Introduction
Steroid-associated osteonecrosis is common as
steroids are a widely-used treatment for a number of diseases.
Steroid-associated osteonecrosis leads to high disability rates as
it results in partial or complete loss of the ability to walk.
However, there remains no ideal treatment for steroid-associated
femoral head necrosis, the mechanisms of which remain to be
elucidated.
Certain researchers have described abnormalities in
the number or in the function of bone progenitor cells in
osteonecrosis (1,2). The current hypothesis is that
osteonecrosis could be associated with an imbalance between
osteoblast formation and necrosis (3,4). In
addition, osteogenic differentiation of mesenchymal stem cells is
attenuated by glucocorticoids (5,6).
MicroRNAs (miRs) are small molecular regulators of gene expression
and serve critical roles in stem cell differentiation (7,8). The
authors previously screened and compared miR expression between
patients with steroid-associated femoral head necrosis and normal
adults (9). miR-23a-3p was the
most significantly upregulated miR in patients with femoral head
necrosis. The authors also previously demonstrated that miR-23a-3p
was significantly downregulated during osteogenic differentiation
(10). Overexpression of
miR-23a-3p inhibited osteogenic differentiation of bone mesenchymal
stem cells (BMSCs), whereas downregulation of miR-23a-3p enhanced
the process (10). The authors
also previously confirmed that low-density
lipoprotein-receptor-related protein 5 (LRP-5) is a direct target
of miR-23a-3p (10). Therefore it
is hypothesized is that inhibiting miR-23a-3p may decrease the
incidence of osteonecrosis. In the present study, the effect of
miR-23a-3p in a rat model of osteonecrosis was investigated.
Materials and methods
Animals
The Animal Experimentation Ethics Committee of
Peking Union Medical College Hospital approved the present study
protocol. Sprague-Dawley male adult rats of between 4 and 12 weeks
old were obtained from Charles River Laboratories (Wilmington, MA,
