Introduction
Non-traumatic osteonecrosis of the femoral head
(NONFH) is a common clinical osteoarthropathy resulting from the
interruption of blood supply to the femoral head following an
insulting event (1). In the past
decade, there has been a steady increase in the worldwide
prevalence of NONFH, owing to the growing risk factors, increased
public awareness of the disease and as a side effect of multiple
medications, for example, corticosteroids (2). A nationwide survey conducted from
2012 to 2013 in China revealed a 0.7% prevalence in tested
subjects, which in translation estimated >8 million NONFH cases
in the Chinese population aged ≥15 years (3).
Despite recent advances in the understanding of
pathophysiologic mechanisms, NONFH remains a debilitating condition
that may result in significant morbidity. The current clinical
treatment modalities of NONFH include nonoperative conservative
approaches, joint-preserving surgical procedures and total
arthroplasty. Nonoperative conservative treatments are usually
indicated in the precollapse disease. This ranges from lifestyle
modifications such as weight-bearing restrictions, to physical
therapies with electrical stimulation, extracorporeal shock wave,
hyperbaric oxygen, and magnetic therapy, and medical management
with nonsteroidal anti-inflammatory drugs or narcotics for
symptomatic relief. Low molecular weight heparin has also been
suggested to prevent the progression of precollapse osteonecrosis
in thrombophilic patients. Dietary regimens and alternative
medicine have also been tried, with their therapeutic efficacy
still in debate (2). Most patients
eventually require surgical interventions such as total hip
arthroplasty (4,5).
Genetic and molecular biology studies have indicated
that NONFH may be closely associated with environmental and genetic
factors (3,6), and its pathological progression may
involve cytokines, nuclear factor kappa-B (NFκ-B) (7,8), and
transforming growth factors (9).
For example, a small clinical study by Gómez-Mont Landerreche et
al (7) demonstrated the use of
pegylated interferon (IFN) in patients with hepatitis C was
associated with bilateral avascular necrosis of the femoral
head.
Transforming growth factor β1 (TGF-β1) is a member
of the TGF-β superfamily of cytokines and serves a crucial role in
diverse cellular processes such as proliferation, differentiation,
apoptosis and immune regulation (10,11).
TGF-β1 has been studied for its implication in the pathogenesis of
NONFH, and has been proposed as a therapeutic target for NONFH
management (9). Overexpression of
TGF-β1 was observed in the transition area between vascular tibia
grafts and irradiated bone in an experimental model of
osteonecrosis (12).
However, there has been no direct investigation of
TGF-β1 in a clinical model of NONFH. The present study determined
the association between TGF-β1 expression, necrotic bone
architecture and remodeling, and systemic inflammation in NONFH.
This may further understanding of the pathophysiology of NONFH, and
provide a mechanistic basis for the clinical development of TGF-β1
targeted therapy for the conservative management of NONFH.
Materials and methods
Patients
Patients (3 males and 17 females) aged between 35
and 56 years were admitted and received hip replacement surgery
between October 2013 and October 2014, and were recruited from the
Department of Orthopaedics at Huainan People's Hospital (Huainan,
China). They had no previous history of trauma and surgery. This
experimental group comprised 20 cases: 16 patients had stage III
NONFH and 4 patients with stage IV NONFH. The control group (9
males and 11 females) aged between 32 and 57 years, comprised 20
cases of fresh femoral neck fractures but with no other chronic
diseases or family history of genetic disorders. The study was
approved by the Ethics Committee of Huainan People's Hospital
(Huainan, China). All participants provided written informed
consent.
Ficat-Arlet classification
The Ficat-Arlet classification was used to identify
the stages of patients (13).
Patients were classified according to the following criteria: i)
Stage 0, no symptoms and normal radiograph (‘suspected stage’); ii)
stage I, normal radiograph or mild diffuse osteoporosis; iii) stage
II, the radiograph revealed signs of reconstruction but no
alterations in the shape of the femoral head and the joint space,
osteoporosis, osteosclerosis and cystic degeneration in the
necrotic area, and a bone marrow core biopsy revealed
histopathological alterations; iv) transitional stage,
characterized by subchondral fracture (crescent sign) and localized
flattening of the femoral head; v) stage III, the radiograph
revealed sclerosis and cystic degeneration inside the femoral head,
collapse of the femoral head, crescent sign and normal joint space;
and vi) stage IV, the radiograph revealed the collapse of the
femoral head and narrowing of the joint space.
