Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor
that occurs in epithelial cells of the nasopharynx and is closely
related to Epstein-Barr virus infection, with an annual incidence
rate of 10–50 out of 100,000 worldwide (1). It is rare in most regions of the
world, and its annual incidence rate is only 0.5–0.6 out of 100,000
in Europe and the United States. The 5-year overall survival of NPC
is 84% of non-metastatic cases with intensity-modulated radiation
therapy (IMRT) and optimal chemotherapy (2,3).
Recently, the phosphoinositide 3 kinase (PI3K)/AKT
signaling transduction pathway has attracted increasing attention
(4). Previous studies have
reported that the PI3K/AKT signaling pathway is important in the
signal transduction of various growth factors, and is closely
related to cellular physiological functions as well as the
incidence and development of certain tumors (5,6). The
products of PI3K3-phosphoinositide, that is 3,4-diphosphoinositide
and 3,4,5-inositol triphosphate, bind to the PH domain of AKT and
induce a conformational change and the subsequent phosphorylation
and activation of AKT, which migrates from the cytoplasm to the
cell membrane, where it directly or indirectly activates its
downstream molecule proteins, such as mammalian target of rapamycin
(mTOR), p70 ribosomal protein S6 kinase (p70S6K), matrix
metalloproteinase (MMP)-2, and nuclear factor-κB (NF-κB) and
insulin-like growth factor I receptor (IGF-IR) (7). The activation of mTOR and p70S6K
initiates protein translation to promote protein synthesis; the
activation of NF-κB promotes the transcription of
apoptosis-inhibiting genes and upregulates the mRNA and protein
expression of IGF-IR and MMP2, thus promoting the invasion and
metastasis of cancer cells (8).
Therefore, the PI3K/AKT signaling pathway may be considered to
serve an important role in cell proliferation, differentiation and
migration.
The NF-κB family of transcription factors promotes
tumorigenesis, and functional proteins encoded by NF-κB may promote
tumor growth; NF-κB may promote tumorigenesis by inhibiting
apoptosis (9). NF-κB is inactive
in patients with head and neck squamous carcinoma, and inhibiting
RelA can increase apoptosis in tumor cells (10). NF-κB may be an important indicator
in the early diagnosis and treatment of tumors (10).
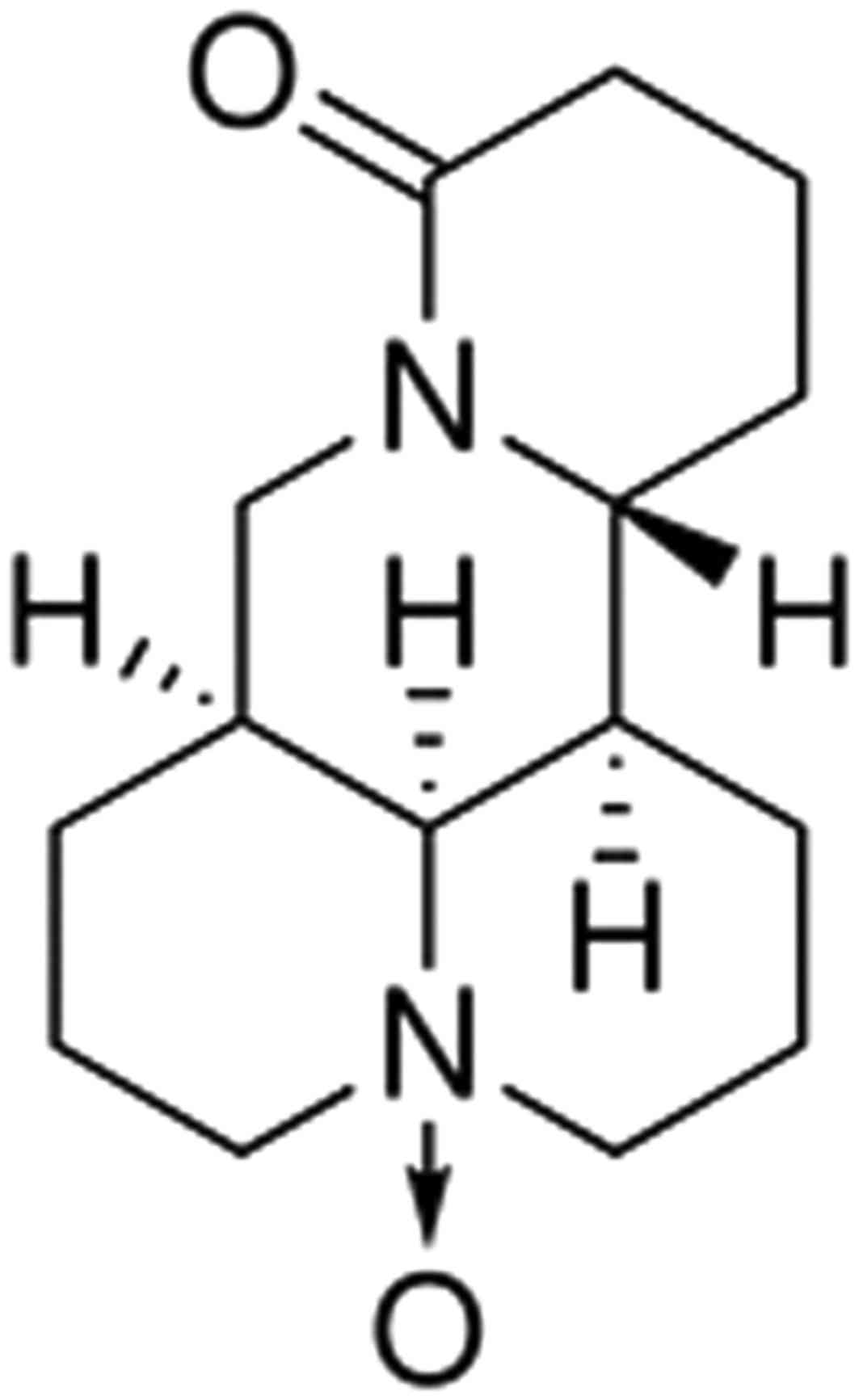
Oxymatrine is a compound (Fig. 1) extracted from radix sophorae
flavescentis that has been previously reported to exhibit
antitumoral effects, mainly by inhibiting the differentiation and
apoptosis of tumor cells, suppressing the proliferation and
metastasis of tumor cells, inhibiting the activity of telomerase,
suppressing tumor angiogenesis, inhibiting drug resistance of tumor
and promoting antitumor immune response of the host (11,12).