USA). BMSCs were isolated from the lower limbs of 4-week old rats.
A total of 18, 12-week old rats (400–450 g) were reared in pairs in
custom-designed plexiglass cages (50×35×20 cm) under standard
laboratory conditions (12-h light/dark cycle; 24–25°C; humidity,
50–55%) with free access to food and water during the study.
Isolation and culture of the
BMSCs
Primary rat BMSCs were harvested by flushing the
bone marrow cavity of the femurs and tibias of 4-week-old rats with
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The cell suspension was plated
and cultured in DMEM supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 U/ml streptomycin at 37°C in a humidified 5% CO2
incubator. The culture medium was replaced and non-adherent cells
were removed every 3–4 days. The cells were treated with trypsin
and were passaged by a ratio of ~1:3 at sub-confluence. The cells
from the third passage were transfected with a lentiviral vector
and used in the following experiments.
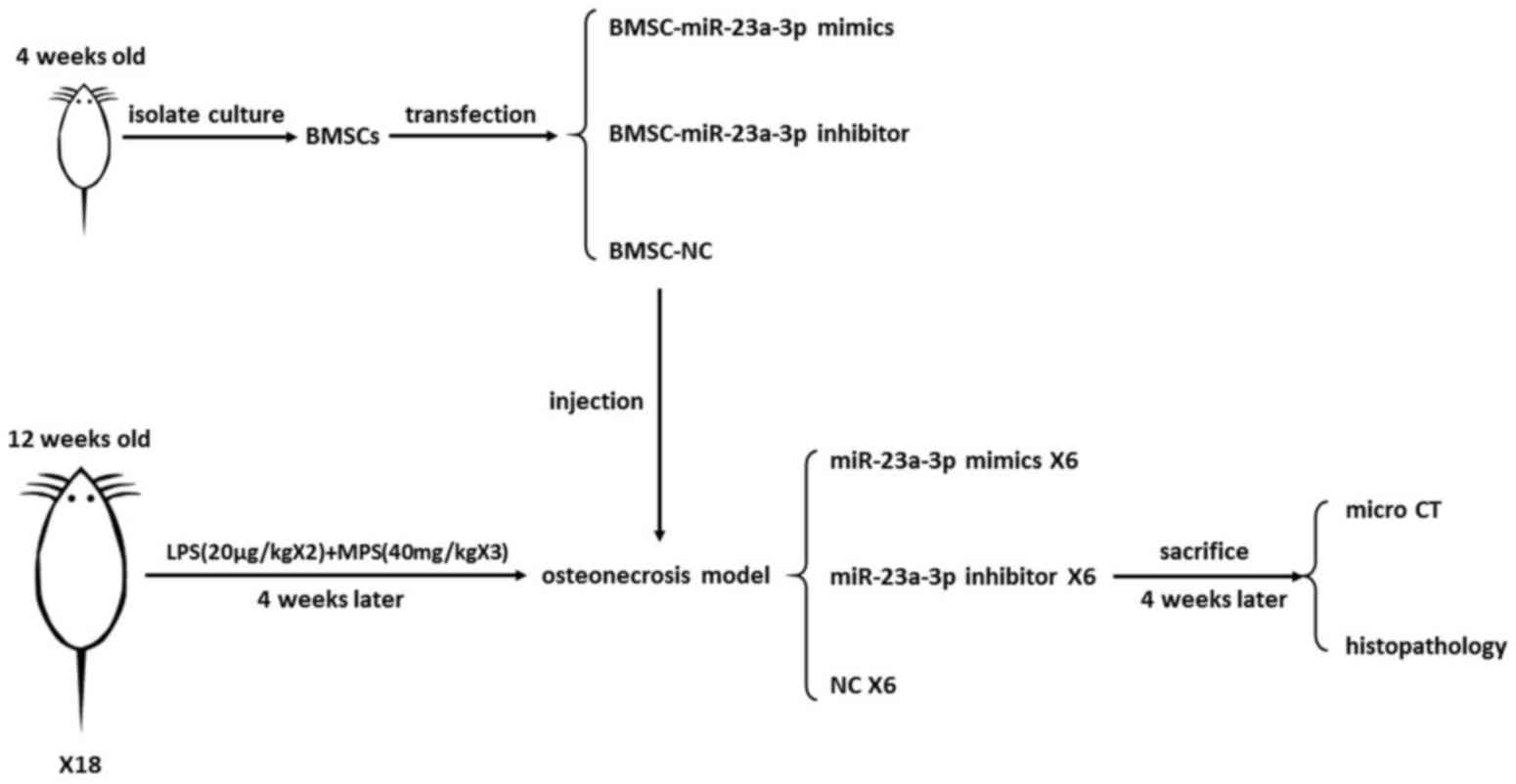
Animal model establishment
In the present study, the steroid-induced
osteonecrosis model was established in rats by two injections of
low-dose (20 µg/kg) lipopolysaccharide (LPS) combined with three
subsequent injections of high-dose methylprednisolone (40 mg/kg).
The 12-week-old rats were administered intraperitoneal injections
of 20 µg/kg LPS (Escherichia coli 055:B5; Merck KGaA,
Darmstadt, Germany) twice with an interval of 24 h. The rats
received three times of intramuscular injection of 40 mg/kg
methylprednisolone sodium succinate (Pfizer, Inc., New York, NY,
USA) on day 3, 4 and 5 at an interval of 24 h (11). The order in which the experiment
was performed is illustrated in Fig.
1.
Transfection of miR-23a-3p in rat
BMSCs (rBMSCs)
Synthetic miR-23a-3p mimic (sequence:
5′-ATCACATTGCCAGGGATTTCC-3′), miR-23a-3p inhibitor (sequence:
5′-GGAAATCCCTGGCAATGTGAT-3′) and negative control (NC, sequence:
5′-TTCTCCGAACGTGTCACGTTTC-3′) were purchased from GenePharma
(Sunnyvale, CA, USA) along with lentiviral green fluorescent
