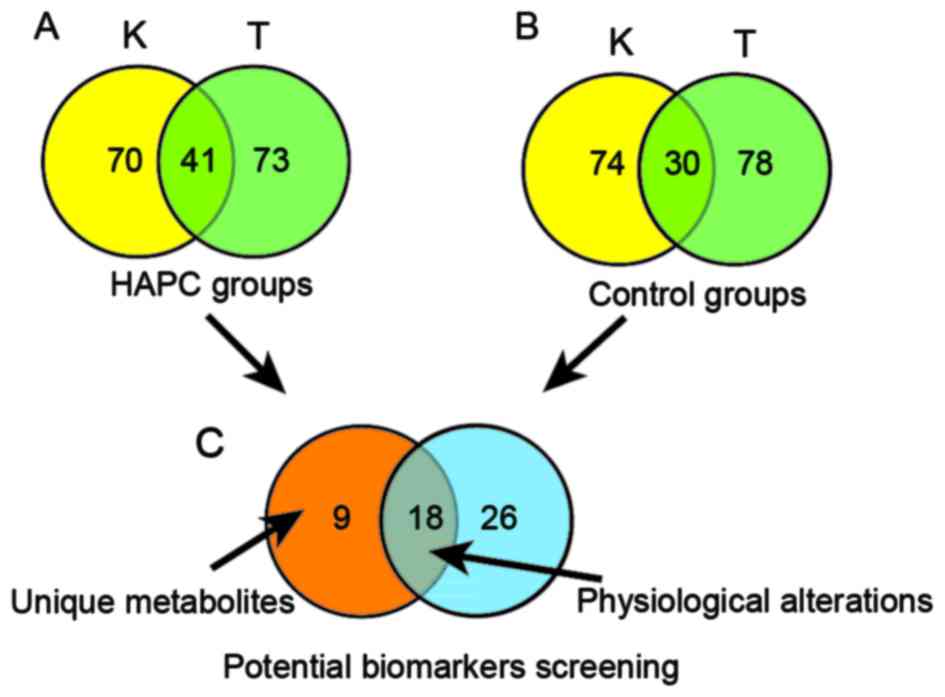
Potential biomarkers screening
To further narrow down the impacts of environmental
factors and raise the accuracy of our selection, a Venn diagram was
executed to focus on the overlapping parts of differential
molecules which were shared by two separating locations and
possessed identical variation tendency. Metabolites shared by HAPC
subjects from two separate locations were exhibited in Fig. 4A. Accordingly, identified in both
control groups of two locations, the most perturbed molecules with
identical alteration trends in physiological alterations were
identified in Fig. 4B.
A significant goal of our study was to screen serum
metabolites which contribute to significant HB reduction for HAPC
subjects. Such biomarkers should meet the criteria of
discriminating HAPC subjects before and after the intervention of
re-oxygenation with none effects on normal physiological
alterations. As shown in Fig. 4C,
9 metabolites in the red part of Fig.
4 varied uniquely in HAPC groups from both locations were
identified. The detailed information of these biomarkers is listed
in Table II. In addition,
physiological changes during re-oxygenation were also showed
Fig. 4C. The detailed information
of these molecules is listed in Table III. The information of
identifying these metabolites from the UPLC-QTOF/MS instrument is
listed in Table IV.
 | Table II.The uniquely significant metabolites
of HAPC subjects in the recovery phases. |
Table II.
The uniquely significant metabolites
of HAPC subjects in the recovery phases.