Collection and processing of
specimens
The femoral heads were collected during total hip
replacement were cut in half along the coronal plane. Subsequently,
a section of bone (1.0×1.0×0.3 cm) was obtained from the necrotic
area and the adjacent non-necrotic area with a chisel, fixed with
4% paraformaldehyde (containing 0.1% DEPC) for 12 h, and placed in
13% EDTA (pH 7.0) for microwave decalcification. After 6–14 days,
complete decalcification was confirmed as no resistance to puncture
by a needle, and the bone sample was embedded in paraffin and cut
into 5-µm thick sections.
Histology and
immunohistochemistry
Hematoxylin and eosin (H&E) staining was
performed to allow pathological examination of tissue sections. The
expression of TGF-β1 was detected by immunohistochemistry. Bone
tissues were embedded in paraffin, cut, and sections were placed
onto slides. Slides were subsequently dewaxed and rehydrated. After
antigen retrieval with 0.01 mol/l sodium citrate (Jing An
Biological Technology, Shanghai, China), slides were blocked with
1% bovine serum albumin (Leagene Biotechnology, Beijing, China),
and incubated at 4°C overnight with anti-TGF-β1 antibody (1:200;
cat no. E-CL-H0109c; Boster Bioloigcal Technology, Pleasanton, CA,
USA). After an overnight incubation, slides were washed with PBS,
and then incubated in the dark at 37°C with a horse radish
peroxidase-conjugated goat anti-mouse secondary antibody (1:50;
R&D Systems, Inc., Minneapolis, MN, USA) for 30 min. After
color development with 3,3′-diaminobenzidine (DAB; Leagene
Biotechnology) and routine hematoxylin nuclear counterstain, the
sections were observed, and images were captured by two associate
chief physicians of the Department of Pathology at Huainan No. 1
People's Hospital. The integrated optical density (IOD) values were
quantified using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Flow cytometry
Flow cytometry followed the manufacturer protocol
(BD Biosciences, Franklin Lakes, NJ, USA). The BD Multitest
antibody kit (cat no. 342417; BD Biosciences) contained fluorescein
isothiocyanate-labeled CD3 (clone SK7), PE-labeled CD8 (clone SK1),
PerCP-labeled CD45 (clone 2D1, HLe-1) and APC-labeled CD4 (clone
SK3). In brief, peripheral blood was collected via venipuncture and
anticoagulated with EDTA (BD Trucoun tube; cat no. 342447). A total