The present study investigated the anticancer effects of oxymatrine
on NPC cell death and the underlying molecular mechanisms of these
effects.
Materials and methods
Cell culture
The human NPC HK-1 cell line was obtained from the
Experimental Center of Capital Medical University (Beijing, China)
and cells were maintained in RPMI-1640 medium (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin in a humidified atmosphere
containing 5% CO2 at 37°C.
Cell proliferation assay
HK-1 cells were seeded (1,000 cells/well) into
96-well plates, incubated overnight and treated with oxymatrine (0,
2, 4, 6 and 8 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
for 1, 2 and 3 days at 37°C. MTT (10 ml; 5 mg/ml; Beyotime
Institute of Biotechnology, Haimen, China) was added and was
incubated in darkness for 4 h at 37°C. The media was removed and
dimethylsulfoxide was added to dissolve the formazan crystals for
20 min; the absorbance was measured using a FluoDia T70 microplate
reader (Photon Technology International, Inc.; Horiba, Ltd.,
Stanmore, UK) at a wavelength of 490 nm.
Flow cytometric analysis
HK-1 cells were seeded (1–2×105
cells/well) into 6-well plates, incubated overnight and treated
with oxymatrine (0, 4, 6 and 8 mg/ml) for 2 days. Cells were
centrifuged at 1,000 × g at 4°C for 10 min and washed using PBS
three times. Cells were stained using an Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) Cell Apoptosis
Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) according
to the manufacturer's protocols, and fluorescence was determined
using a FACSCalibur flow cytometer (BD Biosciences) and analyzed
using Flowjo version 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Caspase-3 and caspase-9
activities
HK-1 cells were seeded (1–2×105
cells/well) into 6-well plates, incubated overnight and treated
with oxymatrine (0, 4, 6 and 8 mg/ml) for 2 days at 37°C. Cell
protein extracts were prepared in radioimmunoprecipitation assay
(RIPA) buffer (Beyotime Institute of Biotechnology) for 15 min. The
protein concentrations were determined using the BCA Protein Assay
kit (Beyotime Institute of Biotechnology). Equal amounts of
proteins (50 µg per condition) were incubated with Caspase-3/9
activities kits (C1115 or C1157; Beyotime Institute of
Biotechnology) for 1–2 h at 37°C. Absorbance was measured using a
FluoDia T70 microplate reader (Photon Technology International,
Inc.; Horiba, Ltd.) at a wavelength of 405 nm and analyzed using
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA).
Western blot analysis
HK-1 cells were seeded (1–2×105
cells/well) into 6-well plates, incubated overnight and treated
with oxymatrine (0, 4, 6 and 8 mg/ml) for 2 days. Protein extracts
were prepared in RIPA for 15 min. Protein concentrations were
determined using the BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Equal amounts of proteins (50 µg) were separated by
8–10% SDS-PAGE and transferred to polyvinyl difluoride membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked in PBS
with 5% skimmed milk powder for 1 h at 37°C and 0.1% Tween-20 and
incubated with primary antibodies against p53 (sc-6243; 1:500;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Bax (sc-493;
1:500), cyclin D (sc-25764; 1:500), PI3K (sc-7174; 1:500),
phosphorylated (p)-AKT (sc-135650; 1:500), p-mTOR p-p70S6K
(sc-8416; 1:500), NF-κB (sc-109; 1:500) and GAPDH (sc-25778; 1:500;
all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight
at 4°C. Following washes with Tris-buffered saline and 0.1%
Tween-20, membranes were incubated with horseradish
peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody
(sc-2005 or sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1
h at 37°C and visualized using the Enhanced Chemiluminescence
Detection reagent (BD Biosciences) and analyzed using ImageLab
version 3.0 (BD Biosciences).