| Molecules | P-value | Q-value | FC | VIP | P-value | Q-value | FC | VIP |
|---|
| ESI+ |
|
|
|
|
|
|
|
|
|
Phosphoribosyl
pyrophosphate | 0.0065 | 0.0039 | 2.0029 | 1.2778 | 0.0002 | 0.00010 | 1.9605 | 1.2735 |
|
L-Tryptophan | 0.0006 | 0.0014 | 0.3693 | 1.3128 | 2.50E-05 | 2.30E-0 | 0.3586 | 1.4052 |
| Glucose
6-phosphate | 0.0051 | 0.0032 | 2.5161 | 1.2682 | 2.22E-06 | 4.40E-0 | 2.4762 | 1.3070 |
|
3,4-Dihydroxyphenylglycol
O-sulfate | 0.0073 | 0.0041 | 2.0014 | 1.2983 | 3.85E-05 | 2.99E-0 | 1.7201 | 1.3754 |
|
LysoPE(0:0/22:2) | 0.0171 | 0.0072 | 2.0335 | 1.2243 | 6.25E-06 | 8.83E-0 | 1.6479 | 1.3319 |
| ESI- |
|
|
|
|
|
|
|
|
|
Uracil | 0.0031 | 0.0024 | 1.5045 | 1.2163 | 2.24E-05 | 2.16E-0 | 4.2566 | 1.4169 |
|
Deoxyribose 5-phosphate | 0.0028 | 0.0024 | 0.2604 | 1.2912 | 4.18E-05 | 3.22E-0 | 0.5764 | 1.3991 |
|
All-trans-retinoic acid | 0.0037 | 0.0027 | 0.0173 | 1.3286 | 3.51E-05 | 2.80E-0 | 0.3724 | 1.3148 |
| LysoPE
(0:0/22:6) | 0.0005 | 0.0014 | 0.4857 | 1.3031 | 0.001220 | 0.00047 | 0.5858 | 1.2854 |
 | Table III.The distinguishing metabolites
enrolled in physiological changes of all subjects during
re-oxygenation. |
Table III.
The distinguishing metabolites
enrolled in physiological changes of all subjects during
re-oxygenation.
| Molecules | P-value | Q-value | FC | VIP | P-value | Q-value | FC | VIP |
|---|
| ESI+ |
|
|
|
|
|
|
|
|
|
Indole | 0.0058 | 0.0029 | 0.6505 | 1.2301 | 5.04E-05 | 3.53E-05 | 0.5517 | 1.2358 |
|
Succinic acid | 0.0003 | 0.0003 | 0.4928 | 1.4292 | 4.28E-07 | 7.52E-07 | 0.4487 | 1.4187 |
|
Hypoxanthine | 0.0003 | 0.0003 | 0.4154 | 1.4173 | 1.07E-08 | 4.29E-08 | 0.4034 | 1.4678 |
|
Phenylpyruvic acid | 0.0003 | 0.0003 | 0.4241 | 1.4370 | 1.85E-08 | 6.60E-08 | 0.3611 | 1.4684 |
|
L-Phenylalanine | 0.0000 | 0.0000 | 7.3158 | 1.5196 | 1.37E-07 | 3.01E-07 | 8.4551 | 1.4653 |
|
3-Phosphoglyceric acid | 0.0000 | 0.0000 | 3.5927 | 1.4906 | 6.90E-11 | 1.15E-09 | 3.0244 | 1.5277 |
|
N-Acetyl-D-glucosamine | 0.0000 | 0.0000 | 0.0891 | 1.4888 | 4.65E-08 | 1.23E-07 | 0.1553 | 1.4811 |
| DHAP
(18:0) | 0.0000 | 0.0001 | 0.2641 | 1.4747 | 3.44E-07 | 6.50E-07 | 0.2308 | 1.4586 |
| PA
(20:4 (5Z,8Z,11Z,14Z)) | 0.0000 | 0.0001 | 3.8687 | 1.4721 | 1.86E-05 | 1.57E-05 | 2.7362 | 1.3258 |
| LysoPC
(P-18:1 (9Z)) | 0.0019 | 0.0013 | 0.2447 | 1.2866 | 1.18E-05 | 1.10E-05 | 0.1747 | 1.3233 |
| LysoPC
(P-18:0) | 0.0016 | 0.0011 | 0.3878 | 1.2779 | 5.53E-08 | 1.41E-07 | 0.2538 | 1.4688 |
|
Tetrahydroaldosterone-3-glucuronide | 0.0022 | 0.0015 | 3.8443 | 1.2379 | 3.53E-06 | 4.31E-06 | 3.7355 | 1.3823 |
|
Bilirubin | 0.0005 | 0.0005 | 0.2375 | 1.2987 | 2.76E-06 | 3.55E-06 | 0.1069 | 1.4108 |
| ESI- |
|
|
|
|
|
|
|
|
|
