Introduction
The incidence of melanoma in men and women has
continued to increase in the last 40 years, despite stable or
decreasing trends for the majority of types ofcancer (1). The use of targeted drugs, including
vemurafenib, dabrafenib and trametinib, and immunotherapeutic
drugs, including ipilimumab, pembrolizumab and nivolumab, for
melanoma treatment were approved by the US Food and Drug
Administration in the last 5 years (2). All of these drugs have been shown to
result in a significant increase in progression-free and overall
survival rates, with long-term benefits in multiple clinical trials
(2). However, these therapies have
several limitations and challenges, including drug resistance,
immune-related adverse events and a lack of predictive biomarkers
(2). Therefore, the development of
novel agents is required to overcome the limitations of currently
used therapeutic agents.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is a member of tumor necrosis factor (TNF)
superfamily. TRAIL can induce the apoptosis of cells in several
types of cancer without causing obvious toxicity to normal cells
(3–5). Currently, recombinant human TRAIL is
being assessed in clinical trials (6). However, limited therapeutic benefit
has been observed and almost 50% of cancer cells, including
melanoma cells, have shown resistance to TRAIL (7). TRAIL resistance has become a major
challenge in TRAIL-based cancer therapy. Reactive oxygen species
(ROS) are known to induce a wide range of responses, which are
dependent on cell type and the levels of ROS within the cells
(8,9). High levels of ROS can lead to
necrotic cell death, whereas low levels of ROS have been shown to
induce apoptotic cell death (8,9). It
has been reported that TRAIL exposure can induce the accumulation
of ROS, activation of p38 mitogen-activated protein kinase (MAPK),
enhancement of the expression of p53 and activation of caspases
with subsequent apoptosis in TRAIL-sensitive cancer cells (10,11).
In TRAIL-resistant cancer cells, natural ROS inducers, including
curcumin (12), sulforaphane
(13), baicalein (14) and icariside II (15), have been reported to overcome TRAIL
resistance in cancer cells.
Juglone (5-hydroxy-1,4-naphthoquinone), is a natural
compound isolated from the roots, leaves, woods and fruits of
walnut trees. It exhibits various pharmacological effects,
including antiviral, antibacterial and antifungal effects (16,17).
Previous studies have shown that juglone is cytotoxic towards cells
in several types of cancer, including human lung cancer (A549)
cells (18), human leukemia
(HL-60) cells (19) and human
cervical carcinoma (HeLa) cells (20). It has been documented that juglone
exerts its cytotoxic effect via the production of ROS (21–23).
In the present study, the effect of juglone on TRAIL-induced
cytotoxicity was examined. It was shown that juglone potentiated
TRAIL-induced apoptosis in melanoma cells, and that these effects