Statistical analysis
Data are expressed as the mean ± standard deviation
(n=3), and statistical analysis was carried out using SPSS version
17.0 (SPSS, Inc.). Statistically significant differences between
groups were determined by one-way analysis of variance and Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Oxymatrine inhibits NPC cell
proliferation
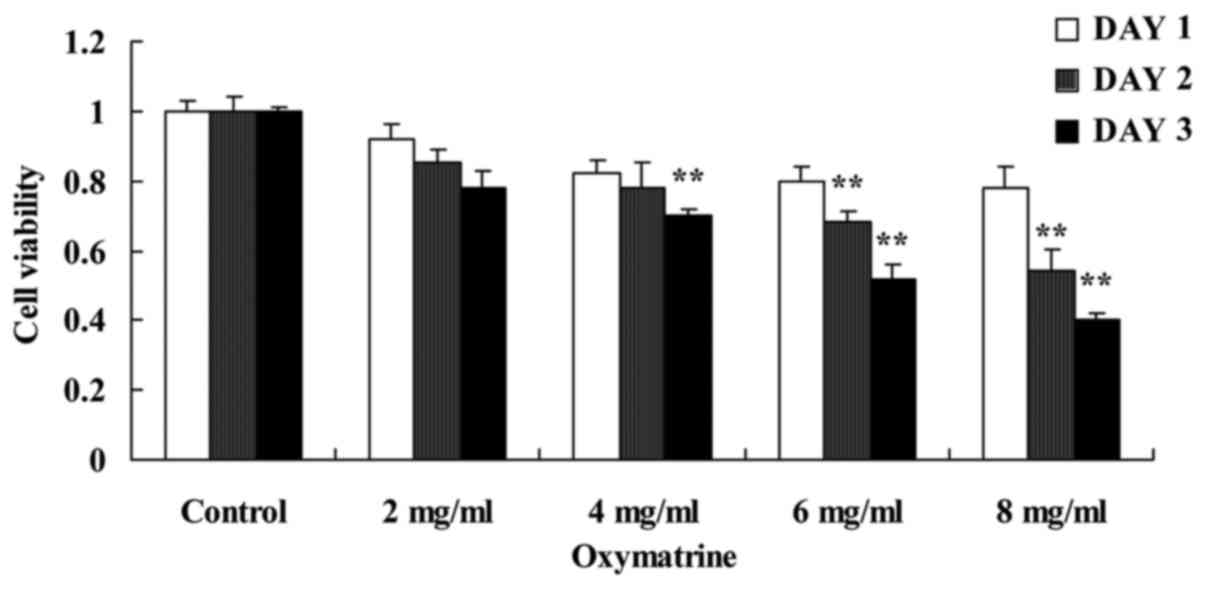
To investigate the putative anticancer effects of
oxymatrine on NPC cell growth, HK-1 cells were treated with various
concentrations of oxymatrine. Results from the MTT assays suggested
that oxymatrine inhibited cell proliferation of HK-1 cell in a
time- and dose-dependent manner (Fig.
2). Treatment with 4, 6 and 8 mg/ml of oxymatrine significantly
inhibited cell proliferation of HK-1 cells, compared with the
untreated Control group (Fig.
2).
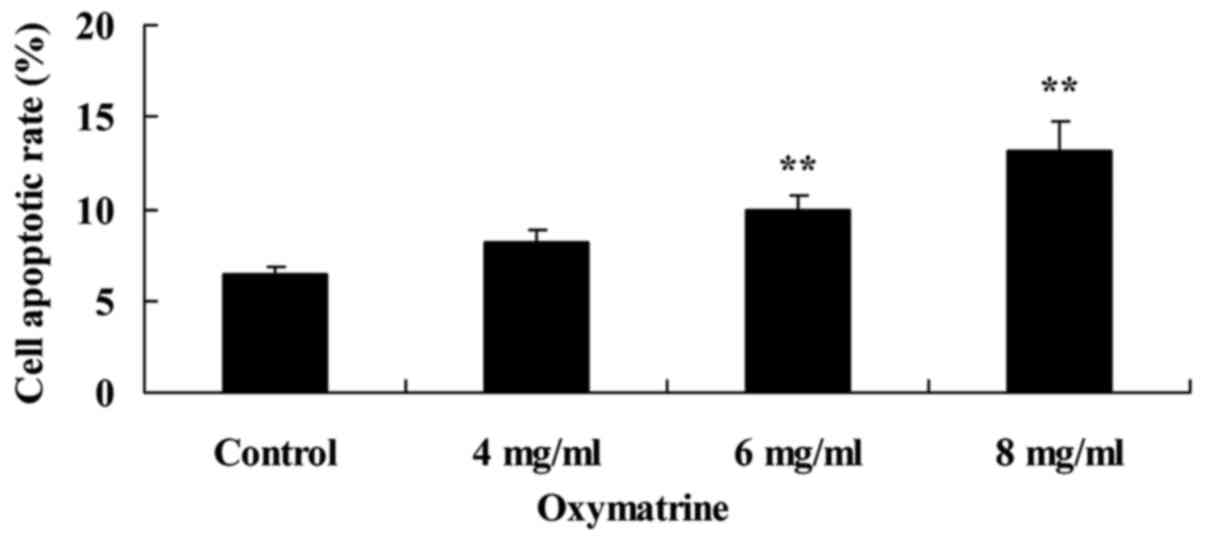
Oxymatrine induces NPC cell
apoptosis
Oxymatrine-induced NPC cell apoptosis was measured
using Annexin V-FITC/PI double staining. The results demonstrated
that 6 and 8 mg/ml of oxymatrine treatment significantly induced
NPC HK-1 cell apoptosis, compared with cells in the untreated
Control group (Fig. 3).