3-Sulfinylpyruvic acid | 0.0000 | 0.0000 | 0.3117 | 1.4857 | 2.74E-05 | 2.11E-05 | 0.4880 | 1.3071 |
|
Tryptamine | 0.0001 | 0.0001 | 0.1339 | 1.4537 | 2.38E-09 | 1.73E-08 | 0.5236 | 1.5287 |
|
L-Dopa | 0.0006 | 0.0005 | 0.5062 | 1.3724 | 6.35E-05 | 4.28E-05 | 0.6258 | 1.2421 |
|
2,3-Diacetoxypropyl
stearate | 0.0000 | 0.0000 | 0.0684 | 1.4906 | 2.53E-06 | 3.39E-06 | 0.3996 | 1.3838 |
|
Ganglioside GA1
(d18:1/9Z-18:1) | 0.0000 | 0.0001 | 0.3729 | 1.3886 | 1.81E-09 | 1.46E-08 | 0.2611 | 1.5264 |
 | Table IV.The information of identifying these
metabolites from the UPLC-QTOF/MS instrument. |
Table IV.
The information of identifying these
metabolites from the UPLC-QTOF/MS instrument.
| Number | Metabolite | tr/min | m/z |
|---|
| ESI+ |
|
|
|
| 1 | Indole | 118.0650 | 5.88417 |
| 2 | Succinic acid | 119.0730 | 5.885 |
| 3 | Hypoxanthine | 137.0150 | 5.88483 |
| 4 | Phenylpyruvic
acid | 165.0100 | 5.885 |
| 5 | Glucose
6-phosphate | 165.0100 | 0.733 |
| 6 |
L-Phenylalanine | 166.0270 | 0.920583 |
| 7 |
3,4-Dihydroxyphenylglycol O-sulfate | 166.0270 | 0.684 |
| 8 | 3-Phosphoglyceric
acid | 187.0700 | 0.854583 |
| 9 | LysoPE
(0:0/22:2) | 187.0700 | 15.6952 |
| 10 | L-Tryptophan | 205.0971 | 5.86033 |
| 11 |
N-Acetyl-D-glucosamine | 222.0290 | 5.8955 |
| 12 | Phosphoribosyl
pyrophosphate | 390.9591 | 0.608417 |
| 13 | DHAP(18:0) | 437.1940 | 14.1175 |
| 14 | PA (20:4 (5Z, 8Z,
11Z, 14Z)) | 487.3570 | 18.3125 |
| 15 | LysoPC (P-18:1
(9Z)) | 506.3590 | 16.5833 |
| 16 | LysoPC
(P-18:0) | 508.3760 | 16.5149 |
| 17 |
Tetrahydroaldosterone-3-glucuronide | 541.3290 | 16.145 |
| 18 | Bilirubin | 585.2710 | 11.2249 |
| ESI- |
|
|
|
| 19 | 3-Sulfinylpyruvic
acid | 149.9950 | 21.9133 |
| 20 | Tryptamine | 159.1140 | 0.796111 |
| 21 | L-Dopa | 196.0220 | 1.34443 |
| 22 | All-trans-retinoic
acid | 299.2016 | 20.4552 |
| 23 | Uracil | 437.1940 | 1.29277 |
| 24 | 2,3-Diacetoxypropyl
stearate | 441.2370 | 14.0312 |
| 25 | Deoxyribose
5-phosphate | 506.3590 | 9.88136 |
| 26 | LysoPE
(0:0/22:6) | 541.3290 | 16.1388 |
| 27 | Ganglioside GA1
(d18:1/9Z-18:1) | 625.3080 | 15.3796 |
Metabolic pathways analysis and
affected networks identification
Metabolic pathway analysis was performed to reveal
alterations of metabolites, which played a common role in the
hosts' response to re-oxygenation. The most disturbed biochemical
pathways involved in normal physiological alterations after
re-oxygenation is phenylalanine metabolism (Fig. 5A). In addition, metabolites set
enrichment analysis on uniquely distinguishing molecules in the
recovery phase of HAPC groups of two locations revealed that
pentose phosphate pathway is the most perturbed pathways with
P<0.05 (Fig. 5B).