were partially mediated through the ROS-p38-p53 pathway.
Materials and methods
Reagents and cell culture
Juglone was purchased from Sigma-Aldrich; Merck
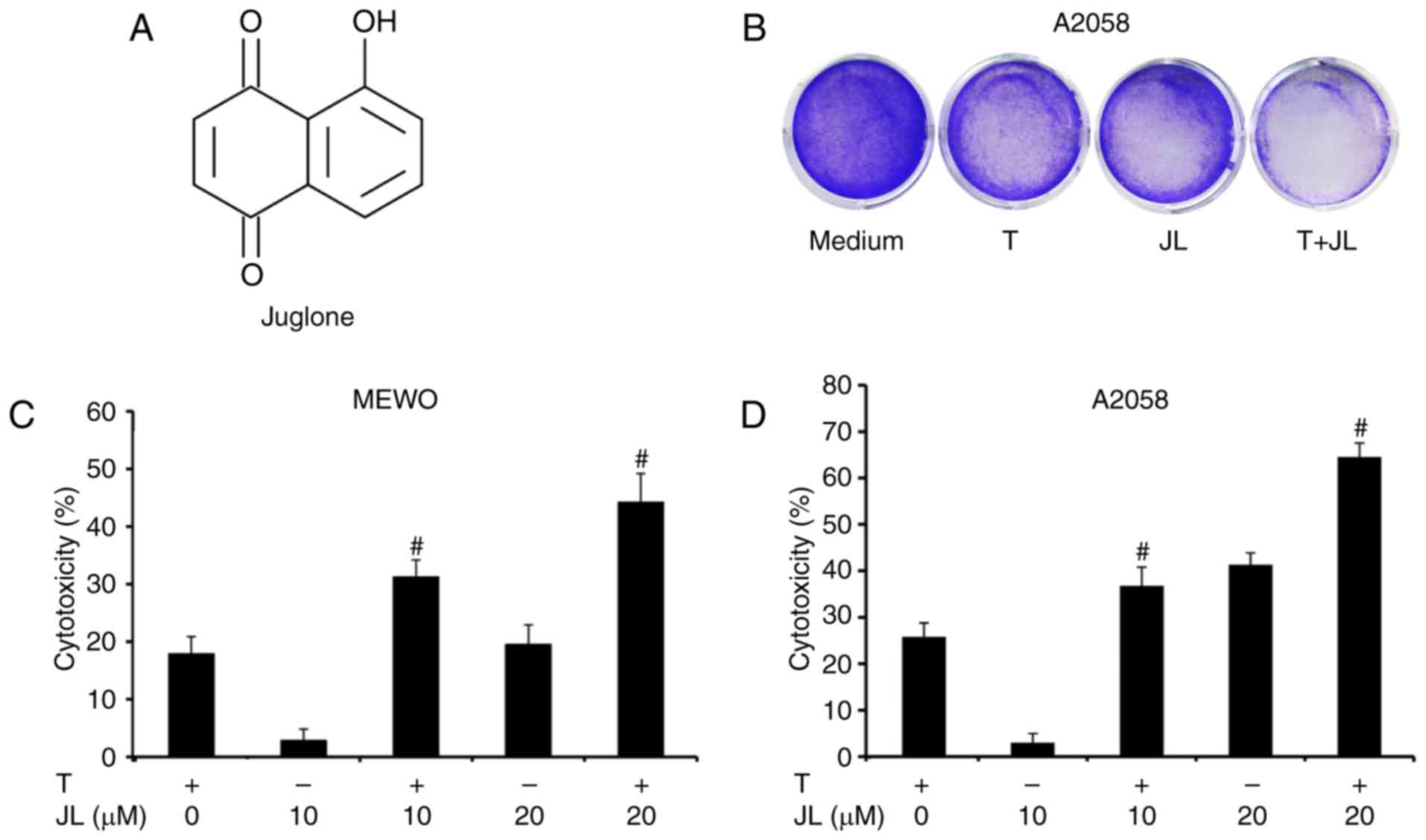
Millipore (Darmstadt Germany; purity>97; CAS 481-39-0; Fig. 1A) and dissolved in dimethyl
sulfoxide (DMSO). The final concentration of DMSO used was <0.1%
(v/v). The human melanoma A2058 and MEWO cells were obtained from
American Type Culture Collection (Manassas, VA, USA) and maintained
in DMEM (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 4 mM L-glutamine, 3.7 g/l sodium bicarbonate, 4.5
g/l glucose and 10% fetal bovine serum (FBS; Invitrogen;Thermo
Fisher Scientific, Inc.). The cells were maintained in a 5%
CO2 humidified incubator at 37°C. Human recombinant
TRAIL was purchased from PeproTech, Inc. (Rocky Hill, NJ, USA).
Propidiumiodide (PI) and RNaseA were supplied by Beyotime Institute
of Biotechnology (Jiangsu, China). The antibodies targeting
phosphorylated (p)-p38 (cat. no. 4511), p38 (cat. no. 8690), p53
(cat. no. 2527), poly(ADP-ribose) polymerase (PARP, cat. no. 9542),
caspase 3 (cat. no. 9662), and GAPDH (cat. no. 5174) were obtained
from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Thiazolylblue tetrazolium bromide (MTT), and N-acetyl-L-cysteine
(NAC) were supplied by Sigma-Aldrich; Merck Millipore.
Cell viability assays
The cytotoxic effects of juglone and/or TRAIL on the
A2058 and MEWO cells were determined using an MTT assay. Cells
(4,000/200 µl/well) were seeded into 96-well plates and pretreated
with juglone (0, 10 or 20 µM) for 6 hat 37°C. The cells were then
washed with PBS, and the medium was replaced, following which the
cells were treated with TRAIL (0 and 25 ng/ml) for 24 h at 37°C.
Subsequently, 20 µl of MTT solution (5 mg/ml) was added to each
well and incubated for 2 h at 37°C. The formazan crystals formed
were dissolved with 100 µl of DMSO and the optical density (OD) was
detected at 570 nm onamicroplate spectrophotometer (BD Biosciences,
SanJose, CA, USA). The cell viability was determined using the
following formula: Ratio (%)=(ODtreatment/ODvehicle control) ×100.
For the crystal violetassay, the cells (2.5×105) were
seeded into 60 mm dishes and exposed to juglone (0 and 20 µM) for 6
h. The cells were then washed with PBS and the medium was replaced,
following which the cells were treated withTRAIL (0 and 25 ng/ml)
for 72 h. The cells were then fixed with 10% formalin for 10 min,
followed by staining with 0.05% crystal violet solution in
distilled water for 30 min. Finally, the crystal violet was
removed, and the cells were washed twice with distilled water.
Cells were visualized using a light microscope and images of them
were captured by a Flatbed Scanner (Canon LiDE 220, Canon Inc.,
Tokyo, Japan).
Cell death assays
The A2058 and MEWO cells were seeded at a density of
2×105 cells/well in 6-well culture plates for 24 h.
