Introduction
The term reactive oxygen species (ROS) refers to all
chemically reactive molecules containing an oxygen atom, including
oxygen radicals and non-radicals. The various types of ROS are
classified as either endogenous mitochondrial factors or
extracellular factors (1).
Hydrogen peroxide (H2O2) is a reactive
byproduct of the electron transport chain in mitochondria (1).
The skin is the largest organ of the body; it
surrounds the body and protects it from the external environment.
Extrinsic stimuli, including ultraviolet (UV) light, pollution and
thermal stress, disrupt skin cell metabolism, thereby disrupting
redox state equilibrium (2).
Although cells employ efficient enzymatic and nonenzymatic
antioxidant mechanisms, excessive ROS production can induce lipid
peroxidation, DNA damage and protein denaturation (1). Proteins associated with conventional
enzyme-mediated antioxidant mechanisms include superoxide dismutase
(SOD), glutathione peroxidase and catalase (CAT) (1). These enzymes are primarily activated
in response to ROS. In H2O2-exposed normal
human dermal fibroblasts (NHDFs), excessive ROS production disrupts
antioxidant defense mechanisms and induces the inflammatory
response (1,3).
In its inactive form in the cytoplasm, nuclear
factor erythroid-derived 2-like 2 (NRF2) interacts with
Kelch-like-ECH-associated protein 1 (4). A disruption in this interaction by
factors, including ROS, activates the NRF2 signaling pathway
(4). Activated NRF2 translocates
to the nucleus where it binds to antioxidant response element
(ARE), and initiates the transcription of genes associated with the
response to oxidative stress, including heme oxygenase-1
(HO-1), SOD and CAT (5–7).
The mammalian Sir2 ortholog, sirtuin 1 (SIRT1), is a
histone deacetylase that targets histones and nonhistone
substrates, including nuclear factor (NF)-κB and p53 (8,9).
Previous studies have demonstrated that SIRT1 is associated with
the regulation of numerous cellular processes, including
inflammation, apoptosis and autophagy (10–12).
Under conditions of adaptive stress, including hypoxia, infection
and caloric restriction, SIRT1 inhibits the proinflammatory
cytokine NF-κB (13). NF-κB serves
a dominant role in inducing the inflammatory response (14,15).
In response to excessive cellular stress, activated NF-κB directly
activates the expression of genes encoding key inflammatory
cytokines, including interleukin (IL)-6 and tumor necrosis factor
(TNF)-α (16).
The use of the plant secondary metabolite rosmarinic
acid (RA) has been studied in pharmaceutical and dietary
supplements in Alzheimer's disease, atopic dermatitis and
cardiovascular disease (17–21).
Previous research has revealed that the effects of RA in these
contexts are mediated by its antioxidant properties. One report
demonstrated that the antioxidant activity of RA is able to inhibit
UV-induced damage in the skin of mice (22). In addition, RA may protect human
melanoma cells from H2O2-induced oxidative
stress (23). Other reports have
also demonstrated that RA protects human keratinocyte HaCaT cells
from UVB-induced oxidative stress (24,25).
However, the effects of RA on NHDFs, which are the key mediators of
skin firmness and elasticity, remain unclear. The present study
aimed to investigate the effects of RA on
H2O2-exposed NHDFs.
Materials and methods
Cell culture and treatment
NHDFs (Lonza Group, Ltd., Basel, Switzerland) and
the NF-κB Luciferase Reporter NIH3T3 Stable Cell Line (Signosis,
Inc., Santa Clara, CA, USA) were cultured in Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and 1% penicillin/streptomycin (Thermo
Fisher Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2. H2O2 (Sigma-Aldrich; Merck
KGaA) was diluted in phosphate-buffered saline (PBS) to obtain a 1
M stock solution. For the H2O2 treatment
experiments, the H2O2 stock solution was
diluted in cell culture media at the indicated concentrations. RA
(Sigma-Aldrich; Merck KGaA) was dissolved in dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA).
Cell viability assay
Cytotoxicity was evaluated using a water-soluble
tetrazolium salt (WST-1) assay (EZ-Cytox Cell Viability Assay kit;
Itsbio, Seoul, Korea). NHDFs were seeded on 96-well culture plates
at a density of 3×103 cells and were incubated for 24 h,
at 37°C. Subsequently, the cells were incubated with RA (0–50 µM)
for 12 h, after which the cytotoxicity of RA was assessed. To
examine the effects of RA against
H2O2-induced cytotoxicity, NHDFs were
incubated with the indicated concentration of RA (10, 20 and 30 µM)
for 12 h prior to H2O2 treatment. After 12 h,
600 µM of H2O2 was added and incubated at
37°C for 2 h. 1/10 volume of WST-1 solution was then added to each
well, and the cells were incubated at 37°C for 1 h. Cell viability
was evaluated by measuring absorbance at 450 nm using an iMark
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
H2O2 scavenging
activity assay
The H2O2 scavenging activity
of RA was evaluated as previously described (26). A solution containing
H2O2 (40 mM in distilled water, pH 7.4) and
varying concentrations of RA (0–1 mg/ml) was incubated at room
temperature for 10 min. Scavenger activity was subsequently
assessed by measuring the absorbance of the
H2O2/RA solution at 230 nm using a UV
spectrophotometer (Shimadzu Corporation, Kyoto, Japan). A solution
lacking H2O2 was used as a control, and
L-ascorbic acid was used as the experimental control treatment. The
experiments were conducted in triplicate at each concentration of
RA. Scavenger activity was calculated using the following formula:
H2O2 scavenging activity (%) = (1 -
sample/control) × 100.
Intracellular ROS scavenging
assay
Intracellular ROS production was evaluated using a
2′7′-dichlorofluorescein diacetate (DCF-DA; Sigma-Aldrich; Merck
KGaA) staining assay. Briefly, cells were incubated with DCF-DA
solution (100 µM) at 37°C for 1 h, after RA and
H2O2 treatment. ROS levels were then analyzed
using flow cytometry. The proportion of fluorescence-positive cells
was measured using a FACSCalibur flow cytometer (BD Biosciences,
San Diego, CA, USA) with excitation and emission filters of 488 and
530 nm, respectively. N-acetyl-cysteine (NAC), ROS scavenger
used as a positive control, was purchased from Sigma-Aldrich; Merck
KGaA.