Validation of findings by comparing
HAPC and Control groups
To explore if our findings also contributed to the
progress of hypoxia-induced erythrocytosis, we comparatively
studied expression patterns of crucial molecules in pentose
phosphate pathway in HAPC and control groups in location T and K,
separately. Further exaggerating variances of these metabolites,
subjects with HB level lower than 20 g/dl and those with HB larger
than 21 g/dl were enrolled in the comparison. As shown in Table V, molecules of 6-phosphogluconic
acid, phosphoenolpyruvic acid, glucose 6-phosphate, phosphoribosyl
pyrophosphate which are the essential participants of pentose
phosphate pathway were elevated in control groups comparing to HAPC
groups. All molecules showed significant alterations at least in
one location. Small samples size may prevent part of differential
metabolites from being apparent.
 | Table V.The essential molecules in pentose
phosphate pathway in HAPC and control groups at high altitude. |
Table V.
The essential molecules in pentose
phosphate pathway in HAPC and control groups at high altitude.
| Metabolites | K_FCa | T_FCb |
|---|
| 6-Phosphogluconic
acid | 1.03 | 1.26c |
| Phosphoenolpyruvic
acid | 1.34c | 1.182 |
| Glucose
6-phosphate | 1.41c | 1.57c |
| Phosphoribosyl
pyrophosphate | 1.152c | 1.02 |
Discussion
Hypoxia-induced erythrocytosis is a severe
complication caused by chronic hypoxemia, which adversely
aggravates organ hypoxia and damage. Without any solid findings of
pre-clinical researches to facilitate recovery of this syndrome,
none pathophysiologic driven therapeutic strategies are available
currently, except for the strategies of re-oxygenation. Therefore,
we conducted metabolomics study based on HAPC model with the aim of
unraveling mechanisms involved in the natural recovery phase of
decreasing HB in plain environment, and searching for potential