Subsequently, the cells were pretreated with juglone (0 and 20 µM)
for 6 h. The cells were then washed with PBS and medium was
replaced, following which the cells were treated with TRAIL (0 and
25 ng/ml) for 24 h. Following incubation, the cells were collected
and fixed in 70% ethanol for 24 h at 4°C. The cells were then
centrifuged at 300 × g at 4°C for 10 min and the cell pellet was
resuspended in 400 µl of PBS containing RNaseA (10 mg/ml; 50 µl)
and PI (2 mg/ml; 10 µl). The mixture was incubated in the dark at
37°C for 30 min and analyzed using a FACSCalibur flow cytometer (BD
Biosciences). The cell death data were analyzed using FlowJo
software V6.0 (Tree Star, Inc., Ashland, OR, USA). The extent of
cell death was determined by evaluating the sub G1 fraction. The
data comprised three replicates.
Western blot analysis
The A2058 cells were pretreated with juglone (0 and
20 µM) for 6 h. The cells were then washed with PBS and medium was
replaced, following which the cells were treated with TRAIL (0 and
25 ng/ml) for 4 h. The cells were then resuspended in lysis buffer
containing 150 mmol/l NaCL, 1% NP-40, 0.5% sodium deoxycholate,
0.1% SDS, and 50 mmol/l Tris-Cl (pH 8.0), 2 µg/ml aprotinin, 2
µg/ml leupeptin, 40 mg/ml of phenylmethylsulfonyl fluoride and 2
mmol/l DTT. The mixture was centrifuged at 10,000 × g at 4°C for 15
min to remove nuclei and cell debris. The supernatants were then
immediately frozen at −80°C until use. The protein concentrations
were determined using a Bradford assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and 30 µg of cellular proteins were
electroblotted onto a PVDF membrane following separation via 10%
SDS-polyacrylamide gel electrophoresis. The immunoblot was blocked
for 1 h with 5% milk at room temperature, followed by incubation
overnight at 4°C with 1:1,000 dilutions of primary antibodies
against p-p38, p38, p53, PARP, caspase 3 or GAPDH. The blots were
washed twice with Tween 20/Tris-buffered saline (TTBS) prior to the
addition of a 1:1,000 dilution of HRP-conjugated secondary antibody
(cat. no. 7074, Cell Signaling Technology Inc., Danvers, MA, USA)
for 1 h at room temperature. The blots were washed again with TTBS,
and developed by enhanced chemiluminescence using Supersignal West
Femto Chemiluminescent substrate (Pierce; Thermo Fisher Scientific,
Inc.). The band intensities were quantified using UN-SCAN-IT gel
analysis software (version 6; Silk Scientific, Orem, UT, USA). The
OD values for the target proteins were calculated as a proportion
of the OD value for GAPDH. The western blot assays were repeated
three times.
Evaluation of ROS
ROS were detected using the cell-permeable
fluorescentprobe 2,-7,-dichlorofluorescein-diacetate
(H2DCFDA; Sigma-Aldrich; Merck Millipore), a
non-fluorescent compound, which is converted into highly
fluorescent dichlorofluorescein (DCF) by cellular peroxides.
Briefly, the A2058 cells were pretreated with juglone (0 and 20 µM)
for 6 h. The cells then were washed with PBS and medium was
replaced, following which the cells were treated with TRAIL (0 and
25 ng/ml) for 4 h. Following treatment, the cells were loaded with
H2DCFDA (10 µM) in serum-free DMEM. Following incubation
at 37°C for 30 min, the cells were washed with PBS and fluorescence
was monitored using flow cytometry at an excitation wave length of
488 nm and emission wavelength of 530 nm. The mean fluorescence
intensity (MFI) data were analyzed using FlowJo software V6.0 (Tree
Star, Inc.) and included three replicates.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed by SPSS Statistics
17.0 software (SPSS Inc., Chicago, IL, USA) using one-way analysis
of variance. For comparisons between two groups, Student's t-test
was used. P<0.05 was considered to indicate a statistically
significant difference.
Results
Juglone sensitizes melanoma cells to
TRAIL-induced cytotoxicity
The present study first examined the cytotoxic
effects of juglone and/or TRAIL in TRAIL-resistant melanoma cells.
As demonstrated bythe crystal violet assay (Fig. 1B) and MTT assay (Fig. 1C and D), the MEWO and A2058
melanoma cells exhibited a low level response to TRAIL treatment.
Juglone (10 and 20 µM) treatment alone induced cytotoxicity in a
dose-dependent manner, whereas a higher level of cytotoxicity was
observed in the juglone and TRAIL combination group, compared with
that inthe groups treated with either TRAIL or juglone alone
(P<0.01).