protein (GFP)-tagged vector LV3-pGLV-h1-GFP-puro. The synthetic
mRNA (1×109 transducing units/ml) was transfected into
BMSCs according to the manufacturer's protocol. The most effective
multiplicity of infection (MOI) was decided according to the pilot
experiment (data not shown). rBMSCs were plated onto 10 cm dishes
at a density of 1×106 cells/dish in 5 ml media and
transfected using a GFP-tagged lentiviral vector
(lenti-23a-mimic-GFP, lenti-23a-inhibitor-GFP and lenti-GFP) at an
MOI of 100 plaque-forming unit/cell in the presence of 5 µl
polybrene (Merck KGaA). Fresh media was added at 24 h following
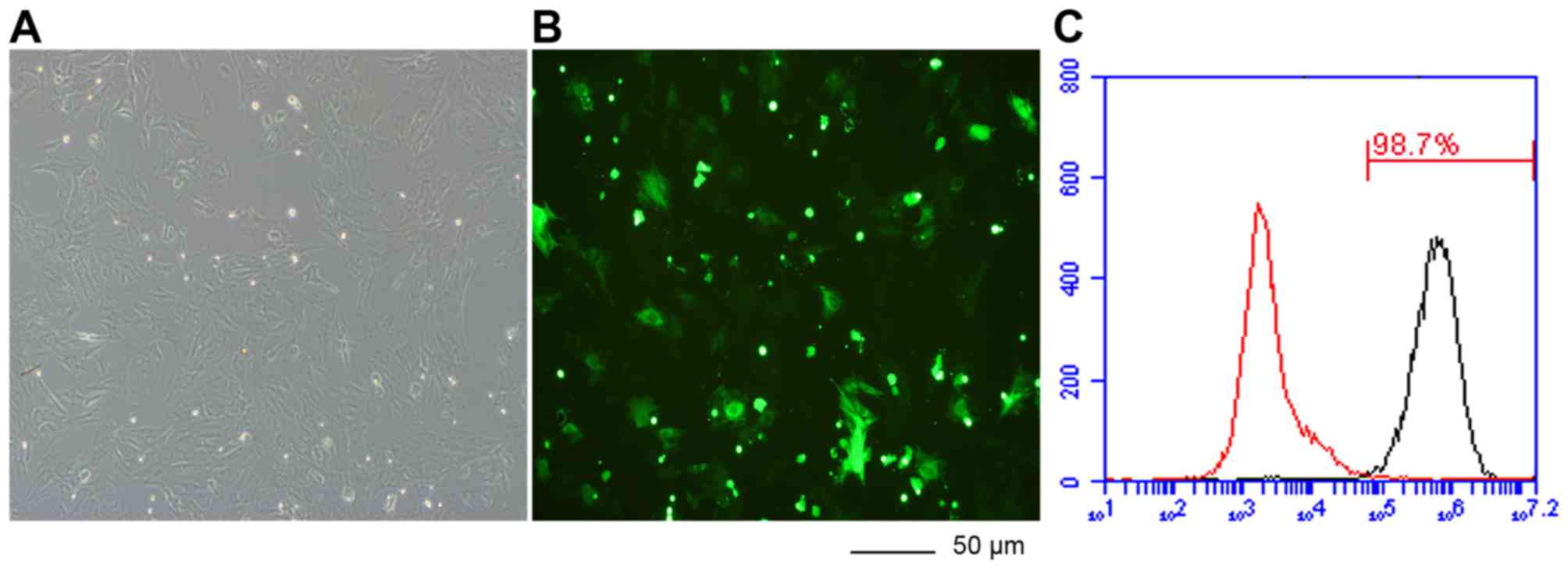
transfection. Transfection efficiency was analyzed by calculating
the number of GFP tagged cells out of the total number of cells
after 72–96 h following transfection. The BMSCs was imaged under a
fluorescence microscope (×200). Transfection rate was measured by
flow cytometry (9) (Fig. 2). The BMSCs of the rats were
isolated and cultured under standard conditions. The transfection
rate was >95%.
Local injection of rBMSCs
The rBMSCs which stably expressed
lenti-23a-mimic-GFP, lenti-23a-inhibitor-GFP or the lenti-GFP
control were harvested. The cells were treated with 2.5% trypsin
and resuspended in PBS. A total of 0.2 ml PBS containing
1×106 cells were prepared from the marrow cavity of the
femur of each animal. The injection technique was practiced in a
preliminary experiment (data not shown). The rats were anesthetized
by chloral hydrate (Pharmacy, Peking Union Medical College
Hospital, Peking, China). The midline approach to the knee was
taken. The skin of the knee was incised and a needle-mounted
syringe was inserted superior to the patellar and parallel to the
shaft of the femur. Once the initial resistance was overcome, the
needle was firmly attached, and could be inferred to be in the
femoral marrow cavity. The cell suspension was injected locally
into the bone marrow cavity from the mid-point of femoral condyles,
4 weeks following the establishment of the animal model.