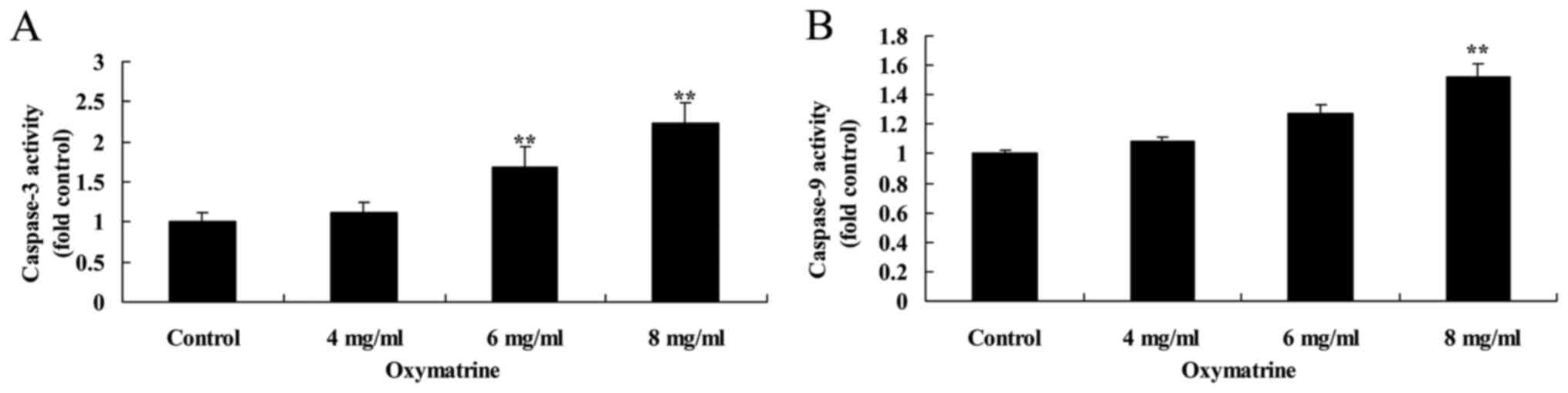
Oxymatrine induces the activity of
caspase-3 and caspase-9 in NPC cells
The effects of oxymatrine treatment on inducing the
activities of caspase-3 and caspase-9 in NPC HK-1 cells were
determined. Oxymatrine exposure increased the activities of both
caspase-3 and caspase-9, compared with the Control group (Fig. 4).
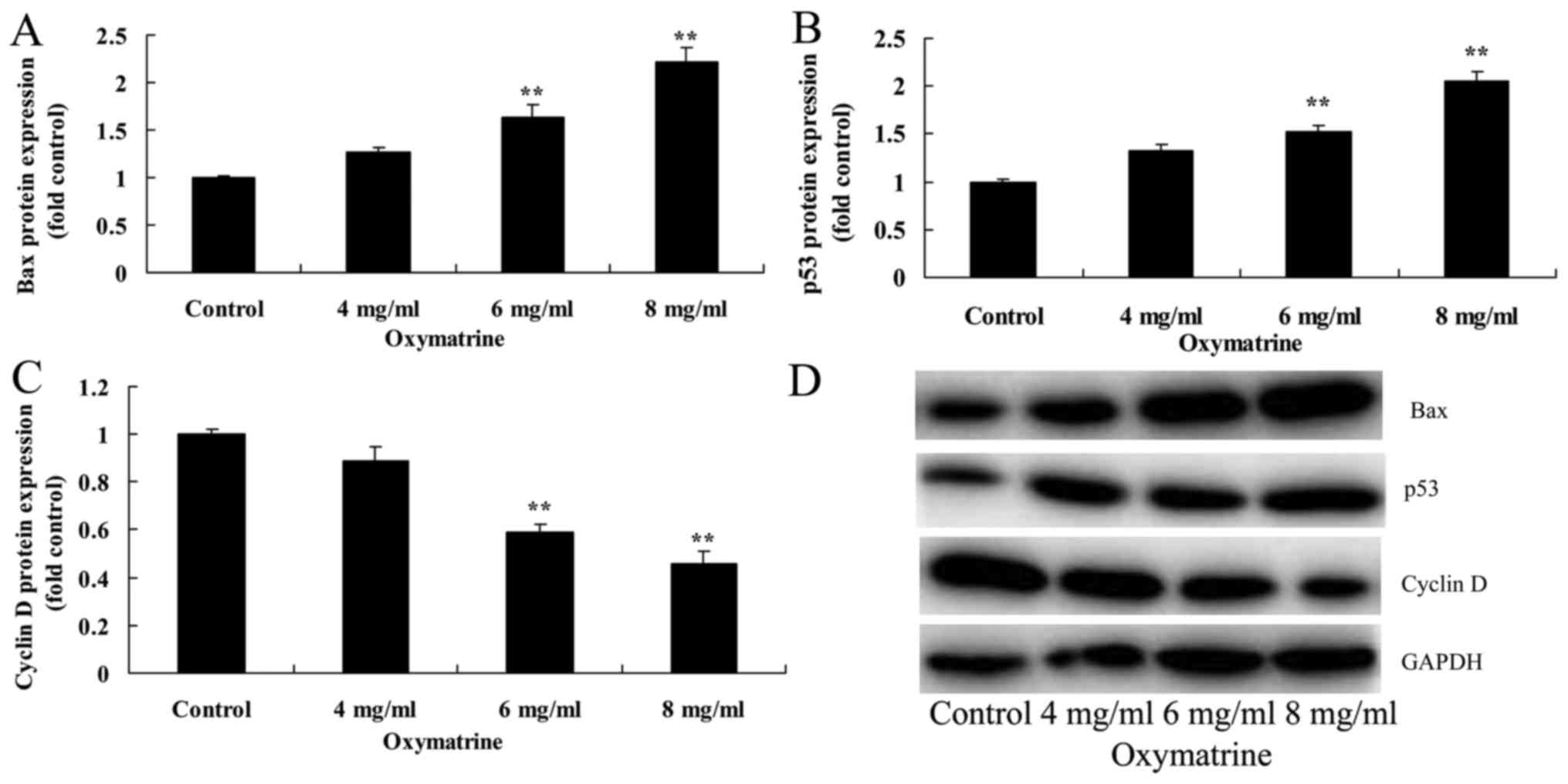
Oxymatrine affects the protein
expression levels of Bax, p53 and cyclin D in NPC cells
The effects of oxymatrine on Bax, p53 and cyclin D
protein expression of NPC HK-1 cells were examined. Compared with
cells in the untreated Control group, oxymatrine treatment at 6 and
8 mg/ml significantly induced the protein expression of Bax and
p53, and suppressed cyclin D protein expression (Fig. 5).