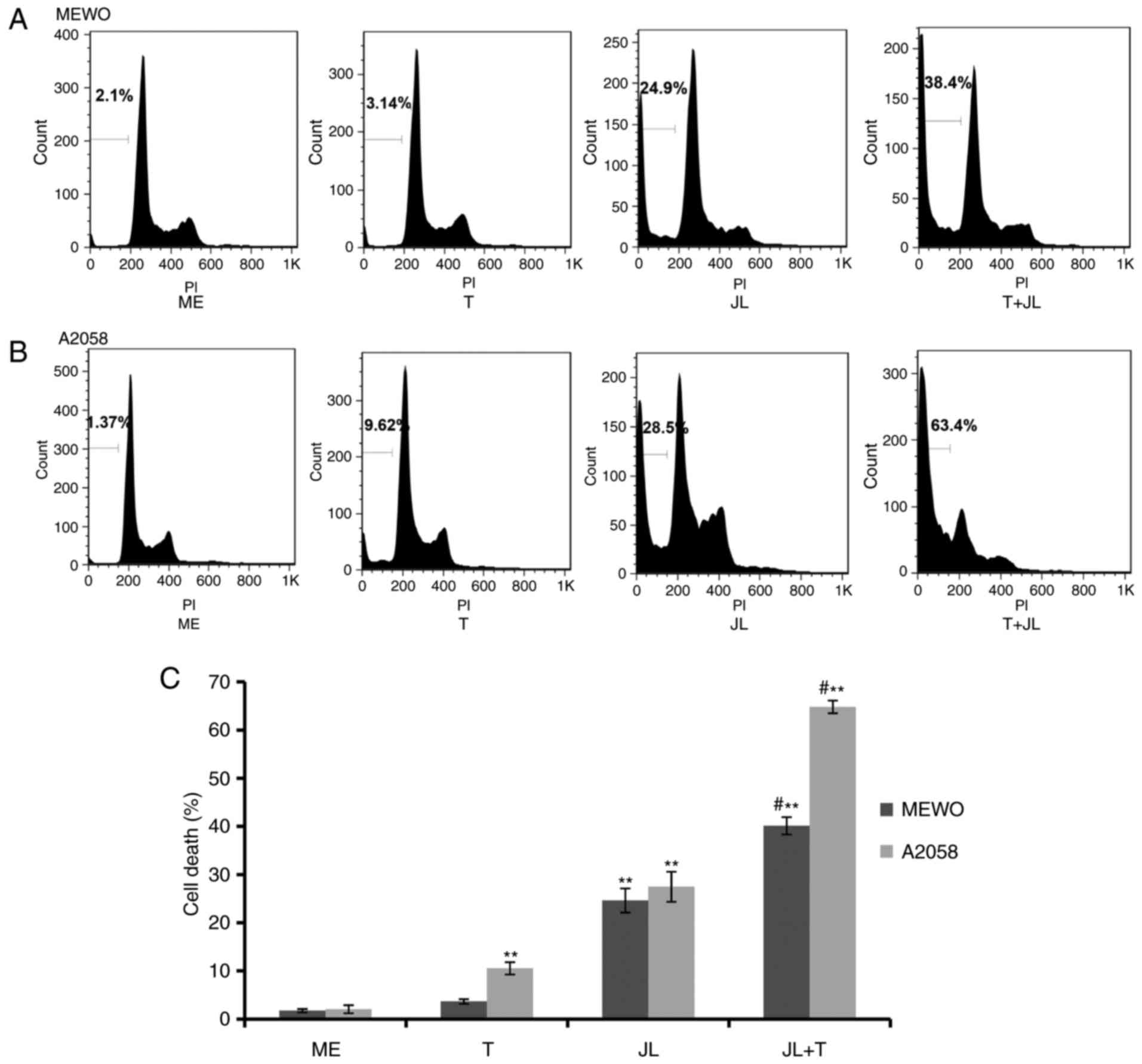
Juglone potentiates TRAIL-induced cell
death
As demonstrated by the flow cytometric analysis
(Fig. 2), MEWO melanoma cells were
not affected by TRAIL-induced cell death (Fig. 2A and C), whereas the A2058 cells
exhibited a minor response to TRAIL-induced cell death (Fig. 2B and C). Treatment with juglone (20
µM) alone significantly induced cell death, however, the
combination of juglone and TRAIL caused a higher rate of cell
death, compared with that in the cells treated with either TRAIL or
juglone alone (Fig. 2;
P<0.01).