therapeutic biomarkers to alleviate erythrocytosis symptom.
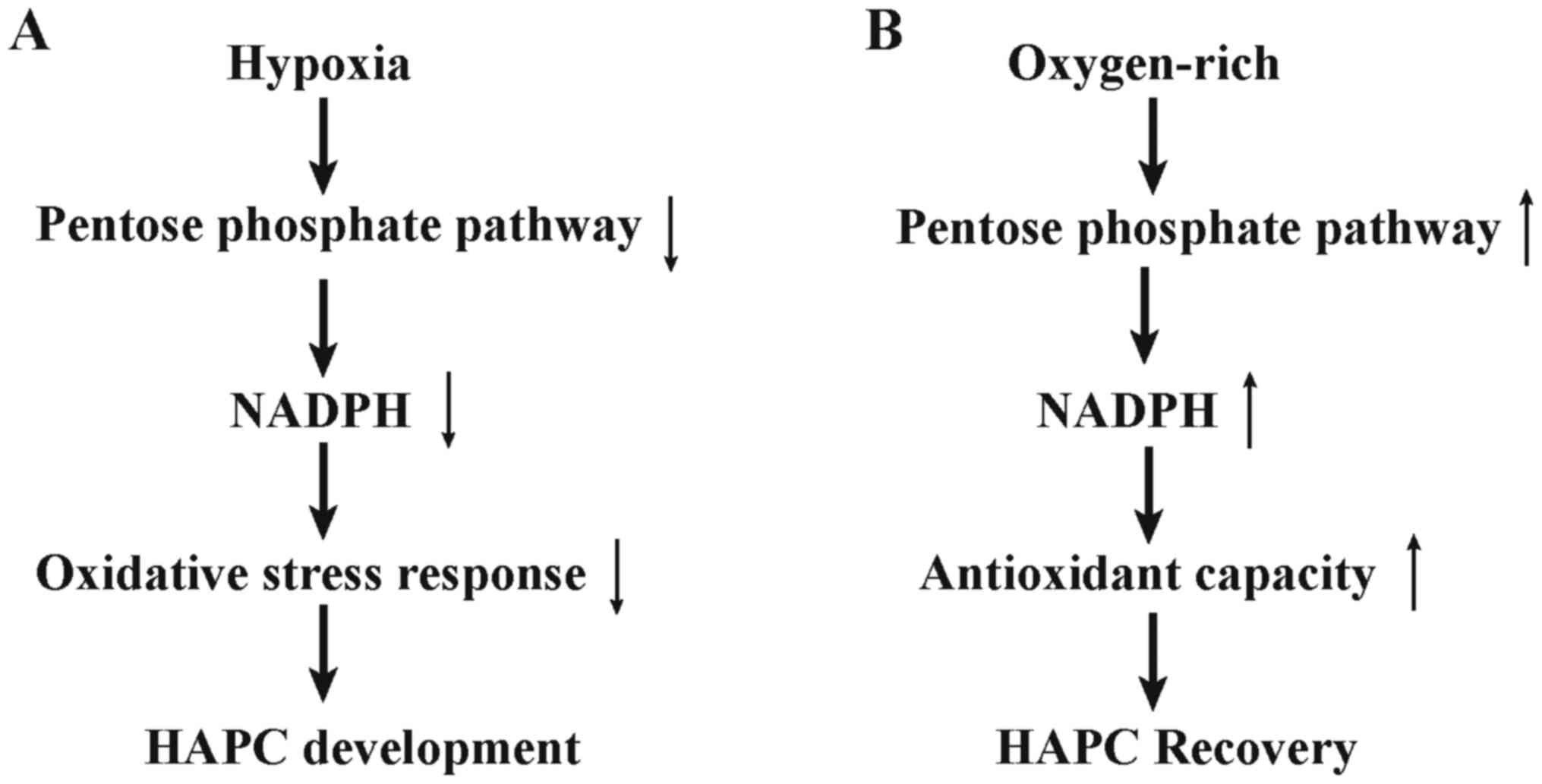
Pentose phosphate pathway (PPP), acted as hexose
monophosphate shunt, forms one of the main antioxidant cellular
defense systems for individuals, and acts as a major provider of
ribose phosphate to regulate cell redox rebalance and proliferative
fate (15). As listed in Fig. 6, our results revealed upregulated
PPP in recovery processes of HAPC. Increased PPP could reduce NADP
to NADPH, which plays an important role in regulating glutathione
(GSH) and further protects against reactive oxygen species (ROS),
repairs oxidized proteins of essential participants in carrying
oxygen in RBC, promotes re-balance between pro-oxidants and
antioxidants (16,17). Further, we validated our findings
by comparing HAPC and control group directly and detected
decreasing pentose phosphate pathway in HAPC subjects, which
implied that PPP may be involved in the progression of
hypoxia-induced erythrocytosis. D'Alessandro et al has
demonstrated that hypoxia blocks metabolic diversion towards the
PPP in RBC cells (18). However,
hypoxia could result in mitochondria and endoplasmic reticulum
dysfunction, over-activate NADPH oxidase, xanthine oxidase, and
uncoupling nitric oxide synthase, which form oxidative stress
responses (19–21). Thus, decreasing PPP impaired the
capacities of RBC to cope with oxidative stress which lead to
protein oxidation, lipid peroxidation, and DNA oxidation, further
decreases ability in carrying oxygen in red blood cells and
pathological hyperplasia of erythrocytes. In addition, decreasing
PPP poses negative effects on GSH homeostasis, which constitutes
another essential antioxidant system, to aggravate oxidative damage
for cells (21). In summary,
increasing PPP pathway may be a potential therapeutic target to
halt the progression and promote the recovery of hypoxia-induced
erythrocytosis.