Micro computed tomography (CT) scan
and quantitative analysis
A total of 4 weeks following the localized injection
of rBMSCs, the rats were sacrificed and the midline of the femoral
condyles was sampled and fixed in 10% buffered neutral formalin
solution until examination. The samples were scanned by Inveon
micro CT manufactured by Siemens AG (Munich, Germany) at a voltage
of 60 kV and a current of 400 µA, with entire scan length of 20 mm
in a spatial resolution of 10 µm. The images were reconstructed
using Inveon analysis workstation (version 2.0; Siemens AG). The
key features for femoral head necrosis diagnosis using micro CT
were fracture of the trabeculae, cystic degeneration, sclerotic
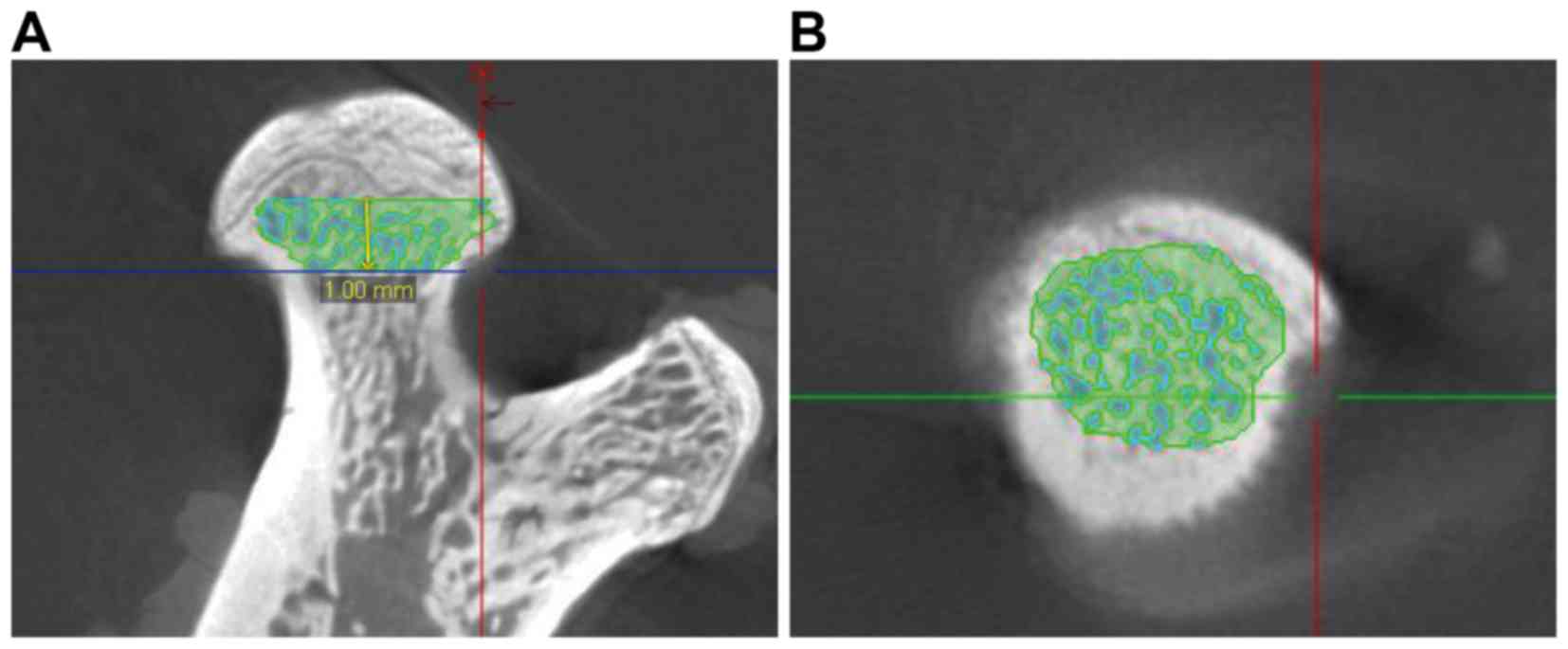
banding or flattening of the femoral head. A total of two
independent researchers performed concordant blind diagnosis. Out
of 50 transverse sections taken, 1 mm below the center of the
epiphyseal line was selected as the region of interest (ROI) for
the analysis and comparison of trabecula parameters (Fig. 3). On the cross-section, the
cortical bone and cancellous bone were separated manually by auto
trace. Then, the trabeculae and bone marrow were separated by the
threshold function. The threshold function could separate the bone
marrow and trabecular bone by adjusting the Min and Max CT
Unit-Hounsfield Unit (HU) to select the bone marrow and trabecular
bone separately according to their different HU. Subsequently, the
bone volume/total volume (BV/TV), bone surface area/bone volume
(BS/BV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and
trabecular spacing (Tb.Sp) in the ROI were calculated respectively
by the workstation.
Histopathology
The femur specimens were fixed in 10% buffered
neutral formalin solution for 72 h at room temperature and followed
by decalcification with 10% EDTA-0.1 M phosphate buffer (pH 7.4).