NF-κB luciferase reporter assay
The NF-κB luciferase reporter assay was conducted as
previously described (27) with
some modifications. Briefly, NF-κB reporter NIH3T3 cells were
seeded in 12-well cell culture plates at a density of
2×105 cells/well. After 24 h incubation, cells were
pretreated RA for 12 h at 10, 20 and 30 µM, and were then treated
with 600 µM H2O2 for 2 h. At the end of the
experiments, the cells were resuspended in Passive Lysis Buffer
(Promega Corporation, Madison, WI, USA). Subsequently, luciferin
(Sigma-Aldrich; Merck KGaA) was added to the cells, and luciferase
activity was measured using a Veritas luminometer (Turner Designs,
Sunnyvale, CA, USA). The luciferase activity was normalized to
β-galactosidase (β-gal) activity, and relative activity is
presented as the percentage activity of the control with standard
deviation. The results represent the mean of three independent
experiments.
Senescence-associated β-gal (SA-β-gal)
activity
β-gal expression was used as a marker of senescence
(28). β-gal expression levels
were determined using an SA-β-gal staining kit (Biovision, Inc.,
Milpitas, CA, USA) according to the manufacturer's instructions.
NHDFs were seeded at a density of 1×106 cells/well in
60-mm cell culture plates and were incubated until cell confluence
reached 90%. Later, cells were pretreated RA for 12 h at 10, 20 and
30 µM, and were then treated with 600 µM H2O2
for 2 h. After treatment, the cells were washed in PBS and
incubated in 0.5 ml fixative solution (4% formaldehyde, 0.5%
glutaraldehyde in PBS buffer, pH 7.2) for 10 min at room
temperature. The fixed cells were incubated in a staining solution
mixture [staining solution (470 µl), staining supplement (5 µl) and
20 mg/ml X-Gal in dimethylformamide (25 µl)] for 24 h at 37°C.
Subsequently, 70% glycerol (1 ml) was added to the cells and blue
SA-β-gal-positive cells were counted under a microscope (Olympus
IX51; Olympus Corporation, Tokyo, Japan).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Before RNA extraction, cells were treated with RA
(10, 20 and 30 µM) for 12 h and H2O2 (600 µM)
for 2 h, in a consecutive manner. Total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RNA purity and
concentration were evaluated using a MaestroNano®
microspectrophotometer (Maestrogen, Las Vegas, NV, USA). Total RNA
was reverse transcribed into cDNA using the oiligo
d(T)23 primer and ProtoScript First Strand cDNA
Synthesis kit (New England BioLabs, Inc., Ipswich, MA, USA)
following the manufacturer's instructions. HO-1, SOD1, CAT,
SIRT1, TNF-α and IL-6 mRNA expression levels were evaluated
using 5× HOT FIREPOL® EvaGreen® qPCR mix
(Solis BioDyne, Tartu, Estonia), a StepOnePlus Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and
gene-specific primers. Each thermo-cycling condition was same in
denaturation (95°C) and extension (72°C), except the annealing
temperature which differed when different primers were used. The Cq
value associated with each gene was normalized to the expression of
the β-actin housekeeping gene. The sequences of the qPCR primers
are provided in Table I. The
2−ΔΔCq method (29) was
used to calculate the relative expression levels of each gene.
 | Table I.Primer sequences for quantitative
polymerase chain reaction. |
Table I.
Primer sequences for quantitative
polymerase chain reaction.
| Gene name | Gene symbol | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| Superoxide
dismutase 1 | SOD1 |
GGGAGATGGCCCAACTACTG |
CCAGTTGACATGCAACCGTT |
| Catalase | CAT |
ATGGTCCATGCTCTCAAACC |
CAGGTCATCCAATAGGAAGG |
| Sirtuin 1 | SIRT1 |
GCAGGTTGCGGGAATCCAA |
GGCAAGATGCTGTTGCAAA |
| Tumor necrosis
factor-α | TNF-α |
CCCAGGGACCTCTCTCTAATC |
GGTTTGCTACAACATGGGCTACA |
| Interleukin-6 | IL-6 |
TAACAGTTCCTGCATGGGCGGC |
AGGACAGGCACAAACACGCACC |
| Actin, beta | β-actin |
GGATTCCTATGTGGGCGACGA |
CGCTCGGTGAGGATCTTCATG |
Analysis of luciferase reporter gene
activity
Transcriptional activity of NRF2 was determined
using an antioxidant ARE Reporter kit (cat. no. 60514; BPS
Bioscience, San Diego, CA, USA). The luciferase activity was
quantitatively assessed according to the manufacturer's
instructions. Briefly, NHDFs were cotransfected with
NRF2-ARE-luciferase reporter plasmid (BPS Bioscience) and a plasmid
that constitutively expressed Renilla luciferase using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
The transfection was conducted according to the manufacturer's
instructions with some modifications. The Lipofectamine reagent was
mixed with serum-free Opti-MEM (Invitrogen; Thermo Fisher
Scientific, Inc.) for 5 min, and then added to the plasmid with
gently flicking for 20 min, at room temperature. The transfection
mixture was added to the cells and incubated at 37°C. After 24 h of
transfection, cells were pretreated for 12 h with the indicated
doses of RA (10, 20 and 30 µM), and were then exposed to 600 µM
H2O2 for 2 h. The cells were re-suspended in
Passive lysis buffer (Promega Corporation) and luciferase activity
was measured using a Dual-Luciferase Reporter Assay system (Promega
Corporation). The luciferase activity of ARE was normalized to
Renilla luciferase activity.