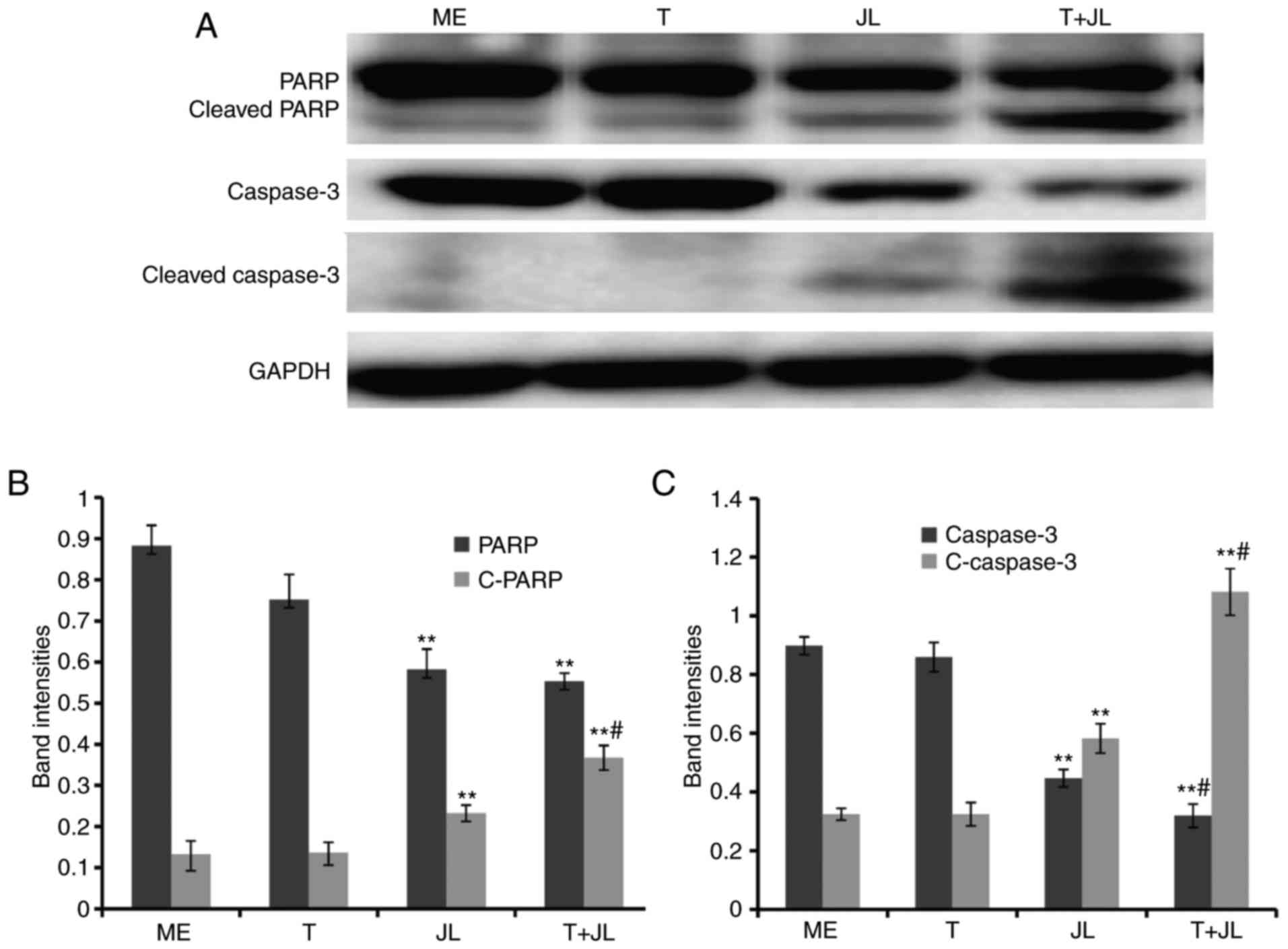
PARP and caspase 3 are the terminal pro-apoptotic
proteins. The cleaved forms of these two proteins are the active
forms. As demonstrated in the results of the western blot analysis
(Fig. 3), no significant changes
in the expression of PARP and caspase 3 were observed in the group
treated with TRAIL alone. However, juglone treatment markedly
increased the expression of cleaved PARP (Fig. 3A and B) and cleaved caspase 3
(Fig. 3A and C). The levels of
cleaved PARP and cleaved caspase 3 in the juglone and TRAIL
combination group were higher, compared with the levels in the
group treated with juglone alone (Fig.
3; P<0.01). Significant decreases in the levels of pro-PARP
and pro-caspase 3 were observed following combined juglone and
TRAIL treatment.
Juglone and TRAIL combination
treatment increases the production of ROS, and activates p38 and
p53
To examine the molecular mechanism underlying
juglone-induced TRAIL sensitization, the present study investigated
whether juglone and/or TRAIL treatment induced the production of
ROS. The ROS levels were determined 6 h following juglone and/or
TRAIL treatment. As demonstrated by the flow cytometric analysis
(Fig. 4A and B), TRAIL treatment
alone did not increase the production of ROS, whereas juglone
treatment alone significantly increased the production of ROS
(P<0.01). The production of ROS was further increased in the
juglone and TRAIL combination group, compared with the cells
treated with juglone alone (P<0.01).