Following decalcification, the tissues were dehydrated in graded
ethanol, embedded in paraffin, cut into 4-µm-thick sections in the
coronal plane. Subsequently the specimens were processed with a
routine hematoxylin and eosin stain at room temperature with
hematoxylin stain for 5–20 min and the eosin for 30 sec, prior to
being evaluated for the degree of osteonecrosis present. All
sections were assessed blind by two independent authors using a
light microscope (Leica Microsystems GmbH, Wetzlar, Germany). The
diagnosis of osteonecrosis was established based on the presence of
empty lacunae or pyknotic nuclei of osteocytes in the bone
trabeculae, accompanied by surrounding bone marrow cell necrosis
(12). If the diagnosis differed
between the two examiners, a third opinion was sought.
Immunohistochemistry
Immunohistochemistry for LRP-5 was performed. The
4-µm-thick sections were deparaffinization with xylene at room
temperature, and rehydrated with graded alcohol, treated to aid
antigen retrieval and incubated with the primary antibody (1:300;
Goat anti-LRP5; Everest Biotech Ltd., Bicester, UK; cat. no.
EB06771) at 4°C overnight. Subsequently, the biotinylated secondary
antibody (rabbit anti-goat; Abcam, Cambridge, UK; cat. no. ab6741;
1:1,000) were applied at room temperature for 1 h and
3,3′-diaminobenzidine tetrahydrochloride substrate was used to
stain the sections. The sections were then treated with hematoxylin
and mounted. All sections were photographed using a light
microscope (Leica Microsystems GmbH) on the same setting. Each
section was imaged three times. Image-Pro Plus (version 6.0; Media
Cybernetics, Inc., PA, USA) was used to measure the mean density of
each photo.
Statistical analysis
The Student's t-test or Fisher's exact test was
performed to compare the variables between the two groups. Two-way
analysis of variance was performed to compare the variables more
than two groups. The least significant difference method was
applied. The parameters were expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS for
Windows (version 17.0; SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Micro CT scan of femoral head
In the present study three rats (two in the
miR-23a-3p mimic group and one in the NC group) succumbed to the
high dose of corticosteroid injection. A single femur sample was
damaged in the miR-23a-3p inhibitor group. The rest of the rats
were alive for the duration of the experiment. Therefore, in the
final analysis, 8, 11 and 10 samples were included in the mimic,
inhibitor and NC groups, respectively. The femoral head of the
miR-23a-3p inhibitor group exhibited a rounded shape and condense
trabeculae without significant cystic degeneration. By contrast,
the femoral head of the mi-23a-3p mimic group exhibited scattered
trabeculae and cystic degeneration (Fig. 4A).
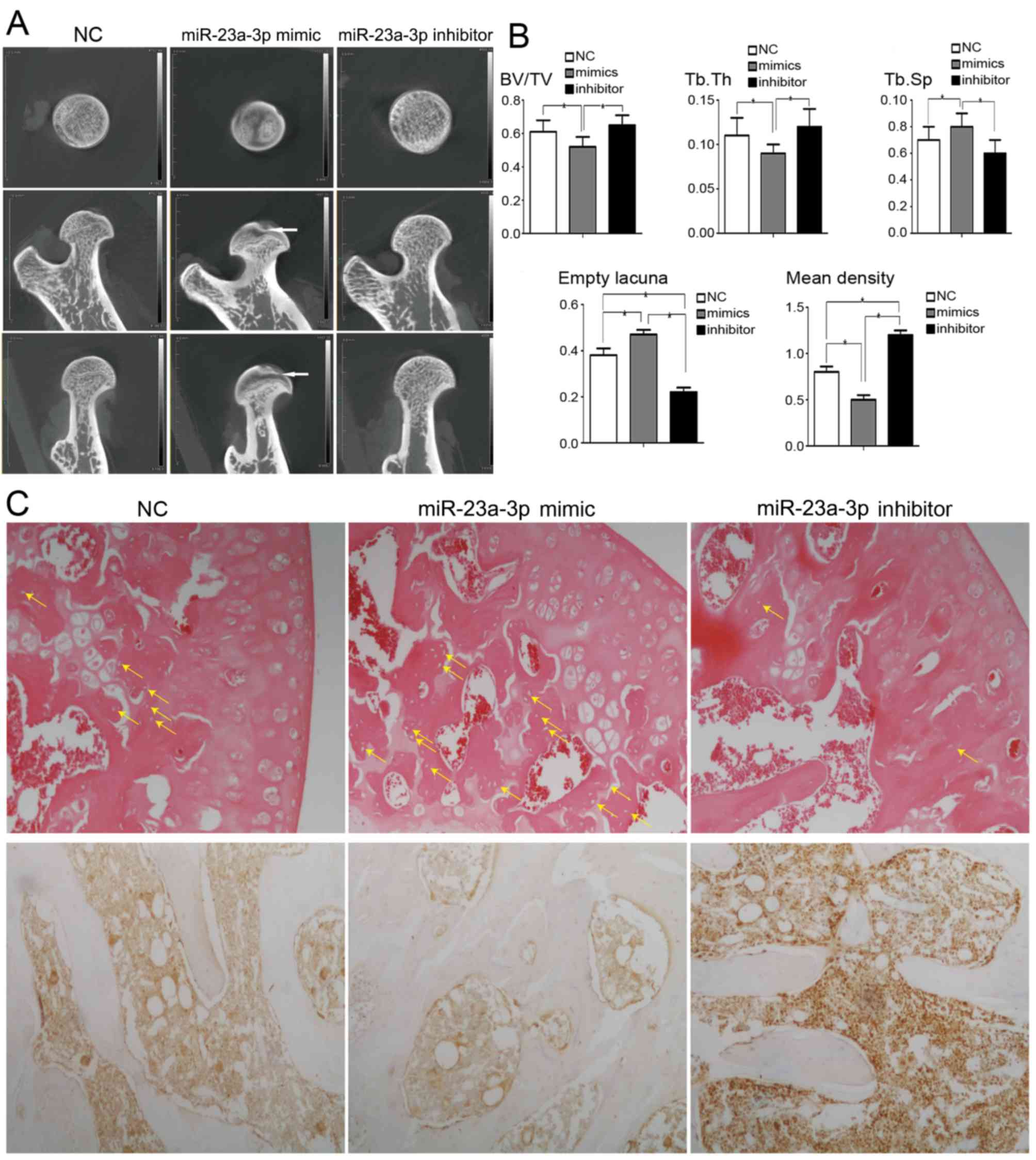 | Figure 4.Quantitative analysis of alterations
in femoral parameters associated with bone thickness and density.