Statistical analysis
All results are presented as the mean ± standard
deviation of three independent experiments. Statistical analysis
was performed using SPSS software version 17.0 (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance followed by Tukey
post hoc test was conducted for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
RA reduces
H2O2-induced cytotoxicity in NHDFs
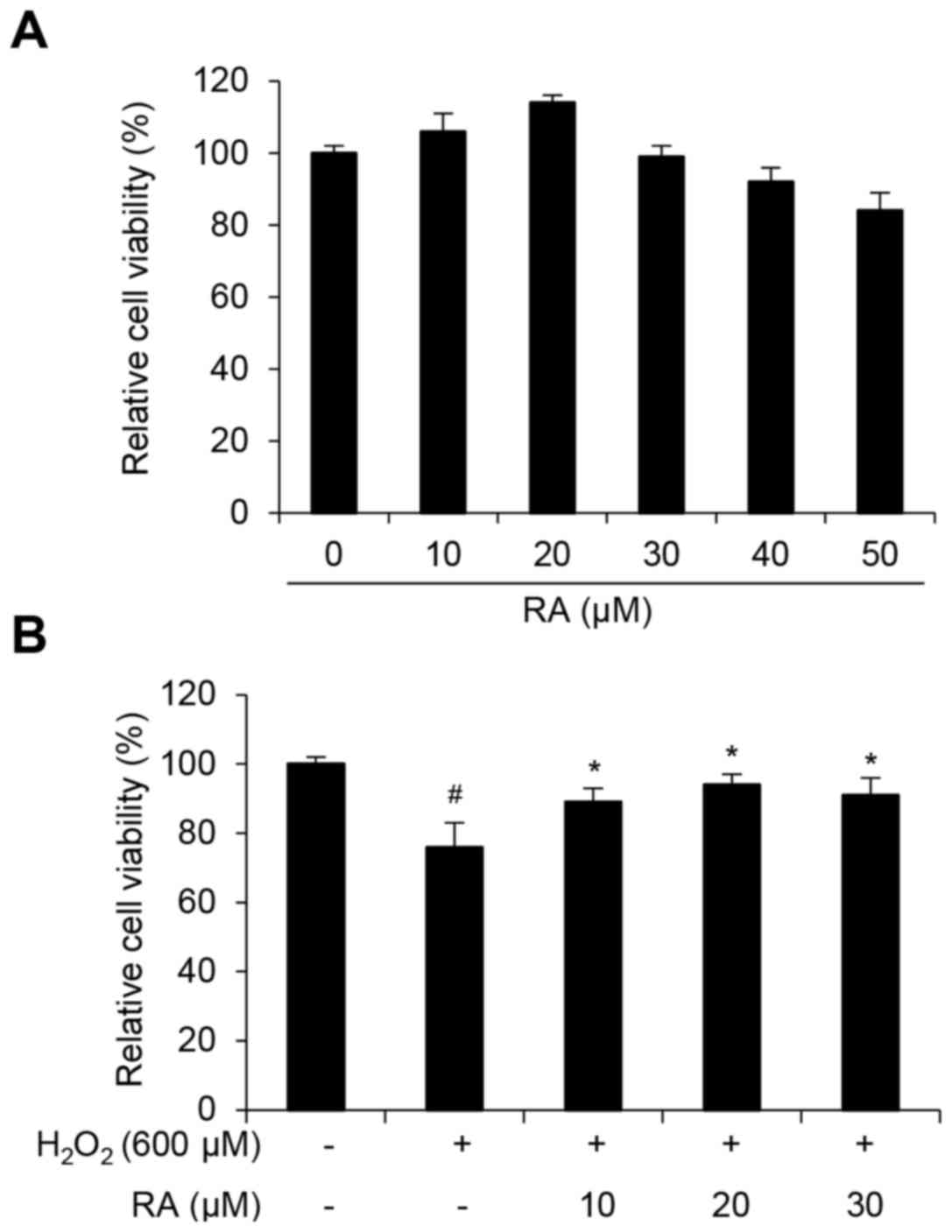
The present study initially determined the dose
range of RA that was not markedly cytotoxic to NHDFs. The cells
were treated with increasing concentrations of RA (0–50 µM) for 12
h. Concentrations of RA <50 µM were not were not significantly
cytotoxic; therefore, 10–30 µM RA was used for subsequent
experiments. To determine if RA affects the viability of NHDFs
exposed to H2O2-induced cellular stress,
NHDFs were sequentially treated with RA and
H2O2, and cytotoxicity was evaluated using a
WST-1 assay. As presented in Fig.
1, cell viability decreased to 76% of the control in cells
treated with 600 µM H2O2 for 12 h.
Pretreatment with 10–30 µM RA for 12 h significantly inhibited
H2O2-induced cytotoxicity (Fig. 1B).