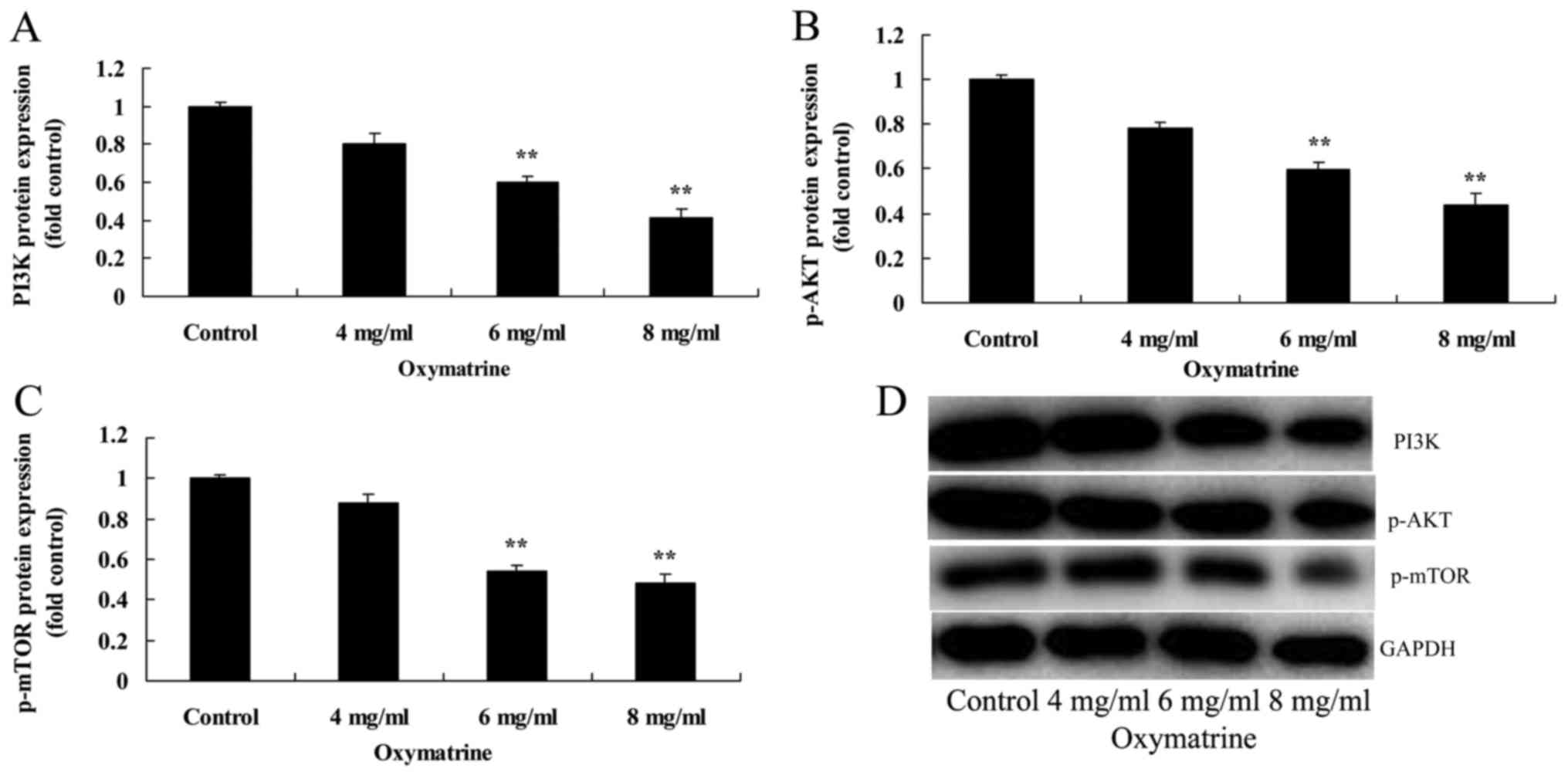
Oxymatrine suppressed the protein
expression levels of PI3K, p-AKT and p-mTOR in NPC cells
To investigate the possible molecular mechanisms
underlying oxymatrine-induced NPC cell apoptosis, PI3K/AKT
signaling was analyzed following treatment with different
concentrations of oxymatrine. Western blotting results suggested
that oxymatrine treatment at 6 and 8 mg/ml significantly suppressed
the protein expressions of PI3K, p-AKT and p-mTOR of HK-1 cell,
compared with the Control group (Fig.
6).