(A) Representative micro computed tomography images of the proximal
femur. miR-23a-3p-inhibitor: The shape of the femoral head was
round with condensed trabeculae. miR-23a-3p-mimic: The femoral head
was flattened and the trabeculae were scattered with cystic
degeneration (white arrow). (B) Bar graphs demonstrating that BV/TV
and Tb.Th were lower in the miR-23a-3p mimic group, and Tb.Sp was
higher in the miR-23a-3p mimic group. The incidence of empty bone
lacunae number was lower in the miR-23a-3p inhibitor group. The
mean density of immunohistochemical images of LRP-5 positive cell
was higher in the miR-23a-3p inhibitor group. *P<0.05. (C) The
average number of empty bone lacuna (yellow arrow) was decreased in
the miR-23a-3p inhibitor group compared with NC and miR-23a-3p
mimic groups (hematoxylin and eosin stained; magnification, ×100).
Representative immunohistochemical images demonstrating a higher
intensity of LRP-5 staining in miR-23a-3p inhibitor group compared
with the NC and miR-23a-3p mimic groups (magnification, ×100)., NC,
negative control; BV/TV, bone volume/ total volume; Tb.Th,
trabecular thickness; Tb.Sp, trabecular spacing; LRP-5, low density
lipoprotein receptor-related protein-5; miR, microRNA. |
Quantitative analysis of the
trabeculae parameters
The quantitative analysis demonstrated that the
BV/TV and Tb.Th were significantly increased in the miR-23a-3p
inhibitor group compared with the miR-23a-3p mimic group. The Tb.Sp
was significantly decreased in the miR-23a-3p inhibitor group
compared with the miR-23a-3p mimic group (Fig. 4B).
Histopathology
The prevalence of osteonecrosis were 18.2% (2/11)
and 75% (6/8) in miR-23a-3p-inhibitor and miR-23a-3p-mimic groups,
respectively (P<0.05, mimic vs. inhibitor). The average number
of empty bone lacunae was significantly less in the miR-23a-3p
inhibitor group. The mean density of immunohistochemical images of
LRP-5 positive cell was significantly higher in the miR-23a-3p
inhibitor group (Fig. 4B and
C).
Discussion
A number of bone diseases, including osteoporosis
and femoral head necrosis may develop if the balance of BMSC
differentiation is disrupted. Understanding the mechanism of
osteogenic differentiation of BMSCs is crucial to getting an
improved insight in the pathogenesis of skeletal disorders and to
increase treatment options. Increasing evidence has indicated that
miRs serve a vital role in the osteogenic differentiation of
BMSCs.
miR-23a, located on chromosome 19p13.12, has been
widely researched in recent years. It has been reported that
miR-23a expression is upregulated in multiple types of cancer,
including hepatocellular carcinoma (13), lung cancer (14) and colorectal cancer (15), indicating that miR-23a participates
in the carcinogenesis and metastasis of cancer. In addition,
miR-23a has been proved to promote myelination in the central
nervous system (16). A recent
study revealed that miR-23a exhibited a significantly higher
expression level in the bone tissue of osteoporotic patients
compared with controls (17).