RA inhibits
H2O2-mediated induction of SA-β-gal activity
in NHDFs
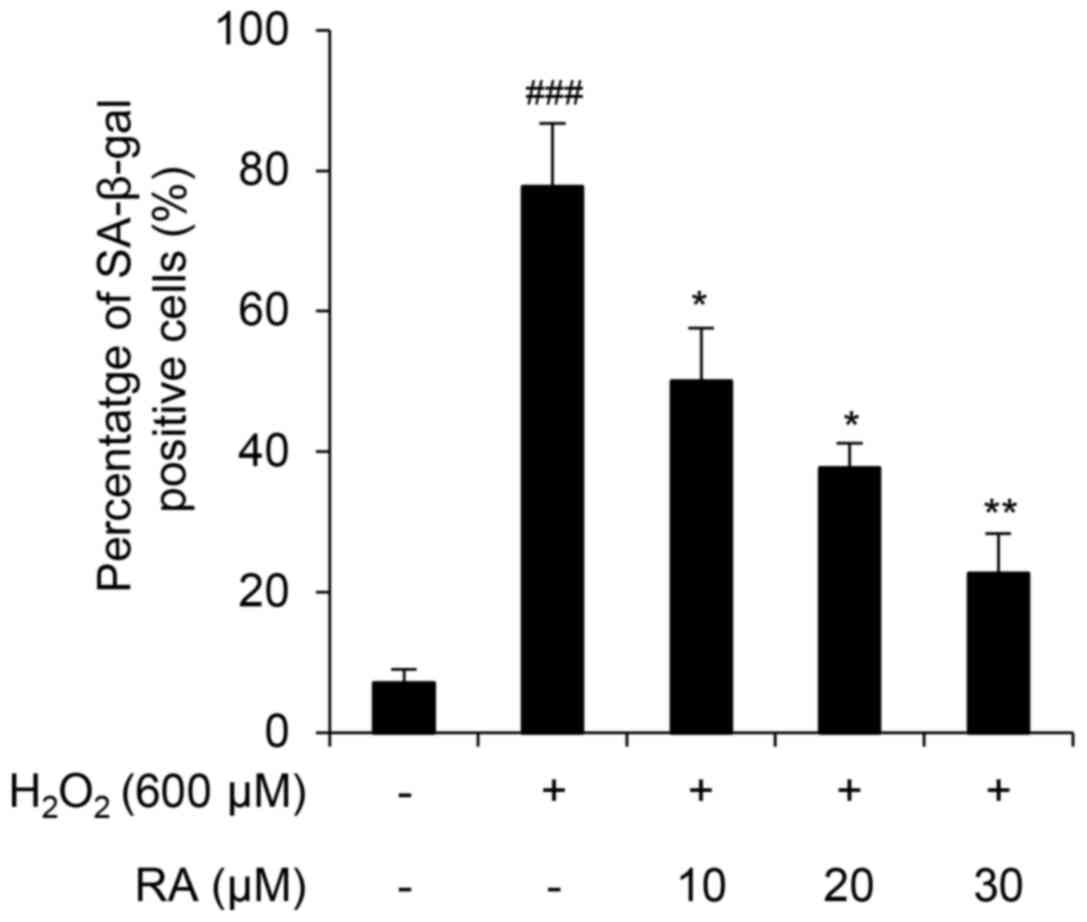
H2O2-mediated ROS activates
endogenous cellular responses to oxidative stress (30). However, when this defense system
collapses, superoxide anion, hydroxyl radical and singlet oxygen
production induces cellular senescence and apoptosis (31–33).
In the present study, H2O2 induced cellular
senescence in NHDFs, as demonstrated by an SA-β-gal assay (Fig. 2). Notably, RA markedly inhibited
cellular senescence in a concentration-dependent manner in
H2O2-exposed NHDFs (Fig. 2), thus suggesting that RA protects
NHDFs from H2O2-mediated induction of
SA-β-gal activity.
RA inhibits
H2O2-induced oxidative stress in NHDFs
Similar to previous studies, the present study
investigated whether the protective effects of RA were associated
with its oxidative properties (23–25).
To evaluate the ROS scavenging activity of RA,
H2O2 scavenging activity, DCF-DA fluorescence
intensity, NRF2 activity and the expression of genes that regulate
oxidative stress (HO-1, SOD1 and CAT) were
assessed. RA showed an increase in H2O2
scavenging activity and ascorbic acid was used as positive control
(Fig. 3A). Although the free
radical scavenging activity of RA was less than the positive
control (L-ascorbic acid), the half maximal inhibitory
concentration of RA was 0.56 mg/ml, and the radical scavenging
activity of RA occurred in a dose-dependent manner. DCF
fluorescence intensity, which is an indicator of intracellular ROS
levels, was evaluated in RA-pretreated NHDFs with or without
H2O2 exposure. DCF intensity increased
>8-fold in H2O2-exposed cells compared
with the nonexposed control group (Fig. 2B). However, this effect was
strongly reduced in NHDFs pretreated with either RA or the positive
control antioxidant NAC (Fig. 3B).
NRF2 serves a key role in the regulation of antioxidant mechanisms
by activating the transcription of genes encoding antioxidant
enzymes, including HO-1, SOD1 and CAT, via the
ARE in the target gene promoter (5–7).
Therefore, the present study analyzed the transcriptional activity
of NRF2 using the ARE-luciferase assay. As presented in Fig. 3C, RA markedly enhanced luciferase
activity in a concentration-dependent manner, thus suggesting that
RA activates NRF2 activity in NHDFs. In addition, the expression
levels of the NRF2 target genes, HO-1, SOD1 and
CAT, were detected. Notably, RA markedly upregulated the
target genes against H2O2-induced oxidative
stress (Fig. 3D). These results
suggested that RA exerts a protective effect on
H2O2-induced oxidative stress in NHDFs via
NRF2-associated antioxidant mechanisms.