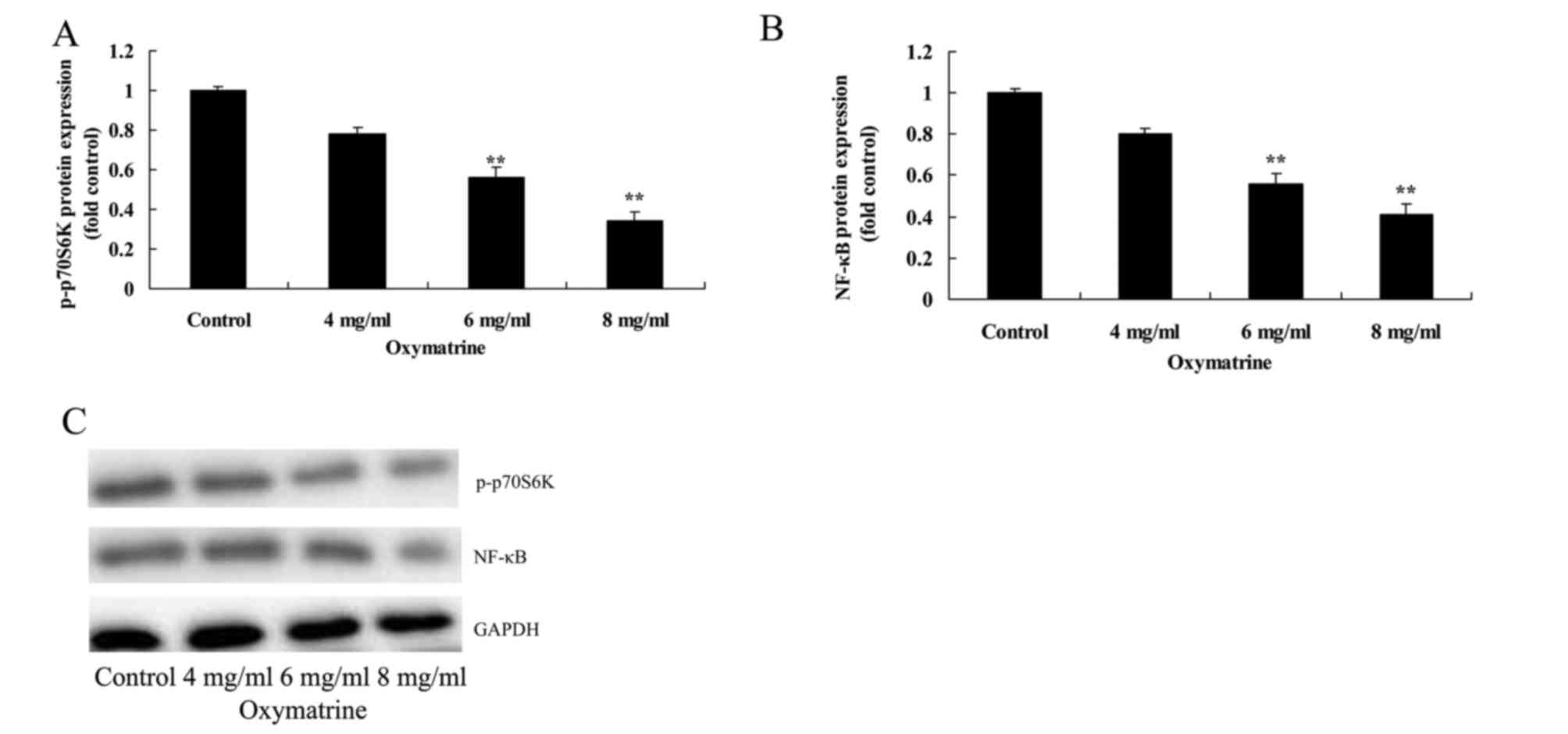
Oxymatrine suppresses the protein
expression levels of p-p70S6K and NF-κB in NPC cells
To further understand the role of p70S6K in
oxymatrine-induced nasopharynx cancer cell apoptosis, p-p70S6K and
NF-κB protein expression levels were analyzed in NPC HK-1 cells
treated with various concentrations of oxymatrine. Compared with
cells in the untreated Control group, oxymatrine treatment at 6 and
8 mg/ml significantly suppressed the protein expression levels of
p-p70S6K and NF-κB (Fig. 7).
Discussion
NPC is a common malignant tumor in southern China,
and is characterized by high racial susceptibility, regional
aggregation and family tendency (13). The cause of NPC remains unknown,
but it is currently considered to be related to genetic factors,
environmental factors and viral infection (14). The clinical symptoms of NPC are
complicated and diverse, are easy to be misdiagnosed or may by
ignored by patients, and include clinical characteristics such as
high degree of malignancy, early cervical lymph node metastasis and
distant metastasis (15). Data
from the present study demonstrated that oxymatrine treatment
significantly inhibited cell proliferation and induced NPC cell
apoptosis in HK-1 cells.
Bcl2 is an apoptosis-regulator that has been
thoroughly studied, and its change in expression not only affects
normal apoptosis of damaged DNA cells and abnormal cells, but also
the apoptosis of tumor cells (16). Bcl2 proteins are highly expressed
in NPC tissues and atypical hyperplasia epithelium, which suggested
that Bcl2 proteins may serve an important role in the early stage
of NPC, and the overexpression of Bcl2 may also be related to the
inhibition of the apoptosis of NPC (17). The present study demonstrated that
oxymatrine increased the expression levels of caspase-3 and
caspase-9 and Bax protein expression of HK-1 cell.
p53 has been reported to have at close relationship
with human tumors, and functions to block cell cycle, inhibit cell
proliferation, induce cell differentiation, initiate apoptosis and
maintain the stability of genome (16). In the present study, higher
concentrations of oxymatrine treatment (6 and 8 mg/ml)
significantly induced the protein expression of p53 in NPC HK-1
cells. A previous study suggested that oxymatrine inhibits prostate
cancer cell proliferation through p53 and Bax/Bcl2 expression
(18).
Cyclin D1 (also known as Bcl-1) is encoded by CCND1,
which is located on chromosome 11q13, comprises 5 exons and 4
introns, and is 15 kb long (19).