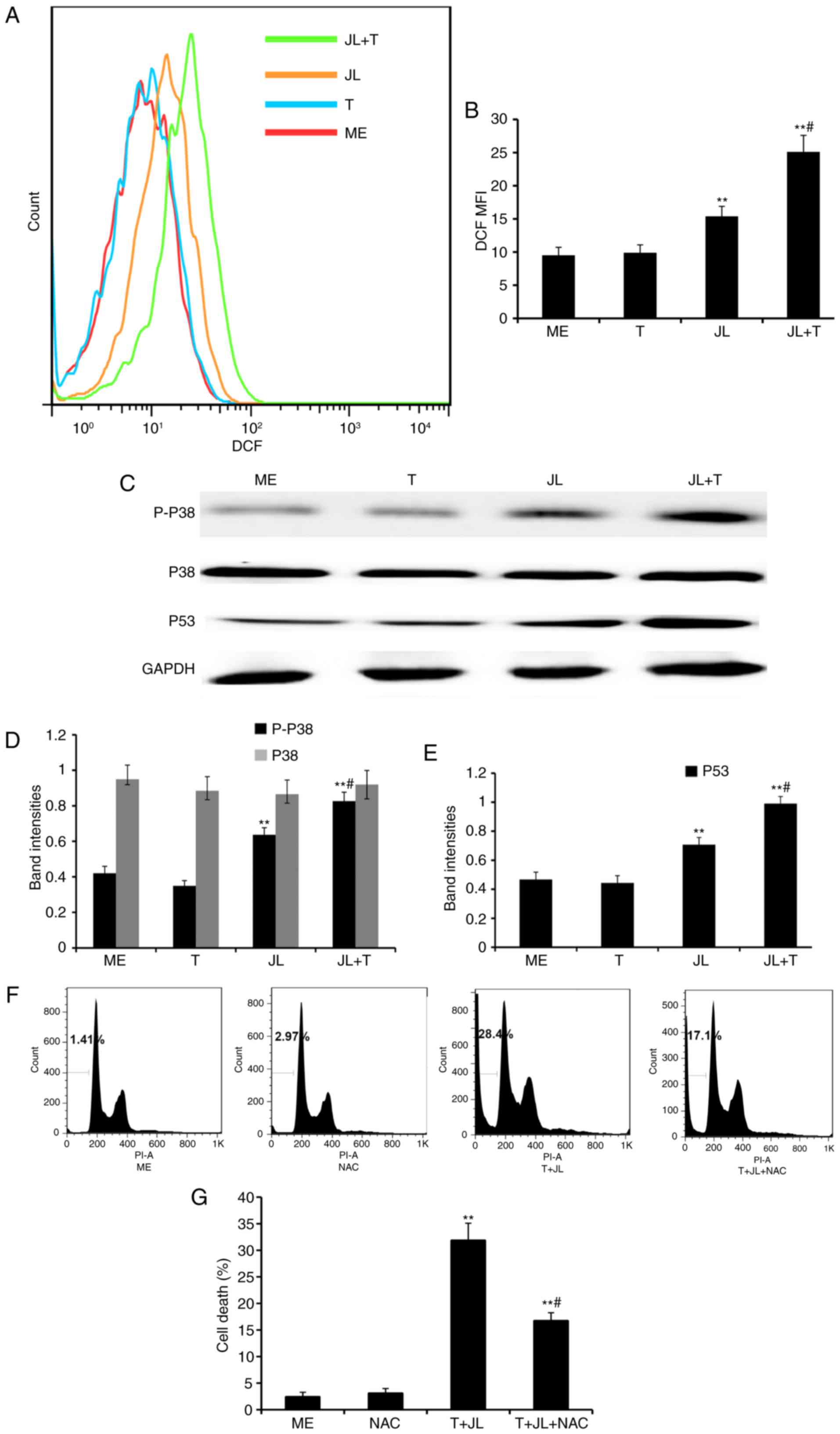 | Figure 4.Juglone in combination with TRAIL
increases the production of ROS, and activates p38 and p53. For the
ROS assay, A2058 cells were pretreated with juglone (0 and 20 µM)
for 6 h, washed with PBS, and treated with TRAIL (0 and 25 ng/ml)
for 4 h. The cells were loaded with H2DCFDA and
fluorescence was monitored by flow cytometry. MFI data were
analyzed using FlowJo software V6.0. (A) Representative images of
ROS MFI and (B) statistical data. For western blot analysis, the
cells were treated with juglone and/or TRAIL for 4 h, total protein
was extracted, and p-p38, p38 and p53 were detected. GAPDH was used
as the loading control. (C) Representative western blot images of
p-p38, total p38 and p53, and quantification of band intensities of
(D) p-p38, total p38 and (E) p53. The band densities for target
protein are shown as a proportion of that for GAPDH. For the cell
death assay, A2058 cells were pretreated with NAC (0 and 2 mM) for
1 h, followed by juglone and TRAIL combination treatment for 16 h.
The fixed cells were stained with PI and analyzed in a FACSCalibur
cytometer, with experiments repeated three times. (F)
Representative images of cell death and (G) statistical analysis.
**P<0.01, vs. ME control; #P<0.01, vs. JLor T
alone. ME, medium; JL, juglone; T/TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; P-, phosphorylated; ROS,
reactive oxygen species; DCF MFI, dichlorofluorescein mean
fluorescence intensity; PI, propidium iodide. |
Previous evidence indicates that ROS induces cell
death via the activation of p38 and p53 (10,11).