of 50 µl fresh blood was mixed with 20 µl antibody cocktail for 15
min at room temperature in the dark. Subsequently, erythrocytes
were lysed with the supplied BD FACS lysis buffer. The mixture was
analyzed with a BD FACSCanto II flow cytometry system. The
CellQuest software CXP version 2.0 (BD Biosciences) was used to
quantify the T-lymphocyte subsets in the peripheral blood.
Statistical analysis
Statistical analyses were performed using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard error. Differences among 3 or more
groups were compared by analysis of variance followed by the
Bonferroni post-hoc test for multiple comparisons. Differences
among 2 groups were compared by unpaired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Radiological findings
Radiograph A revealed that In the experimental
group, patients with stage IV NONFH had the typical manifestations
of the disease, including deformation and collapse of the femoral
head, narrowing of the joint space, loss of the articular
cartilage, and formation of acetabular osteophytes. Radiograph B
revealed that in the control group, there were signs of bone
discontinuity and soft tissue incarcerated between the two ends of
fracture, including a femoral neck fracture (data not shown).
Histological findings
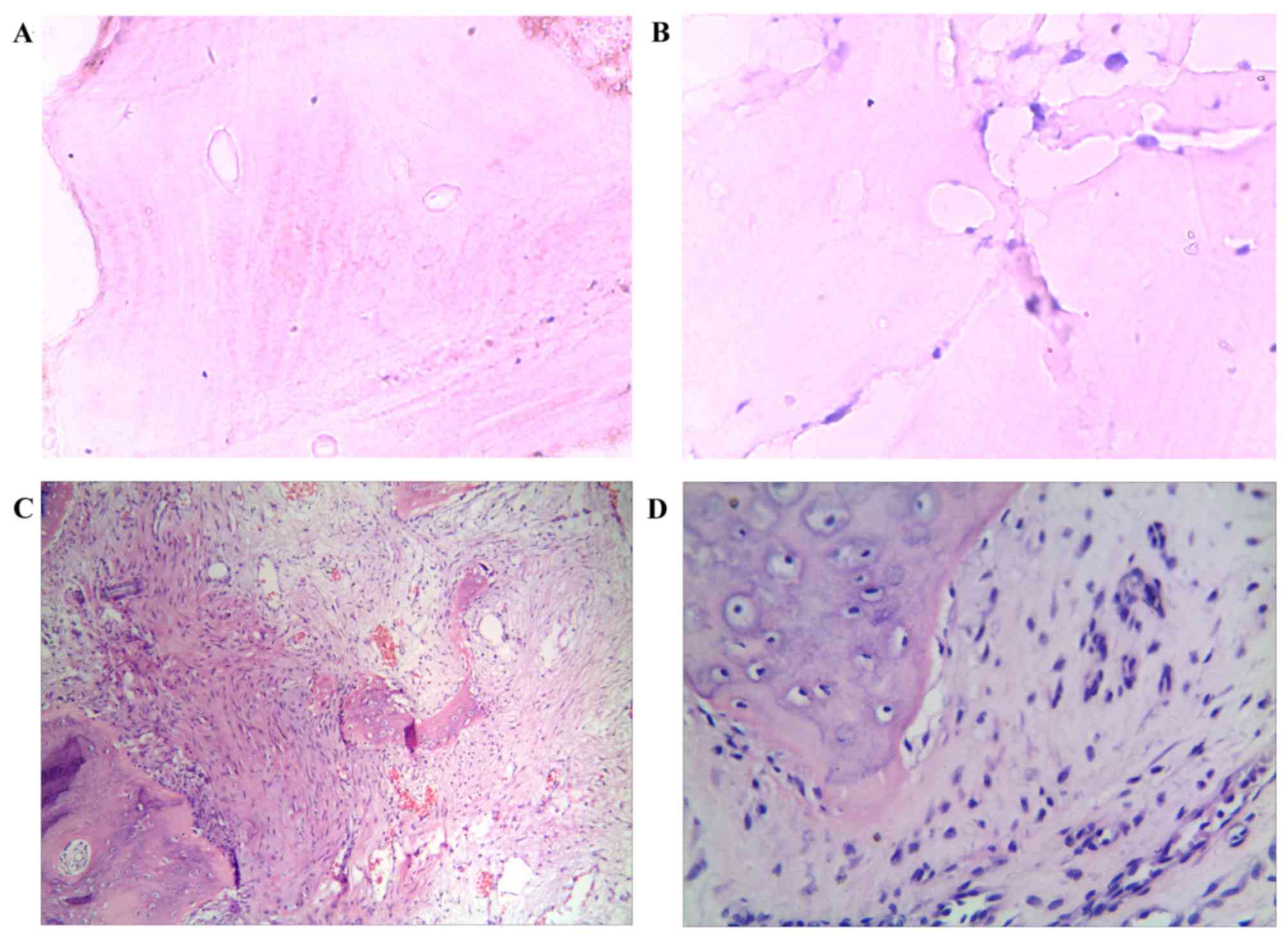
The control group cartilage was thick, there was
active osteogenesis, the bone trabeculae was regularly arranged,
the circumferential lamellae appeared to be closely and regularly
arranged in parallel, the osteocyte lacunae were located between or
within the lamellae and were dispersively arranged, there was no
osteonecrosis or proliferation of fibrous tissues (Fig. 1A and B). In the experimental group,
the cartilage was thin, the subchondral trabeculae became thin with
increased spacing and collapse, the absence of osteocyte lacunae
was evident and the circumferential lamellae were not arranged in
order. This was accompanied by the dissolution or fracture of
lamellae, the necrosis and disintegration of osteocytes, and
karyopyknosis and karyorrhexis were visible (Fig. 1C and D). In the surrounding
tissues, hyperemia and edema were present and lymphocyte and plasma
cell infiltration, bone marrow necrosis and proliferation of
fibrous granulation tissues were also evident (Fig. 1C and D).