The authors previously identified that miR-23a was
involved in the osteogenesis of BMSCs. Inhibiting miR-23a enhanced
the osteogenic differentiation of BMSCs (10). In the present study, it was
demonstrated that the injection of a miR-23a-3p inhibitor could
decrease the incidence of osteonecrosis in a rat model. The authors
previously demonstrated that miR-23a was partially complementary to
a site in the 3′ untranslated region of LRP-5 (10). LRP-5 expression was increased
during the osteogenesis of BMSCs, while miR-23a-3p expression was
reduced. Furthermore, miR-23a-3p overexpression resulted in
downregulation of LRP-5, whereas functional inhibition of
miR-23a-3p upregulated LRP-5, suggesting that LRP-5 was regulated
by miR-23a during osteogenic differentiation. Immunohistochemical
staining also demonstrated increased intensity of LRP-5 staining in
the miR-23a-3p inhibitor group compared with the NC and miR-23a-3p
mimic group, which was consistent with the previous cell
experiment. LRP-5 is a single-pass transmembrane protein belonging
to the LRP family. LRP-5 is closely associated with homologue
LRP-6, which is another co-receptor for the canonical Wnt signaling
pathway. LRP-5 serves a crucial role in bone formation. Mice
lacking LRP-5 exhibit a decrease in bone mass, while LRP-5
activation increases bone mass (18). LRP-5 variants were proved to be
associated with low peak bone mass and osteoporosis, which may
control bone formation by inhibiting serotonin synthesis in the
duodenum (19).
Increasing evidence indicates that miR serves a
vital role in the development of various bone diseases. However,
the biological role of miR in the pathogenesis of non-traumatic
osteonecrosis of femoral head (ONFH) remains to be completely
investigated. Wu et al (20) used miR microarray chip analysis,
and revealed that 22 miRs were upregulated and 17 miRNAs were
downregulated in the non-traumatic ONFH samples compared with the
femoral neck fracture samples. Wang et al (21) also described abnormal expression of
miR in the serum of patients with ONFH. Yamasaki et al
(22) demonstrated that that
mature miR-210 was expressed around the necrotic area. In addition,
von Willebrand factor and vascular endothelial growth factor were
also highly expressed in the miR-210 expressing cells. Jia et
al (23) further studied the
level of mature miR-17-5p and demonstrated that it was
significantly decreased in non-traumatic osteonecrotic samples
compared with the osteoarthritis samples. miR-17-5p modulated
osteoblastic differentiation and cell proliferation by targeting
Mothers against decapentaplegic homolog 7 in non-traumatic
osteonecrosis (23). To the best
of the authors' knowledge, the present study was the first study to
investigate the role of miR in femoral head necrosis in an animal
model. miR may provide a novel and alternative approach for
understanding the mechanism underlying steroid-associated necrosis
of the femoral head.
In conclusion, inhibition of miR-23a caused a lower
incidence of osteonecrosis, and higher expression of BV/TV and
Tb.Th. miR-23a-3p may be used as a potential biomarker and
therapeutic target of femoral head necrosis in the future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81272009).
References
|
1
|
Hernigou P, Beaujean F and Lambotte JC:
Decrease in the mesenchymal stem-cell pool in the proximal femur in
corticosteroid-induced osteonecrosis. J Bone Joint Surg Br.
81:349–355. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hernigou P and Beaujean F: Abnormalities
in the bone marrow of the iliac crest in patients who have
osteonecrosis secondary to corticosteroid therapy or alcohol abuse.