As demonstrated by western blot analysis (Fig. 4C-E), treatment with TRAIL alone did
notactivated p38 and p53, whereas treatment with juglone alone
significantly increased the levels of p-p38 and p53 (P<0.01).
The expression levels of p-p38 and p53 were further increased in
the juglone and TRAIL combination group, as compared with the group
treated with juglone alone (P<0.01). No significant change was
observed in the protein expression of total p38 following juglone
and/or TRAIL treatment.
NAC pretreatment partially reverses
juglone and TRAIL combination-induced cell death
As demonstrated by the flow cytometric analysis
(Fig. 4F and G), treatment with
juglone in combination with TRAIL for 16 h resulted in a
significant increase in the rate of cell death (28.4%), compared
with that in the medium control group (1.41%), whereas pretreatment
with NAC, a ROS scavenger, partially reversed the juglone and TRAIL
combination-induced cell death (P<0.01; 14.7, vs. 28.4%).
Discussion
Intrinsic or acquired resistance to TRAIL in several
types of cancer cell has become a major challenge in TRAIL-based
cancer therapy. In the present study, it was found that juglone
sensitized melanoma cells to TRAIL-induced cytotoxicity, which was
accompanied by increases in the levels of cleaved PARP and cleaved
caspase 3.
It has been reported that TRAIL exposure can induce
the accumulation of ROS and activation of p38 MAPK, enhance the
expression of p53 and activate caspases in TRAIL-sensitive cancer
cells. In the present study, it was found that TRAIL exposure did
not induce the production of ROS, activation of p38 or increase in
p53 in the TRAIL-resistant melanoma cells. However juglone in
combination with TRAIL markedly increased the production of ROS and
activation of p38, and increased the expression of p53, compared
with the groups treated with either juglone or TRAIL alone.
Pretreatment with NAC, a ROS scavenger, significantly reduced the
cytotoxicity of juglone in combination with TRAIL, which further
supported the hypothesis that ROS is involved in juglone-induced
TRAIL sensitization.
The resistance of several types of cancer cell to
TRAIL is partially due to decreased levels or mutations of TRAIL
receptors, including death receptor 4 (DR4) and DR5 (24,25).
The sensitivity of cancer cells to TRAIL can be partially restored
by treatment with subtoxic concentrations of chemotherapeutic drugs
through the upregulation of DR4 and DR5 (26,27).
Several studies have shown that DR4 and DR5 can be upregulated by
ROS and MAPKs, including extracellular signal-regulated kinases1/2,
p38 MAPK and c-Jun NH2-terminal kinase (28–30).
As juglone could increase the production of ROS and increase p-p38
and p53 protein levels, further investigations are required to
determine the effects of juglone on DR4 and DR5 TRAIL
receptors.
In conclusion, the present study demonstrated that
juglone, a natural product from walnut trees, potentiated
TRAIL-induced apoptosis in melanoma cells and that these effects
were partially mediated through the ROS-p38-p53 pathway. These
findings suggest that juglone maybe a potential sensitizer for
TRAIL in the treatment of melanoma.
Acknowledgements
This study was funded by grants from the National
Natural Science Foundation of China (grant no. 81673917) and the
Shandong Provincial Natural Science Foundation (grant no.
ZR2014HM069, ZR2014HM002).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu Z, Liu W and Gotlieb V: The rapidly
evolving therapies for advanced melanoma-Towards immunotherapy,
molecular targeted therapy, and beyond. Crit Rev Oncol Hematol.
99:91–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeda K, Stagg J, Yagita H, Okumura K and
Smyth MJ: Targeting death-inducing receptors in cancer therapy.
Oncogene. 26:3745–3757. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S: The promise of cancer therapeutics
targeting the TNF-related apoptosis-inducing ligand and TRAIL
receptor pathway. Oncogene. 27:6207–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falschlehner C, Ganten TM, Koschny R,
Schaefer U and Walczak H: TRAIL and other TRAIL receptor agonists
as novel cancer therapeutics. Adv Exp Med Biol. 647:195–206. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellail AC, Qi L, Mulligan P, Chhabra V
and Hao C: TRAIL agonists on clinical trials for cancer therapy:
The promises and the challenges. Rev Recent Clin Trials. 4:34–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L and Fang B: Mechanisms of
resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther.
12:228–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kannan K, Holcombe RF, Jain SK,
Alvarez-Hernandez X, Chervenak R, Wolf RE and Glass J: Evidence for
the induction of apoptosis by endosulfan in a human T-cell leukemic
line. Mol Cell Biochem. 205:53–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kannan K and Jain SK: Oxidative stress and
apoptosis. Pathophysiology. 7:153–163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee MW, Park SC, Yang YG, Yim SO, Chae HS,
Bach JH, Lee HJ, Kim KY, Lee WB and Kim SS: The involvement of
reactive oxygen species (ROS) and p38 mitogen-activated protein
(MAP) kinase in TRAIL/Apo2L-induced apoptosis. FEBS Lett.