Immunohistochemical findings
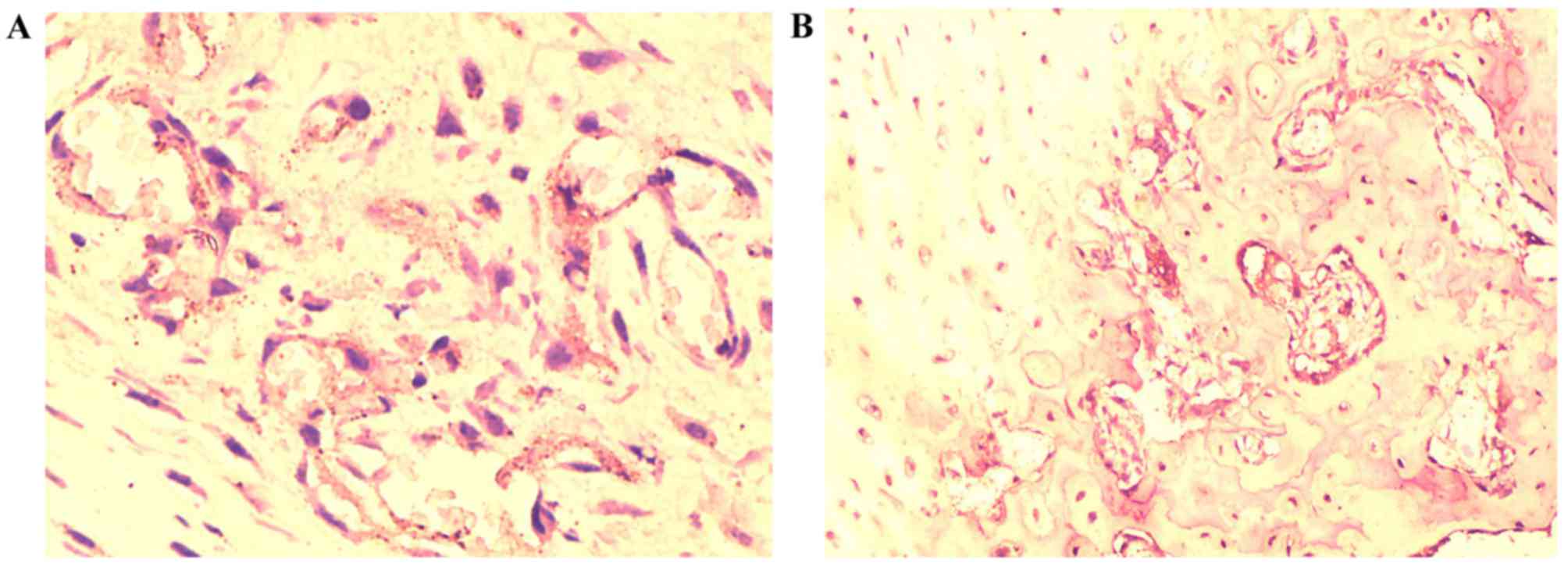
Following immunohistochemical staining, the brown
granules inside the femoral head were visible and were considered
to indicate specific TGF-β1 expression. TGF-β1 expression was
present in experimental and control groups. In the experimental
group, expression was markedly reduced in the necrotic area and was
present only in the surrounding area, whereas in the control group,
TGF-β1 expression was primarily located in the cytoplasm, nucleus
and plasma membrane of osteocytes (Fig. 2). The positive expression area in
the experimental group was reduced compared with the control group
(Fig. 2).
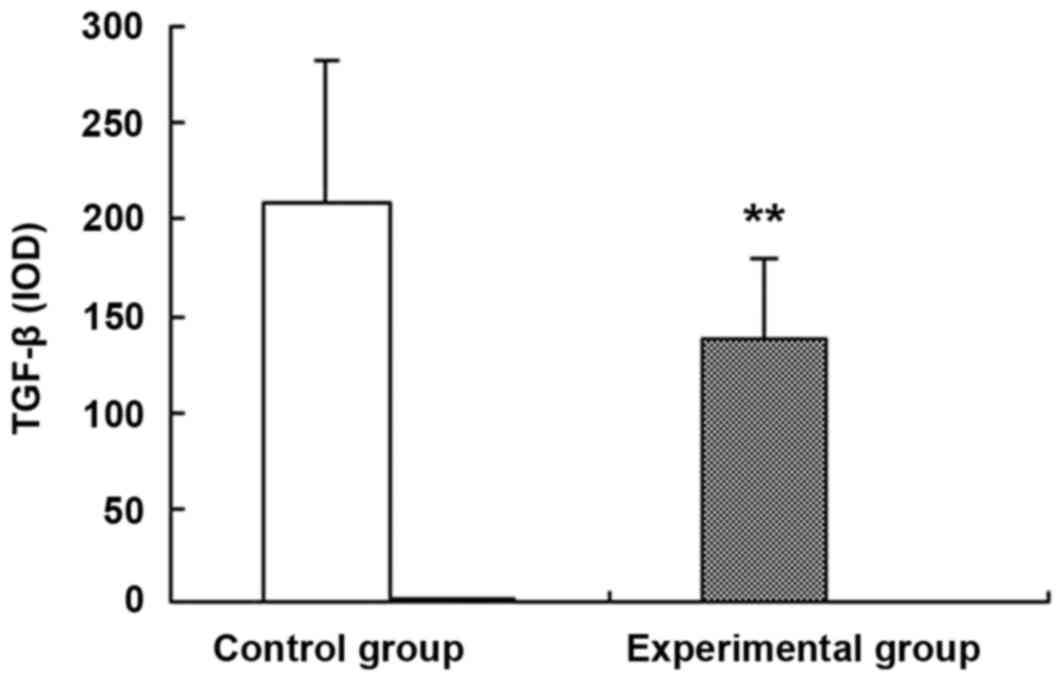
Image-Pro Plus software was used to perform a
semi-quantitative analysis of the IOD values of TGF-β1 expression
(Fig. 3). The mean IOD value in
the control group was 209±73, whereas the experimental group was
137±43; therefore, the expression of TGF-β1 in the experimental
group was significantly reduced compared with the control group
(P<0.01; Fig. 3).