Cyclin D1 is a nuclear protein that serves an important role in the
regulation of the G1 stage of the cell cycle. Overexpression of
cyclin D1 may lead to the disorder of regulation and detection
point at G1/S stage, thus accelerating cell cycle progression and
inducing irreversible abnormal proliferation, which results in
disordered cell proliferation and regulation (20). As a regulatory factor of cell
cycle, cyclin D1 has a close relationship with cell proliferation,
it participates in the regulation of cell cycle, and reflects
proliferative activity of cells to a certain extent, and the
proliferative activity of tumor cells is one of crucial factors
affecting the radiosensitivity of NPC (21). Overall, the results of the present
study suggested that oxymatrine significantly suppressed the
protein expression of cyclin D of HK-1 cell. Another study reported
that oxymatrine enhances the antitumor activity of oxaliplatin on
colon cancer cell growth by downregulating cyclin D expression and
the PI3K/AKT/mTOR pathway (22).
Previous studies have indicated that the biological
function of the PI3K/AKT signaling pathway is closely related to
the survival of tumor cells, glucose metabolism and the incidence
and development of tumors (5,23).
There are extensive and in-depth studies on the effects of PI3K/AKT
signaling in the occurrence and development, as well as resistance
to radiotherapy, chemotherapy and drugs, of malignant tumors
(5,23). One previous study reported that AKT
was highly expressed and the activity of AKT kinase was increased
in ovarian cancer tissues, which was associated with the biological
behaviors of malignant tumors (24). Other studies have demonstrated that
the incidence and development of prostate cancer, cervical cancer,
non-small cell lung cancer and other malignant tumors are closely
related to the activation of the PI3K/AKT signaling pathway
(5,25). In the present study, oxymatrine
treatment significantly reduced the protein expression levels of
PI3K, p-AKT, p-mTOR and p-p70S6K in NPC HK-1 cells.
NF-κB belongs to a family of tumorigenesis proteins,
and several functional proteins encoded by NF-κB may promote tumor
growth (10). The cyclin D1
promoter region contains two NF-κB binding sites, and the
activation of NF-κB promotes conversion function of the expression
of cyclin D1 and G1/S, which accelerates cell cycle progression
(26). NF-κB may also promote
tumorigenesis by inhibiting apoptosis (27). In vitro experiments
demonstrated that specifically inhibiting the activity of NF-κB was
able to block the cell cycle in glioma cell lines and induce the
apoptosis of tumor cells, whereas in vivo experiments
confirmed that NF-κB inhibited the growth of glioma cells in rats
(27). The results of the present
study revealed that oxymatrine treatment suppressed the protein
expression of NF-κB in NPC HK-1 cells. A previous study
demonstrated that oxymatrine inhibited epithelial-mesenchymal
transition of colorectal cancer cells through the regulation of
NF-κB signaling (11).
In conclusion, the results of the present study
demonstrated that oxymatrine significantly inhibited cell
proliferation, induced NPC cell apoptosis, increased caspase-3 and
caspase-9 activities, promoted p53 and Bax protein expression and
suppressed cyclin D protein expression in NPC HK-1 cells through
the regulation of PI3K/AKT and NF-κB pathway. Therefore, these
findings may provide a novel approach for the development of
oxymatrine, which is derived from TCM herb, as a potential NPC
therapy.
References
|
1
|
Casanova M, Özyar E, Patte C, Orbach D,
Ferrari A, Veyrat-Follet C, Errihani H, Pan J, Zhang L, Shen L, et
al: International randomized phase 2 study on the addition of
docetaxel to the combination of cisplatin and 5-fluorouracil in the
induction treatment for nasopharyngeal carcinoma in children and
adolescents. Cancer Chemother Pharmacol. 77:289–298. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP,
Tang LL, Guo Y, Lin AH, Zeng XF and Ma J: Preliminary results of a
prospective randomized trial comparing concurrent chemoradiotherapy
plus adjuvant chemotherapy with radiotherapy alone in patients with
locoregionally advanced nasopharyngeal carcinoma in endemic regions
of china. Int J Radiat Oncol Biol Phys. 71:1356–1364. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li JG, Yuan X, Zhang LL, Tang YQ, Liu L,
Chen XD, Gong XC, Wan GF, Liao YL, Ye JM and Ao F: A randomized
clinical trial comparing prophylactic upper versus whole-neck
irradiation in the treatment of patients with node-negative
nasopharyngeal carcinoma. Cancer. 119:3170–3176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q,
Luo X, Chen Y, Deng X, Liang Z, et al: miR-3188 regulates
nasopharyngeal carcinoma proliferation and chemosensitivity through
a FOXO1-modulated positive feedback loop with
mTOR-p-PI3K/AKT-c-JUN. Nat Commun. 7:113092016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng D, Zhu G, Liao S, Yi W, Luo G, He J,
Pei Z, Li G and Zhou Y: Dysregulation of the PI3K/Akt signaling
pathway affects cell cycle and apoptosis of side population cells
in nasopharyngeal carcinoma. Oncol Lett. 10:182–188.