J Bone Joint Surg Am. 79:1047–1053. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang JK, Ho ML, Yeh CH, Chen CH and Wang
GJ: Osteogenic gene expression decreases in stromal cells of
patients with osteonecrosis. Clin Orthop Relat Res. 453:286–292.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JS, Lee JS, Roh HL, Kim CH, Jung JS
and Suh KT: Alterations in the differentiation ability of
mesenchymal stem cells in patients with nontraumatic osteonecrosis
of the femoral head: Comparative analysis according to the risk
factor. J Orthop Res. 24:604–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cárcamo-Orive II, Gaztelumendi A, Delgado
J, Tejados N, Dorronsoro A, Fernández-Rueda J, Pennington DJ and
Trigueros C: Regulation of human bone marrow stromal cell
proliferation and differentiation capacity by glucocorticoid
receptor and AP-1 crosstalk. J Bone Miner Res. 25:2115–2125. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rauch A, Seitz S, Baschant U, Schilling
AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A,
Schmidt-Ullrich R, et al: Glucocorticoids suppress bone formation
by attenuating osteoblast differentiation via the monomeric
glucocorticoid receptor. Cell Metab. 11:517–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gangaraju VK and Lin H: MicroRNAs: Key
regulators of stem cells. Nat Rev Mol Cell Biol. 10:116–125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lakshmipathy U and Hart RP: Concise
review: MicroRNA expression in multipotent mesenchymal stromal
cells. Stem Cells. 26:356–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li T, Li H, Li T, Fan J, Zhao RC and Weng
X: MicroRNA expression profile of dexamethasone-induced human bone
marrow-derived mesenchymal stem cells during osteogenic
differentiation. J Cell Biochem. 115:1683–1691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Li H, Wang Y, Li T, Fan J, Xiao K,
Zhao RC and Weng X: microRNA-23a inhibits osteogenic
differentiation of human bone marrow-derived mesenchymal stem cells
by targeting LRP5. Int J Biochem Cell Biol. 72:55–62. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tong P, Wu C, Jin H, Mao Q, Yu N, Holz JD,
Shan L, Liu H and Xiao L: Gene expression profile of
steroid-induced necrosis of femoral head of rats. Calcif Tissue
Int. 89:271–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto T, Irisa T, Sugioka Y and Sueishi
K: Effects of pulse methylprednisolone on bone and marrow tissues:
Corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum.
40:2055–2064. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao L, Zhao J, Dai X, Wang Y, Ma R, Su Y,
Cui H, Niu J, Bai S, Xiao Z, et al: Correlation between miR-23a and
onset of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol.
38:318–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mengru C, Masahiro S, Chie S, Hideaki M,
Kazuhiro K, Yuji M, Rintaro N, Akinobu Y, Li C and Akihiko G:
MiR-23a regulates TGF-es TGF- Yuji M, Rintaro N, Akinobu Y, Li C
and Akihiko G: MiR-23a regulates TGF-s TGF-y 38: 318–323, 2014.
Oncology. 41:869–875. 2012.
|
|
15
|
Jahid S, Sun J, Edwards RA, Dizon D,
Panarelli NC, Milsom JW, Sikandar SS, Gümüs ZH and Lipkin SM:
miR-23a promotes the transition from indolent to invasive
colorectal cancer. Cancer Discov. 2:540–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin ST, Huang Y, Zhang L, Heng MY, Ptácek
LJ and Fu YH: MicroRNA-23a promotes myelination in the central
nervous system. Proc Natl Acad Sci USA. 110:pp. 17468–17473. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seeliger C, Karpinski K, Haug AT, Vester
H, Schmitt A, Bauer JS and van Griensven M: Five freely circulating
miRNAs and bone tissue miRNAs are associated with osteoporotic
fractures. J Bone Miner Res. 29:1718–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui Y, Niziolek PJ, MacDonald BT, Zylstra
CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon
RA, et al: Lrp5 functions in bone to regulate bone mass. Nat Med.
17:684–691. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yadav VK, Ryu JH, Suda N, Tanaka KF,
Gingrich JA, Schütz G, Glorieux FH, Chiang CY, Zajac JD, Insogna
KL, et al: Lrp5 controls bone formation by inhibiting serotonin
synthesis in the duodenum. Cell. 135:825–837. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu X, Zhang Y, Guo X, Xu H, Xu Z, Duan D
and Wang K: Identification of differentially expressed microRNAs
involved in non-traumatic osteonecrosis through microRNA expression
profiling. Gene. 565:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Qian W, Wu Z, Bian Y and Weng X:
Preliminary screening of differentially expressed circulating
microRNAs in patients with steroid-induced osteonecrosis of the
femoral head. Mol Med Rep. 10:3118–3124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamasaki K, Nakasa T, Miyaki S, Yamasaki
T, Yasunaga Y and Ochi M: Angiogenic microRNA-210 is present in
cells surrounding osteonecrosis. J Orthop Res. 30:1263–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia J, Feng X, Xu W, Yang S, Zhang Q, Liu
X, Feng Y and Dai Z: MiR-17-5p modulates osteoblastic
differentiation and cell proliferation by targeting SMAD7 in
non-traumatic osteonecrosis. Exp Mol Med. 46:e1072014. View Article : Google Scholar : PubMed/NCBI
|