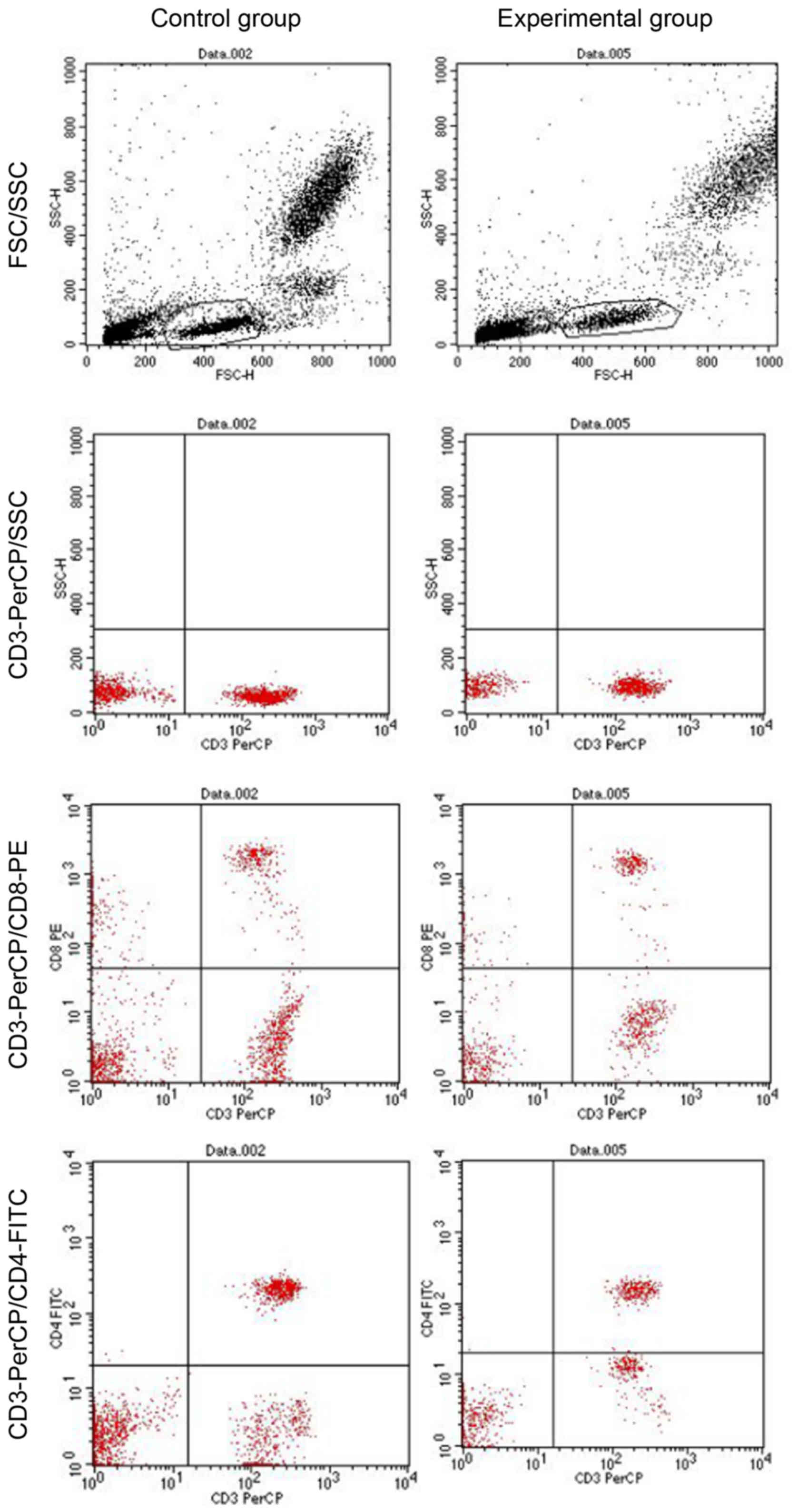
Detection of T-lymphocyte subsets
CellQuest software was used to detect the
percentages of CD3+, CD4+ and CD8+
cells and the ratio of CD4+ to CD8+ cells.
The findings revealed that the experimental group had reduced
CD3+ and CD4+ cell populations (P<0.05,
P<0.01), an increased CD8+ population (P<0.05) and
a reduced ratio of CD4+ to CD8+ cells,
compared with the control group (P<0.01; Fig. 4 and Table I).
 | Table I.Quantitation of flow cytometry. |
Table I.