In this study, we also identified disturbed
phenylalanine metabolism in physiological changes during the
process of re-oxygenation in all groups. Originated from exogenous
supply via food, phenylalanine is an essential amino acid, which
human body is unable to synthetize. The significantly elevated
phenylalanine level observed in our enrolled subjects, was far from
diagnostic standards of Phenylketonuria, and none symptoms of
Phenylketonuria could be detected in these participants in our
study. Tyrosine and phenylpyruvic acid are the major downstream
metabolites of phenylalanine and maintain homeostasis in
individuals (22). However, none
identical alterations in serum tyrosine was observed in our study
comparing to that of phenylalanine while phenylpyruvic acid was
significantly impaired after the process of re-oxygenation
(23). These paradoxical phenomena
may due to increasing oxidative stress caused by re-oxygenation
with elevated ROS and RNS, which further interfere the endogenous
synthesis of antioxidants and influence metabolic enzyme activities
(24). Previous reports have
identified that the conversion from phenylalanine to tyrosine is
catalyzed by the enzyme phenylalanine hydroxylase, which could be
impaired by oxidative stress (25,26).
On the other hand, conversion from phenylalanine to phenylpyruvic
acid exhibited a NAD(H)-dependent pattern, which was impaired by
increasing oxidative stress and decreased significantly during
re-oxygention (27,28). Therefore, the metabolism of
phenylalanine to phenylpyruvic acid and tyrosine may contribute to
the accumulation of phenylalanine.
The elevated phenylalanine expression detected in
our study posed both advantages and disadvantages during
re-oxygenation process. On one hand, oxidative stress would be
promoted with the rising phenylalanine expression by increasing
endogenous synthesis of reactive species, free radicals and
interfering with the endogenous synthesis of enzymatic antioxidants
(29). On the other hand,
phenylalanine exhibits anti-hypertension and anti-cardiovascular
remodeling by decreasing intracellular [Ca2+] and cardiovascular
cells growth, which formed the basis for the unbeneficial
structural reconstruction under prolonged hypoxic exposure
(30). In addition, phenylalanine
has been demonstrated to be involved in regulating metabolic
conditions. Lin et al revealed that elevated phenylalanine
was correlating to lower level of glucose in serum, with a
significant insulinotropic effect, and contributed to the
establishment of effective energy metabolic pathways (23). Increasing phenylalanine was also
demonstrated to promote pulmonary function, with high correlation
to FVC1 (31). Generally, as a
physiologically regulated metabolite, appropriately higher
expression of phenylalanine could alleviate the structural
alterations and promote the recovery of metabolic patterns after
the long-living in chronic hypoxia environment. The effects on
oxidation-promoting may be reduced by enhancing the conversion from
phenylalanine to meta-L-tyrosine (32). Phenylalanine warrants further
studies in the process of hypoxia/re-oxygenation.
There are several limitations needed to be
reconsidered in this study. Firstly, a relative small sample size
may prevent parts of distinguishing metabolites from being
apparent. Thus, instead of comparing subjects suffering
hypoxia-induced erythrocytosis with control groups directly, we
established a self-control experiment to improve efficiency and
accuracy of our study. Secondly, the homogeneity of enrolled
participants was not prerequisite for this study and heterogeneous
population should be recruited, such as subjects in diverse sexes
and age periods. Therefore, a larger metabolomics study combining
validation experiments to verify our findings by comparing the HAPC
with controls directly is now conducting by our group.
In conclusion, this is the first study in providing
a comprehensive description of metabolic profiling in the recovery
phase of hypoxia-induced polycythemia, and tracing along for half a
year. Elevated pentose phosphate pathway may be involved in the
recovery of HAPC, while downregulated pentose phosphate pathway
could contribute to the progress of hypoxia-induced erythrocytosis.