2015.PubMed/NCBI
|
|
6
|
Viana LR and Gomes-Marcondes MC: A
leucine-rich diet modulates the tumor-induced down-regulation of
the MAPK/ERK and PI3K/Akt/mTOR signaling pathways and maintains the
expression of the ubiquitin-proteasome pathway in the placental
tissue of NMRI mice. Biol Reprod. 92:492015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong CH, Loong HH, Hui CW, Lau CP, Hui EP,
Ma BB and Chan AT: Preclinical evaluation of the PI3K-mTOR dual
inhibitor PF-04691502 as a novel therapeutic drug in nasopharyngeal
carcinoma. Invest New Drugs. 31:1399–1408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tasioudi KE, Sakellariou S, Levidou G,
Theodorou D, Michalopoulos NV, Patsouris E, Korkolopoulou P and
Saetta AA: Immunohistochemical and molecular analysis of
PI3K/AKT/mTOR pathway in esophageal carcinoma. APMIS. 123:639–647.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin SY, Kim CG, Jung YJ, Lim Y and Lee
YH: The UPR inducer DPP23 inhibits the metastatic potential of
MDA-MB-231 human breast cancer cells by targeting the
Akt-IKK-NF-κB-MMP-9 axis. Sci Rep. 6:341342016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng H, Dai W, Cheung AK, Ko JM, Kan R,
Wong BW, Leong MM, Deng M, Kwok TC, Chan JY, et al: Whole-exome
sequencing identifies multiple loss-of-function mutations of NF-κB
pathway regulators in nasopharyngeal carcinoma. Proc Natl Acad Sci
USA. 113:pp. 11283–11288. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang L and Huang J: Oxymatrine inhibits
epithelial-mesenchymal transition through regulation of NF-κB
signaling in colorectal cancer cells. Oncol Rep. 36:1333–1338.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin B, Li D and Zhang L: Oxymatrine
mediates Bax and Bcl-2 expression in human breast cancer MCF-7
cells. Pharmazie. 71:154–157. 2016.PubMed/NCBI
|
|
13
|
Peng PJ, Ou XQ, Chen ZB, Liao H, Peng YL,
Wang SY, Zhang HY and Lin Z: Multicenter phase II study of
capecitabine combined with nedaplatin for recurrent and metastatic
nasopharyngeal carcinoma patients after failure of cisplatin-based
chemotherapy. Cancer Chemother Pharmacol. 72:323–328. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Sun Y, Liang SB, Zong JF, Li WF,
Chen M, Chen L, Mao YP, Tang LL, Guo Y, et al: Progress report of a
randomized trial comparing long-term survival and late toxicity of
concurrent chemoradiotherapy with adjuvant chemotherapy versus
radiotherapy alone in patients with stage III to IVB nasopharyngeal
carcinoma from endemic regions of China. Cancer. 119:2230–2238.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhao L, Huang P, Wu J, Wang F,
Huang Y and Zhang L: Open-label, single-arm phase II study of
pemetrexed in the treatment of patients with recurrent or
metastatic nasopharyngeal carcinoma who have had prior
platinum-based chemotherapy. Cancer Chemother Pharmacol.
70:611–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bourouba M, Benyelles-Boufennara A, Terki
N, Baraka-Kerboua E, Bouzid K and Touil-Boukoffa C: Epidermal
growth factor receptor (EGFR) abundance correlates with p53 and
Bcl-2 accumulation and patient age in a small cohort of North
African nasopharyngeal carcinoma patients. Eur Cytokine Netw.
22:38–44. 2011.PubMed/NCBI
|
|
17
|
Kao CL, Cho J, Lee YZ, Cheng YB, Chien CY,
Hwang CF, Hong YR, Tseng CN and Cho CL: Ethanolic extracts of
pluchea indica induce apoptosis and antiproliferation effects in
human nasopharyngeal carcinoma cells. Molecules. 20:11508–11523.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu C, Huang W, Guo Y, Xia P, Sun X, Pan X
and Hu W: Oxymatrine inhibits the proliferation of prostate cancer
cells in vitro and in vivo. Mol Med Rep. 11:4129–4134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu M, Cheung CC, Chow C, Lun SW, Cheung ST
and Lo KW: Overexpression of PIN1 enhances cancer growth and
aggressiveness with cyclin D1 induction in EBV-associated
nasopharyngeal carcinoma. PLoS One. 11:e01568332016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao D, Wu Y, Pu X, Chen H, Luo S, Li B,
Ding C, Huang GL and He Z: Cyclin D1 G870A polymorphism and risk of
nasopharyngeal carcinoma: A case-control study and meta-analysis.
PLoS One. 9:e1132992014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng G, Cao RB, Li YH, Zou ZW, Huang J and
Ding Q: Alterations of cell cycle control proteins SHP-1/2, p16,
CDK4 and cyclin D1 in radioresistant nasopharyngeal carcinoma
cells. Mol Med Rep. 10:1709–1716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Bi T, Wang Z, Wu G, Qian L, Gao Q
and Shen G: Oxymatrine synergistically enhances antitumor activity
of oxaliplatin in colon carcinoma through PI3K/AKT/mTOR pathway.
Apoptosis. 21:1398–1407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu X, Zhen Y, Yang H, Wang H, Zhou Y, Wang
E, Marincola FM, Mai C, Chen Y, Wei H, et al: Loss of connective
tissue growth factor as an unfavorable prognosis factor activates
miR-18b by PI3K/AKT/C-Jun and C-Myc and promotes cell growth in
nasopharyngeal carcinoma. Cell Death Dis. 4:e6342013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuen JW, Chung GT, Lun SW, Cheung CC, To
KF and Lo KW: Epigenetic inactivation of inositol polyphosphate
4-phosphatase B (INPP4B), a regulator of PI3K/AKT signaling pathway
in EBV-associated nasopharyngeal carcinoma. PLoS One.
9:e1051632014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J: Roles of the PI3K/Akt pathway in
Epstein-Barr virus-induced cancers and therapeutic implications.
World J Virol. 1:154–161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu DD, Zhang J, Deng W, Yip YL, Lung HL,
Tsang CM, Law WT, Yang J, Lau VM, Shuen WH, et al: Significance of
NF-κB activation in immortalization of nasopharyngeal epithelial
cells. Int J Cancer. 138:1175–1185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin ML, Lu YC, Chung JG, Wang SG, Lin HT,
Kang SE, Tang CH, Ko JL and Chen SS: Down-regulation of MMP-2
through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin
leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol
Carcinog. 49:783–797. 2010.PubMed/NCBI
|