Quantitation of flow cytometry.
| Group | n | CD3+
(%) | CD4+
(%) | CD8+
(%) |
CD4+/CD8+ |
|---|
| Experimental
group | 20 | 62.4±9.3a | 31.4±7.9b | 37.1±5.2a | 0.9±0.4b |
| Control group | 20 | 76.7±5.9 | 42.3±4.6 | 32.7±5.3 | 1.3±0.3 |
Discussion
The typical pathological features of NONFH include
decreased density of the femoral head, cystic degeneration of the
bone, fracture of the trabecular structure and deformity and
collapse of the femoral head (14). However, bone injuries that may not
be detected by radiographic examinations are often present during
the initial stage of NONFH. At this stage, cells in the local
tissues initiate the complex mechanism underlying repair via the
production of various cytokines including IL-17A, IFN-γ and TNF-α
(15). The molecular mechanism
underlying these events remains to be elucidated and when it fails
to prevent the progression of bone injuries, structural damage and
dysfunction of the bone will occur due to local osteonecrosis that
increases with the progression of NONFH. The structural injury to
the femoral head deteriorates with such progression and self-repair
functions will be limited (14–17).
The pathological alterations of avascular necrosis of the femoral
head contribute to a sequential process that deteriorates and
progresses rapidly.
As aforementioned, the healing and repair of bone
tissue following trauma is a complex and continuous process that
involves multiple cytokines such as interferons and transforming
growth factors, and an array of intracellular signaling pathways,
including the NF-κB and TGF-β/bone morphogenic protein signaling
(18–22).
TGF-β1 is a member of TGF-β superfamily, the primary
biological functions of which include the regulation of cell
growth, immune activity and extracellular matrix components
(23–25). Previous studies have confirmed that
promoting the secretion and synthesis of extracellular matrix
components is a primary mechanism responsible for the effect of
TGF-β1 on osteogenesis, in which TGF-β1 may regulate osteoblast
proliferation and differentiation, stimulate new bone formation,
inhibit matrix metalloproteinase activity, and prevent the
degradation of matrix macromolecules, thereby promoting bone
formation (26–30). In addition, TGF-β1 may be involved
in the early process of callus formation.
Therefore, TGF-β1 is an important cell signaling
factor in the regulation of the trauma-healing processes, which is
involved in the growth and differentiation of osteocytes and the
synthesis of the extracellular matrix. The extracellular matrix is
a structural complex formed by collagens, non-collagenous
glycoproteins, hyaluronan and proteoglycans. It is not only a
scaffold for embedded cells, but it is also a reservoir for various
growth factors and cytokines necessary for cell activation and
turnover. Thus, the extracellular matrix is critical for the
development of cartilage and bone, and the repair and healing
process following a bone fracture.
TGF-β1 is involved in almost all trauma-healing
processes and it is widely distributed in the bone tissues,
platelets and cartilage; therefore, it may additionally be involved
in the process of new bone formation following avascular necrosis
of the bone. The results of the present study suggested that TGF-β1
was expressed in the control group, which may indicate good bone
repair capacity. By comparison, the expression of TGF-β1 in the
necrotic areas of NONFH was significantly decreased. This was
likely a reflection of diminished expression of TGF-β1, or that few
cells remained variable in the necrotic area. In either case,
TGF-β1 downregulation was associated with a reduced bone repair
capacity. The experimental group had significantly downregulated
TGF-β expression compared with the control group (P<0.01).
Therefore, TGF-β may have a crucial role in the formation of new
bone in the control group, whereas it was significantly inhibited
in the experimental group. The histopathological findings
additionally revealed that there was new bone formation and reduced
osteonecrosis and trabecular destruction in the control group,
whereas in the experimental group, novel bone tissues were
negligible and there was severe osteocyte necrosis, trabecular
destruction and extensive lymphocyte infiltration. The findings of
the present study revealed that the control group had active TGF-β1
expression. Therefore, patients in the control group retained bone
repair capacity following traumatic osteonecrosis of the femoral
head, whereas the experimental group had reduced bone tissue
regeneration capacity and their bone repair capacity was absent.
Furthermore, the present study determined that the experimental
group had numerous fibrous granulation tissues surrounding the
necrotic femoral head, whereas the control group had few fibrous
granulation tissues; this provided further evidence that patients
with NONFH had reduced bone repair capacity.
T lymphocytes serve an important role in the
regulation and maintenance of the immune system and clinically, the
immune status of patients may be assessed by quantification of the
T-lymphocyte subsets, which is additionally important for the
diagnosis and prognosis of patients' condition. T lymphocytes may
be divided into different subsets according to their surface
markers. The CD4+ cell population contains helper T
cells. The CD8+ cell population contains suppressor T
cells, which are also termed cytotoxic T cells. The two subsets
suppress and aid each other to maintain the balance of immune
functions. In the present study, T-lymphocyte subsets in the
peripheral blood were detected by the flow cytometry. It was
determined that the percentage of CD3+ T cells in the
experimental group peripheral blood was reduced compared with the
control group. The percentage of CD4+ cells in the
experimental group was decreased compared with the control group.