In addition, alterations in phenylalanine metabolism participate in
the individuals' reaction to re-oxygenation.
Acknowledgements
The present study was supported by the Key Projects
in the Military Science and Technology Pillar Program during the
Thirteen 5-year Plan Period (AWS14C007), by National Natural
Science Foundation of China (J1310001).
References
|
1
|
Chen Y, Jiang C, Luo Y, Liu F and Gao Y:
Interaction of CARD14, SENP1 and VEGFA polymorphisms on
susceptibility to high altitude polycythemia in the Han Chinese
population at the Qinghai-Tibetan Plateau. Blood Cells Mol Dis.
57:13–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tashi T, Feng T, Koul P, Amaru R, Hussey
D, Lorenzo FR, RiLi G and Prchal JT: High altitude genetic
adaptation in Tibetans: No role of increased hemoglobin-oxygen
affinity. Blood Cells Mol Dis. 53:27–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al-Sheikh M, Moradkhani K, Lopez M,
Wajcman H and Préhu C: Disturbance in the HIF-1alpha pathway
associated with erythrocytosis: Further evidences brought by
frameshift and nonsense mutations in the prolyl hydroxylase domain
protein 2 (PHD2) gene. Blood Cells Mol Dis. 40:160–165. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frey H, Moreth K, Hsieh LT, Zeng-Brouwers
J, Rathkolb B, Fuchs H, Gailus-Durner V, Iozzo RV, de Angelis MH
and Schaefer L: A novel biological function of soluble biglycan:
Induction of erythropoietin production and polycythemia. Glycoconj
J. 34:393–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azad P, Zhao HW, Cabrales PJ, Ronen R,
Zhou D, Poulsen O, Appenzeller O, Hsiao YH, Bafna V and Haddad GG:
Senp1 drives hypoxia-induced polycythemia via GATA1 and Bcl-xL in
subjects with Monge's disease. J Exp Med. 213:2729–2744. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang C, Cui J, Liu F, Gao L, Luo Y, Li P,
Guan L and Gao Y: Mitochondrial DNA 10609T promotes hypoxia-induced
increase of intracellular ROS and is a risk factor of high altitude
polycythemia. PLoS One. 9:e877752014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Köhler D and Dellweg D: Polycythemia.
Dtsch Med Wochenschr. 135:2300–2303. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian H, Lam SM and Shui G: Metabolomics, a
powerful tool for agricultural research. Int J Mol Sci. 17:pii:
E18712016. View Article : Google Scholar
|
|
9
|
Lima AR, Mde L Bastos, Carvalho M and de
Pinho P Guedes: Biomarker discovery in human prostate cancer: An
update in metabolomics studies. Transl Oncol. 9:357–370. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McClain DA, Abuelgasim KA, Nouraie M,
Salomon-Andonie J, Niu X, Miasnikova G, Polyakova LA, Sergueeva A,
Okhotin DJ, Cherqaoui R, et al: Decreased serum glucose and
glycosylated hemoglobin levels in patients with Chuvash
polycythemia: A role for HIF in glucose metabolism. J Mol Med
(Berl). 91:59–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao WT, Liu B, Chen J, Cui JH, Gao YX,
Liu FY, Xu G, Sun BD, Zhang EL, Yuan ZB, et al: Metabolite
modulation in human plasma in the early phase of acclimatization to
hypobaric hypoxia. Sci Rep. 6:225892016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smith CA, O'Maille G, Want EJ, Qin C,
Trauger SA, Brandon TR, Custodio DE, Abagyan R and Siuzdak G:
METLIN: A metabolite mass spectral database. Ther Drug Monit.
27:747–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wishart DS, Jewison T, Guo AC, Wilson M,
Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, et al: HMDB
3.0-the human metabolome database in 2013. Nucleic Acids Res.