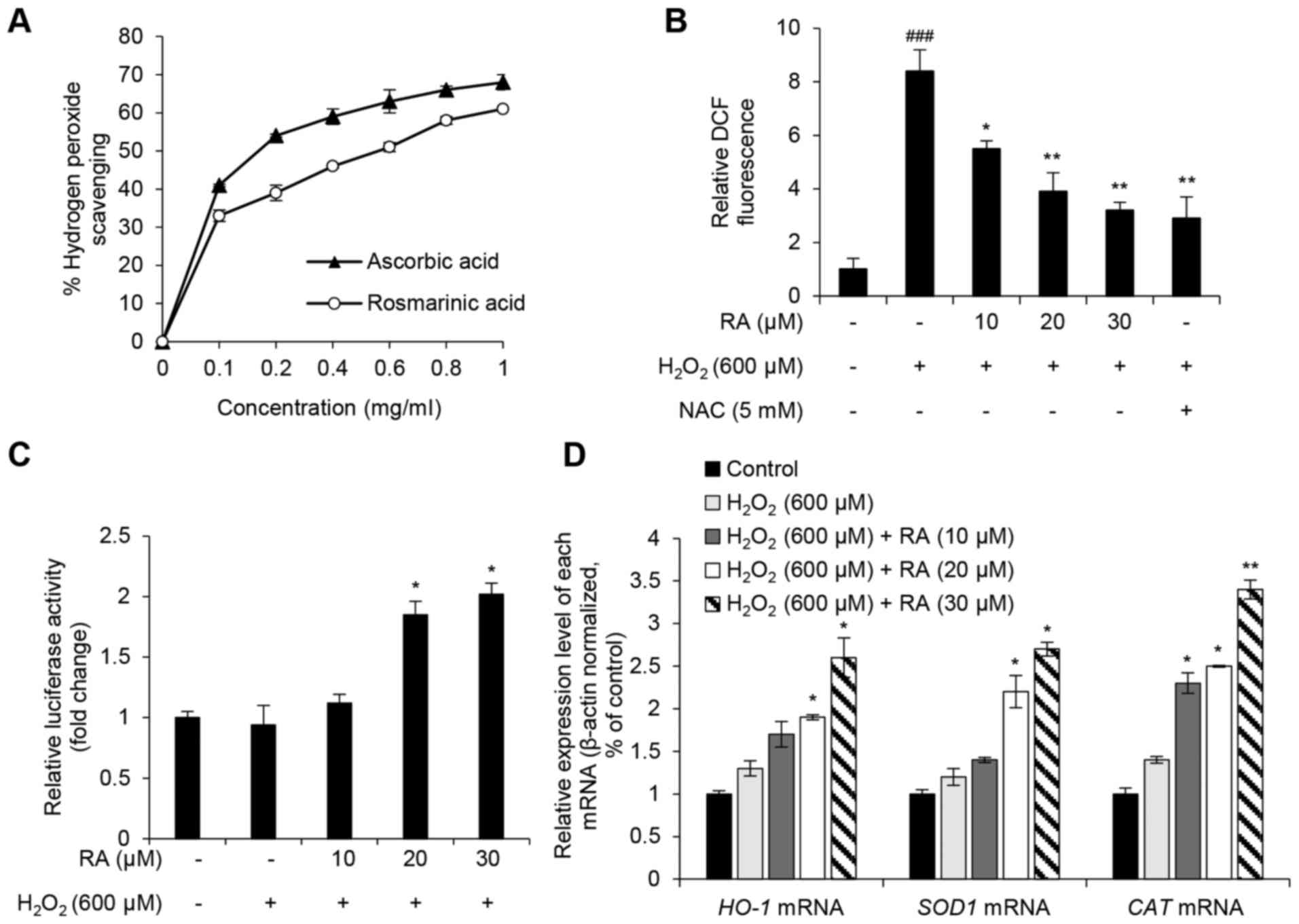 | Figure 3.Antioxidative effects of RA in
H2O2-exposed NHDFs. (A)
H2O2 scavenging activity of ≥1 mg/ml RA.
L-ascorbic acid was used as the positive control. (B) Intracellular
ROS scavenging activity of RA in H2O2-exposed
NHDFs. NAC was used as the positive control. (C) Effects of RA on
nuclear factor erythroid-derived 2-like 2 transcriptional activity
in H2O2-treated NHDFs. Transcriptional
activity was evaluated using an antioxidant response
element-luciferase reporter assay. (D) Effects of RA on the
expression of antioxidant genes in
H2O2-exposed NHDFs. HO-1, SOD1
and CAT mRNA expression levels were evaluated using
quantitative polymerase chain reaction. Data are presented as the
mean ± standard deviation from triplicate experiments.
###P<0.001 compared with non-treated control cells;
*P<0.05, **P<0.01 compared with
H2O2-treated cells. CAT, catalase;
DCF, 2′7′-dichlorofluorescein; H2O2, hydrogen
peroxide; HO-1, heme oxygenase-1; NAC, N-acetyl-cysteine;
NHDFs, normal human dermal fibroblasts; RA, rosmarinic acid;
SOD1, superoxide dismutase. |
RA inhibits the
H2O2-induced inflammatory response in
NHDFs
The mammalian Sir2 ortholog, SIRT1, is a histone
deacetylase that targets histones and nonhistone substrates,
including NF-κB and p53 (8,9,34).
Previous studies have demonstrated that SIRT1 is involved in the
regulation of numerous cellular processes, including inflammation,
apoptosis and autophagy, and that SIRT1 expression is downregulated
in response to oxidative stress (10–12,35).
Therefore, the present study evaluated the expression levels of
SIRT1 and various inflammatory cytokines, including NF-κB, TNF-α
and IL-6 in NHDFs. SIRT1 levels were decreased in NHDFs
exposed to 600 µM H2O2, whereas RA inhibited
this effect in a concentration-dependent manner (Fig. 4A). To determine the effects of
H2O2 and RA on NF-κB expression, an NF-κB
luciferase reporter stable NIH-3T3 cell line was used. As presented
in Fig. 4B, relative luciferase
activity in H2O2-exposed NHDFs increased
5.71±0.42-fold compared with the control group (P<0.05).
Notably, pretreatment with 20 and 30 µM RA significantly inhibited
this effect (P<0.05). To further evaluate the effects of
H2O2 and RA on the activity of NF-κB, the
expression levels of the NF-κB target genes, TNF-α and
IL-6, were determined. H2O2
upregulated TNF-α and IL-6 expression, whereas RA
significantly inhibited this effect in a concentration-dependent
manner (Fig. 4C). Taken together,
these results indicated that RA exerts an anti-inflammatory effect
on NHDFs under conditions of excessive oxidative stress.