Additionally, the percentage of CD8+ cells in the
experimental group was increased. The
CD4+/CD8+ ratio in the experimental group was
reduced compared with the control group, which may suggest damaged
immune functions and abnormal generation or function of T cells in
patients with NONFH.
Regardless of the cause of NONFH, chronic
inflammatory reactions were present in the experimental group and
the alterations in T-lymphocyte subsets may be an indication of
various inflammatory reactions. Therefore, abnormal percentages of
CD3+, CD4+ and CD8+ cells, and the
altered ratio of CD4+ to CD8+ cells suggested
that chronic inflammatory reactions and immune regulation were
present in patients with NONFH. Additionally, important events in
NONFH pathology occur due to various factors; therefore, the
detection of T-lymphocyte subsets may be crucial for successful
clinical treatment and prognosis. It would be of interest to
incorporate heathy control femoral heads, perhaps harvested
postmortem, for a pairwise comparison in a future study. This would
allow further delineation in the differences in TGF-β1 expression,
histological alterations, and systemic T-lymphocyte mediated
inflammation observed in the current study.
In conclusion, the present study revealed that
decreased TGF-β1 expression was associated with altered bone
architecture and remodeling, and systemic immune functions in adult
patients with NONFH. TGF-β1 is a cytokine involved in various
processes. The present study supports the notion that exogenous
introduction of TGF-β1, either by direct intraarticular injection
or targeted overexpression, may be an effective treatment for bone
injuries and bone repair at certain stages of NONFH. However,
substantial future work is required to understand the complex
underlying mechanism of mutual regulations of multiple cytokines,
as is the complete decipher of principles underlying bone
self-repair and regeneration in NONFH.
Acknowledgements
The present study was supported by the Science and
Technology Plan Project of Huainan (grant no. 2015012), and the
Doctoral Fund Project of Anhui University of Science and Technology
(grant no. 11740).
References
|
1
|
Mont MA, Jones LC and Hungerford DS:
Nontraumatic osteonecrosis of the femoral head: Ten years later. J
Bone Joint Surg Am. 88:1117–1132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mont MA, Cherian JJ, Sierra RJ, Jones LC
and Lieberman JR: Nontraumatic osteonecrosis of the femoral head:
Where do we stand today? A ten-year update. J Bone Joint Surg Am.
97:1604–1627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang
BJ, Gao XH, Guo YM, Xu YQ, Wei YS, et al: Prevalence of
nontraumatic osteonecrosis of the femoral head and its associated
risk factors in the Chinese population: Results from a nationally
representative survey. Chin Med J (Engl). 128:2843–2850. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan G, Kang PD and Pei FX: Glucocorticoids
affect the metabolism of bone marrow stromal cells and lead to
osteonecrosis of the femoral head: A review. Chin Med J (Engl).
125:134–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zalavras CG and Lieberman JR:
Osteonecrosis of the femoral head: Evaluation and treatment. J Am
Acad Orthop Surg. 22:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pouya F and Kerachian MA: Avascular
necrosis of the femoral head: Are any genes involved? Arch Bone Jt
Surg. 3:149–155. 2015.PubMed/NCBI
|
|
7
|
Gómez-Mont Landerreche JG, Gil-Orbezo F,
Morales-Dominguez H, Navarrete-Álvarez M, Trueba-Davalillo C and
Capuano-Tripp P: Nontraumatic causes of bilateral avascular
necrosis of the femoral head: Link between hepatitis C and
pegylated interferon. Acta Ortop Mex Mex and pegylated interferon.
Acta Ortop Mex. 29:172–175. 2015.(In Spanish). PubMed/NCBI
|
|
8
|
Farrier AJ, Franco LC Sanchez, Shoaib A,
Gulati V, Johnson N, Uzoigwe CE and Choudhury MZ: New
anti-resorptives and antibody mediated anti-resorptive therapy.