512:313–318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamy V, Bousserouel S, Gossé F, Minker C,
Lobstein A and Raul F: Lupulone triggers p38 MAPK-controlled
activation of p53 and of the TRAIL receptor apoptotic pathway in
human colon cancer-derived metastatic cells. Oncol Rep. 26:109–114.
2011.PubMed/NCBI
|
|
12
|
Park S, Cho DH, Andera L, Suh N and Kim I:
Curcumin enhances TRAIL-induced apoptosis of breast cancer cells by
regulating apoptosis-related proteins. Mol Cell Biochem. 383:39–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim H, Kim EH, Eom YW, Kim WH, Kwon TK,
Lee SJ and Choi KS: Sulforaphane sensitizes tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma
cells to TRAIL-induced apoptosis through reactive oxygen
species-mediated up-regulation of DR5. Cancer Res. 66:1740–1750.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taniguchi H, Yoshida T, Horinaka M, Yasuda
T, Goda AE, Konishi M, Wakada M, Kataoka K, Yoshikawa T and Sakai
T: Baicalein overcomes tumor necrosis factor-related
apoptosis-inducing ligand resistance via two different
cell-specific pathways in cancer cells but not in normal cells.
Cancer Res. 68:8918–8927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du J, Wu J, Fu X, Tse AK, Li T, Su T and
Yu ZL: Icairiside II overcomes TRAIL resistance of melanoma cells
through ROS-mediated downregulation of STAT3/cFLIP signaling.
Oncotarget. 7:52218–52229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omar S, Lemonnier B, Jones N, Ficker C,
Smith ML, Neema C, Towers GH, Goel K and Arnason JT: Antimicrobial
activity of extracts of eastern North American hardwood trees and
relation to traditional medicine. J Ethnopharmacol. 73:161–170.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vardhini SR: Exploring the antiviral
activity of juglone by computational method. J Recept Signal
Transduct Res. 34:456–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XB, Zou CL, Duan YX, Wu F and Li G:
Activity guided isolation and modification of juglone from Juglans
regia as potent cytotoxic agent against lung cancer cell lines. BMC
Complement Altern Med. 15:3962015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu HL, Yu XF, Qu SC, Zhang R, Qu XR, Chen
YP, Ma XY and Sui DY: Anti-proliferative effect of Juglone from
Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing
apoptosis through the mitochondria-dependent pathway. Eur J
Pharmacol. 645:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang W, Liu A, Li Y, Zhao X, Lv S, Zhu W
and Jin Y: Anticancer activity and mechanism of juglone on human
cervical carcinoma HeLa cells. Can J Physiol Pharmacol.
90:1553–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seshadri P, Rajaram A and Rajaram R:
Plumbagin and juglone induce caspase-3-dependent apoptosis
involving the mitochondria through ROS generation in human
peripheral blood lymphocytes. Free Radic Biol Med. 51:2090–2107.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu HL, Yu XF, Qu SC, Qu XR, Jiang YF and
Sui da Y: Juglone, from Juglans mandshruica Maxim, inhibits growth
and induces apoptosis in human leukemia cell HL-60 through a
reactive oxygen species-dependent mechanism. Food Chem Toxicol.
50:590–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jha BK, Jung HJ, Seo I, Suh SI, Suh MH and
Baek WK: Juglone induces cell death of Acanthamoeba through
increased production of reactive oxygen species. Exp Parasitol.
159:100–106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kischkel FC, Lawrence DA, Chuntharapai A,
Schow P, Kim KJ and Ashkenazi A: Apo2L/TRAIL-dependent recruitment
of endogenous FADD and caspase-8 to death receptors 4 and 5.
Immunity. 12:611–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daniel PT, Wieder T, Sturm I and
Schulze-Osthoff K: The kiss of death: Promises and failures of
death receptors and ligands in cancer therapy. Leukemia.
15:1022–1032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mühlethaler-Mottet A, Bourloud KB,
Auderset K, Joseph JM and Gross N: Drug-mediated sensitization to
TRAIL-induced apoptosis in caspase-8-complemented neuroblastoma
cells proceeds via activation of intrinsic and extrinsic pathways
and caspase-dependent cleavage of XIAP, Bcl-xL and RIP. Oncogene.
23:5415–5425. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lacour S, Micheau O, Hammann A, Drouineaud
V, Tschopp J, Solary E and Dimanche-Boitrel MT: Chemotherapy
enhances TNF-related apoptosis-inducing ligand DISC assembly in
HT29 human colon cancer cells. Oncogene. 22:1807–1816. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prasad S, Kim JH, Gupta SC and Aggarwal
BB: Targeting death receptors for TRAIL by agents designed by
Mother Nature. Trends Pharmacol Sci. 35:520–536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhardwaj A and Aggarwal BB:
Receptor-mediated choreography of life and death. J Clin Immunol.
23:317–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song JJ and Lee YJ: Differential cleavage
of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation
of the MAPK superfamily. Cell Signal. 20:892–906. 2008. View Article : Google Scholar : PubMed/NCBI
|