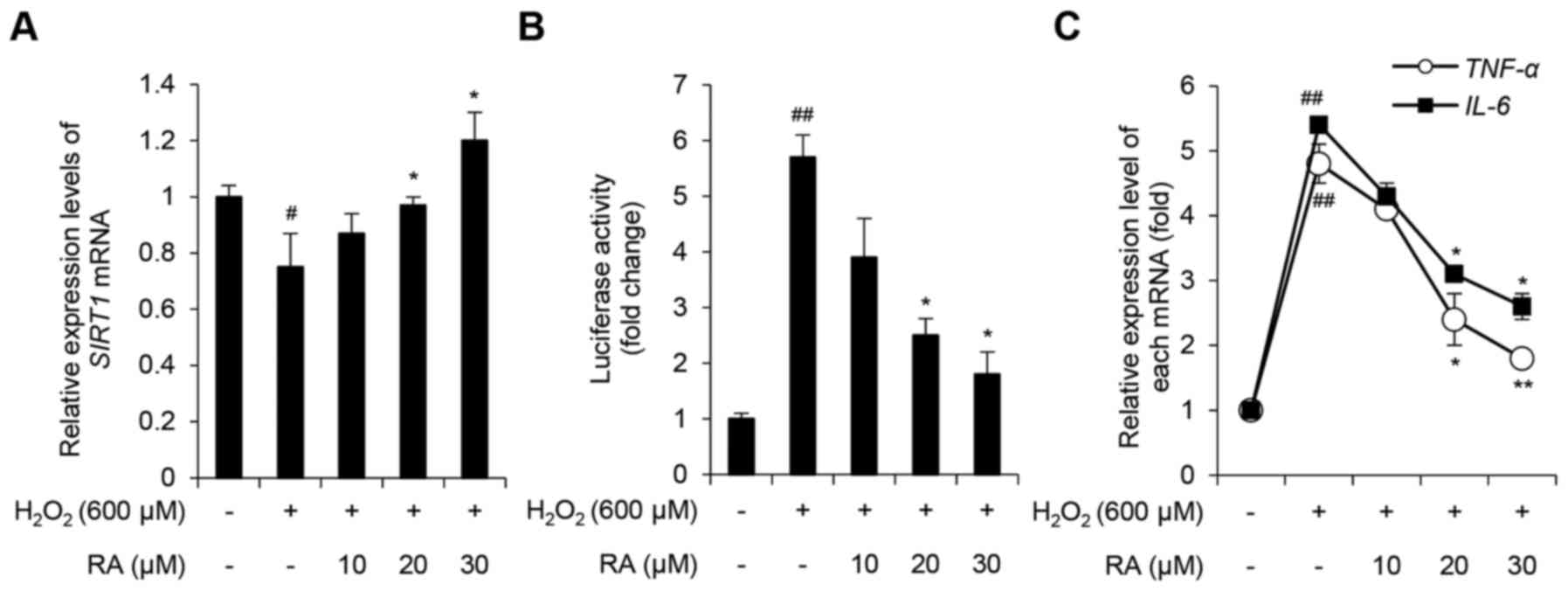 | Figure 4.Effects of RA on the
H2O2-induced inflammatory response in NHDFs.
(A) Effects of RA on SIRT1 expression in
H2O2-exposed NHDFs. SIRT1 expression
was evaluated using qPCR. (B) Effects of RA on NF-κB activity in
fibroblasts. NF-κB activity was evaluated using an NF-κB-luciferase
reporter assay. (C) Effects of RA on the expression of genes
associated with inflammation in H2O2-exposed
NHDFs. TNF-α and IL-6 expression levels were
evaluated using qPCR. Data are presented as the mean ± standard
deviation from triplicate experiments. #P<0.05,
##P<0.01 compared with non-treated control cells;
*P<0.05, **P<0.01 compared with
H2O2-treated cells.
H2O2, hydrogen peroxide; IL-6,
interleukin-6; NF-κB, nuclear factor-κB; NHDFs, normal human dermal
fibroblasts; qPCR, quantitative polymerase chain reaction; RA,
rosmarinic acid; SIRT1, sirtuin 1; TNF-α, tumor
necrosis factor-α. |
Discussion
Numerous studies have demonstrated that ROS exist in
all living tissues, and are essential for various cellular
functions, including epithelial cell proliferation and wound
healing (36), and dermal
fibroblast migration (37).
However, excess ROS can trigger deleterious effects, such as
apoptosis and cellular senescence (3). There is a marked decreased in
antioxidant levels in aged skin, and treating aged skin with
antioxidants, such as resveratrol and vitamin E, can partially
rejuvenate aged skin (38). These
findings suggested that ROS may be the primary cause for the
appearance of aging skin. H2O2 is a type of
ROS, which is generated in response to UV light and numerous
pollutants (39). The histological
alterations in aged skin are markedly visible in the dermis layer,
where dermal fibroblasts are the primary cell type (40). In addition, a reduction in CAT
activity and an increase in H2O2 levels have
been observed in dermal fibroblasts from aged human skin compared
with in dermal fibroblasts from younger human skin (41). In a previous study, treatment with
exogenous CAT rescued the reduction in intracellular CAT protein
activity and the increase in H2O2 levels in
aged fibroblasts, and also reduced collagenase matrix
metalloproteinase 1 expression (41). These data indicated that
suppressing H2O2 generation in dermal
fibroblasts may inhibit the skin aging process. Notably, in the
present study, H2O2 exposure strongly
enhanced cytotoxicity and SA-β-gal activity, which are similar to
the effects observed in tissues from older individuals (42). However, RA pretreatment markedly
protected NHDFs from H2O2-induced
cytotoxicity and SA-β-gal activation. In addition, the
H2O2 scavenging capacity of RA was
investigated, as was the intracellular H2O2
scavenging capacity of RA in NHDFs. The results demonstrated that
RA exhibited substantial H2O2 scavenging
activity and inhibited H2O2-induced
intracellular ROS production. Taken together, these data suggested
that RA may be considered a potential ingredient in anti-aging skin
products.
The results of the present study prompted the
hypothesis that RA may regulate intracellular antioxidant activity
in NHDFs. Although RA has exhibited antioxidant activity in
keratinocytes (24,25), there are differences in the
oxidative stress response between keratinocytes and dermal
fibroblasts (43). To test the
hypothesis, ARE luciferase assays and RT-qPCR were conducted to
evaluate the expression of the antioxidant-induced NRF2 target
genes HO-1, SOD1 and CAT. RA rescued
H2O2-mediated inhibition of NRF2
transcriptional activity and the consequential decrease in NRF2
target gene expression. Consistent with these findings, a previous
study demonstrated that RA inhibits UVB-induced ROS production and
the decrease in protein levels encoded by NRF2 target genes in
HaCaT keratinocytes (24). In
addition, a previous report demonstrated that the NRF2-inducer
tanshinone I exerted a protective effect against UV radiation in
human skin cells and reconstructed human skin (44). Furthermore, NRF2 depletion
induces damage to the extracellular matrix (45), a hallmark of skin aging (46). Taken together, these findings
indicated that RA may inhibit H2O2 via a
NRF2-associated antioxidant mechanism in NHDFs.