Bone Joint J. 98-B:1–165. 2016. View Article : Google Scholar :
|
|
9
|
Mont MA, Jones LC, Einhorn TA, Hungerford
DS and Reddi AH: Osteonecrosis of the femoral head. Potential
treatment with growth and differentiation factors. Clin Orthop
Relat Res. (355 Suppl): S314–S335. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Letterio JJ and Roberts AB: Regulation of
immune responses by TGF-beta. Annu Rev Immunol. 16:137–161. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massagué J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schultze-Mosgau S, Lehner B, Rödel F,
Wehrhan F, Amann K, Kopp J, Thorwarth M, Nkenke E and Grabenbauer
G: Expression of bone morphogenic protein 2/4, transforming growth
factor-beta1, and bone matrix protein expression in healing area
between vascular tibia grafts and irradiated bone-experimental
model of osteonecrosis. Int J Radiat Oncol Biol Phys. 61:1189–1196.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mouzas OD, Zibis AH, Bonotis KS,
Katsimagklis CD, Hadjigeorgiou GM, Papaliaga MN, Dimitroulias AP
and Malizos KN: Psychological distress, personality traits and
functional disability in patients with osteonecrosis of the femoral
head. J Clin Med Res. 6:336–344. 2014.PubMed/NCBI
|
|
14
|
Ficat RP: Idiopathic bone necrosis of the
femoral head. Early diagnosis and treatment. J Bone Joint Surg Br.
67:3–9. 1985.PubMed/NCBI
|
|
15
|
Zhang H, Xiao F, Liu Y, Zhao D, Shan Y and
Jiang Y: A higher frequency of peripheral blood activated B cells
in patients with non-traumatic osteonecrosis of the femoral head.
Int Immunopharmacol. 20:95–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng L, Wang W, Ni J, Li Z and Xiao T:
The association of eNOS gene polymorphism with avascular necrosis
of femoral head. PLoS One. 9:e875832014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hong YC, Luo RB, Lin T, Zhong HM and Shi
JB: Efficacy of alendronate for preventing collapse of femoral head
in adult patients with nontraumatic osteonecrosis. Biomed Res Int.
2014:7165382014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakaguchi M, Tanaka T, Fukushima W, Kubo T
and Hirota Y; Idiopathic ONF Multicenter Case-Control Study Group,
: Impact of oral corticosteroid use for idiopathic osteonecrosis of
the femoral head: A nationwide multicenter case-control study in
Japan. J Orthop Sci. 15:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lykissas MG, Gelalis ID, Kostas-Agnantis
IP, Vozonelos G and Korompilias AV: The role of hypercoagulability
in the development of osteonecrosis of the femoral head. Orthop Rev
(Pavia). 4:e172012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong JM, Kim TH, Kim HJ, Park EK, Yang EK
and Kim SY: Genetic association of angiogenesis- and
hypoxia-related gene polymorphisms with osteonecrosis of the
femoral head. Exp Mol Med. 42:376–385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seamon J, Keller T, Saleh J and Cui Q: The
pathogenesis of nontraumatic osteonecrosis. Arthritis.
2012:6017632012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Plaas A, Velasco J, Gorski DJ, Li J, Cole
A, Christopherson K and Sandy JD: The relationship between
fibrogenic TGFβ1 signaling in the joint and cartilage degradation
in post-injury osteoarthritis. Osteoarthritis Cartilage.
19:1081–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Finnson KW, McLean S, Di Guglielmo GM and
Philip A: Dynamics of transforming growth factor beta signaling in
wound healing and scarring. Adv Wound Care (New Rochelle).
2:195–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Penn JW, Grobbelaar AO and Rolfe KJ: The
role of the TGF-β family in wound healing, burns and scarring: A
review. Int J Burns Trauma. 2:18–28. 2012.PubMed/NCBI
|
|
26
|
Bastian O, Pillay J, Alblas J, Leenen L,
Koenderman L and Blokhuis T: Systemic inflammation and fracture
healing. J Leukoc Biol. 89:669–673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang H, Gao F, Li X, Wang J, Liu H and
Zheng Z: TGF-β1 antagonizes TNF-α induced up-regulation of matrix
metalloproteinase 3 in nucleus pulposus cells: Role of the ERK1/2
pathway. Connect Tissue Res. 56:461–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rivas M Noval, Weatherly K, Hazzan M,
Vokaer B, Dremier S, Gaudray F, Goldman M, Salmon I and Braun MY:
Reviving function in CD4+ T cells adapted to persistent systemic
antigen. J Immunol. 183:4284–4291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumarasinghe DD, Sullivan T, Kuliwaba JS,
Fazzalari NL and Atkins GJ: Evidence for the dysregulated
expression of TWIST1, TGFβ1 and SMAD3 in differentiating
osteoblasts from primary hip osteoarthritis patients.
Osteoarthritis Cartilage. 20:1357–1366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng W, Ying WZ, Aaron KJ and Sanders PW:
Transforming growth factor-β mediates endothelial dysfunction in
rats during high salt intake. Am J Physiol Renal Physiol.
309:F1018–F1025. 2015. View Article : Google Scholar : PubMed/NCBI
|