41(Database Issue): D801–D807. 2013.PubMed/NCBI
|
|
14
|
Xia J, Sinelnikov IV, Han B and Wishart
DS: MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic
Acids Res. 43(W1): W251–W257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Riganti C, Gazzano E, Polimeni M, Aldieri
E and Ghigo D: The pentose phosphate pathway: An antioxidant
defense and a crossroad in tumor cell fate. Free Radic Biol Med.
53:421–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Zwieten R, Verhoeven AJ and Roos D:
Inborn defects in the antioxidant systems of human red blood cells.
Free Radic Biol Med. 67:377–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karasawa T, Saito T, Ueno Y, Sugimoto M
and Soga T: Metabolome analysis of erythrocytes from patients with
chronic hepatitis C reveals the etiology of ribavirin-induced
hemolysis. Int J Med Sci. 10:1575–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
D'Alessandro A, Gevi F and Zolla L: Red
blood cell metabolism under prolonged anaerobic storage. Mol
Biosyst. 9:1196–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou L, Chen P, Peng Y and Ouyang R: Role
of oxidative stress in the neurocognitive dysfunction of
obstructive sleep apnea syndrome. Oxid Med Cell Longev.
2016:96268312016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim M, Han CH and Lee MY: NADPH oxidase
and the cardiovascular toxicity associated with smoking. Toxicol
Res. 30:149–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rauchová H, Vokurková M and Koudelová J:
Hypoxia-induced lipid peroxidation in the brain during postnatal
ontogenesis. Physiol Res. 61 Suppl 1:S89–S101. 2012.PubMed/NCBI
|
|
22
|
Gostner JM, Becker K, Kurz K and Fuchs D:
Disturbed amino acid metabolism in HIV: Association with
neuropsychiatric symptoms. Front Psychiatry. 6:972015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin X, Zhao L, Tang S, Zhou Q, Lin Q, Li
X, Zheng H and Gao H: Metabolic effects of basic fibroblast growth
factor in streptozotocin-induced diabetic rats: A 1H NMR-based
metabolomics investigation. Sci Rep. 6:364742016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stevens JP, Churchill T, Fokkelman K,
Haase E, Idikio H, Korbutt G, Bigam DL and Cheung PY: Oxidative
stress and matrix metalloproteinase-9 activity in the liver after
hypoxia and reoxygenation with 21% or 100% oxygen in newborn
piglets. Eur J Pharmacol. 580:385–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuchs JE, Huber RG, von Grafenstein S,
Wallnoefer HG, Spitzer GM, Fuchs D and Liedl KR: Dynamic regulation
of phenylalanine hydroxylase by simulated redox manipulation. PLoS
One. 7:e530052012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang W and Fang BS: Construction and
evaluation of a novel bifunctional phenylalanine-formate
dehydrogenase fusion protein for bienzyme system with cofactor
regeneration. J Ind Microbiol Biotechnol. 43:577–584. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vilaseca MA, Farré C and Ramón F:
Phenylalanine determined in plasma with use of phenylalanine
dehydrogenase and a centrifugal analyzer. Clin Chem. 39:129–131.
1993.PubMed/NCBI
|
|
29
|
Rocha JC and Martins MJ: Oxidative stress
in phenylketonuria: Future directions. J Inherit Metab Dis.
35:381–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao G, Li Z and Gu T: Antihypertension
and anti-cardiovascular remodeling by phenylalanine in
spontaneously hypertensive rats: Effectiveness and mechanisms. Chin
Med J (Engl). 114:270–274. 2001.PubMed/NCBI
|
|
31
|
Førli L, Pedersen JI, Bjørtuft, Vatn M,
Kofstad J and Boe J: Serum amino acids in relation to nutritional
status, lung function and energy intake in patients with advanced
pulmonary disease. Respir Med. 94:868–874. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Molnár GA, Kun S, Sélley E, Kertész M,
Szélig L, Csontos C, Böddi K, Bogár L, Miseta A and Wittmann I:
Role of tyrosine isomers in acute and chronic diseases leading to
oxidative stress-A Review. Curr Med Chem. 23:667–685. 2016.
View Article : Google Scholar : PubMed/NCBI
|