The present study also demonstrated that RA
inhibited the H2O2-induced inflammatory
response in NHDFs. Oxidative stress is associated with inflammation
in skin tissue (47), and
upregulation of the inflammatory response is associated with
numerous skin diseases, including atopic dermatitis and psoriasis
(48). SIRT1 serves critical roles
in apoptosis, senescence, autophagy, gene silencing and
inflammation (11,12,48,49).
Under excessive ROS conditions, SIRT1 is inactivated and cannot
suppress the activity of NF-κB, which is a protein that serves a
key role in inflammatory responses by activating the transcription
of proinflammatory cytokines, such as TNF-α and IL-6 (16). In the present study, RA inhibited
H2O2-mediated SIRT1 downregulation in
NHDFs, and NF-κB activity was decreased in RA-treated NHDFs.
Furthermore, RA significantly downregulated the expression levels
of TNF-α and IL-6 in a dose-dependent manner. NF-κB
activation is regarded as a potential biomarker of aging (14); an increase in NF-κB activity is
correlated with tissue aging and age-associated degenerative
diseases (15).
In conclusion, the findings of the present study
suggested that RA may inhibit senescence and inflammatory responses
in NHDFs, which are effects associated with aging skin. To gain
insight into the effects of RA on human skin, further studies
should focus on the efficacy of RA in reversing the signs of aging
skin and evaluate the precise molecular mechanisms by which RA
mediates this effect in vivo.
Acknowledgements
The present study was supported by a grant from the
Ministry of Trade, Industry and Energy (MOTIE), Korea Institute for
Advancement of Technology (KIAT) through the Encouragement Program
for The Industries of Economic Cooperation Region (grant no.
R0003962) and a grant from the Marine Biotechnology Program (grant
no. 20150184) funded by the Ministry of Oceans and Fishers,
Republic of Korea.
References
|
1
|
Poljsak B, Dahmane RG and Godić A:
Intrinsic skin aging: The role of oxidative stress. Acta
Dermatovenerol Alp Pannonica Adriat. 21:33–36. 2012.PubMed/NCBI
|
|
2
|
Ganceviciene R, Liakou AI, Theodoridis A,
Makrantonaki E and Zouboulis CC: Skin anti-aging strategies.
Dermatoendocrinol. 4:308–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davalli P, Mitic T, Caporali A, Lauriola A
and D'Arca D: ROS, cell senescence, and novel molecular mechanisms
in aging and age-related diseases. Oxid Med Cell Longev.
2016:35651272016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kansanen E, Kuosmanen SM, Leinonen H and
Levonen AL: The Keap1-Nrf2 pathway: Mechanisms of activation and
dysregulation in cancer. Redox Biol. 1:45–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Itoh K, Chiba T, Takahashi S, Ishii T,
Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et
al: An Nrf2/small Maf heterodimer mediates the induction of phase
II detoxifying enzyme genes through antioxidant response elements.
Biochem Biophys Res Commun. 236:313–322. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buendia I, Michalska P, Navarro E, Gameiro
I, Egea J and Leon R: Nrf2-ARE pathway: An emerging target against
oxidative stress and neuroinflammation in neurodegenerative
diseases. Pharmacol Ther. 157:84–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo J, Nikolaev AY, Imai S, Chen D, Su F,
Shiloh A, Guarente L and Gu W: Negative control of p53 by Sir2alpha
promotes cell survival under stress. Cell. 107:137–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaziri H, Dessain SK, Ng Eaton E, Imai SI,
Frye RA, Pandita TK, Guarente L and Weinberg RA: hSIR2(SIRT1)
functions as an NAD-dependent p53 deacetylase. Cell. 107:149–159.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu A, Ying Z and Gomez-Pinilla F:
Oxidative stress modulates Sir2alpha in rat hippocampus and
cerebral cortex. Eur J Neurosci. 23:2573–2580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon MH, Jeong JK, Lee YJ, Seol JW,
Jackson CJ and Park SY: SIRT1, a class III histone deacetylase,
regulates TNF-α-induced inflammation in human chondrocytes.
Osteoarthritis Cartilage. 21:470–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takayama K, Ishida K, Matsushita T, Fujita
N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T and Fujioka H:
SIRT1 regulation of apoptosis of human chondrocytes. Arthritis
Rheum. 60:2731–2740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salminen A, Kauppinen A, Suuronen T and
Kaarniranta K: SIRT1 longevity factor suppresses NF-kappaB-driven
immune responses: Regulation of aging via NF-kappaB acetylation?
Bioessays. 30:939–942. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balistreri CR, Candore G, Accardi G,
Colonna-Romano G and Lio D: NF-κB pathway activators as potential
ageing biomarkers: Targets for new therapeutic strategies. Immun
Ageing. 10:242013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tilstra JS, Clauson CL, Niedernhofer LJ
and Robbins PD: NF-κB in Aging and Disease. Aging Dis. 2:449–465.
2011.PubMed/NCBI
|
|
16
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vladimir-Knezevic S, Blazekovic B, Kindl
M, Vladic J, Lower-Nedza AD and Brantner AH: Acetylcholinesterase
inhibitory, antioxidant and phytochemical properties of selected
medicinal plants of the Lamiaceae family. Molecules. 19:767–782.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang AH, Kim TH, Kim GD, Kim JE, Kim HJ,
Kim SS, Jin YH, Park YS and Park CS: Rosmarinic acid attenuates
2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice.
Int Immunopharmacol. 11:1271–1277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karthik D, Viswanathan P and Anuradha CV:
Administration of rosmarinic acid reduces cardiopathology and blood
pressure through inhibition of p22phox NADPH oxidase in
fructose-fed hypertensive rats. J Cardiovasc Pharmacol. 58:514–521.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim GD, Park YS, Jin YH and Park CS:
Production and applications of rosmarinic acid and structurally
related compounds. Appl Microbiol Biotechnol. 99:2083–2092. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khojasteh A, Mirjalili MH, Hidalgo D,
Corchete P and Palazon J: New trends in biotechnological production
of rosmarinic acid. Biotechnol Lett. 36:2393–2406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanchez-Campillo M, Gabaldon JA, Castillo
J, Benavente-García O, Del Baño MJ, Alcaraz M, Vicente V, Alvarez N
and Lozano JA: Rosmarinic acid, a photo-protective agent against UV
and other ionizing radiations. Food Chem Toxicol. 47:386–392. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoo SM and Kang JR: Antioxidation effects
of rosmarinic acid on human skin melanoma cells treated with
hydrogen peroxide. J Korean Soc Appl Bi. 52:247–251. 2009.
View Article : Google Scholar
|
|
24
|
Fernando PM, Piao MJ, Kang KA, Ryu YS,
Hewage SR, Chae SW and Hyun JW: Rosmarinic acid attenuates cell
damage against UVB radiation-induced oxidative stress via enhancing
antioxidant effects in human HaCaT cells. Biomol Ther (Seoul).
24:75–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vostálová J, Zdarilová A and Svobodová A:
Prunella vulgaris extract and rosmarinic acid prevent UVB-induced
DNA damage and oxidative stress in HaCaT keratinocytes. Arch
Dermatol Res. 302:171–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oyedemi SO, Bradley G and Afolayan AJ:
In-vitro and -vivo antioxidant activities of aqueous extract of
Strychnos henningsii Gilg. Afr J Pharm Pharmaco. 4:70–78. 2010.
|
|
27
|
Martinez-Outschoorn UE, Trimmer C, Lin Z,
Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S,
Martinez-Cantarin MP, Capozza F, et al: Autophagy in cancer
associated fibroblasts promotes tumor cell survival: Role of
hypoxia, HIF1 induction and NFκB activation in the tumor stromal
microenvironment. Cell Cycle. 9:3515–3533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Debacq-Chainiaux F, Erusalimsky JD,
Campisi J and Toussaint O: Protocols to detect
senescence-associated beta-galactosidase (SA-betagal) activity, a
biomarker of senescent cells in culture and in vivo. Nat Protoc.
4:1798–1806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: Signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Korycka-Dahl MB and Richardson T:
Activated oxygen species and oxidation of food constituents. CRC
Crit Rev Food Sci Nutr. 10:209–241. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Halliwell B and Gutteridge JM: Oxygen free
radicals and iron in relation to biology and medicine: Some
problems and concepts. Arch Biochem Biophys. 246:501–514. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ou X, Lee MR, Huang X, Messina-Graham S
and Broxmeyer HE: SIRT1 positively regulates autophagy and
mitochondria function in embryonic stem cells under oxidative
stress. Stem Cells. 32:1183–1194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huo Y, Qiu WY, Pan Q, Yao YF, Xing K and
Lou MF: Reactive oxygen species (ROS) are essential mediators in
epidermal growth factor (EGF)-stimulated corneal epithelial cell
proliferation, adhesion, migration, and wound healing. Exp Eye Res.
89:876–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shi HX, Cheng Y, Ye JJ, Cai P, Zhang J, Li
R, Yang Y, Wang Z, Zhang H, Lin C, et al: bFGF promotes the
migration of human dermal fibroblasts under diabetic conditions
through reactive oxygen species production via the
PI3K/Akt-Rac1-JNK pathways. Int J Biol Sci. 11:845–859. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Farris P, Yatskayer M, Chen N, Krol Y and
Oresajo C: Evaluation of efficacy and tolerance of a nighttime
topical antioxidant containing resveratrol, baicalin, and vitamin e
for treatment of mild to moderately photodamaged skin. J Drugs
Dermatol. 13:1467–1472. 2014.PubMed/NCBI
|
|
39
|
Peus D, Vasa RA, Meves A, Pott M, Beyerle
A, Squillace K and Pittelkow MR: H2O2 is an
important mediator of UVB-induced EGF-receptor phosphorylation in
cultured keratinocytes. J Invest Dermatol. 110:966–971. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gilchrest BA: Skin aging and photoaging:
An overview. J Am Acad Dermatol. 21:610–613. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shin MH, Rhie GE, Kim YK, Park CH, Cho KH,
Kim KH, Eun HC and Chung JH: H2O2
accumulation by catalase reduction changes MAP kinase signaling in
aged human skin in vivo. J Invest Dermatol. 125:221–229. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee BY, Han JA, Im JS, Morrone A, Johung
K, Goodwin EC, Kleijer WJ, DiMaio D and Hwang ES:
Senescence-associated beta-galactosidase is lysosomal
beta-galactosidase. Aging Cell. 5:187–195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marionnet C, Pierrard C, Lejeune F, Sok J,
Thomas M and Bernerd F: Different oxidative stress response in
keratinocytes and fibroblasts of reconstructed skin exposed to non
extreme daily-ultraviolet radiation. PLoS One. 5:e120592010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tao S, Justiniano R, Zhang DD and Wondrak
GT: The Nrf2-inducers tanshinone I and dihydrotanshinone protect
human skin cells and reconstructed human skin against solar
simulated UV. Redox Biol. 1:532–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saw CL, Yang AY, Huang MT, Liu Y, Lee JH,
Khor TO, Su ZY, Shu L, Lu Y, Conney AH and Kong AN: Nrf2 null
enhances UVB-induced skin inflammation and extracellular matrix
damages. Cell Biosci. 4:392014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Panich U, Sittithumcharee G, Rathviboon N
and Jirawatnotai S: Ultraviolet radiation-induced skin aging: The
role of DNA damage and oxidative stress in epidermal stem cell
damage mediated skin aging. Stem Cells Int. 2016:73706422016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wagener FA, Carels CE and Lundvig DM:
Targeting the redox balance in inflammatory skin conditions. Int J
Mol Sci. 14:9126–9167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rahman I, Kinnula VL, Gorbunova V and Yao
H: SIRT1 as a therapeutic target in inflammaging of the pulmonary
disease. Prev Med. 54 Suppl:S20–S28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yao H and Rahman I: Perspectives on
translational and therapeutic aspects of SIRT1 in inflammaging and
senescence. Biochem Pharmacol. 84:1332–1339. 2012. View Article : Google Scholar : PubMed/NCBI
|